The Effect of Nitrogen and Potassium Interaction on the Leaf Physiological Characteristics, Yield, and Quality of Sweet Potato
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site Overview
2.2. Experimental Design
2.3. Measurement Content and Method
2.3.1. Metabolic Enzyme Assay
2.3.2. Determination of Photosynthetic Characteristics
2.3.3. Root Quality and Yield Determination
2.4. Statistical Analysis
3. Results
3.1. Leaf Metabolic Enzyme Activity Analysis
3.1.1. Leaf Soluble Protein Analysis
3.1.2. Leaf GS Analysis
3.2. Leaf Photosynthetic Characteristics Analysis
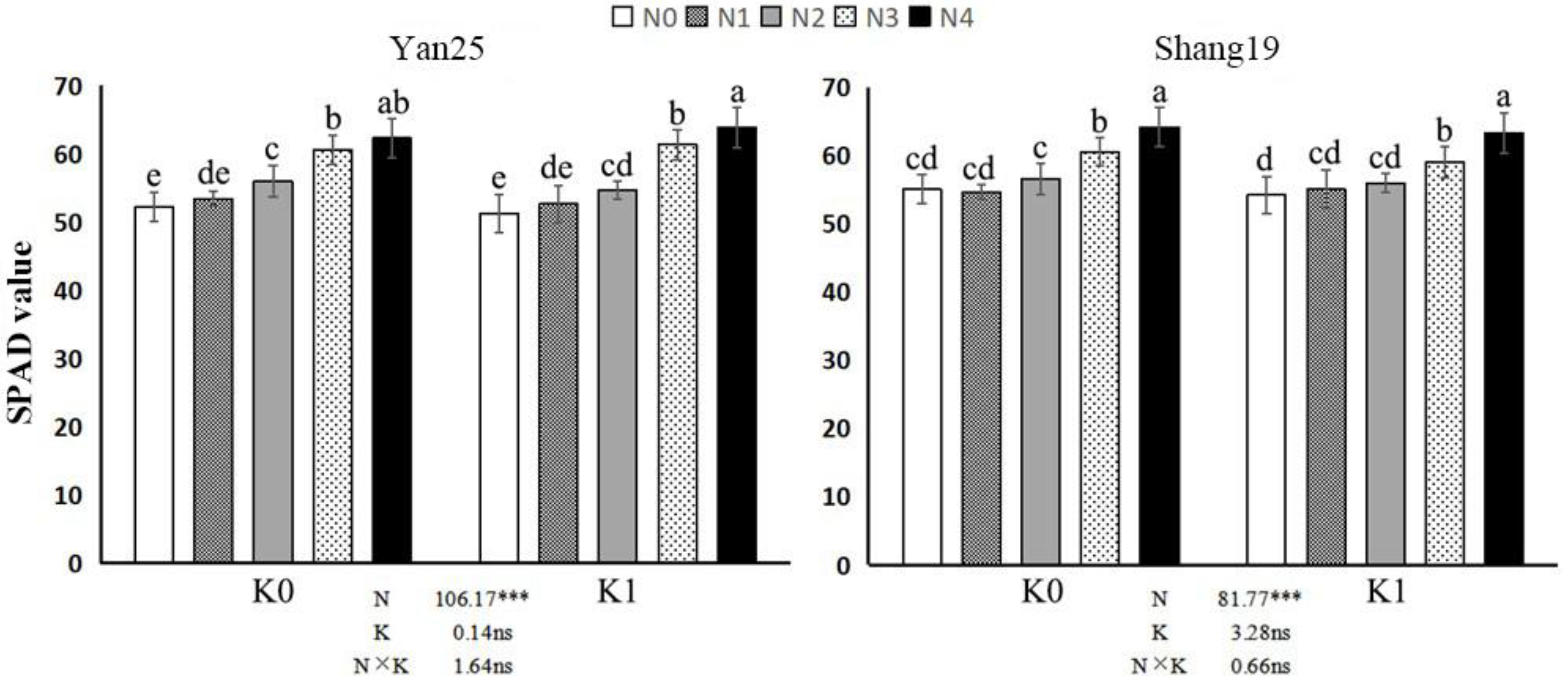
3.2.1. SPAD Value
3.2.2. Leaf Photosynthetic Characteristics
3.3. Yield and Quality Analysis
3.3.1. Quality Analysis
3.3.2. Yield Analysis
4. Discussion
4.1. Effects of Combined Application of N and K on Metabolic Enzymes of Sweet Potato Leaves
4.2. Effects of Combined Application of N and K on Photosynthetic Characteristics of Sweet Potato
4.3. Effects of Combined Application of N and K on Nutrient Composition and Yield of Sweet Potato Roots
4.4. Effects of Photosynthetic Characteristics of Sweet Potato on Root Tuber Yield and Quality under N and K Combination Conditions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Wang, Q.; Wang, Y. The main nutrient components and health care function of sweet potato. Hortic. Seed 2003, 23, 162–166. [Google Scholar]
- Huang, X.; Liu, L.; He, H.; Guo, S.; He, W.; Zhang, X.; Li, H.; Yang, H. Effects of plastic film mulching on agronomic traits and yield of fresh-eating sweet potato variety Pushu 32. Mod. Agric. Sci. Technol. 2023, 4, 29–31. [Google Scholar]
- Coskun, D.; Britto, D.T.; Kronzucker, H.J. The nitrogen-potassium intersection: Membranes, metabolism, and mechanism. Plant Cell Environ. 2017, 40, 2029–2041. [Google Scholar] [CrossRef] [PubMed]
- Soratto, R.P.; Job, A.L.G.; Fernandes, A.M.; Assunção, N.S.; Fernandes, F.M. Biomass accumulation and nutritional requirements of potato as affected by potassium supply. J. Soil. Sci. Plant Nutr. 2020, 20, 1051–1066. [Google Scholar] [CrossRef]
- Liu, C.; Wang, M.; Sun, H.; Yu, L.; Sun, Z.; Qiang, B. Effects of nitrogen levels on nitrogen metabolism enzymes activities, nitrogen absorption and nutritional quality of kidney bean leaves. J. Northeast. Agric. Sci. 2020, 45, 16–21. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X.; Liu, B.; Zhang, H.; Guo, X. Heterosis of Enzymes Involved in Nitrogen Metabolism at Different Growth Stages in Hybrid Millet. Acta Agric. Boreali-Occident. Sin. 2013, 22, 75–79. [Google Scholar]
- Meng, W.; Wang, D.; Yu, Z. Effects of nitrogen application rate on enzyme activities related to nitrogen metabolism and grain protein quality in wheat. J. Plant Nutr. Fert. 2012, 18, 10–17. [Google Scholar]
- Zhao, P.; He, J.; Xiong, S.; Ma, X. Effects of nitrogen forms on flag leaf enzyme activity, grain protein and yield of special wheat. J. China Agric. Univ. 2010, 15, 29–34. [Google Scholar]
- Runyon, J.R.; Sunilkumar, B.A.; Nilsson, L.; Rascon, A.; Bergenståhl, B. The effect of heat treatment on the soluble protein content of oats. J. Cereal Sci. 2015, 65, 119–124. [Google Scholar] [CrossRef]
- Liu, N.; Yan, Z.; Fan, Y.; Song, B.; Jia, H.; Yang, J. Effect of different nitrogen application levels on the content of soluble protein and key enzyme activities in nitrogen metabolism of sugar beet. Chin. Agric. Sci. Bull. 2015, 31, 149–154. [Google Scholar]
- Calatayud, A.; Iglesias, D.J.; Talón, M.; Barreno, E. Effects of long-term ozone exposure on citrus: Chlorophyll a fluorescence and gas exchange. Photosynthetica 2006, 44, 548–554. [Google Scholar] [CrossRef]
- Xiong, D.; Yu, T.; Liu, X.; Li, Y.; Peng, S.; Huang, J. Heterogeneity of photosynthesis within leaves is associated with alteration of leaf structural features and leaf N content per leaf area in rice. Funct. Plant Biol. 2015, 42, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Kunz, H.H.; Gierth, M.; Herdean, A.; Satoh-Cruz, M.; Kramer, D.M.; Spetea, C.; Schroeder, J.I. Plastidial transporters KEA1, -2, and -3 are essential for chloroplast osmoregulation, integrity, and pH regulation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 7480–7485. [Google Scholar] [CrossRef] [PubMed]
- Laterre, R.; Pottier, M.; Remacle, C.; Boutry, M. Photosynthetic trichomes contain a specific rubisco with a modified pH-dependent activity. Plant Physiol. 2017, 173, 2110–2120. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shi, C.; Chai, S.; Wang, C.; Ren, G.; Jiang, Y.; Si, C. Effect of different potassium application time on the vigor of photosynthate transportations of edible sweet potato (Ipomoea batata L.). J. Plant Nutr. Fert. 2015, 21, 171–180. [Google Scholar]
- Du, X.; Zhang, X.; Xi, M.; Kong, L. Split application enhances sweetpotato starch production by regulating the conversion of sucrose to starch under reduced nitrogen supply. Plant Physiol. Biochem. 2020, 151, 743–750. [Google Scholar] [CrossRef]
- Lamaro, G.P.; Tsehaye, Y.; Girma, A.; Vannini, A.; Fedeli, R.; Loppi, S. Evaluation of Yield and Nutraceutical Traits of Orange-Fleshed Sweet Potato Storage Roots in Two Agro-Climatic Zones of Northern Ethiopia. Plants 2023, 12, 1319. [Google Scholar] [CrossRef]
- Lamaro, G.P.; Tsehaye, Y.; Girma, A.; Vannini, A.; Fedeli, R.; Loppi, S. Essential Mineral Elements and Potentially Toxic Elements in Orange-Fleshed Sweet Potato Cultivated in Northern Ethiopia. Biology 2023, 12, 266. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Yang, J.; Fan, W.; Yang, N.; Yin, M.; Zhang, P. Advances in storage root development and regulation in sweetpotato (Lpomoea batata L.). Plant Physiol. J. 2017, 53, 749–757. [Google Scholar] [CrossRef]
- Mortley, D.G.; Bonsi, C.K.; Hill, W.A.; Morris, C.E.; Williams, C.S.; Davis, C.F.; Williams, J.W.; Levine, L.H.; Petersen, B.V.; Wheeler, R.M. Influence of microgravity environment on root growth, soluble sugars, and starch concentration of sweet potato stem cuttings. J. Am. Soc. Hortic. Sci. 2008, 133, 327–332. [Google Scholar] [CrossRef]
- Ankumah, R.O.; Khan, V.; Mwamba, K.; Kpomblekou-A, K. The influence of source and timing of nitrogen fertilizers on yield and nitrogen use efficiency of four sweet potato cultivars. Agric. Ecosyst. Environ. 2003, 100, 201–207. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, L.; Shi, C.; Zhang, C.; Liu, H.; Zhang, H. Effects of potassium application period on the plant traits and yield of sweet potato. Acta Agric. Boreali-Occident. Sin. 2010, 19, 82–85. [Google Scholar]
- Feng, J.; Shao, Z.; Wang, B.; Ma, Q.; Wang, Y.; Hou, W.; Gao, Q. Effects of nitrogen and potassium combined application on canopy structure, light energy allocation and utilization and yield formation of rice. J. Jilin Agric. Univ. 2023, 1–13. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Q.; Shi, Y.; Li, H. Interactive effects of nitrogen and potassium on photosynthesis product distribution and accumulation of sweet potato. Sci. Agric. Sin. 2017, 50, 2706–2716. [Google Scholar]
- Sun, Z.; Tian, C.; Chen, L.; Wang, H.; Zheng, J.; Zhao, F. Interactive effects of nitrogen and potassium on the stem and leaves growth, yield formation and dry matter distribution of sweet potato. Soil. Fert. Sci. China 2021, 4, 186–191. [Google Scholar]
- Zhang, L.; Fan, J. Plant Physiology Experiment Tutorial; China Agricultural University Press: Beijing, China, 2007. [Google Scholar]
- Li, C.; Ma, F.; Zhao, Y.; Li, W. Effects of nitrogen forms on key enzyme activities and related products of nitrogen and sugar metabolism in sugar beet. Acta Agron. Sin. 2003, 29, 128–132. [Google Scholar]
- Chen, X.; Li, H.; Zhang, A.; Shi, X.; Tang, Z.; Wei, M.; Shi, C. Effects of paclobutrazol on photosynthesis and starch accumulation of edible sweet potato under different nitrogen levels. Acta Agron. Sin. 2012, 38, 1728–1733. [Google Scholar]
- Hirel, B.; Bertin, P.; Quilleré, I.; Bourdoncle, W.; Attagnant, C.; Dellay, C.; Gouy, A.; Cadiou, S.; Retailliau, C.; Falque, M.; et al. Towards a better understanding of the genetic and physiological basis for nitrogen use efficiency in maize. Plant Physiol. 2001, 125, 1258–1270. [Google Scholar] [CrossRef]
- Weng, B.; Zheng, X.; Zhao, T.; Xu, G.; Wang, J.; Ye, H. Analysis of protein content and nitrogen metabolism related enzyme activity in peanut leaves at different growth stages. J. Plant Resour. Environ. 2014, 23, 65–70. [Google Scholar]
- Liu, L.; Zou, D. Effects of nitrogen application rate on glutamine synthetase activity and yield of rice grain. J. Northeast. Agric. Univ. 2009, 40, 1–4. [Google Scholar] [CrossRef]
- Jiao, F.; Wang, P.; Zhai, R. Effects of potassium fertilizer on the activity of nitrate reductase and glutamine synthetase, yield and quality of soybean. J. Heilongjiang Bayi Agric. Univ. 2009, 21, 12–15. [Google Scholar]
- Geng, Y.; Li, G.; Cao, X.; Li, C.; Cao, G. Effects of different levels of nitrogen and potassium nutrition on nitrogen metabolism of spring maize. J. Maize Sci. 2009, 17, 101–104. [Google Scholar]
- Zhu, R.; Mu, Y.; Kang, J.; Zhao, J.; Wu, H. Effects of different nitrogen application rates on chlorophyll content and fluorescence characteristics of high temperature spring wheat after anthesis. J. South. Agric. 2017, 48, 609–615. [Google Scholar]
- Ma, Z.; Du, H.; Liu, R.; Yan, Z. Effects of zinc fertilizer on dry matter accumulation, physiological characteristics and tuber nutritional quality of potato. J. Arid. Land Resour. Environ. 2017, 31, 148–153. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, J.; Wang, X. Study on nitrogen nutrition diagnosis technology of sugar beet by SPAD meter. Chin. Agric. Sci. Bull. 2015, 31, 92–98. [Google Scholar]
- Sekhar, K.M.; Kota, V.R.; Reddy, T.P.; Rao, K.V.; Reddy, A.R. Amelioration of plant responses to drought under elevated CO2 by rejuvenating photosynthesis and nitrogen use efficiency: Implications for future climate-resilient crops. Photosynth. Res. 2021, 150, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.J.; Xu, X.M.; Zhu, Y.M.; He, W.C.; Jin, M.H.; Liu, Y.H.; Lu, G.Q.; Lv, Z.F. Changes in dilution curves of critical nitrogen concentration in sweetpotato under different potassium conditions. Field Crops Res. 2023, 303, 109130. [Google Scholar] [CrossRef]
- Zheng, S.; Yang, S.; Li, S.; Yuan, J. Effects of nitrogen fertilizer level on photosynthesis and chlorophyll fluorescence characteristics of potato. J. Southwest. Univ. 2013, 35, 1–9. [Google Scholar] [CrossRef]
- Wei, Q.; Cao, M.; Shi, Y.; Chen, B. Effects of nitrogen levels on photosynthetic characteristics and yield of potato during the whole growth period. Genom. Appl. Biol. 2017, 36, 324–330. [Google Scholar] [CrossRef]
- Wei, M.; Tang, Z.; Chen, X.; Li, H.; Zhang, A.; Shi, X. Effects of different nitrogen levels on photosynthesis and growth characteristics of leaf-vegetable sweetpotato. Jiangsu J. Agric. Sci. 2014, 30, 87–91. [Google Scholar]
- Chen, G.; Gao, S.; Zhang, X. Effects of potassium application and water supplement on photosynthetic characteristics and yield of dryland potato. J. Gansu Agric. Univ. 2009, 44, 74–78. [Google Scholar]
- Fan, Y.; Sun, J.; Gao, J.; Liu, J.; Su, Z.; Hu, S.; Wang, Z.; Yu, X. Effects of potassium fertilizer on photosynthesis, fluorescence characteristics and potassium absorption efficiency of maize. Mol. Plant Breed. 2023, 21, 337–348. [Google Scholar] [CrossRef]
- Tang, Z.; Li, H.; Zhang, A.; Shi, X.; Wei, M.; Chen, X.; Ding, Y. Responses of photosynthetic characteristics of sweet potato leaves and main root traits to nitrogen supply forms. J. Plant Nutr. Fert. 2013, 19, 1494–1501. [Google Scholar]
- Thompson, M.V.; Holbrook, N.M. Application of a single-solute non-steady-state phloem model to the study of long-distance assimilate transport. J. Theor. Biol. 2003, 220, 419–455. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Wang, Z.; Zhao, B.; Guo, F.; Yu, S. Effects of potassium nutrition on parenchyma cell microstructure, 14C assimilate distribution and yield of sweetpotato storage roots. J. Plant Nutr. Fertil. 2002, 3, 335–339. [Google Scholar]
- Zheng, Y. Effects of potassium on accumulation and distribution of assimilates in sweet potato. Soil. Fert. Sci. China 2004, 4, 14–16. [Google Scholar]
- Zhao, G.; Chang, X.; Liu, L.; Yang, Y.; Chi, Z.; Yang, L.; Li, Z. Effects of nitrogen application rate on yield and processing quality of different strong gluten wheat varieties. Acta Agron. Sin. 2006, 47, 723–727. [Google Scholar]
- Yao, L.; Tan, X.; Jiang, X. Effects of different nitrogen application rates on growth and yield of edible sweet potato. J. Anhui Agric. Sci. 2015, 43, 33–35. [Google Scholar] [CrossRef]
- Cao, Y.; Zhao, J.; Zhao, H. Effects of potassium application rate on soluble sugar and yield of sweet corn. Crops 2009, 5, 64–65. [Google Scholar]
- Du, M. Effects of Potassium Fertilizer Application on Yield and Quality in Vegetable Soybean; Northeast Agricultural University: Harbin, China, 2013. [Google Scholar]
- Hou, M.; Zhang, Y.; Liu, Y.; Wang, X.; Tang, W.; Yan, H.; Ma, D.; Li, Q. Effects of different application periods of potassium fertilizer on yield and quality of edible sweet potato. Acta Agric. Boreali-Sin. 2014, 29, 368–372. [Google Scholar]
- Gao, L.; Fang, Z.; Shi, Y. Effects of nitrogen application rate on yield, quality and nitrogen utilization of fresh-eating sweetpotato. Acta Agric. Boreali-Sin. 2014, 29, 189–194. [Google Scholar]
- Wu, H.; Hou, S.; Liu, Q.; Li, H. Effects of nitrogen application rate and planting density on population development and yield formation of sweet potato. J. Jiangsu Norm. Univ. Nat. Sci. Ed. 2019, 37, 35–39. [Google Scholar]
- Tang, Z.; Li, H.; Zhang, A.; Shi, X.; Zhu, H.; Sun, J. Effects of long-term located fertilization on root yield and main quality of sweetpotato. Acta Agric. Univ. Zhejiangensis 2010, 22, 57–61. [Google Scholar]
- Wang, J.D.; Hou, P.F.; Zhu, G.P. Potassium partitioning and redistribution as a function of K-use efficiency under K deficiency in sweet potato (Ipomoea batatas L.). Field Crops Res. 2017, 211, 147–154. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Z.; Liang, T.; Zhang, X.; Liu, L.; Shi, C. Effects of potassium humate on potassium absorption, utilization and root yield of edible sweetpotato varieties. Plant Nutr. Fert. Sci. 2008, 3, 520–526. [Google Scholar]
- Wang, M.; Fang, Z.; Liang, B.; Zeng, L.; Li, J. Effects of combined application of nitrogen and potassium on yield and quality of fresh-eating sweet potato in high fertility soil. Acta Agric. Boreali-Sin. 2016, 31, 199–204. [Google Scholar]
- Simkin, A.J.; López-Calcagno, P.E.; Raines, C.A. Feeding the world: Improving photosynthetic efficiency for sustainable crop production. J. Exp. Bot. 2019, 70, 1119–1140. [Google Scholar] [CrossRef]
- Battie-Laclau, P.; Laclau, J.P.; Beri, C.; Mietton, L.; Muniz, M.R.; Arenque, B.C.; de Cassia Piccolo, M.; Jordan-Meille, L.; Bouillet, J.P.; Nouvellon, Y. Photosynthetic and anatomical responses of Eucalyptus grandis leaves to potassium and sodium supply in a field experiment. Plant Cell Environ. 2014, 37, 70–81. [Google Scholar] [CrossRef]
- Xiong, D.; Liu, X.; Liu, L.; Douthe, C.; Li, Y.; Peng, S.; Huang, J. Rapid responses of mesophyll conductance to changes of CO2 concentration, temperature and irradiance are affected by N supplements in rice. Plant Cell Environ. 2015, 38, 2541–2550. [Google Scholar] [CrossRef]

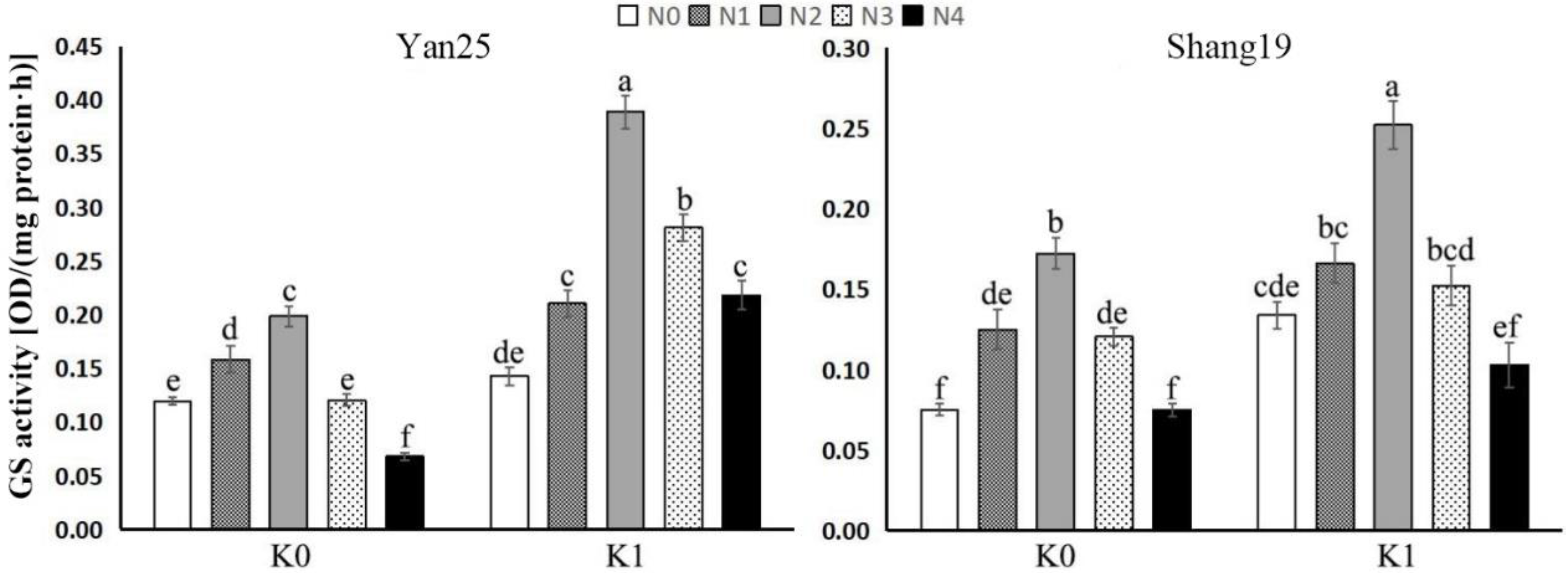
| Varieties | ANOVA | F Value | |
|---|---|---|---|
| Soluble Protein | GS Enzyme | ||
| Yan25 | N | 63.1 *** | 75.1 *** |
| K | 23.0 ** | 302.0 *** | |
| N × K | 1.3 ns | 24.2 *** | |
| Shang19 | N | 46.9 *** | 30.3 *** |
| K | 109.4 *** | 61.0 *** | |
| N × K | 1.5 ns | 10.9 ** | |
| K Treatments | N Treatments | Pn/(μmol/m2·s) | Gs/(mol/m2·s) | Ci/(μmol/mol) | Tr/(mmol/m2·s) |
|---|---|---|---|---|---|
| N0 | 20.01 ± 0.99 c | 0.46 ± 0.02 bc | 256.8 ± 4.48 ef | 3.47 ± 0.42 d | |
| N1 | 20.82 ± 0.21 bc | 0.47 ± 0.04 bc | 280.1 ± 5.58 bc | 3.82 ± 0.40 cd | |
| K0 | N2 | 21.40 ± 0.72 bc | 0.48 ± 0.03 abc | 289.5 ± 3.30 ab | 4.43 ± 0.31 bc |
| N3 | 22.68 ± 0.44 ab | 0.48 ± 0.02 abc | 298.3 ± 3.08 a | 4.92 ± 0.07 ab | |
| N4 | 21.66 ± 0.41 bc | 0.43 ± 0.03 c | 289.8 ± 2.23 ab | 5.14 ± 0.13 ab | |
| N0 | 20.56 ± 0.76 bc | 0.49 ± 0.03 abc | 245.6 ± 3.35 f | 5.02 ± 0.19 ab | |
| N1 | 21.22 ± 0.65 bc | 0.53 ± 0.01 ab | 260.2 ± 2.1 de | 5.38 ± 0.17 a | |
| K1 | N2 | 22.49 ± 0.82 ab | 0.50 ± 0.01 abc | 280.7 ± 8.00 bc | 5.34 ± 0.13 a |
| N3 | 24.76 ± 0.64 a | 0.56 ± 0.03 a | 289.6 ± 5.50 ab | 5.53 ± 0.20 a | |
| N4 | 22.37 ± 0.84 ab | 0.45 ± 0.01 bc | 273.4 ± 2.30 cd | 5.62 ± 0.22 a | |
| ANOVA | F value | ||||
| N | 7.21 * | 2.90 ns | 27.79 *** | 41.85 *** | |
| K | 5.74 * | 6.47 * | 21.40 ** | 6.43 * | |
| N × K | 0.463 ns | 0.575 ns | 0.61 ns | 2.46 ns | |
| K Treatments | N Treatments | Pn/(μmol/m2·s) | Gs/(mol/m2·s) | Ci/(μmol/mol) | Tr/(mmol/m2·s) |
|---|---|---|---|---|---|
| N0 | 15.51 ± 0.65 c | 0.39 ± 0.04 b | 269.67 ± 6.49 cd | 4.86 ± 0.19 e | |
| N1 | 15.88 ± 0.65 c | 0.40 ± 0.03 b | 280.4 ± 3.20 bc | 5.04 ± 0.12 de | |
| K0 | N2 | 17.30 ± 0.95 abc | 0.45 ± 0.02 ab | 288.5 ± 6.18 ab | 5.11 ± 0.12 de |
| N3 | 19.24 ± 0.63 ab | 0.47 ± 0.04 ab | 297.6 ± 2.50 a | 5.24 ± 0.21 cde | |
| N4 | 16.77 ± 0.90 bc | 0.46 ± 0.02 ab | 274.9 ± 4.30 bcd | 5.81 ± 0.27 abc | |
| N0 | 17.17 ± 0.65 abc | 0.44 ± 0.03 ab | 262.2 ± 2.40 d | 5.60 ± 0.28 bcd | |
| N1 | 17.88 ± 0.67 abc | 0.45 ± 0.02 ab | 272.3 ± 4.10 cd | 5.98 ± 0.09 ab | |
| K1 | N2 | 18.63 ± 1.11 ab | 0.48 ± 0.02 ab | 277.6 ± 3.30 bc | 6.08 ± 0.19 ab |
| N3 | 19.59 ± 0.81 a | 0.49 ± 0.01 a | 283.2 ± 3.93 bc | 6.14 ± 0.13 ab | |
| N4 | 17.21 ± 0.40 abc | 0.47 ± 0.03 a | 271.5 ± 4.20 cd | 6.41 ± 0.19 a | |
| ANOVA | F value | ||||
| N | 4.98 * | 3.194 ns | 9.62 ** | 5.02 * | |
| K | 5.66 * | 4.418 ns | 10.79 * | 48.18 *** | |
| N × K | 0.46 ns | 0.382 ns | 0.461 ns | 1.05 ns | |
| K Treatments | N Treatments | Yan25 | N Treatments | Shang19 | ||||
|---|---|---|---|---|---|---|---|---|
| Starch/% | Soluble Sugar/% | Protein/% | Starch/% | Soluble Sugar/% | Protein/% | |||
| N0 | 43.2 ± 1.06 f | 20.7 ± 0.82 ab | 3.4 ± 0.34 d | N0 | 55.4 ± 1.2 d | 12.2 ± 0.51 ab | 3.2 ± 0.07 d | |
| N1 | 45.7 ± 0.85 e | 19.1 ± 0.23 cd | 3.5 ± 0.16 cd | N1 | 59.7 ± 1.02 c | 11.5 ± 0.47 bc | 3.5 ± 0.13 d | |
| K0 | N2 | 48.2 ± 0.99 d | 17.3 ± 0.33 e | 3.9 ± 0.11 c | N2 | 62.1 ± 0.52 c | 8.9 ± 0.48 ef | 3.9 ± 0.14 cd |
| N3 | 51.3 ± 0.57 c | 14.8 ± 0.13 g | 4.5 ± 0.10 b | N3 | 63.2 ± 0.45 c | 8.2 ± 0.82 fg | 4.7 ± 0.58 ab | |
| N4 | 52.2 ± 0.99 c | 14.3 ± 0.23 g | 4.8 ± 0.14 ab | N4 | 64.1 ± 1.37 c | 7.2 ± 0.12 g | 5.1 ± 0.06 a | |
| N0 | 48.7 ± 0.53 d | 21.6 ± 0.58 a | 3.4 ± 0.12 d | N0 | 62.8 ± 1.78 c | 13.0 ± 0.25 a | 3.8 ± 0.12 cd | |
| N1 | 51.3 ± 0.61 c | 20.0 ± 0.43 bc | 3.6 ± 0.18 cd | N1 | 63.6 ± 0.33 c | 11.6 ± 0.33 bc | 4.3 ± 0.46 bc | |
| K1 | N2 | 54.6 ± 0.60 b | 18.7 ± 0.18 d | 4.0 ± 0.12 c | N2 | 67.5 ± 0.65 b | 10.4 ± 0.42 cd | 4.6 ± 0.03 abc |
| N3 | 59.7 ± 0.46 a | 17.6 ± 0.35 e | 4.9 ± 0.07 a | N3 | 70.3 ± 0.26 a | 10.3 ± 0.42 d | 4.9 ± 0.31 ab | |
| N4 | 60.1 ± 0.73 a | 15.8 ± 0.38 f | 5.1 ± 0.12 a | N4 | 71.2 ± 0.65 a | 9.5 ± 0.33 de | 5.3 ± 0.13 a | |
| ANOVA | F value | |||||||
| N | 118.1 *** | 132.2 *** | 125.9 *** | N | 143.0 *** | 44.8 *** | 17.16 *** | |
| K | 358.4 *** | 60.7 *** | 7.7 * | K | 383.8 *** | 35.6 *** | 12.17 ** | |
| N × K | 3.2 ns | 3.1 ns | 3.2 ns | N × K | 3.2 ns | 3.2 ns | 2.2 ns | |
| Varieties | K Treatments | N Treatments | The Number of Tubers Per Plant | Root Fresh Weight/(t/ha−1) | Dry Rate/% | Root Dry Weight/(t/ha−1) | Vine Length/mm | Stem Diameter/mm |
|---|---|---|---|---|---|---|---|---|
| Yan25 | N0 | 3.11 ± 0.42 c | 31.08 ± 1.03 d | 28.12 ± 0.40 bc | 8.73 ± 0.16 fg | 272.83 ± 3.93 e | 11.55 ± 0.11 c | |
| N1 | 3.89 ± 0.42 bc | 34.20 ± 0.95 c | 29.79 ± 0.23 abc | 10.19 ± 0.21 de | 276.67 ± 5.33 e | 11.63 ± 0.21 bc | ||
| K0 | N2 | 4.67 ± 0.27 ab | 37.80 ± 1.02 b | 27.65 ± 1.50 c | 10.50 ± 0.29 cd | 296.50 ± 5.20 d | 12.06 ± 0.29 abc | |
| N3 | 4.33 ± 0.47 ab | 37.20 ± 0.37 b | 30.63 ± 1.07 ab | 11.40 ± 0.51 bc | 334.33 ± 9.03 bc | 12.45 ± 0.47 abc | ||
| N4 | 3.67 ± 0.27 bc | 33.53 ± 0.68 c | 28.77 ± 0.91 abc | 9.64 ± 0.11 def | 363.67 ± 9.67 a | 12.07 ± 0.49 abc | ||
| N0 | 4.00 ± 0.54 bc | 28.80 ± 0.55 d | 28.62 ± 0.23 abc | 8.24 ± 0.22 g | 252.50 ± 6.91 f | 11.96 ± 0.10 bc | ||
| N1 | 4.44 ± 0.57 ab | 33.83 ± 0.45 c | 27.34 ± 0.74 c | 9.25 ± 0.13 ef | 268.50 ± 5.33 ef | 12.51 ± 0.28 abc | ||
| K1 | N2 | 5.22 ± 0.31 a | 39.13 ± 0.92 ab | 30.41 ± 0.78 ab | 11.9 ± 0.59 ab | 297.00 ± 1.73 d | 12.55 ± 0.33 abc | |
| N3 | 5.11 ± 0.57 a | 40.73 ± 0.42 a | 30.84 ± 0.21 a | 12.56 ± 0.21 a | 316.67 ± 3.17 c | 12.75 ± 0.14 ab | ||
| N4 | 4.44 ± 0.42 ab | 39.37 ± 0.66 ab | 31.16 ± 0.12 a | 12.27 ± 0.16 ab | 336.67 ± 3.53 b | 12.92 ± 0.50 a | ||
| ANOVA | F value | |||||||
| N | 6.26 ** | 49.89 *** | 3.50 * | 41.58 *** | 76.51 *** | 2.21 ns | ||
| K | 13.13 ** | 10.98 * | 2.04 ns | 15.63 ** | 15.21 ** | 8.22 * | ||
| N × K | 0.212 ns | 9.18 ** | 3.80 * | 11.75 ** | 1.68 ns | 0.33 ns | ||
| Varieties | K Treatments | N Treatments | The Number of Tubers Per Plant | Root Fresh Weight/(t/ha−1) | Dry Rate/% | Root Dry Weight/(t/ha−1) | Vine Length/mm | Stem Diameter/mm |
|---|---|---|---|---|---|---|---|---|
| Shang19 | N0 | 3.33 ± 0.27 e | 26.10 ± 0.79 de | 35.42 ± 0.60 cd | 9.24 ± 0.12 e | 236.33 ± 3.87 d | 12.17 ± 0.61 c | |
| N1 | 4.00 ± 0.27 d | 30.54 ± 0.48 bc | 35.73 ± 0.21 c | 10.91 ± 0.11 c | 291.33 ± 2.33 c | 12.48 ± 0.20 c | ||
| K0 | N2 | 4.67 ± 0.27 bc | 30.53 ± 0.66 bc | 39.15 ± 0.65 a | 12.05 ± 0.16 b | 293.00 ± 4.20 c | 13.36 ± 0.27 c | |
| N3 | 4.11 ± 0.16 cd | 30.84 ± 0.63 b | 38.11 ± 0.22 ab | 11.75 ± 0.17 b | 402.00 ± 7.00 a | 13.63 ± 0.40 bc | ||
| N4 | 3.78 ± 0.16 de | 27.54 ± 0.23 de | 36.79 ± 0.10 bc | 10.13 ± 0.11 cd | 405.00 ± 6.78 a | 15.10 ± 0.97 b | ||
| N0 | 4.11 ± 0.16 cd | 25.33 ± 0.99 e | 37.54 ± 0.79 b | 9.36 ± 0.02 de | 254.33 ± 2.33 d | 12.38 ± 0.30 c | ||
| N1 | 4.89 ± 0.16 ab | 28.18 ± 0.95 cd | 37.55 ± 0.48 b | 10.58 ± 0.33 c | 297.33 ± 7.87 c | 12.69 ± 0.26 c | ||
| K1 | N2 | 5.33 ± 0.54 a | 36.25 ± 1.01 a | 34.12 ± 0.66 de | 12.37 ± 0.39 ab | 306.00 ± 7.20 c | 17.40 ± 0.49 a | |
| N3 | 4.67 ± 0.27 bc | 37.59 ± 1.05 a | 36.63 ± 0.63 bc | 13.12 ± 0.54 a | 348.33 ± 11.13 b | 13.74 ± 0.60 bc | ||
| N4 | 4.11 ± 0.16 cd | 37.21 ± 0.38 a | 33.78 ± 0.23 e | 12.57 ± 0.19 ab | 348.75 ± 6.55 b | 15.11 ± 0.13 b | ||
| ANOVA | F value | |||||||
| N | 13.67 *** | 41.34 *** | 5.20 * | 47.43 *** | 125.87 *** | 12.31 ** | ||
| K | 29.00 *** | 61.34 *** | 14.35 ** | 24.18 ** | 12.69 * | 5.92 * | ||
| N × K | 0.64 ns | 22.50 *** | 21.70 *** | 9.25 ** | 44.75 *** | 7.20 * | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, X.; Jin, M.; Wang, S.; Xu, X.; Deng, L.; Zhang, Z.; Zhao, X.; Yu, J.; Zhu, Y.; Lu, G.; et al. The Effect of Nitrogen and Potassium Interaction on the Leaf Physiological Characteristics, Yield, and Quality of Sweet Potato. Agronomy 2024, 14, 2319. https://doi.org/10.3390/agronomy14102319
Shu X, Jin M, Wang S, Xu X, Deng L, Zhang Z, Zhao X, Yu J, Zhu Y, Lu G, et al. The Effect of Nitrogen and Potassium Interaction on the Leaf Physiological Characteristics, Yield, and Quality of Sweet Potato. Agronomy. 2024; 14(10):2319. https://doi.org/10.3390/agronomy14102319
Chicago/Turabian StyleShu, Xing, Minghuan Jin, Siyu Wang, Ximing Xu, Lijuan Deng, Zhi Zhang, Xu Zhao, Jing Yu, Yueming Zhu, Guoquan Lu, and et al. 2024. "The Effect of Nitrogen and Potassium Interaction on the Leaf Physiological Characteristics, Yield, and Quality of Sweet Potato" Agronomy 14, no. 10: 2319. https://doi.org/10.3390/agronomy14102319







