Biological Characterization and Fungicide Sensitivity of Dactylobotrys graminicola Causing Oat Spikelet Rot in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal and Antimicrobial Agents
2.2. Biological Characterization of D. graminicola Strain GY-18 In Vitro
2.3. Antimicrobial Activity to D. graminicola In Vitro
2.4. Evaluation of Fungicides against Oat Spikelet Rot under Artificial Inoculation Conditions
2.5. Statistical Analysis
3. Results
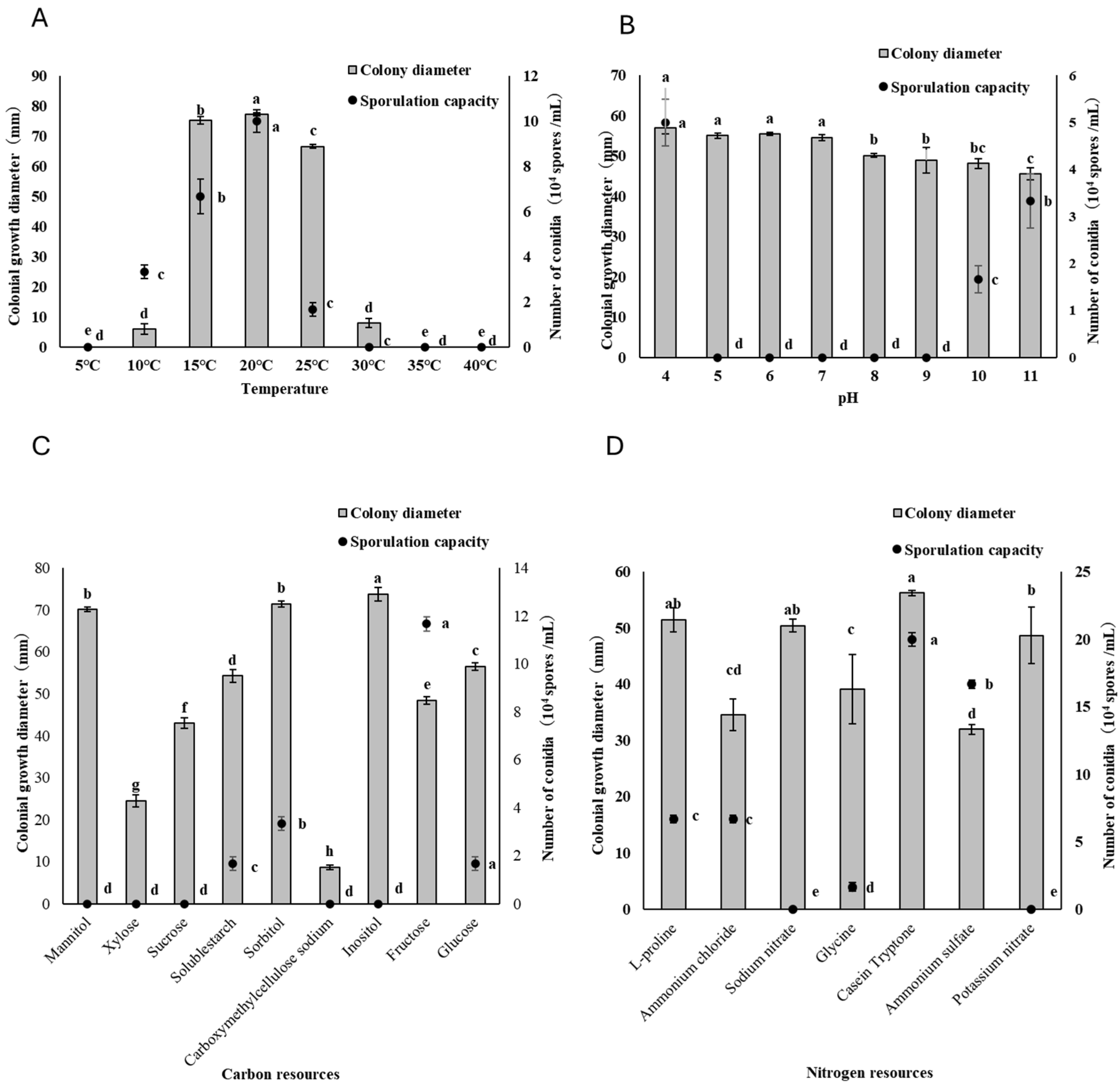
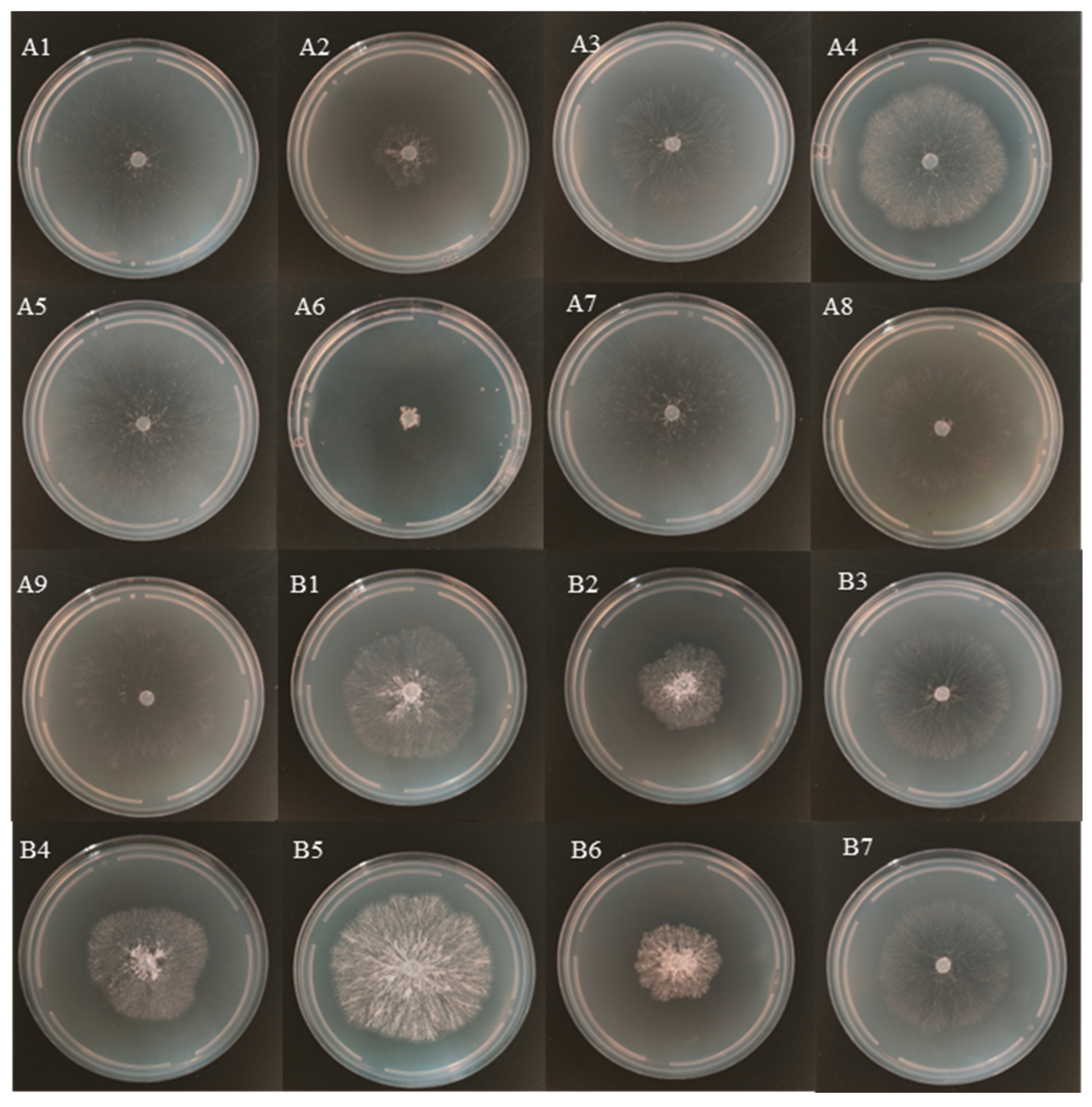
3.1. Effect of Temperature and pH
3.2. Utilization of Different Carbon and Nitrogen Sources
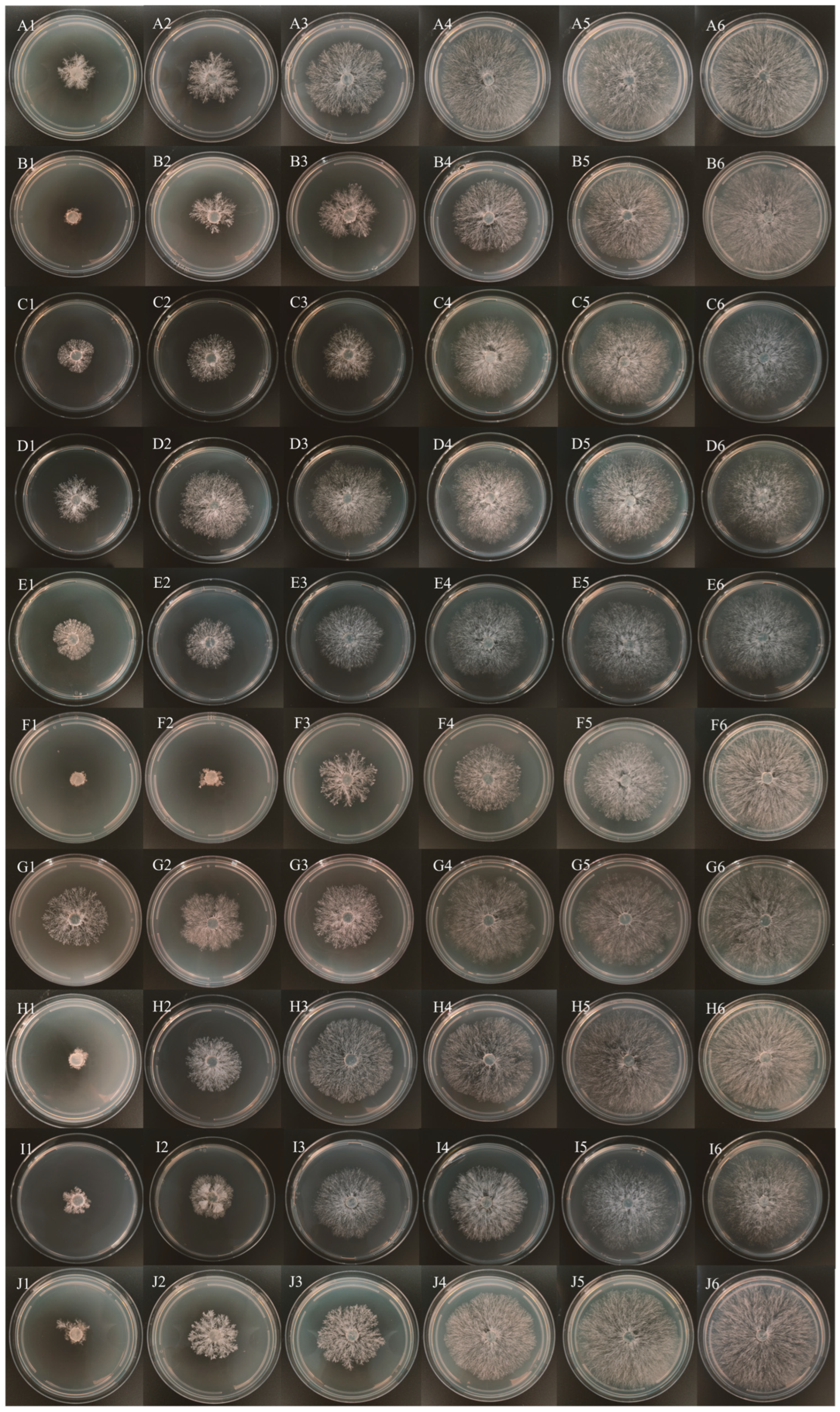
3.3. Fungicide Assays
3.4. Efficacy Assays
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kamal, N.; Tsardakas Renhuldt, N.; Bentzer, J.; Gundlach, H.; Haberer, G.; Juhász, A.; Lux, T.; Bose, U.; Tye-Din, J.A.; Lang, D.; et al. The mosaic oat genome gives insights into a uniquely healthy cereal crop. Nature 2022, 606, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, T.; Wei, X.; Kamran, M.; White, J.; Zhao, G.; Li, C. Evaluation of different antimicrobial agents for laboratory and field against Pantoea agglomerans, the causative agent of bacterial leaf blight disease on oat (Avena sativa). Plant Pathol. 2023, 72, 1585–1594. [Google Scholar] [CrossRef]
- Wang, C.; Liu, J.; Li, S.; Zhang, T.; Shi, X.; Zeng, Z.; Lei, Y.; Chu, Q. Diagnosing the climatic and agronomic dimensions of rain-fed oat yield gaps and their restrictions in North and Northeast China. Sustainability 2019, 11, 2104. [Google Scholar] [CrossRef]
- Ye, X.; Gan, Z.; Wan, Y.; Xiang, D.; Wu, X.; Wu, Q.; Zou, L. Progress and Prospects of Breeding Research on Forage Oats. J. Grass Ind. 2023, 32, 160. [Google Scholar]
- Liu, W.; Yang, C.; Zhang, X.; Bai, J.; Yang, X.; Li, T.; Zhou, H. Nutritional quality and correlation analysis of oat in different regions of China. ACS Agric. Sci. Technol. 2019, 12, 151–155. [Google Scholar]
- Liu, L.; Ma, M.; Liu, Z.; Zhang, L.; Zhou, J. Community structure of fungal pathogens causing spikelet rot disease of naked oat from different ecological regions of China. Sci. Rep. 2021, 11, 1243. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, S.; Zhao, G.; Wen, C.; Liu, Y.; Ma, F. A new disease of oat and barley—Sheath rot. Gansu Agric. Sci. Technol. 2010, 10, 3–4. [Google Scholar]
- Du, C.; Zhang, H.; Yao, Q. Analysis of genetic diversity of barley sheath rot fungi in Qinghai Province. J. Qinghai Univ. 2023, 41, 33–39. [Google Scholar]
- He, S.; Wen, C.; Wang, S.; Zhao, G.; Wang, S.; Liu, Y.; Ma, Y. A new genus of asexual mycorrhizal fungi causing sheath rot of barley and oats. Gramineae Finger Glucosporum. J. Mycol. 2015, 34, 331–340. [Google Scholar]
- Chen, L.; Lin, R.; Wang, F.; Pang, Y.; Li, X.; Zhao, A.; Zhang, Y.; Zhang, J.; Li, W.; He, S.; et al. Genetic diversity and pathogenicity of Aspergillus oryzae on seedling barley. Sci. Agric. Sin. 2020, 53, 213–224. [Google Scholar]
- Lurwanu, Y.; Wang, Y.; Abdul, W.; Zhan, J.; Yang, L. Temperature-mediated plasticity regulates the adaptation of Phytophthora infestans to azoxystrobin fungicide. Sustainability 2020, 12, 1188. [Google Scholar] [CrossRef]
- Shcherbakova, L.; Mikityuk, O.; Arslanova, L.; Stakheev, A.; Erokhin, D.; Zavriev, S.; Dzhavakhiya, V. Studying the ability of thymol to improve fungicidal effects of tebuconazole and difenoconazole against some plant pathogenic fungi in seed or foliar treatments. Front. Microbiol. 2021, 12, 629429. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, Y.; Huang, Y.; Cai, L.; Meng, J.; Song, G.; Li, Z. Identification and characterization of the pathogen Didymella segeticola causing tobacco leaf spot. J. Plant Prot. 2023, 50, 757–766. [Google Scholar]
- Guo, C.; Wei, H.; Guo, M.; He, S.; Jin, S.; Chen, H.; Wang, X.; Guo, J. Isolation, identification and biological characteristics of Fusarium verticillioides from maize ear rot samples in Gansu province. Acta Phytopathol. Sin. 2014, 44, 17–25. [Google Scholar]
- Wang, S.; He, S.; Zhuo, Q.; Wen, C. Biological characteristics of Dactylobotrys graminicola and fungicides screening test in vitro. J. Wheat Crops 2017, 37, 281–286. [Google Scholar]
- Tang, X.; Gong, Y.; Gu, Z.; Guo, X.; Cao, P.; Yi, B.; Wang, W.; Ji, D.; Pasquali, M.; Baccelli, L.; et al. Biological characterization and in vitro fungicide screenings of a new causal agent of wheat Fusarium head blight in Tibet, China. Front. Microbiol. 2022, 13, 941734. [Google Scholar] [CrossRef]
- Li, T.; Xu, Y.; Zhang, X.; Wu, X.; Zhang, Y.; Xuan, Y.; Wang, S. Virulence Characterization of Puccinia graminis f. sp. avenae and Resistance of Oat Cultivars in China. Plant Dis. 2022, 106, 901–905. [Google Scholar] [CrossRef]
- Zhao, J.; Kebede, A.; Menzies, J.; Paczos-Grzęda, E.; Chong, J.; Mitchell Fetch, J.; McCartney, C. Chromosomal location of the crown rust resistance gene Pc98 in cultivated oat (Avena sativa L.). Theor. Appl. Genet. 2020, 133, 1109–1122. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, G.; Yang, C.; Man, Y. Primary study on biotic stress tolerance of oats in tibetan plateau. Acta Agrestia Sin. 2007, 15, 582–587. [Google Scholar]
- Pan, Y.; Niu, K.; Miao, P.; Zhao, G.; Ju, Z.; Chai, J.; Yang, J.; Cui, X.; Zhang, R. Genome-wide analysis of the SWEET gene family and its response to powdery mildew and leaf spot infection in the common oat (Avena sativa L.). Preprint 2024. [Google Scholar]
- Berlezi, J.; Carvalho, I.; Silva, J.; Loro, M.; Sfalcin, I.; Pradebon, L.; Port, E.; Ourique, R.; Roza, J. Selection of white oat genotypes for contrasting fungicide management conditions. Pesqui. Agropecuária Bras. 2023, 58, e03084. [Google Scholar] [CrossRef]
- Nan, Z. Establishing sustainable management system for disease of pasture crops in China. Acta Prataculturae Sin. 2000, 9, 1–9. [Google Scholar]
- Satapute, P.; Kamble, M.; Adhikari, S.; Jogaiah, S. Influence of triazole pesticides on tillage soil microbial populations and metabolic changes. Sci. Total Environ. 2019, 651, 2334–2344. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wicaksono, W.; Berg, G.; Cernava, T. Bacterial communities in the plant phyllosphere harbour distinct responders to a broadspectrum pesticide. Sci. Total Environ. 2021, 751, 141799. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Amores, J.; Michel, S.; Löschenberger, F.; Buerstmayr, H. Dissecting the Contribution of Environmental Influences, Plant Phenology, and Disease Resistance to Improving Genomic Predictions for Fusarium Head Blight Resistance in Wheat. Agronomy 2020, 10, 2008. [Google Scholar] [CrossRef]
- Yang, L.; Nkurikiyimfura, O.; Pan, Z.; Wang, Y.; Waheed, A.; Chen, R.; Burdon, J.; Sui, Q.; Zhan, J. Plant diversity ameliorates the evolutionary development of fungicide resistance in an agricultural ecosystem. J. Appl. Ecol. 2021, 58, 2566–2578. [Google Scholar] [CrossRef]
- Xin, W.; Mao, Y.; Lu, F.; Li, T.; Wang, J.; Duan, Y.; Zhou, M. In vitro fungicidal activity and in planta control efficacy of coumoxystrobin against Magnaporthe oryzae. Pestic. Biochem. Physiol 2020, 162, 78–85. [Google Scholar] [CrossRef]
- Okorski, A.; Milewska, A.; Pszczółkowska, A.; Karpiesiuk, K.; Kozera, W.; Dąbrowska, J.; Radwińska, J. Prevalence of Fusarium fungi and Deoxynivalenol Levels in Winter Wheat Grain in Different Climatic Regions of Poland. Toxins 2022, 14, 102. [Google Scholar] [CrossRef]
- Ren, C.; Liang, G.; Liu, W.; Liu, K.; Duan, J. Screening and adaptive evaluation of early-maturing oats in the alpine region of the Qinghai-Tibetan Plateau. J. Grass Ind. 2023, 32, 116–129. [Google Scholar]
- Jiang, S.; Zhou, D.; Cai, L. Study on the evaluation of quality grade and fertility analysis of arable land in Shandan County. Res. Land Nat. Resour. 2023, 139, 23–28. [Google Scholar]
- Wang, X.; Yu, C. Identification of the pathogen, biological characterization and indoor virulence determination of the fungus of Yuanbao maple. J. Plant Pathol. 2024, 54, 26–35. [Google Scholar]
- Ding, S.; Shao, X.; Li, J.; Ahammed, G.; Yao, Y.; Ding, J.; Hu, Z.; Yu, J.; Shi, K. Nitrogen forms and metabolism affect plant defence to foliar and root pathogens in tomato. Plant Cell Environ. 2021, 44, 1596–1610. [Google Scholar] [CrossRef]
- Li, S.; Pang, J.; Fang, X. Progress of research on nitrogen, phosphorus and potassium nutrition affecting plant root diseases. J. Plant Physiol. 2023, 59, 2011–2017. [Google Scholar]
- Sharma, R.; Ahir, R.; Yadav, S.; Sharma, P.; Ghasolia, R. Effect of nutrients and plant extracts on Alternaria blight of tomato caused by Alternaria alternata. J. Plant Dis. Prot. 2021, 128, 951–960. [Google Scholar] [CrossRef]
- Nelson, R. International plant pathology: Past and future contributions to global food security. Phytopathology 2020, 110, 245–253. [Google Scholar] [CrossRef]
- Gautier, C.; Pinson-Gadais, L.; Richard-Forget, F. Fusarium mycotoxins enniatins: An updated review of their occurrence, the producing Fusarium species, and the abiotic determinants of their accumulation in crop harvests. J. Agric. Food Chem. 2020, 68, 4788–4798. [Google Scholar] [CrossRef]
- Hay, W.; Anderson, J.; McCormick, S.; Hojilla-Evangelista, M.; Selling, G.; Utt, K.; Bowman, M.; Doll, K.; Ascherl, K.; Berhow, M.; et al. Fusarium head blight resistance exacerbates nutritional loss of wheat grain at elevated CO2. Sci. Rep. 2022, 12, 15. [Google Scholar] [CrossRef]


| Fungicide Name | Abbreviation | Formulation | Registration Number | Plate Concentration (mg/L) |
|---|---|---|---|---|
| 32.5% Difenoconazole Azoxystrobin | DA | SC | PD20150707 | 10, 1, 0.1, 0.01, 0.001 |
| 30% Difenoconazole Propiconazole | DP | EC | PD20211385 | 0.625, 0.125, 0.025, 0.005, 0.001 |
| 70% Mancozeb | MAN | WP | PD20060179 | 24, 12, 6, 3, 1.5 |
| 0.25% Fludioxonil | FLU | SD | PD20150099 | 0.540, 0.18, 0.06, 0.02, 0.007 |
| 68% Metalaxyl-M Mancozeb | MMM | WG | PD20080846 | 32, 16, 8, 4, 2 |
| 20% Pydiflumetofen | PYD | SC | PD20220035 | 0.081, 0.027, 0.009, 0.003, 0.001 |
| 50% Kresoxim-methyl | KM | WG | PD20070124 | 1.62, 0.54, 0.18, 0.06, 0.02 |
| 35% Metalaxyl-M Fludioxonil | MMF | SD | PD20171139 | 0. 128, 0.064, 0.032, 0.016, 0.008 |
| 15% Triadimefon | TRI | WP | PD20040283 | 160, 80, 40, 20, 10 |
| 10% Trifloxystrobin &20% Tebuconazole | TT | SC | PD20184323 | 10, 1, 0.1, 0.01, 0.001 |
| Agent a | EC50 (mg/L) | Regression Equation b | R2 |
|---|---|---|---|
| DA | 0.750 | y = 0.54x + 5.0673 | 0.986 |
| DP | 0.050 | y = 0.7178x + 5.9392 | 0.977 |
| MAN | 8.280 | y = 1.176x + 3.9197 | 0.950 |
| FLU | 0.310 | y = 0.7056x + 5.3617 | 0.999 |
| MMM | 13.960 | y = 1.073x +3.7708 | 0.996 |
| PYD | 0.005 | y = 1.3237x + 8.0904 | 0.958 |
| KM | 189.230 | y = 1.2884x + 2.0665 | 0.916 |
| MMF | 0.040 | y = 2.323x + 8.3689 | 0.988 |
| TRI | 46.060 | y = 1.5726x + 2.3843 | 0.984 |
| WT | 0.250 | y = 0.4121x + 5.2474 | 0.998 |
| Agent a | Disease Spikelet Incidence (%) | Control Effects (%) |
|---|---|---|
| DA | 8.05 ± 1.70 d | 87.39 ± 2.27 a |
| DP | 16.67 ± 4.61 c | 73.93 ± 6.36 b |
| MAN | 22.00 ± 6.00 c | 65.43 ± 9.09 b |
| FLU | 27.67 ± 7.77 b | 56.41 ± 12.45 c |
| MMM | 21.00 ± 4.36 c | 67.12 ± 5.66 b |
| PYD | 7.33 ± 1.15 d | 88.52 ± 1.28 a |
| KM | 8.00 ± 2.00 d | 87.42 ± 3.03 a |
| MMF | 19.67 ± 4.04 c | 69.24 ± 5.02 b |
| TRI | 10.33 ± 2.51 d | 83.84 ± 3.45 a |
| TT | 6.00 ± 2.00 d | 90.66 ± 2.72 a |
| Water | 63.67 ± 3.21 a | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, R.; Wang, N.; Chen, Z.; Wang, S.; Lin, K.; Zhang, Y. Biological Characterization and Fungicide Sensitivity of Dactylobotrys graminicola Causing Oat Spikelet Rot in China. Agronomy 2024, 14, 2314. https://doi.org/10.3390/agronomy14102314
Jia R, Wang N, Chen Z, Wang S, Lin K, Zhang Y. Biological Characterization and Fungicide Sensitivity of Dactylobotrys graminicola Causing Oat Spikelet Rot in China. Agronomy. 2024; 14(10):2314. https://doi.org/10.3390/agronomy14102314
Chicago/Turabian StyleJia, Ruifang, Na Wang, Zhengqiang Chen, Shengze Wang, Kejian Lin, and Yuanyuan Zhang. 2024. "Biological Characterization and Fungicide Sensitivity of Dactylobotrys graminicola Causing Oat Spikelet Rot in China" Agronomy 14, no. 10: 2314. https://doi.org/10.3390/agronomy14102314
APA StyleJia, R., Wang, N., Chen, Z., Wang, S., Lin, K., & Zhang, Y. (2024). Biological Characterization and Fungicide Sensitivity of Dactylobotrys graminicola Causing Oat Spikelet Rot in China. Agronomy, 14(10), 2314. https://doi.org/10.3390/agronomy14102314






