Experimental Designs and Statistical Analyses for Rootstock Trials
Abstract
:1. Introduction
2. Development of Modern Statistical Methods for Agricultural Research
2.1. Early Estimates of Variation
2.2. Annual Crops
2.3. Fruit Trees
2.4. Common Experimental Designs
2.5. Using Additional Information
2.6. Advances in Statistical Software and Data Analysis
2.6.1. Statistical Models Commonly Used in Agricultural Research
2.6.2. Statistical Software Development
3. Rootstock Classification, Introduction, and Evaluation
3.1. Apple Rootstock Classification at East Malling
3.2. Development of Malling Merton Rootstocks
3.3. Error-Controlling Designs for European Rootstock Trials
3.4. Experimental Designs in North America
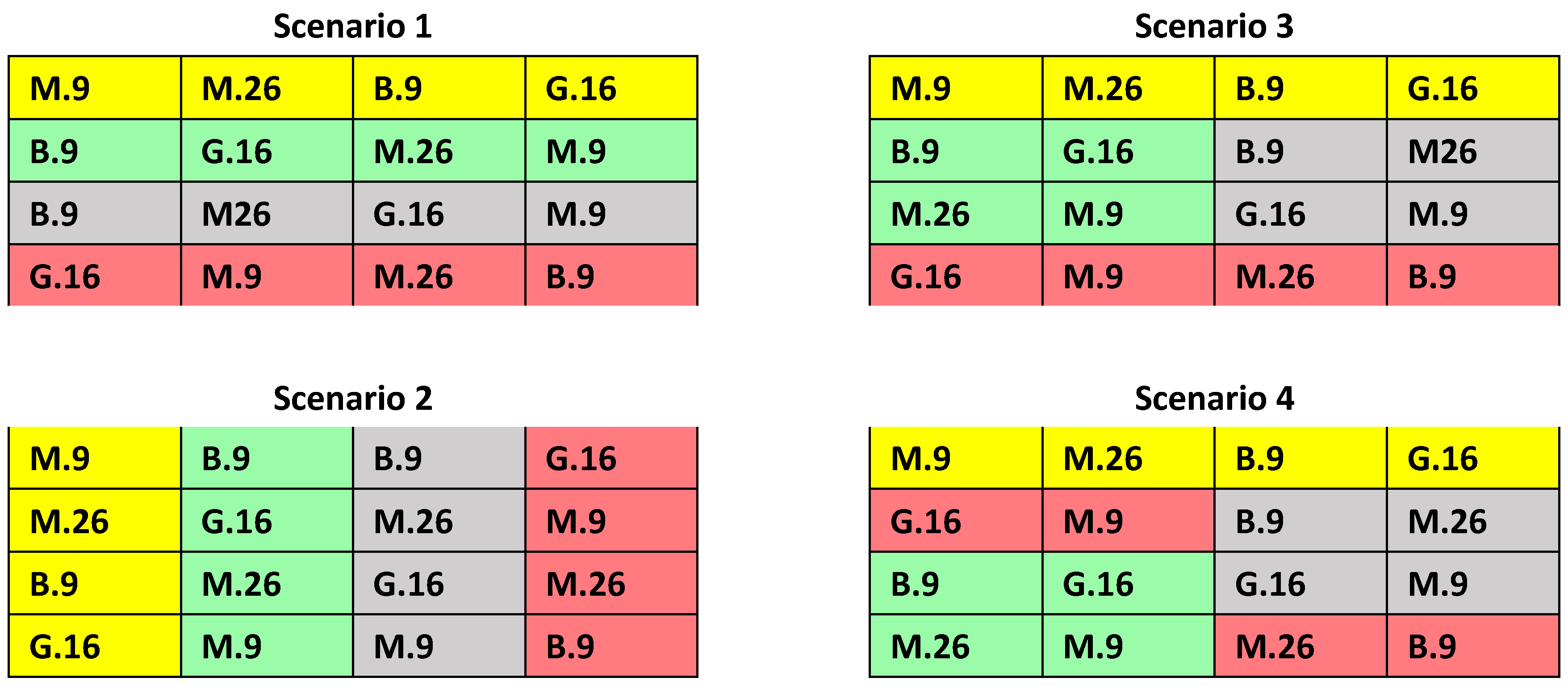
3.5. Factorial Experiments
4. Analysis of Fruit and Tree Measurements
4.1. Field Measurements
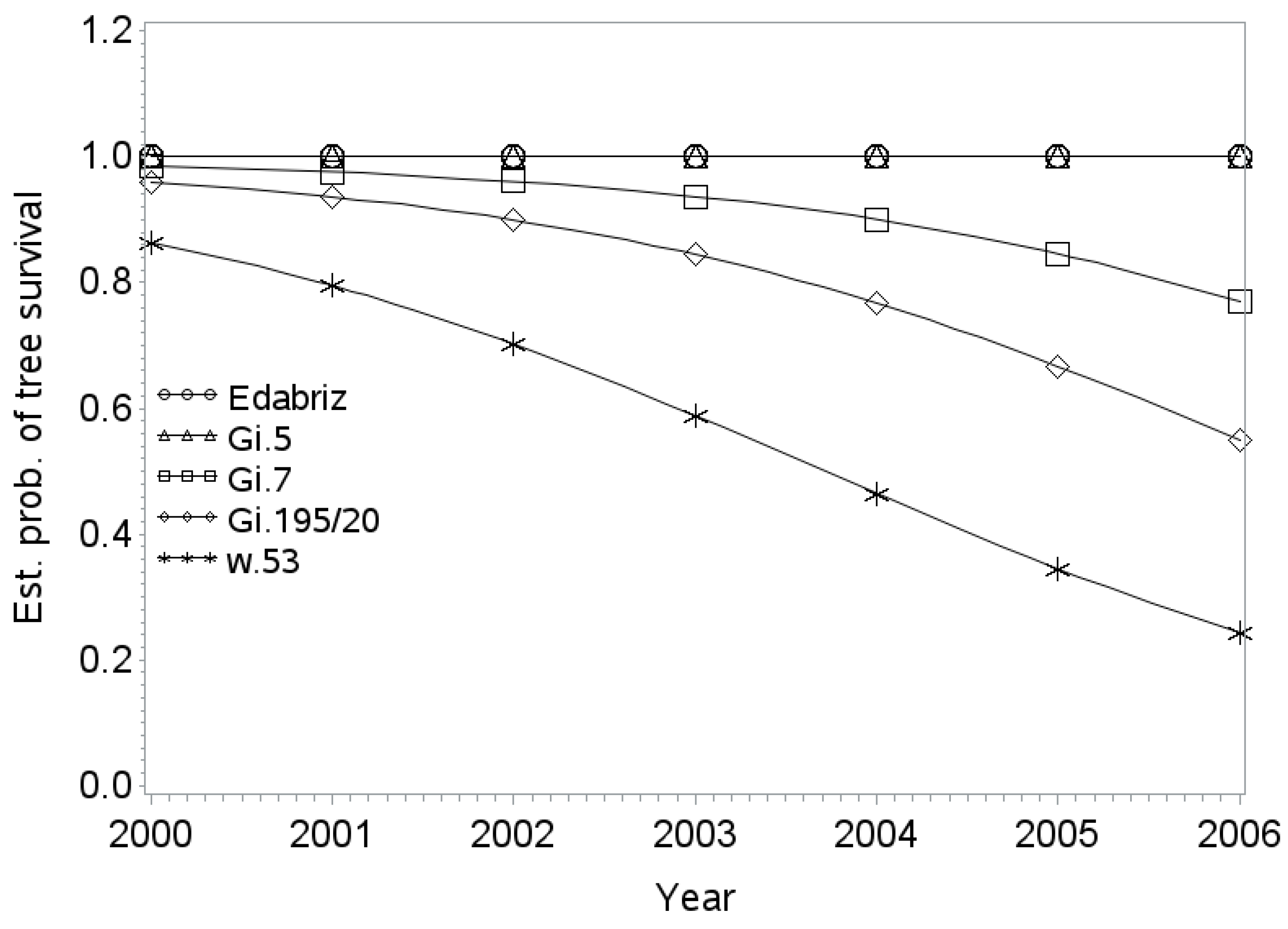
Tree Survival
4.2. Tree Size: Most Researchers Record Tree Height and Spread Each Year
4.3. Trunk Cross-Sectional Area (TCSA)
4.3.1. Accounting for Initial Tree Size
4.3.2. Accounting for Cropping
4.3.3. Accounting for Correlated Errors
4.3.4. Yield and Fruit Weight
4.3.5. Yield Efficiency and Crop Density
4.3.6. Root Suckers and Burrknots
4.3.7. Biennial Bearing
4.4. Evaluating Fruit Maturity and Quality
4.5. Duration of the Trial
5. Designing Future Rootstock Trials
5.1. Choice of Experimental Design: CRD vs. RCBD
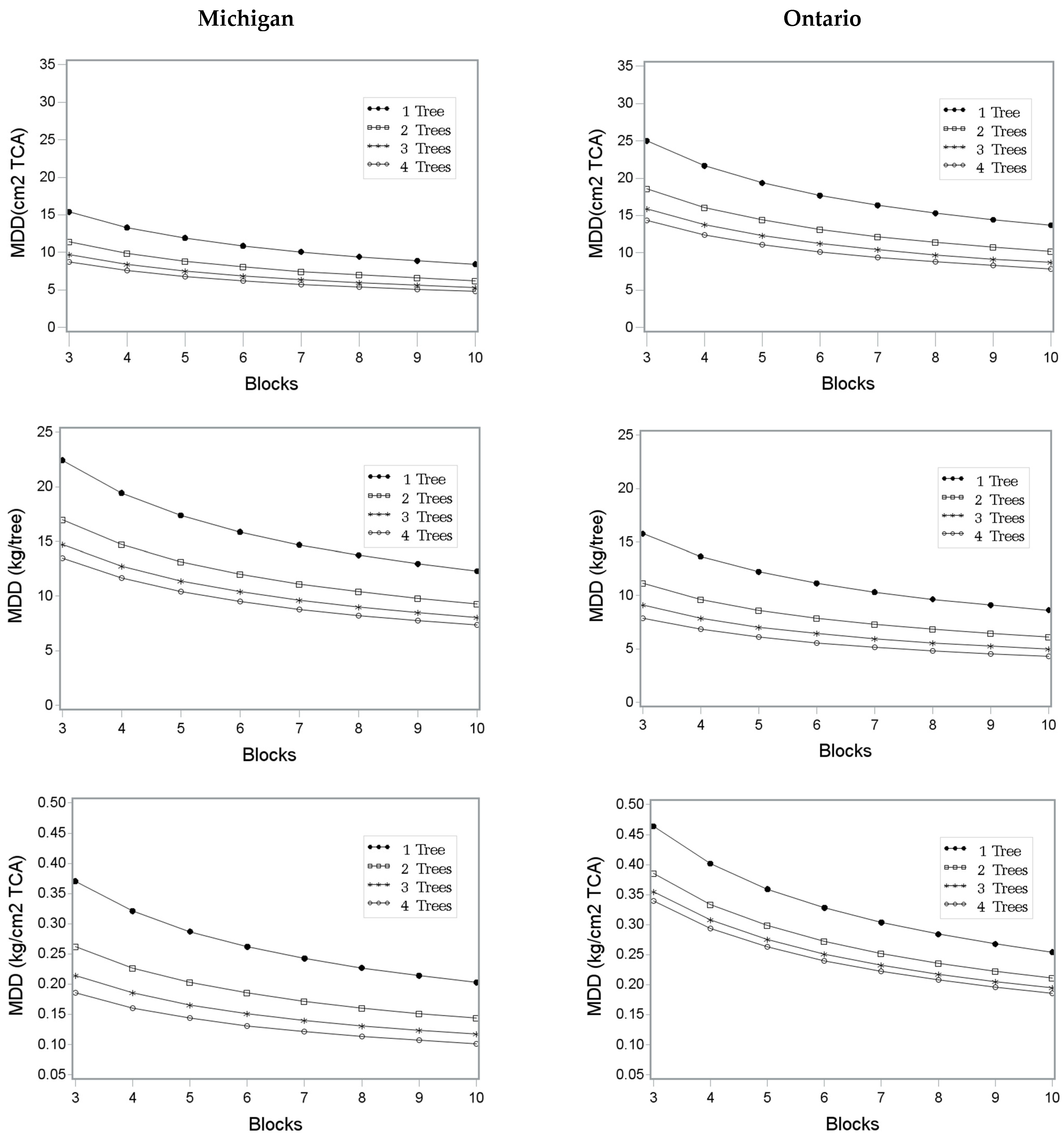
5.2. Sample Size Estimates
6. Multilocation Trials
6.1. Factorial Structure Ignored
6.2. Physical Slicing the Data
6.3. Slicing without Separating the Data
6.4. Stability Analysis
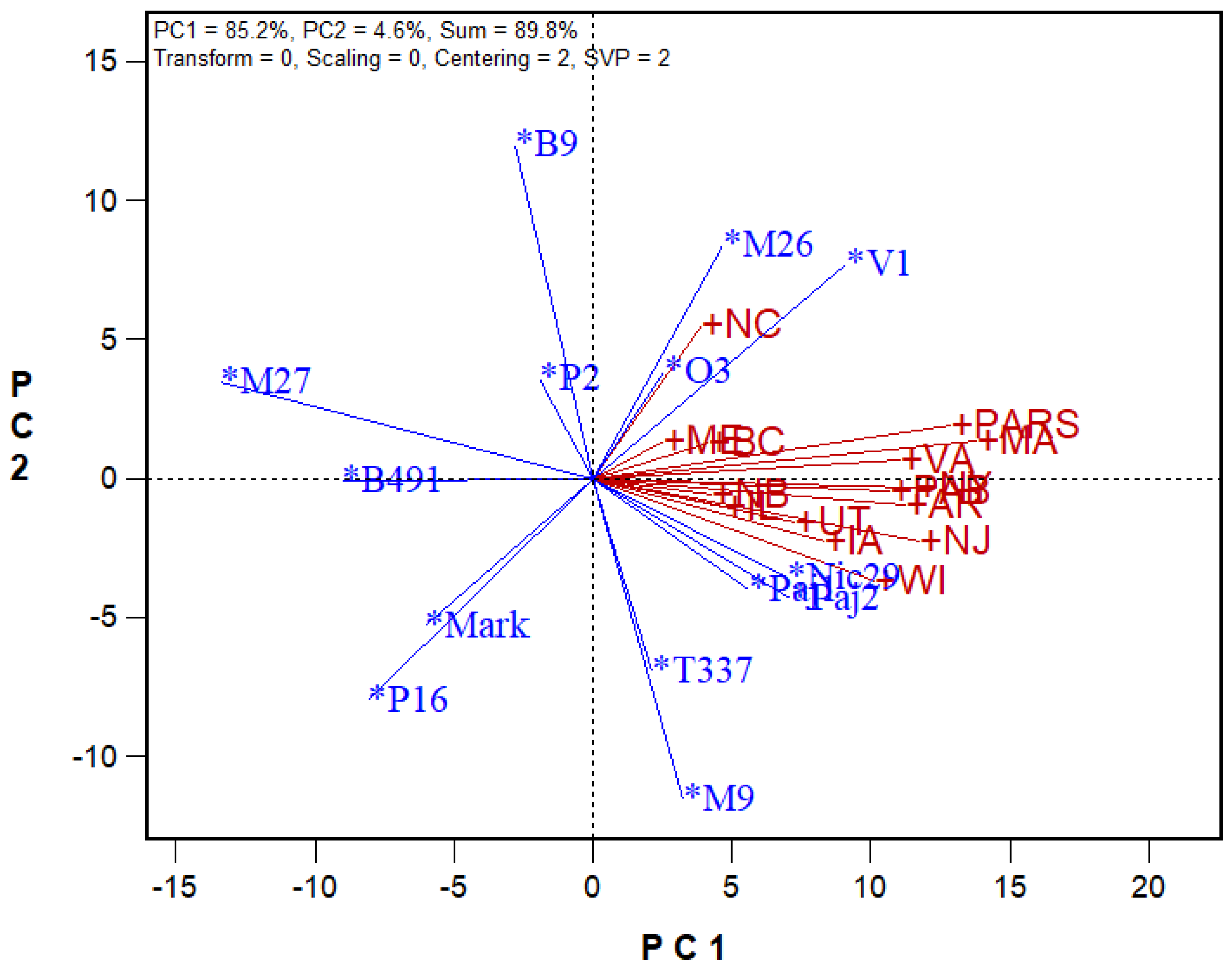
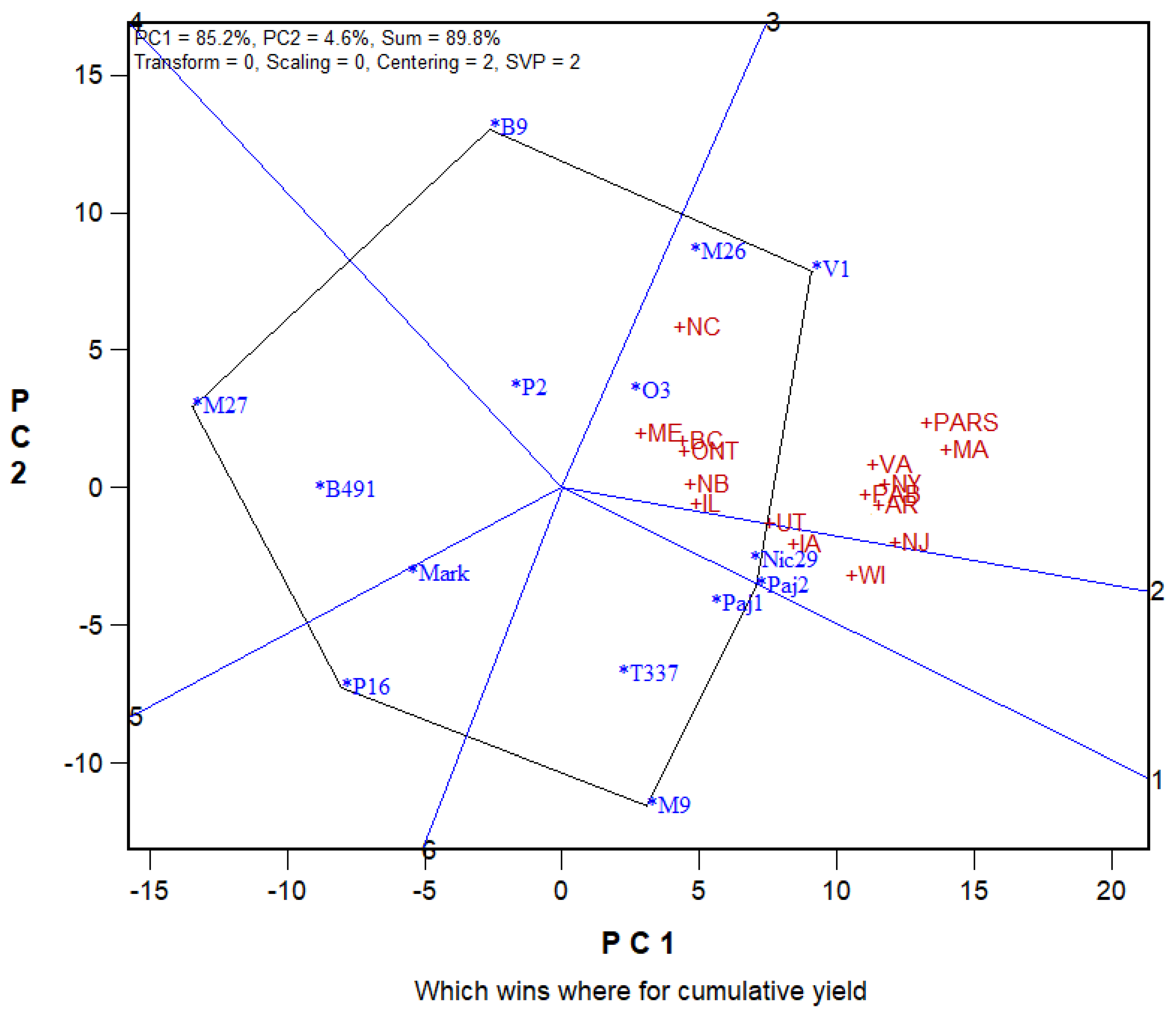
6.5. Genotype-Genotype × Environment Biplot (GGE Biplots)
7. Final Suggestions
Funding
Conflicts of Interest
References
- International Fruit Tree Association. Available online: https://ifruittree.org/ifta-history/ (accessed on 5 September 2024).
- NC-140. Growth and production of ‘Starkspur Supreme Delicious’ on 9 rootstocks in the NC-140 cooperative planting. Fruit Var. J. 1987, 41, 31–39. [Google Scholar]
- Cowgill Jr, W.P.; Autio, W.R.; Hoover, E.E.; Marini, R.P.; Domoto, P.A. NC-140 multi-state research project: Improving economic and environmental sustainability in tree-fruit production through changes in rootstock use. J. Amer. Pomol. Soc. 2017, 71, 34–46. [Google Scholar]
- NC-140. Performance of the NC-140 cooperative apple rootstock planting: II. A 10-year summary of TCA, yield, and yield efficiency at 31 sites. Fruit Var. J. 1996, 50, 225–235. [Google Scholar]
- Perry, R.; Reighard, G.; Ferree, D.; Barden, J.; Beckman, T.; Brown, G.; Cummins, J.; Durner, E.; Greene, G.; Johnson, S.; et al. Performance of the 1984 NC-140 cooperative peach rootstock planting. J. Amer. Pomol. Soc. 2000, 54, 6–10. [Google Scholar]
- Marini, R.P.; Barritt, B.H.; Barden, J.A.; Cline, J. Performance of ‘Gala’ apple on eight dwarf rootstocks: Ten-year summary of the 1990 NC-140 rootstocks trial. J. Amer. Pomol. Soc. 2001, 55, 197–204. [Google Scholar]
- Marini, R.P.; Barritt, B.H.; Barden, J.A.; Cline, J.; Hoover, E.E.; Granger, R.L.; Kushad, M.M.; Parker, M.; Perry, R.L.; Robinson, T.; et al. Performance of Ten Apple Orchard Systems: Ten -Year Summary of the 1990 NC-140 Systems Trial. J. Amer. Pom. Soc. 2001, 55, 222–238. [Google Scholar]
- Schneider, G.W.; Chaplin, C.E.; Martin, D.C. Effects of apple rootstock, tree spacing, and cultivar on fruit and tree size, yield, and foliar mineral composition. J. Amer. Soc. Hortic. Sci. 1987, 103, 230–232. [Google Scholar] [CrossRef]
- Marini, R.P.; Autio, W.R.; Black, B.; Cline, J.; Crassweller, R.M.; Domoto, P.A.; Hampson, C.; Moran, R.; Quezada, R.A.; Robinson, T.; et al. The influence of crop density on annual trunk growth of ‘Golden Delicious’ apple trees on three rootstocks at 11 locations. J. Amer. Pomol. Soc. 2012, 66, 183–195. [Google Scholar]
- Meland, M. Performance of six European plum cultivars on four plum rootstocks growing in a northern climate. Acta Agric. Scand. Sect. B Soil Plant Sci. 2010, 60, 381–387. [Google Scholar] [CrossRef]
- Boyce, B.R.; Hopp, R.J. Semidwarf Apple Trees: Performance of Several Rootstock-Interstock Combinations; CABI: Egham, UK, 1963; p. 633. [Google Scholar]
- Ystaas, J.; Frøynes, O. Performance of five plum rootstocks over 17 years to five commercial important plum cultivars in Norway. Nor. J. Agric. Sci. 1993, 7, 267–274. Available online: https://scholar.google.com/scholar?hl=en&as_sdt=0%2C39&q=ystaas+performance+of+five+plum+rootstocks&btnG= (accessed on 8 May 2024).
- Bielsa, B.; Rubio-Cabetas, M.J.; Felipe, A.J.; Gómez-Aparisi, J.; Socias, R. Rootstock Trial of Eight GxN Interspecific Hybrids in Almond. CIHEAM. 2016. Available online: https://citarea.cita-aragon.es/citarea/bitstream/10532/3585/1/2017_016.pdf (accessed on 30 November 2023).
- Kviklys, D.; Kvikliene, N.; Bite, A.; Lepsis, J.; Univer, T.; Univer, N.; Uselis, N.; Lanauskas, J.; Buskiene, L. Baltic fruit rootstock studies: Evaluation of 12 apple rootstocks in North-East Europe. Hort. Sci. 2012, 39, 1–7. [Google Scholar] [CrossRef]
- Lanauskas, J.; Uselis, N.; Kviklys, D.; Kvikliene, N.; Buskiene, L. Rootstock effect on the performance of sweet cherry. Hortic. Sci. 2012, 39, 55–60. [Google Scholar] [CrossRef]
- Layne, R.E.C. Prunus rootstock affect long-term orchard performance of ‘Redhaven’ peach on Brookston clay loam. HortScience 1994, 29, 167–171. [Google Scholar] [CrossRef]
- Reighard, G.; Bridges, W.; Archbold, D.; Atucha, A.; Autio, W.; Beckman, T.; Black, B.; Chavez, D.J.; Coneva, E.; Day, K.; et al. Nine-year rootstock performance of the NC-140 ‘Redhaven’ peach trial across 13 states. J. Amer. Pomol. Soc. 2020, 74, 45–56. [Google Scholar]
- Mestre, L.; Reig, G.; Betran, J.A.; Moreno, M. Influence of plum rootstocks on agronomic performance, leaf mineral nutrition and fruit quality of ‘Catherina’ peach cultivar in heavy-calcareous soil conditions. Span. J. Agric. Rept. 2017, 15, e0901. [Google Scholar] [CrossRef]
- Font i Forcada, C.; Gogorcena, Y.; Moreno, M.A. Agronomical and fruit quality traits of two peach cultivars on peach-almond hybrid rootstocks growing on Mediterranean conditions. Sci. Hortic. 2012, 140, 157–163. [Google Scholar] [CrossRef]
- DeJong, T.M.; Johnson, R.S.; Doyle, J.F.; Basile, B.; Marsal, J.; Ramming, D.; Bryla, D. Growth, yield and physiological behavior of size-controlling peach rootstocks developed in California. Acta Hortic. 2004, 658, 449–455. [Google Scholar] [CrossRef]
- Russo, N.L.; Robinson, T.L.; Fazio, G.; Aldwinckle, H.S. Field evaluation of 64 apple rootstocks for orchard performance and fire blight resistance. HortScience 2007, 42, 1517–1527. [Google Scholar] [CrossRef]
- Autio, W.R.; Robinson, T.L.; Barritt, B.H.; Cline, J.A.; Crassweller, R.M.; Embree, C.G.; Garcia, M.E.; Greene, G.M.; Hoover, E.E.; Johnson, R.S.; et al. Performance of ‘Fuji’ and ‘McIntosh’ apple trees after 5 years as affected by several dwarf rootstocks in the 1999 NC-140 Apple Rootstock Trial. J. Amer. Pomol. Soc. 2005, 59, 202–214. [Google Scholar]
- Autio, W.R.; Robinson, T.L.; Blatt, S.; Cocran, D.; Francescato, P.; Hoover, E.E.; Kushad, M.; Lang, G.; Lordan, J.; Miller, D.; et al. Budagovsky, Geneva, Pillnitz, and Malling apple rootstocks affect ‘Honeycrisp’ performance over eight years in the 2010 NC-140 ‘Honeycrisp’ apple rootstock trial. J. Amer. Pomol. Soc. 2020, 74, 182–195. [Google Scholar]
- Autio, W.R.; Robinson, T.L.; Black, B.; Crassweller, R.M.; Fallahi, E.; Hoying, S.; Parker, M.L.; Parra Quezada, R.; Reig, G.; Wolfe, D. Budagovsky, Geneva,, Pillnitz, and Malling apple rootstocks affect ‘Fuji’ performance over eight years in the 2010 NC-140 ‘Fuji’ apple rootstock trial. J. Amer. Pomol. Soc. 2020, 74, 196–209. [Google Scholar]
- Cline, J.A.; Black, B.; Coneva, E.; Cowgill, W.; Crassweller, R.; Fallahi, E.; Kon, T.; Muehlbauer, M.; Reighard, G.L.; Ouellette, D.R. Performance of ‘Fuji’ apple trees on several size-controlling rootstocks in the 2014 NC-140 rootstock trial after eight years. J. Amer. Pomol. Soc. 2023, 77, 226–243. [Google Scholar]
- Roper, T.; Black, B.; Stasiak, M.; Marini, R.; Cline, J.; Robinson, T.; Lang, G.; Anderson, L.; Anderson, R.; Freer, J.; et al. Performance of ‘Montmorency’ sour cherry (Prunus cerasus L.) on size-controlling rootstocks at six NC-140 trial locations in North American. J. Am. Pomol. Soc. 2019, 73, 168–177. [Google Scholar]
- Pászti, E.M.; Bujdosó, G.; Mendel, Á. Vegetative characteristics of three apricot cultivars grafted on six different rootstocks. Horticulture 2022, 8, 1004. [Google Scholar] [CrossRef]
- Wright, S.P. Multivariate Analysis Using the MIXED Procedure. SUGI 23. 1998. Available online: https://support.sas.com/resources/papers/proceedings/proceedings/sugi23/Stats/p229.pdf (accessed on 29 July 2024).
- Webster, D.H.; Brown, G.L. Trunk growth of apple tree as affected by crop load. Can. J. Plant Sci. 1980, 60, 1383–1391. [Google Scholar] [CrossRef]
- Westwood, M.N.; Roberts, A.N. The relationship between trunk cross-sectional area and weight of apple trees. J. Am. Soc. Hortic. Sci. 1970, 95, 28–30. [Google Scholar] [CrossRef]
- Strong, D.; Azarenko, A.N. Relationship between trunk cross-sectional area, harvest index, total tree dry weight and yield components of ‘Starkspur Supreme Delicious’ apple trees. J. Am. Pomol. Soc. 2000, 54, 22–27. [Google Scholar]
- Pearce, S.C. The statistical interpretation of vigor measurements of fruit trees. J. Pomol. Hortic. Sci. 1943, 20, 111–115. [Google Scholar]
- Vyvyan, M.C. Interrelation of scion and rootstock in fruit-trees: I. weights and relative weights of young trees formed by the reciprocal unions, as scion and rootstock, of three apple rootstock varieties: M.IX, M.IV, and M.XII. 1955. Ann. Bot. New Ser. 1955, 19, 401–423. [Google Scholar] [CrossRef]
- Vyvyan, M.C. An analysis of growth and of form in young apple trees: I. relative growth and net assimilation rates in 1- and 2-year-old trees of the apple rootstock-variety M.XIII. Ann. Bot. 1957, 21, 479–497. [Google Scholar] [CrossRef]
- Pearce, S.C. Some problems of experimental design and technique with perennial crops. Biometrics 1956, 12, 330–337. [Google Scholar] [CrossRef]
- Marini, R.P.; Barden, J.A.; Cline, J.A.; Perry, R.L.; Robinson, T. Effect of apple rootstocks on average ‘Gala’ fruit weight at four locations after adjusting for crop load. J. Am. Soc. Hortic. Sci. 2002, 127, 749–753. [Google Scholar] [CrossRef]
- Autio, W.; Robinson, T.; Archbold, D.; Cowgill, W.; Hampson, C.; Quezada, R.P.; Wolfe, D. ‘Gala’ Apple Trees on Supporter 4, P.14, and Different Strains of B.9, M.9 and M.26 Rootstocks: Final 10-Year Report on the 2002 NC-140 Apple Rootstock. J. Am. Pomol. Soc. 2013, 67, 62–71. [Google Scholar]
- Marini, R.P.; Anderson, J.L.; Autio, W.R.; Barritt, B.H.; Cline, J.; Cowgill, W.P.; Crassweller, R.C.; Garner, R.M.; Gauss, A.; Godin, R.; et al. Performance of ‘Gala’ apple Tees on 18 dwarfing rootstocks: A ten-year summary of the 1994 NC-140 rootstock t. J. Am. Pomol. Soc. 2006, 60, 69–83. [Google Scholar]
- Marini, R.P.; Autio, W.R.; Black, B.; Cline, J.; Cowgill, W.R.; Crassweller, R.M.; Domoto, P.A.; Hampson, C.; Moran, R.; Quezada, R.A.; et al. Summary of the NC-140 apple physiology trial: The relationship between ‘Golden Delicious’ fruit weight and crop density on three rootstocks at 12 locations as influenced by three dwarfing rootstocks. J. Am. Pomol. Soc. 2012, 66, 78–90. [Google Scholar]
- Marini, R.P. Estimating mean fruit weight and mean fruit value for apple trees: Comparison of two sampling methods with the true mean. J. Am. Soc. Hortic. Sci. 2001, 126, 503–510. [Google Scholar] [CrossRef]
- Marini, R.P.; Schupp, J.R.; Baugher, T.A.; Crassweller, R. Sampling apple trees to accurately estimate mean fruit weight and fruit size distribution. HortScience 2019, 54, 1017–1022. [Google Scholar] [CrossRef]
- Pearce, S.C. The Measurement of Fruit Crops by Sampling; CABI: Egham, UK, 1948; pp. 77–82. [Google Scholar]
- Dorsey, M.J.; McMunn, R.L. A comparison of different methods of taking samples of apples in experimental plots. Proc. Am. Soc. Hortic. Sci. 1938, 36, 619–626. [Google Scholar]
- Reighard, G.L.; Beckmans, T.; Belding, R.; Black, B.; Byers, P.; Cline, J.; Cowgill, W.; Godin, R.; Johnson, R.S.; Kamas, J.; et al. Six-year performance of 14 Prunus Rootstocks at 11 sites in the 2001 NC-140 peach trial. J. Am. Pomol. Soc. 2011, 65, 26–41. [Google Scholar]
- Barden, J.A.; Marini, R.P. Rootstock effects on growth and fruiting of a spur-type and a standard strain of ‘Delicious’ over eighteen years. Fruit Var. J. 1919, 53, 115–125. [Google Scholar]
- Reig, G.; Lordan, J.; Hoying, S.; Fargione, M.; Donahue, D.J.; Francescatto, P.; Acimovic, D.; Fazio, G.; Robinson, T. Long term performance of ‘Delicious’ apple trees on Geneva® rootstocks and trained to four high-density systems under New York state climatic conditions. HortScience 2020, 55, 1538–1550. [Google Scholar] [CrossRef]
- DeJong, T.M.; Johnson, R.S.; Doyle, J.F.; Ramming, D. Labor costs may be reduced … research yields size-controlling rootstocks for peach production. Calif. Agr. 2005, 59, 80–83. [Google Scholar] [CrossRef]
- Barden, J.A.; Marini, R.P. Comparison of methods to express growth, size, and productivity of apple trees. J. Am. Pomol. Soc. 2001, 55, 251–256. [Google Scholar]
- Vanneste, J.L.; Eden-Greene, S. Migration of Erwinia amylovora in host plant tissues. In Fire Blight: The Disease and Its Causative Agent, Erwinia amylovora; Vanneste, J.L., Ed.; CAB International: Wallingford, UK, 2000; pp. 73–87. [Google Scholar]
- Costante, J.F.; Lord, W.J. Progress report: Response of interstem apple trees to planting depth. Compact Fruit Tree 1981, 13, 31–33. [Google Scholar] [CrossRef]
- Costante, J.F.; Lord, W.J.; Howard, D.; Connington, L. Influences of planting depth on growth, root suckering, and yield on interstem apple trees. HortScience 1983, 18, 913–915. [Google Scholar] [CrossRef]
- Frey, B.R.; Lieffers, V.J.; Landhäusser, S.M.; Comeau, P.G.; Greenway, K.J. An analysis of sucker regeneration of trembling aspen. Can. J. For. Res. 2003, 33, 1169–1179. [Google Scholar] [CrossRef]
- Rom, R.C.; Brown, S.A. Burrknot characteristics of six clonal apple rootstocks. Fruit Var. J. 1973, 27, 84–86. [Google Scholar]
- Rom, R.; Carlson, R.F. Rootstocks for Fruit Crops; Wiley: New York, NY, USA, 1987; p. 494. [Google Scholar]
- Marini, R.P. Effects of growing location on fruit tree root suckers. J. Am. Pomol. Soc. 2020, 74, 210–219. [Google Scholar]
- Marini, R.P.; Parker, M.L.; Barden, J.A.; Unrath, C.R. The effect of eight dwarf rootstocks on burrknot development on ‘Gala’ apple trees at two locations. J. Am. Pomol. Soc. 2003, 57, 93–96. [Google Scholar]
- Hoblyn, T.N.; Grubb, N.H.; Painter, A.C.; Waters, B.L. Studies in biennial bearing. J. Pomol. Hortic. Sci. 1936, 14, 39–76. [Google Scholar] [CrossRef]
- Jonkers, H. Biennial bearing in apple and pear: A literature survey. Sci. Hortic. 1979, 11, 303–317. [Google Scholar] [CrossRef]
- Barritt, B.H.; Konishi, B.S.; Dilley, M.A. Performance of three apple cultivars with 23 dwarfing rootstocks during 8 seasons in Washington. Fruit Var. J. 1995, 49, 158–170. [Google Scholar]
- Ferree, C.G.; Hirst, P.M.; Schmid, J.C.; Dotson, P.E. Performance of three apple cultivars with 22 dwarfing rootstocks during 8 seasons in Ohio. Fruit Var. J. 1995, 49, 171–178. [Google Scholar]
- Marini, R.P.; Autio, W.R.; Black, B.; Cline, J.; Cowgill, W.R.; Crassweller, R.M.; Domto, P.A.; Hampson, C.; Moran, R.; Quezada, R.A.; et al. Return bloom on ‘Golden Delicious’ apple trees as affected by previous season’s crop density on three rootstocks at 11 locations. J. Am. Pomol. Soc. 2013, 67, 73–79. [Google Scholar]
- Beckman, T.G.; Okie, W.R.; Meyers, S.C. Rootstocks affect bloom date and fruit maturation of ‘Redhaven’ peach. J. Am. Soc. Hortic. Sci. 1992, 117, 377–379. [Google Scholar] [CrossRef]
- Orazem, P.; Stampar, F.; Hudina, M. Fruit quality of Redhaven and Royal glory peach cultivars on seven different rootstocks. J. Agric. Food Chem. 2011, 59, 9394–9401. [Google Scholar] [CrossRef]
- Remorini, D.; Tavarini, S.; Del’Innocneti, E.; Loreti, F.; Massai, R.; Guidi, L. Effect of rootstocks and harvesting time on the nutritional quality of peel and flesh of peach fruits. Food Chem. 2008, 110, 361–367. [Google Scholar] [CrossRef]
- Giorgi, M.; Capocasa, F.; Scalzo, J.; Murri, G.; Battino, M.; Mezzetti, B. The rootstock effects on plant adaptability, production, fruit quality, and nutrition in the peach (cv.‘Suncrest’). Sci. Hortic. 2005, 107, 36–42. [Google Scholar] [CrossRef]
- Autio, W.R. Rootstocks affect ripening and other qualities of ‘Delicious’ apple. J. Am. Soc. Hortic. Sci. 1991, 116, 378–382. [Google Scholar] [CrossRef]
- Barden, J.A.; Marini, M.E. Maturity and quality of ‘Delicious’ apples as influenced by rootstock. J. Am. Soc. Hortic. Sci. 1992, 117, 547–550. [Google Scholar] [CrossRef]
- Hewetson, F.N. growth and yield of McIntosh apple trees as influenced by the use of various intermediate stem pieces. Proc. Am. Soc. Hortic. Sci. 1944, 45, 181–186. [Google Scholar]
- Larson, F.E.; Fitts, R.; Olsen, K.L. Rootstock influence on ‘Delicious’ and ‘Golden Delicious’ apple fruit quality at harvest and after storage. Sci. Hortic. 1985, 26, 339–349. [Google Scholar] [CrossRef]
- Lord, W.J.; Greene, D.W.; Damon, R.A.; Baker, J.H. Effects of stempiece and rootstock combinations on growth, leaf mineral concentrations, yield, and fruit quality of ‘Empire’ apple trees. J. Am. Soc. Hortic. Sci. 1985, 110, 422–425. [Google Scholar] [CrossRef]
- Perry, R.L.; Dilley, D.R. The influence of interstem on ripening indices of ‘Empire’ apples. Compact Fruit Tree 1984, 17, 50–54. [Google Scholar]
- Barden, J.A.; Marini, R.P. Growth and fruiting of a spur-type and a standard strain of ‘Golden Delicious’ on several rootstocks over eighteen years. Fruit Var. J. 1997, 51, 165–175. [Google Scholar]
- Serra, S.; Leisso, R.; Giordani, L.; Kalcsits, L.; Musacchi, S. Crop load influences fruit quality, nutritional balance, and return bloom in ‘Honeycrisp’ apple. HortScience 2016, 51, 236–244. [Google Scholar] [CrossRef]
- Al-Hinai, Y.K.; Roper, T.R. Rootstock effects on growth and quality of ‘Gala’ apples. HortScience 2004, 39, 1231–1233. [Google Scholar] [CrossRef]
- Autio, W.R.; Hayden, R.A.; Micke, W.C.; Brown, G.R. Rootstock affects ripening, color, and shape of ‘Starkspur Supreme Delicious’ apples in the 1984 NC-140 cooperative planting. Fruit Var. J. 1996, 50, 45–53. [Google Scholar]
- Baldassi, C.; Berim, A.; Roeder, S.; Losciale, P.; Serra, S.; Gang, D.R.; Musacchi, S. Rootstock and crop load effects on ‘Honeycrisp’ photosynthetic performance and carbohydrate accumulation. Plants 2023, 12, 4035. [Google Scholar] [CrossRef]
- Barritt, B.H.; Konishi, B.S.; Dilley, M.A. Tree size, yield and biennial bearing relationships with 40 apple rootstocks and three scion cultivars. Acta Hortic. 1997, 451, 105–112. [Google Scholar] [CrossRef]
- Milliken, G.A.; Johnson, D.E. Analysis of Messy Data, Vol. III: Analysis of Covariance; Chapman and Hall/CRC: New York, NY, USA, 2001; p. 622. [Google Scholar] [CrossRef]
- Marini, R.P.; Ward, D. Using analysis of covariance with unequal slopes to increase efficiency and information obtained from designed experiments. J. Am. Pomol. Soc. 2012, 66, 91–100. [Google Scholar]
- Marini, R.P.; Autio, W.R.; Black, B.; Cline, J.; Cowgill, W.P.; Crassweller, R.M.; Hampson, C.; Kushad, M.M.; Moran, R.; Parker, M.; et al. Time required for classifying rootstock vigor in multi-location rootstock trials. J. Am. Pomol. Soc. 2016, 70, 82–91. [Google Scholar]
- Tukey, R.B.; Klackle, R.L.; McClintock, J.A. Twelve years performance of East Malling rootstocks at Lafayette, Indiana. Proc. Am. Soc. Hortic. Sci. 1954, 64, 146–155. [Google Scholar]
- Trout, J.R.; Marini, R.P. Estimating sample size to achieve efficient experiment designs. HortScience 1983, 19, 355–358. [Google Scholar] [CrossRef]
- Marini, R.P.; Moran, R.; Hampson, C.; Kushad, M.; Perry, R.L.; Robinson, T.L. Effect of dwarf rootstocks on average ‘Gala’ fruit weight in six locations over three years. J. Am. Pomol. Soc. 2008, 62, 129–136. [Google Scholar]
- Autio, W.; Robinson, T.; Black, B.; Blatt, S.; Cochran, D.; Cowgill, W.; Hampson, C.; Hoover, E.; Lang, G.; Miller, D.; et al. Budagovsky, Geneva, Pillnitz, and Malling apple rootstocks affect ‘Honeycrisp’ performance over the first five years in the 2010 NC-140 ‘Honeycrisp’ apple rootstock trial. J. Am. Pomol. Soc. 2017, 71, 149–166. [Google Scholar]
- Reighard, G.; Bridges, W.; Archbold, D.; Atucha, A.; Autio, W.; Beckman, T.; Black, B.; Chavez, D.J.; Coneva, E.; Day, K.; et al. 2018, Rootstock performance in the 2009 NC-140 peach trial across 11 states. Acta Hortic. 2018, 1228, 181–186. [Google Scholar] [CrossRef]
- Reighard, G.; Andersen, R.; Anderson, J.; Autio, W.; Beckman, T.; Baker, T.; Belding, R.; Brown, G.; Byers, P.; Cowgill, W.; et al. Growth and yield of Redhaven peach on 19 rootstocks at 20 North American locations. J. Am. Pomol. Soc. 2004, 58, 174–202. [Google Scholar]
- Marini, R.P. A note on the analysis and interpretation of designed experiments with factorial treatment structure. J. Am. Pomol. Soc. 2022, 76, 27–35. [Google Scholar]
- Gimeno, V.; Syvertsen, J.P.; Nieves, M.; Simón, I.; Martínez, V.; García-Sánchez, F. Additional nitrogen fertilization affects salt tolerance of lemon trees on different rootstocks. Sci. Hortic. 2009, 121, 298–305. [Google Scholar] [CrossRef]
- McClymont, L.; Goodwin, I.; Whitfield, D.; O’Connell, M.; Turpin, S. Effects of Rootstock, Tree Density and Training System on Early Growth, Yield and Fruit Quality of Blush Pear. HortScience 2021, 56, 1408–1415. [Google Scholar] [CrossRef]
- Mass, F. Evaluation of Pyrus and quince rootstocks for high density pear orchards. Sci. Work. Lith. Inst. Hortic. Lith. Univ. Agric. 2006, 25, 13–26. [Google Scholar] [CrossRef]
- Jiménez, S.; Pinochet, J.; Gogorcena, Y.; Betrán, J.A.; Moreno, M.A. Influence of different vigour cherry rootstocks on leaves and shoots mineral composition. Sci. Hortic. 2007, 112, 73–79. [Google Scholar] [CrossRef]
- Rato, A.E.; Agulheiro, A.C.; Barroso, J.M.; Riquelme, F. Soil and rootstock influence on fruit quality of plums (Prunus domestica L.). Sci. Hortic. 2008, 118, 218–222. [Google Scholar] [CrossRef]
- Hampson, C.R.; Quamme, H.A.; Brownlee, R.T. Canopy growth, yield, and fruit quality of ‘Royal Gala’ apple trees grown for eight years in five tree training systems. HortScience 2002, 37, 627–631. [Google Scholar] [CrossRef]
- Autio, W.R.; Robinson, T.L.; Black, B.; Bradshaw, T.; Cline, J.A.; Crassweller, R.M.; Embree, C.G.; Hoover, E.E.; Hoying, S.A.; Iungerman, K.A.; et al. Performance of ‘Fuji’ and ‘McIntosh’ apple trees after 10 years as affected by several dwarf rootstocks in the 1999 NC-140 apple rootstock trial. J. Am. Pomol. Soc. 2011, 65, 2–20. [Google Scholar]
- Lin, C.S.; Binns, M.R. A method of analyzing cultivar x location x year experiments: A new stability parameter. Theoret. Appl. Genet. 1988, 76, 425–430. [Google Scholar] [CrossRef]
- Marini, R.P. Rootstock x site interaction and stability analysis of apple rootstocks across sites in two NC-140 multilocation apple rootstock trials. J. Am. Pomol. Soc. 2020, 74, 220–230. [Google Scholar]
- Dia, M.; Wehner, T.C.; Hassell, R.; Prince, D.S.; Boyhan, G.E.; Olson, S.; King, S.; Davis, A.R.; Tolla, G.E. Genotype x environment interaction and stability analysis for watermelon fruit yield in the United States. Crop Sci. 2016, 56, 1645–1661. [Google Scholar] [CrossRef]
- Yan, W.; Tinker, A. Biplot analysis of multienvironment trial data: Principles and applications. Can. J. Plant Sci. 2006, 86, 623–645. [Google Scholar] [CrossRef]
- Marini, R.P. Graphical analysis of rootstock x site interaction for two NC-140 multi-location apple rootstock trials using GGEbiplot. J. Am. Pomol. Soc. 2020, 74, 231–244. [Google Scholar]
- Gates, C.E. A user’s guide to misanalyzing planned experiments. HortScience 1991, 26, 1262–1265. [Google Scholar] [CrossRef]
- Allison, D.B.; Brown, A.W.; George, B.J.; Kaiser, K.A. Reproducibility: A tragedy of errors. Nature 2016, 530, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuis, S.; Forstmann, B.U.; Wagenmakers, E.J. Erroneous analyses of interactions in neuroscience: A problem of significance. Nat. Neurosci. 2011, 14, 1105–1107. [Google Scholar] [CrossRef]
- Hatton, R.G. Results of research on fruit trees stocks at East Malling. J. Pomol. 1920, 3, 1–10. [Google Scholar]
- Hatton, R.G. The influence of different rootstocks upon the vigor and productivity of the variety budded or grafted thereon. J. Pomol. Hortic. Sci. 1927, 4, 1–28. [Google Scholar]
- Russel, E.J. Rothamsted and its experiment station. Agric. Hist. 1942, 16, 161–183. Available online: http://www.jstor.org/stable/3739533 (accessed on 9 February 2024).
- Mahalanobis, P.C. Professor Ronald Aylmer Fisher. Biometrics 1964, 20, 238–252. [Google Scholar] [CrossRef]
- Watson, D.J. Field experimentation at Rothamsted in the years 1926–1936. Rothamsted Expt. Stat. Rept. 1937, 64–74. [Google Scholar] [CrossRef]
- East Malling Res. Stat. Ann. Rept. for 1924. The Station. 1925. p. 16. Available online: https://books.google.com/books?hl=en&lr=&id=zKtPAAAAIAAJ&oi=fnd&pg=PA29&dq=t+n+hoblyn++biography&ots=QkfS10Ivax&sig=6jgyRD9ds7ROCLBHMi47ePI0TAU (accessed on 6 October 2023).
- Gower, J.C. Statistics and agriculture. J. Royal Stat. Soc. 1988, 151, 179–200. [Google Scholar] [CrossRef]
- Hatton, R.G. The elimination of sources of error in field experiments: The standardization of fruit tree stocks. Annu. Rep. East Malling Res. Stn. 1931, 13–21. [Google Scholar]
- Hoblyn, T.N. The relationship between the experimental and the demonstration plot and their relative value to the investigator, the county officer and the fruit grower. Ann. Rept. E. Malling Res. Stat. 1929 1930, 41–55. [Google Scholar]
- Tukey, J.W. Comparing individual means in the analysis of variance. Biometrics 1949, 5, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Scheffé, H. A method for judging all contrasts in the analysis of variance. Biometrika 1953, 40, 87–104. [Google Scholar] [CrossRef]
- Duncan, D.B. Multiple range and multiple F test. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Marini, M.; Hinkelmann, K.; Marini, R. Least squares means comparisons for interaction means in a two-factor study in apple rootstock trials. HortScience 2000, 35, 433. [Google Scholar] [CrossRef]
- Schabenberger, O.; Gregoire, T.G.; Weyerhaeuser, J.P.; Kong, F. Collections of simple effects and their relationship to main effects and interactions in factorials. Amer. Stat. 2000, 54, 210–214. [Google Scholar] [CrossRef]
- Parolini, G. In pursuit of a science of agriculture: The role of statistics in field experiments. HPLS 2015, 37, 261–281. [Google Scholar] [CrossRef]
- Student. The probable error of a mean. Biometrika 1908, 6, 1–25. [Google Scholar] [CrossRef]
- Cowles, M.; Davis, C. On the origins of the. 05 level of statistical significance. Amer. Psychol. 1982, 37, 553–558. [Google Scholar] [CrossRef]
- Love, H.H. The Importance of the probable error concept in the interpretation of experimental results. Agron. J. 1923, 15, 217–224. [Google Scholar] [CrossRef]
- Mercer, W.B.; Hall, A.D. The experimental error of field trials. J. Agric. Sci. 1911, 4, 107–132. [Google Scholar] [CrossRef]
- Anthony, R.D.; Waring, J.H. Methods of Interpreting Yield Records in Apple Fertilization Experiments; Pennsylvania State University: University Park, PA, USA, 1922; Bull 173; Available online: https://www.google.com/books/edition/Bulletin/iPsmAQAAMAAJ?hl=en&gbpv=1&dq=Anthony+and+Waring+pennsylvania+agricultural+experiment+station+bulletin+&pg=PA2&printsec=frontcover (accessed on 12 January 2024).
- Verdooren, L.R. History of the statistical design of agricultural experiments. J. Agric. Biol. Environ. Stat. 2020, 25, 457–486. [Google Scholar] [CrossRef]
- Fisher, R.A. The arrangement of field experiments. J. Minist. Agric. Great Br. 1926, 33, 503–513. [Google Scholar]
- Fisher, R.A. Statistical Methods for Research Workers, 1st ed.; Oliver & Boyd: Edinburgh, Scotland, 1925. [Google Scholar]
- Radhakrishna, R.R.A. Fisher: The founder of modern statistics. Inst. Math. Stat. 1992, 7, 34–48. [Google Scholar]
- Fisher, R.A. The Design of Experiments; Oliver and Boyd: Edinburgh, Scotland, 1935; p. 274. Available online: https://archive.org/details/in.ernet.dli.2015.502684/page/n5/mode/2up (accessed on 22 September 2023).
- Hoblyn, T.N. Field Experiments in Horticulture; Imperial Bureau of Fruit Production: East Malling, UK, 1931; p. 2. [Google Scholar]
- Freeman, G.H. The analysis and interpretation of experimental results. Inc. Stat. 1961, 11, 33–56. [Google Scholar] [CrossRef]
- Pearce, S.C. Field experimentation with fruit trees and other perennial plants. Tech. Commun. Bur. Hort. Plant. Crops East Malling. 1953, 23, 131, Commonwealth Agricultural Bureaux. [Google Scholar]
- Pearce, S.C. Biological Statistics: An Introduction; McGraw-Hill, Inc.: New York, NY, USA, 1965; p. 212. [Google Scholar]
- Batchelor, L.D.; Reed, H.S. Relationship of the variability of yields of fruit trees to the accuracy of field trials. J. Agric. Res. 1918, 12, 245–287. Available online: https://babel.hathitrust.org/cgi/pt?id=chi.57143034&seq=3 (accessed on 31 October 2023).
- Hoblyn, T.N.; Pearce, S.C.; Freeman, G.H. Some considerations in the design of successive experiments in fruit plantations. Int. Biom. Soc. 1954, 10, 503–515. [Google Scholar] [CrossRef]
- Parker, E.R.; Batchelor, L.D. Variation in the yields of fruit trees in relation to the planning of future experiments. Hilgardia 1932, 7, 81–161. Available online: https://hilgardia.ucanr.edu/fileaccess.cfm?article=152060&p=MOTOLM (accessed on 6 November 2023). [CrossRef]
- Marini, R.P.; Trout, J.R. Sampling procedures for minimizing variation in peach fruit quality. J. Am. Soc. Hortic. Sci. 1984, 109, 361–364. [Google Scholar] [CrossRef]
- Marini, R.P. Sample size estimates for peach tree growth and yield experiments. J. Am. Soc. Hortic. Sci. 1985, 110, 604–608. [Google Scholar] [CrossRef]
- Hedrick, U.P. Fertilizing Apple Orchards; New York State Agricultural Experiment Station: Geneva, NY, USA, 1923; Circ. 66. [Google Scholar]
- Anthony, R.D. Planning and analyzing apple orchard experiments by the use of “Student’s method”. Proc. Am. Soc. Hortic. Sci. 1926, 23, 71–73. [Google Scholar]
- Pickering, M.A. Experimental errors in horticultural work. J. Bd. Agr. Sup. 1911, 7, 38–42. [Google Scholar]
- Munson, W.M. Experiments in orchard culture. Maine Agric. Exp. Sta. Bul. 1903, 89, 1–24. [Google Scholar]
- Harris, J.A. Practical universality of field heterogeneity as a factor influencing plot yields. J. Agric. Res. 1920, 19, 279–314. [Google Scholar]
- Pearce, S.C.; Taylor, J. The purposes and design of calibration trials. Annu. Rep. East Malling Res. Stn. 1949 1950, A33, 83–90. [Google Scholar]
- Hudina, M.; Veberic, R. The effect of rootstock in long-term performance of the peach cultivar ‘Redhaven’. Acta Hortic. 2022, 1352, 349–356. [Google Scholar] [CrossRef]
- Menegatti, R.D.; Souza, A.G.; Bianchi, V.J. Different environments and doses of controlled-release fertilizer in peach rootstocks production. Adv. Hortic. Sci. 2020, 34, 157–166. [Google Scholar]
- Bielsa, B.; Rubio-Cabetas, M.J.; Felipe, A.J.; Gómez-Aparisi, J.; Socias, R. Rootstock trial of eight GxN interspecific hybrids in almond. In Options Méditerranéennes. Series A: Mediterranean Seminars. ENA, École Nationale d’Agriculture de Meknès; CIHEAM: Zaragoza, Spain, 2016; pp. 183–186. Available online: http://om.ciheam.org/om/pdf/a119/00007388.pdf (accessed on 14 February 2024).
- Minas, I.S.; Anthony, B.M.; Pieper, J.R.; Sterle, D.G. Large-scale and accurate non-destructive visual to near infrared spectroscopy-based assessment of the effect of rootstock on peach fruit internal quality. Eur. J. Agron. 2023, 143, 126706. [Google Scholar] [CrossRef]
- Piestrzeniewicz, C.; Sadowski, A.; Dziuban, R. Performance of ‘Rubin’ apple trees on nineteen rootstocks after four years. Latvian J. Agron. 2006, 9, 98–102. [Google Scholar]
- Fallahi, E.; Richardson, D.G.; Westwood, M.N. Quality of apple fruit from a high density orchard as influenced by rootstocks, fertilizers, maturity, and storage. J. Am. Soc. Hortic. Sci. 1985, 110, 71–74. [Google Scholar] [CrossRef]
- Lentner, M.; Bishop, T. Experimental Design and Analysis; Valley Book Comp: Blacksburg, VA, USA, 1993; p. 585. [Google Scholar]
- Jones, B.; Montgomery, D.C. Design of Experiments: A Modern Approach, 1st ed.; Wiley: Hoboken, NJ, USA, 2020; p. 272. [Google Scholar]
- Clarke, G.M.; Kempson, R.E. Introduction to the Design and Analysis of Experiments; Wiley: Hoboken, NJ, USA, 1997; p. 344. [Google Scholar]
- Bose, R.C.; Nair, K.R. Partially balanced incomplete block designs. Sankhyā Indian J. Stat. 1939, 1, 337–372. Available online: http://library.isical.ac.in:8080/jspui/bitstream/10263/270/1/39.05.pdf (accessed on 14 November 2023). [CrossRef]
- Stringer, J.K.; Smith, A.B.; Cullis, B.R. Spatial analysis of agricultural field experiments. In Design and Analysis of Experiment; Hinkelmann, K., Ed.; Special Designs and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; Volume 3, pp. 109–136. [Google Scholar]
- Yates, F. A new method of arranging variety trials involving a large number of varieties. J. Agric. Sci. 1936, 26, 424–455. [Google Scholar] [CrossRef]
- Hinkelmann, K.; Kempthorne, O. Design and Analysis of Experiments: Introduction to Experimental Design; Wiley & Sons, Inc.: New York, NY, USA, 1994; Volume 1, p. 495. [Google Scholar]
- Addelmann, S. The generalized randomized block design. Am. Stat. 1969, 23, 35–36. [Google Scholar] [CrossRef]
- Marini, R.P.; Black, B.; Crassweller, R.M.; Domoto, P.A.; Hampson, C.; Moran, R.; Robinson, T.; Stasiak, M.; Wolfe, D. Performance of ‘Golden Delicious’ apple on 23 rootstocks at eight locations: A ten-year summary of the 2003 NC-140 dwarf rootstock trial. J. Am. Pomol. Soc. 2014, 68, 54–68. [Google Scholar]
- Pearce, S.C. Experimenting with blocks of natural size. Int. Biom. Soc. 1963, 20, 699–706. [Google Scholar] [CrossRef]
- Little, R.C.; Milliken, G.A.; Stroup, W.W.; Wolfinger, R.D.; Schabenberger, O. SAS® for Mixed Models, 2nd ed.; SAS Inst.: Cary, NC, USA, 2006; pp. 154–157. [Google Scholar]
- Askari-Khorasgani, O.; Jafarpour, M.; Hadad, M.M.; Pessarakli, M. Fruit yield and quality characteristics of ‘Shahmiveh’ pear cultivar grafted on six rootstocks. J. Plant Nutr. 2019, 42, 323–332. [Google Scholar] [CrossRef]
- Ikinci, A.; Bolat, I.; Ercisli, S.; Kodad, O. Influence of rootstocks on growth, yield, fruit quality and leaf mineral element contents of pear cv. ‘Santa Maria’ in semi-arid conditions. Biol. Res. 2014, 47, 71. [Google Scholar] [CrossRef]
- Pietranek, A.; Jadczuk, E. Growth and bearing of ‘Jonagold’ apple trees as affected by rootstock and type of nursery trees used for planting. Latv. J. Agron. 2006, 9, 103–108. [Google Scholar]
- Tomala, K.; Slowinska, I. The effect of rootstock on the physiological status and the storage ability of ‘Elsa’ apples. Latv. J. Agron. 2006, 9, 162–167. [Google Scholar]
- Univer, N.; Tiirmaa, K.; Univer, T. Effect of rootstock on growth and early bearing of five apple cultivars in Estonia. Latv. J. Agron. 2006, 9, 167–171. [Google Scholar]
- Gur, A.; Zamet, D.; Arad, E. A pear rootstock trial in Isreal. Sci. Hortic. 1978, 8, 249–264. [Google Scholar] [CrossRef]
- Havlicek, L.L.; Peterson, N.L. Robustness of the t test: A guide for researchers on effect of violations of assumptions. Psychol. Rept. 1974, 34, 1095–1114. [Google Scholar] [CrossRef]
- Glass, G.V.; Peckham, P.D.; Sanders, J.R. Consequences of failure to meet assumptions underlying the fixed effects analyses of variance and covariance. Rev. Ed. Res. 1972, 42, 237–288. [Google Scholar] [CrossRef]
- Pearce, S.C.; Moore, C.S. Reduction of experimental error in perennial crops, using adjustment by neghbouring plots. Expt. Agric. 1976, 12, 267–272. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis, 2nd ed.; Cambridge University Press: New York, NY, USA, 1952; p. 318. [Google Scholar] [CrossRef]
- Rencher, A.C.; Schaalje, G.B. Linear Models in Statistics; Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; p. 671. [Google Scholar]
- McCullgh, P.; Nelder, J.A. Generalized Linear Models, 2nd ed.; Chapman & Hall/CRC: New York, NY, USA, 1989; p. 511. [Google Scholar]
- Fisher, R.A. Two new properties of mathematical likelihood. Proc. R. Soc. London. Ser. A Contain. Pap. A Math. Phys. Character 1934, 144, 285–307. [Google Scholar]
- Nelder, J.A.; Wedderburn, R.W.M. Generalized linear models. J. Royal Stat. Soc. 1972, 135, 370–384. [Google Scholar] [CrossRef]
- Nelder, J.A. Present position and potential developments: Some personal views statistical computing. J. Royal Stat. Soc. 1984, 147, 151–160. [Google Scholar] [CrossRef]
- Rosen, S. Electronic computers: A historical survey. ACM Comput. Surv. (CSUR) 1969, 1, 7–36. [Google Scholar] [CrossRef]
- Johnson, L. Oral History of Wilfred, J.; (Wil) Dixon and Linda Glassner, Recorded March 27, 1986; Computer History Museum: Mountain View, CA, USA, 2007; p. 19. Available online: http://archive.computerhistory.org/resources/access/text/2012/04/102658169-05-01-acc.pdf (accessed on 30 August 2024).
- Gower, J.C. The development of statistical computing at Rothamsted. Int. Stat. Rev. 2015, 83, 357–370. [Google Scholar] [CrossRef]
- Kramer, M.H.; Paparozzi, E.T.; Stroup, W.W. Statistics in a horticultural journal: Problems and solutions. J. Am. Soc. Hortic. Sci. 2016, 141, 400–406. [Google Scholar] [CrossRef]
- Venables, W.N.; Smith, D.M.; R Core Team. An Introduction to R. 2024. Available online: https://cran.r-project.org/doc/manuals/r-release/R-intro.pdf (accessed on 10 September 2024).
- Littell, R.C.; Milliken, G.A.; Stroup, W.W.; Wolfinger, R.D. SAS System for Mixed Models, 1st ed.; SAS Institute Inc.: Cary, NC, USA, 1996. [Google Scholar]
- Schabenberger, O. Introducing the GLIMMIX procedure for generalized linear mixed models. In Proceedings of the GLIMMIX Procedure for Generalized Linear Mixed Model, SUGI, Cary, NC, USA, 10–13 April 2005; SAS Institute: Cary, NC, USA, 2004; Volume 30, pp. 1–20. Available online: https://www.lexjansen.com/wuss/2005/data_analysis_and_statistics/das-sas_introducing_the_glimmix_procedure.pdf (accessed on 10 September 2024).
- Littell, R.C.; Stroup, W.W.; Freund, R.J. SAS for Linear Models, 4th ed.; SAS Institute Inc.: Cary, NC, USA, 2002; p. 466. [Google Scholar]
- SAS/STAT Software: The GENMOD Procedure, version 6.09; SAS Inst., Inc.: Cary, NC, USA, 1993.
- Spengler, R.N. Origins of the apple: The role of megafaunal mutualism in the domestication of Malus and rosaceous trees. Front. Plant Sci. 2019, 10, 617. [Google Scholar] [CrossRef] [PubMed]
- Mudge, K.; Janick, J.; Scofield, S.; Goldschmidt, E.E. A history of grafting. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons Inc.: New York, NY, USA, 2009; Volume 35, pp. 437–493. [Google Scholar]
- Tukey, H.B. Dwarfed Fruit Trees; The MacMillan Company: New York, NY, USA, 1964; p. 562. [Google Scholar]
- Marini, R.P.; Fazio, G. Apple rootstocks: History, physiology, management, and breeding. In Horticultural Reviews; Warrington, I., Ed.; John Wiley & Sons Inc.: New York, NY, USA, 2018; Volume 44, pp. 197–312. [Google Scholar]
- Hatton, R.G. Paradise apple stocks. J. Roy. Hortic. Soc. 1917, 42, 361–399. [Google Scholar]
- Bunyard, E.A. The history of the Paradise stocks. J. Pomol. 1920, 1, 166–176. [Google Scholar] [CrossRef]
- Hatton, R.G. Paradise apple stocks their fruit and blossom described. J. R. Hortic. Soc. 1919, 44, 89–94. [Google Scholar]
- Preston, A.P. The control of fruitfulness behaviour by the use of rootstocks. Ann. Appl. Biol. 1956, 44, 511–517. [Google Scholar] [CrossRef]
- Preston, A.P. Five New Apple Rootstocks; CABI: Egham, UK, 1953; pp. 169–170. [Google Scholar]
- Hatton, R.G. Apple rootstock studies effect of layered stocks upon the vigour and cropping of certain scions. J. Pomol. Hortic. Sci. 1935, 13, 293–350. [Google Scholar] [CrossRef]
- Tydeman, H.M. A Description and Classification of the Malling-Merton and Malling XXV Apple Rootstocks; CABI: Egham, UK, 1952; pp. 55–63. [Google Scholar]
- Ritter, C.M.; Tukey, L.D. Growth and fruiting of various apple varieties in response to several clonal rootstocks. Bull. Pa. Agric. Exp. Stn. 1959, 649, 1–21. [Google Scholar]
- Pickering, S.U. The experimental error in horticultural work. J. Board Agric. Sup. 1911, 7, 38–47. [Google Scholar]
- Witt, A.W.; Garner, R.J. Peach Stock Trials; CABI: Egham, UK, 1931; pp. 22–31. [Google Scholar]
- Hatton, R.G.; Amos, J.; Witt, A.W. Plum rootstocks: Their varieties, propagation, and influence upon cultivated varieties worked thereon. J. Pomol. Hortic. Sci. 1929, 7, 63–99. [Google Scholar] [CrossRef]
- Hoblyn, T.N. The layout and conduct of two manurial trials of raspberries: Together with the deductions which can validly be drawn. J. Pomol. Hortic. Sci. 1931, 9, 303–330. [Google Scholar] [CrossRef]
- Hatton, R.G. The behavior of certain pears on various quince rootstocks. J. Pomol. Hortic. Sci. 1929, 7, 216–233. [Google Scholar]
- Tydeman, H.M. Trials with New Quince Rootstocks; CABI: Egham, UK, 1949; pp. 68–73. [Google Scholar]
- Loreti, F.; Massai, R.; Fei, C.; Cinelli, F.; Cecconi, B. Evaluation of eleven dwarfing apple rootstocks: Preliminary results. Acta Hort. 2001, 557, 155–161. [Google Scholar] [CrossRef]
- Preston, A.P. Apple Rootstock Studies: The M.IX Crosses; CABI: Egham, UK, 1954; pp. 89–94. [Google Scholar]
- Webster, A.D.; Wertheim, S.J. Comparisons of species and hybrids rootstocks for European plum cultivars. J. Hortic. Sci. 1993, 68, 861–869. [Google Scholar] [CrossRef]
- Şahin, S.; Yiğit, T.; Erdoğan, A. Determination of effects of some clonal rootstocks on morphological and pomological properties of ‘Hacıhaliloğlu’ and ‘Kabaaşı’ apricot cultivars. Acta Hortic. 2020, 1290, 225–230. [Google Scholar] [CrossRef]
- Moreno, M.A.; Adrada, R.; Aparicio, J.; BetráN, S. Performance of ‘Sunburst’ sweet cherry grafted on different rootstocks. J. Hortic. Sci. Biotechnol. 2001, 76, 167–173. [Google Scholar] [CrossRef]
- Tabakov, S.G.; Yordanov, A.I.; Petrov, M.N. Study of the influence of five rootstocks on the growth and productivity of three plum cultivars grown in Bulgaria. Acta Hortic. 2021, 1322, 131–138. [Google Scholar] [CrossRef]
- Czynczyk, A.; Omiecinska, B. Effect of new rootstocks of Polish, Russian and Czechoslovakian breeds and two depths of planting of trees with interstems on growth and cropping of 3 apple cultivars. Acta Hortic. 1989, 243, 71–78. [Google Scholar] [CrossRef]
- Parry, M.S. Trials of Dwarfing Quince Rootstocks with Comice and Conference Pears. J. Hortic. Sci. 1981, 56, 139–143. [Google Scholar] [CrossRef]
- Hudina, M.; Fajt, N.; Ătampar, F. Influence of rootstock on orchard productivity and fruit quality in peach cv. ‘Redhaven’. J. Hortic. Sci. Biotechnol. 2006, 81, 1064–1068. [Google Scholar] [CrossRef]
- McKenzie, D.W. Apple rootstock trials Jonathan on East Malling, Merton and Malling-Merton rootstocks. J. Hortic. Sci. 1964, 39, 69–77. [Google Scholar] [CrossRef]
- Glenn, E.M. Plum rootstock trials at East Malling. J. Hortic. Sci. 1961, 36, 28–39. [Google Scholar] [CrossRef]
- Avery, D.J. Effects of fruiting on the growth of apple trees on four rootstock varieties. New Phytol. 1970, 69, 19–30. [Google Scholar] [CrossRef]
- Tubbs, F.R. Rootstock/scion relations in horticultural crop physiology. Sci. Hortic. 1974, 2, 221–230. [Google Scholar] [CrossRef]
- Greenslade, R.M.; Pearce, S.C. Field sampling for the comparison of infestation of strawberry crops by the aphis (Capitophorus fragariae Theob). J. Pomol. Hortic. Sci. 1940, 17, 308–317. [Google Scholar]
- Zeiger, D.; Tukey, H.B. An Historical Review of the Malling Apple Rootstocks in America; CABI: Egham, UK, 1960; p. 75. [Google Scholar]
- Anthony, R.D.; Yerks, G.E. The influence of clone roots on the variability of young apple trees. Proc. Am. Soc. Hortic. Sci. 1928, 25, 169–171. [Google Scholar]
- Shaw, J.K. The Malling clonal stocks in relation to McIntosh and Wealthy. Proc. Am. Soc. Hortic. Sci. 1936, 33, 346–349. [Google Scholar]
- Anthony, R.D.; Clarke, W.S., Jr. Performance of clonal understocks at the Pennsylvania State College. Proc. Am. Soc. Hortic. Sci. 1946, 48, 212–226. [Google Scholar]
- Clarke, W.S.; Anthony, R.D. An orchard test of Mazzard and Mahaleb cherry rootstocks. Proc. Am. Soc. Hortic. Sci. 1946, 48, 200–226. [Google Scholar]
- Yerks, G.E.; Sudds, R.H. Growth and fruitfulness of three apple varieties on French crab seedling and on a clonal stock. Proc. Am. Soc. Hortic. Sci. 1937, 35, 363–368. [Google Scholar]
- Gourley, J.H.; Howlett, F.S. Malling stocks at the Ohio Agricultural Experiment Station. Proc. Am. Soc. Hortic. Sci. 1946, 48, 241–244. [Google Scholar]
- Brase, K.D. Growth behavior of four apple varieties on Manchurian crab seedling rootstocks in the nursery. Proc. Am. Soc. Hortic. Sci. 1946, 48, 236–240. [Google Scholar]
- Sudds, R.H. Can apple trees on clonal rootstocks be recommended for commercial orchards in the Shenandoah Valley? Proc. Am. Soc. Hortic. Sci. 1946, 48, 245–248. [Google Scholar]
- Yerks, G.E.; Aldrich, W.W. Behavior of apple varieties on certain clonal stocks. J. Am. Soc. Hortic. Sci. 1946, 48, 227–235. [Google Scholar]
- Koehler, K. Iowa State University statistics department. In Strength in Numbers: The Rising of Academic Statistics Departments in the U.S.; Agresti, A., Meng, Eds.; Springer Science and Business Media: New York, NY, USA, 2013; pp. 111–127. [Google Scholar] [CrossRef]
- Agresti, A.; Meng, X.L. Statistics as an academic discipline. In Stgength in Numbers: The Rising of Academic Statistics Departments in the U.S.; Agresti, A., Meng, X.-L., Eds.; Springer Science and Business Media: New York, NY, USA, 2013; pp. 1–8. [Google Scholar] [CrossRef]
- Gibbons, J.D.; Freund, R.J. Organizations for statistical consulting at colleges and universities. Am. Stat. 1980, 34, 140–145. [Google Scholar] [CrossRef]
- Olein, W.C.; Ferree, D.C.; Bishop, B.L. Long-term performance potential and stability across 10 environments for nine apple rootstocks tested in the 1980-81 NC-140 trial. Fruit Var. J. 1991, 45, 208–213. [Google Scholar]
- Carlson, R.F.; Tukey, H.B. Fourteen-year orchard performance of several apple varieties on East Malling rootstocks in Michigan (second report). Proc. Am. Soc. Hortic. Sci. 1959, 74, 47–53. [Google Scholar]
- Tukey, R.B.; Langston, R.; Cline, R.A. Influence of rootstock, bodystock, and interstock on the nutrient content of apple foliage. Proc. Am. Soc. Hortic. Sci. 1962, 80, 73–78. [Google Scholar]
- Longley, R.P. the variability of trunk cross sectional area and yields of Fameuse and McIntosh apple trees grown on three clonal and two seedling rootstocks. Proc. Am. Soc. Hortic. Sci. 1960, 76, 11–15. [Google Scholar]
- Longley, R.P. Growth and yield of trees during the first five years of three varieties of apples on Malling-Merton rootstocks 104, 106, 109 and 111. Proc. Am. Soc. Hortic. Sci. 1963, 83, 74–76. [Google Scholar]
- Cline, J.A.; Black, B.; Coneva, E.; Cowgill, W.; Crassweller, R.; Fallahi, E.; Kon, T.; Muehlbauer, M.; Reighard, G.L.; Ouellette, D.R. Early performance of ‘Fuji’ apple trees on several size-controlling rootstocks in the 2014 NC-140 rootstock trial. J. Am. Pomol. Soc. 2021, 75, 203–213. [Google Scholar]
- Westwood, M.N.; Reimer, R.C.; Quackenbush, V.L. Long term yield as related to ultimate tree size of three pear varieties grown on rootstocks of five Pyrus species. Proc. Am. Soc. Hortic. Sci. 1963, 82, 103–108. [Google Scholar]
- Rubauskis, E.; Skrivele, M.; Dimza, I.; Berlands, V. Rootstock effects on growth on apple growing and yields, as influenced by mulching and fertigation. Acta Hortic. 2004, 658, 251–256. [Google Scholar] [CrossRef]
- Autio, W.R.; Anderson, J.L.; Barden, J.A.; Brown, G.R.; Crassweller, R.M.; Domoto, P.A.; Erb, A.; Ferree, D.C.; Gaus, A.; Hirst, P.M.; et al. Performance of ‘Golden Delicious’, ‘Jonagold’, ‘Empire’, and ‘Rome Beauty’ apple trees on five rootstocks over ten years in the 1990 NC-140 cultivar/rootstock trial. J. Am. Pomol. Soc. 2001, 55, 131–137. [Google Scholar]
- Hilgeman, R.H.; Rodney, D.R.; Dunlap, J.A. rootstock evaluations for lemons on two soil types in Arizona. Proc. Am. Soc. Hortic. Sci. 1966, 88, 280–290. [Google Scholar]
- Lord, W.J.; Damon, R.A., Jr.; Robinson, D.E. Comparative responses of three apple rootstocks to soil-incorporated simazine. J. Am. Soc. Hortic. Sci. 1970, 95, 737–739. [Google Scholar] [CrossRef]
- Embleton, T.W.; Labanavskes, C.K.; Bitters, W.P. The influence of certain rootstocks on the concentration of boron, iron, manganese, and other elements in lemon leaves, and on boron toxicity symptoms. Proc. Am. Soc. Hortic. Sci. 1962, 80, 285–290. [Google Scholar]
- Childers, N.F. Modern Fruit Science, 4th ed.; Rutgers University: New Brunswick, NJ, USA, 1970; p. 960. [Google Scholar]
| Iowa | Kentucky | |||||||
|---|---|---|---|---|---|---|---|---|
| Rootstock | Survival at 10 Yrs. | Cum. Yld. (kg/Surviving Tree) | Avg. Life Span (Yrs) | Cum. Yld. (kg/Tree Planted) | Survival at 10 Yrs. | Cum. Yld. (kg/Surviving Tree) | Avg. Life Span (Yrs) | Cum. Yld. (kg/Tree Planted) |
| B.9 | 100 | 74 ab z | 10 | 74 ab | 50 b | 87 a | 6.9 a | 43 a |
| G.41 | 100 | 133 b | 10 | 133 b | 88 a | 307 bcd | 9.0 b | 266 c |
| G.16 | 100 | 119 b | 10 | 119 b | 50 b | 298 bcd | 6.0 a | 150 b |
| M.9 T337 | 100 | 115 b | 10 | 115 b | 75 a | 295 bc | 8.8 b | 243 c |
| B.10 | 100 | 107 b | 10 | 107 b | 100 a | 310 bcd | 10.0 b | 310 cd |
| G.935 | 100 | 149 b | 10 | 149 b | 25 c | 296 bc | 5.4 a | 133 b |
| M.9 pajam 2 | 100 | 117 b | 10 | 117 b | 88 a | 347 d | 9.5 b | 310 d |
| J-TE-H | 100 | 123 b | 10 | 123 b | 100 a | 333 cd | 10.0 b | 333 d |
| M.26 | 100 | 117 b | 10 | 117 b | 75 a | 287 b | 8.6 b | 228 c |
| PiAu 51-4 | 100 | 94 ab | 10 | 94 ab | 100 a | 441 e | 10.0 b | 440 e |
| PiAu 56-83 | 100 | 61 a | 10 | 61 a | 100 a | 425 e | 10.0 b | 425 e |
| Stock | TCSA 2003 | TCSA 2008 | Increase 2003–2008 | ANCOVA |
|---|---|---|---|---|
| P I 514 | 4.19 a z | 56.32 a | 52.1 a | 48.6 a y |
| P I 5683 | 3.45 ab | 52.53 a | 49.1 a | 48.4 a |
| J THE | 3.08 bc | 29.20 b | 26.1 b | 26.8 b |
| B. 62396 | 2.74 bcd | 26.9 bc | 19.9 b | 21.9 b |
| G.935 | 2.56 cde | 21.48 bc | 18.9 b | 21.6 b |
| G.16 | 2.61 cde | 19.49 bcd | 16.8 bc | 18.9 b |
| M.9 P2 | 2.88 bcd | 25.69 bc | 22.8 b | 24.3 b |
| g.41 | 2.22 de | 18.60 cd | 16.4 bc | 20.3 b |
| M.26 | 1.84 ef | 23.61 bc | 21.8 b | 27.2 b |
| T337 | 1.84 ef | 18.77 cd | 16.9 bc | 22.3 b |
| B.9 | 1.10 f | 11.0 d | 9.9 c | 18.1 b |
| p-values from ANCOVA | ||||
| Rootstock | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| Covariate | - - - | - - - | - - - | 0.0015 |
| Rootstock × covariate | - - - | - - - | - - - | 0.8564 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marini, R.P. Experimental Designs and Statistical Analyses for Rootstock Trials. Agronomy 2024, 14, 2312. https://doi.org/10.3390/agronomy14102312
Marini RP. Experimental Designs and Statistical Analyses for Rootstock Trials. Agronomy. 2024; 14(10):2312. https://doi.org/10.3390/agronomy14102312
Chicago/Turabian StyleMarini, Richard P. 2024. "Experimental Designs and Statistical Analyses for Rootstock Trials" Agronomy 14, no. 10: 2312. https://doi.org/10.3390/agronomy14102312






