Growing Tomato Seedlings Suitable for Mechanical Grafting under Regulated Light Regime
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatment Design
2.2. Growth Measurements
2.2.1. Determination of Morphological Traits and Fresh and Dry Biomass
dry biomass) × seedling dry biomass
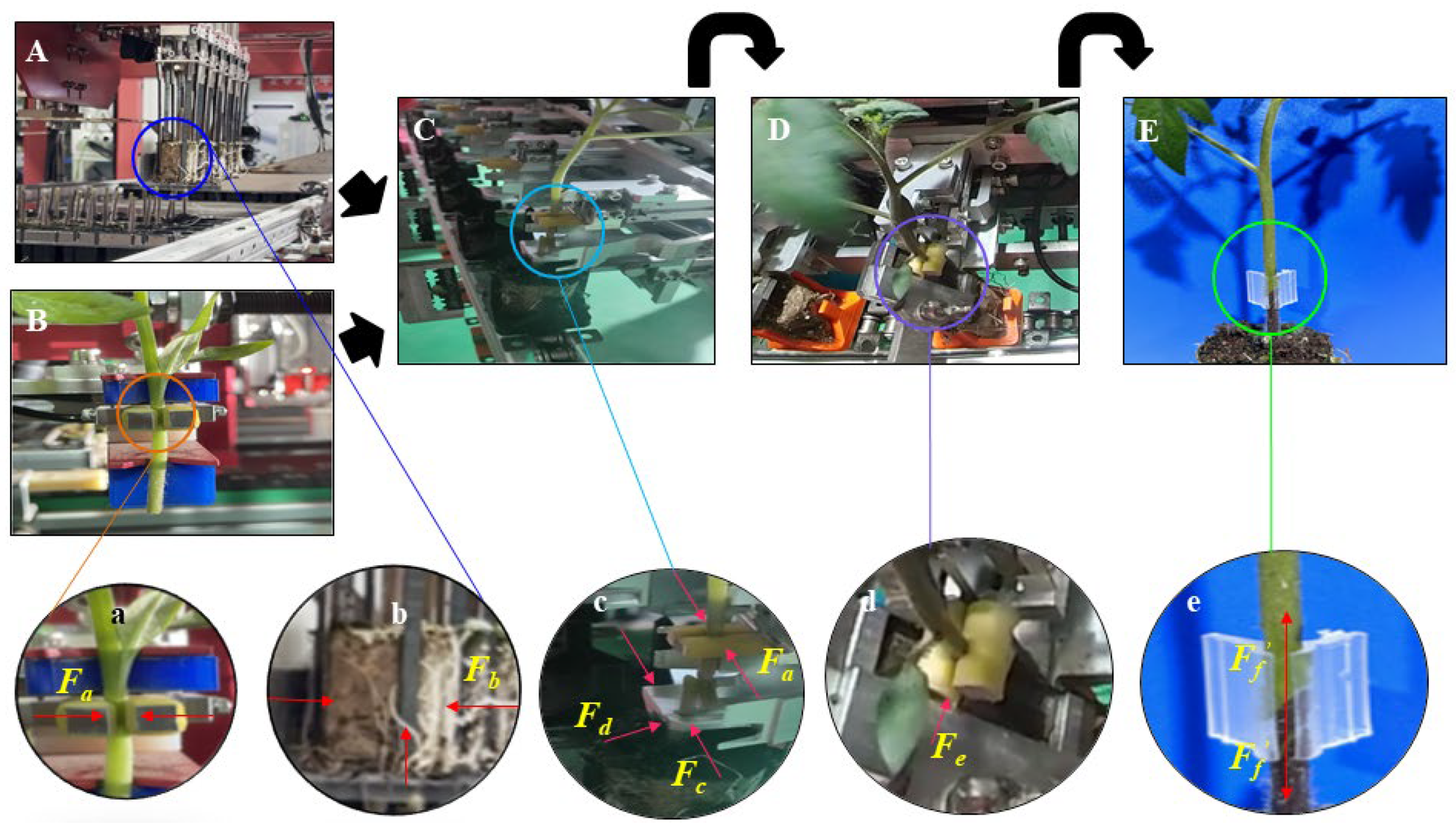
2.2.2. Measurement of Mechanical Properties
2.3. Statistical Analysis
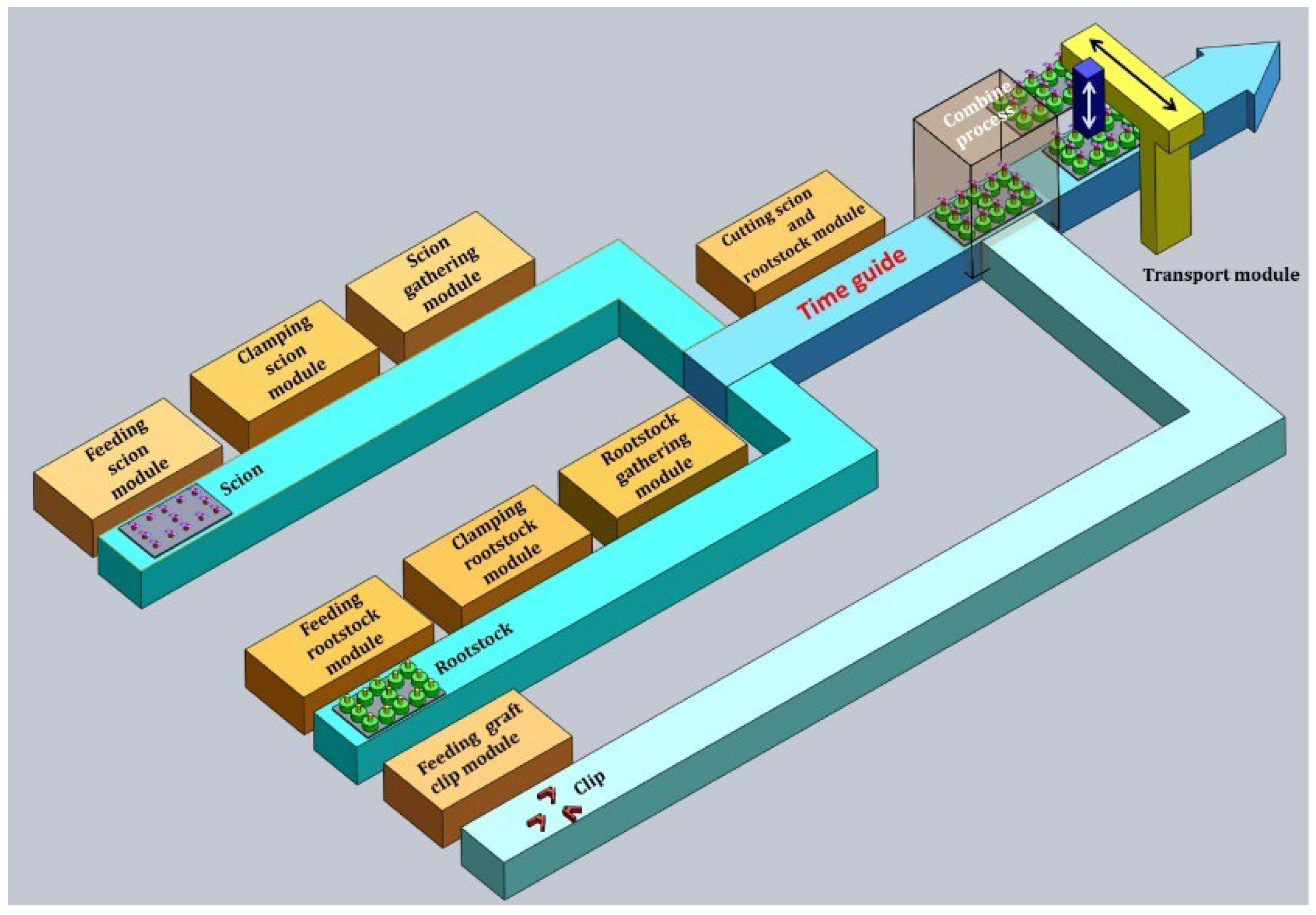
2.4. Grafting Experiment Comparing Commercially Available Seedlings and Light-Regulated Seedlings
3. Results
3.1. Single-Factor Experiments
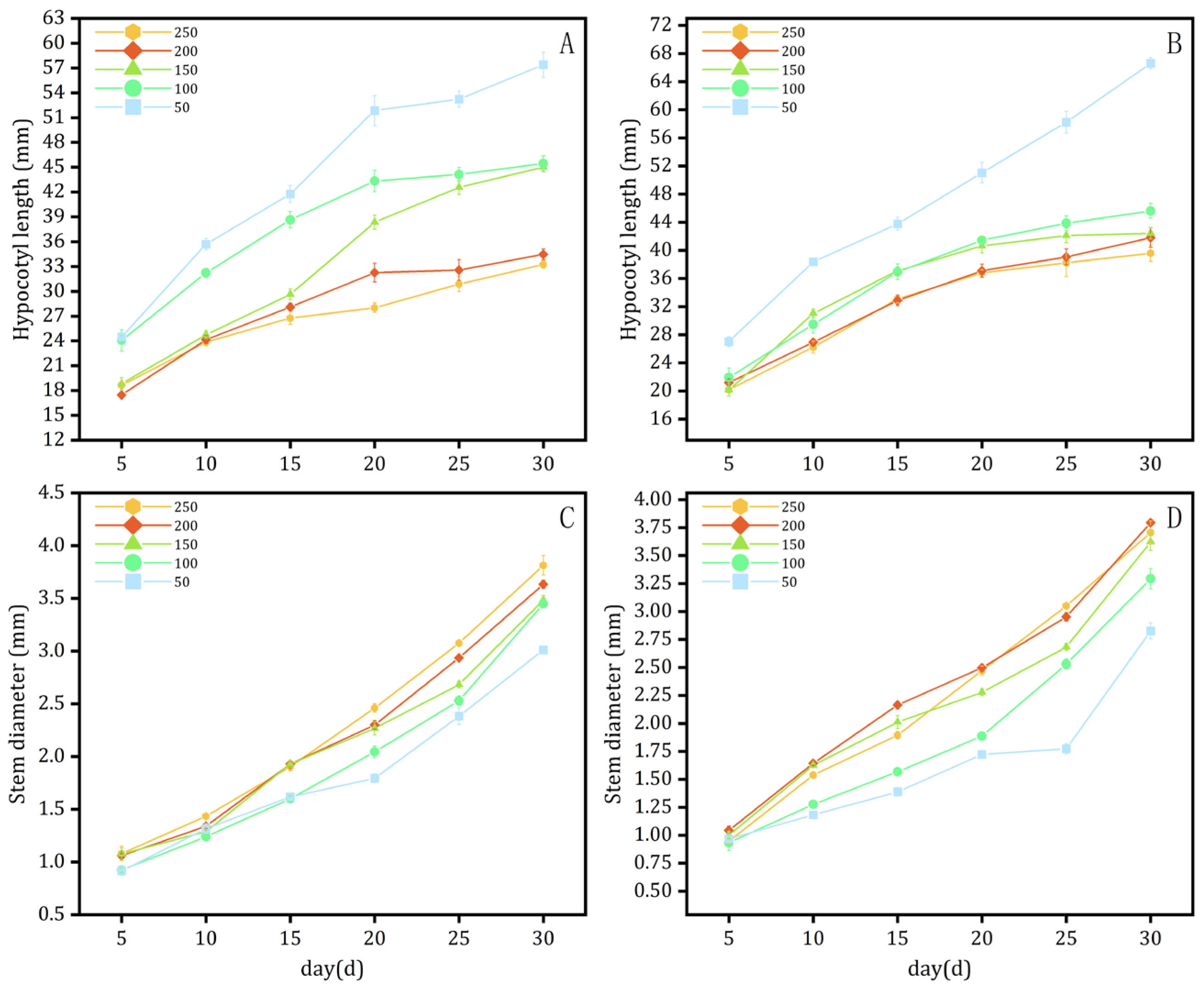
3.1.1. Light Intensity Experiment
Effects on Morphology and Longevity
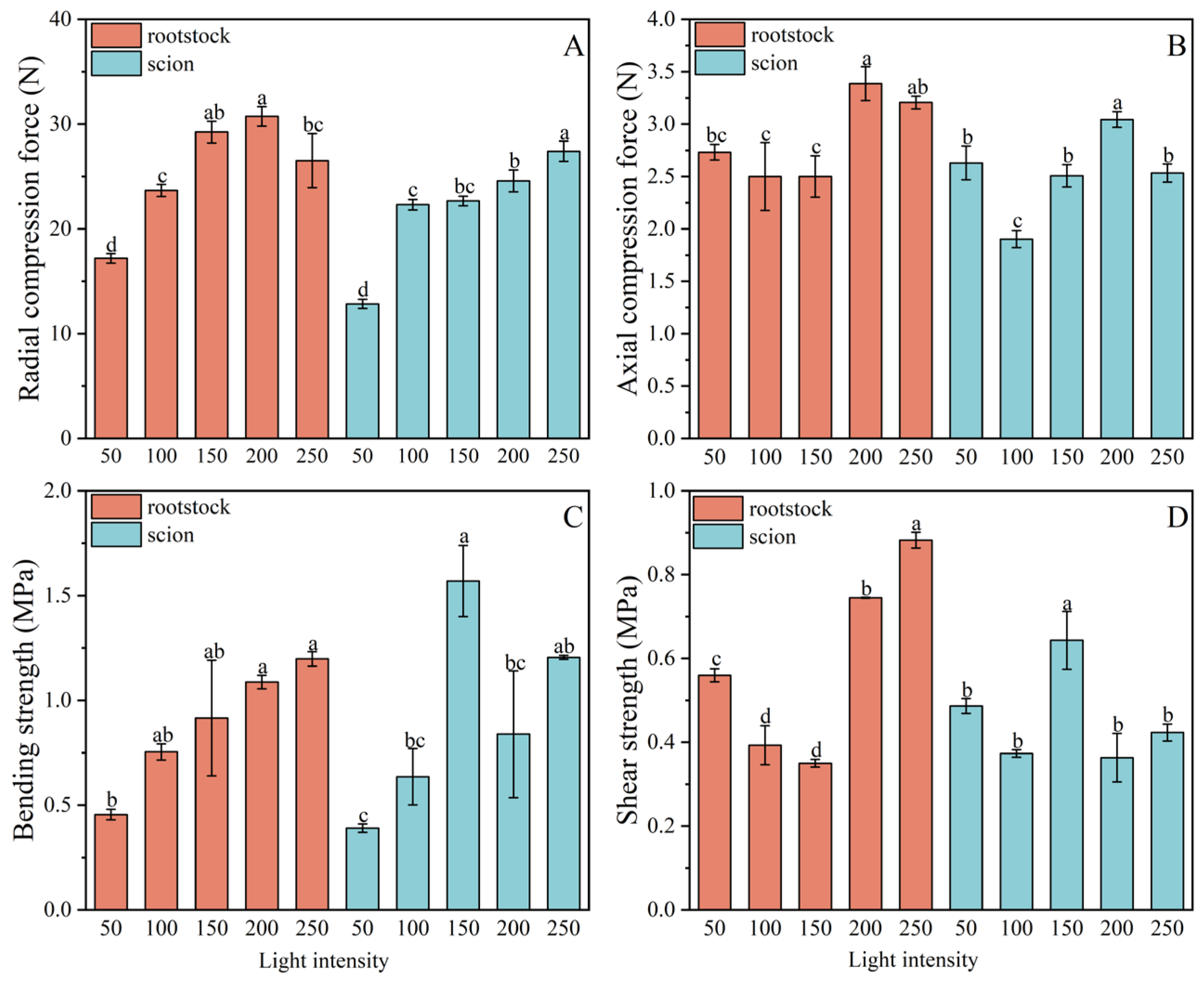
Effect of Light Intensities on the Mechanical Properties of Grafted Seedlings
Summary
3.1.2. Photoperiodic Experiment
Photoperiodic Effect on Morphology and Longevity
Effect on Mechanical Properties
Summary
3.2. Multifactor Experiments
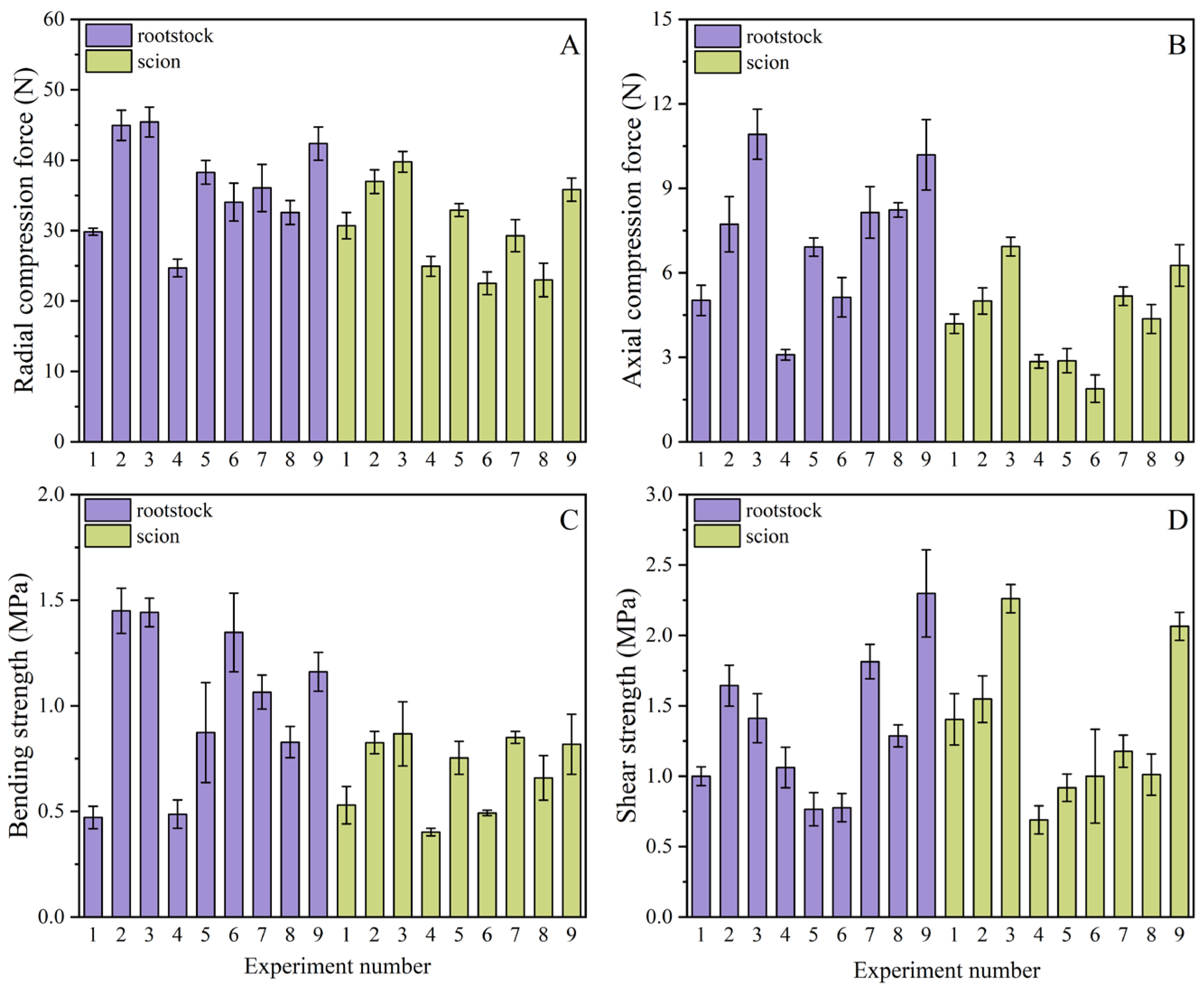
3.2.1. Light Formulation Experimental Design
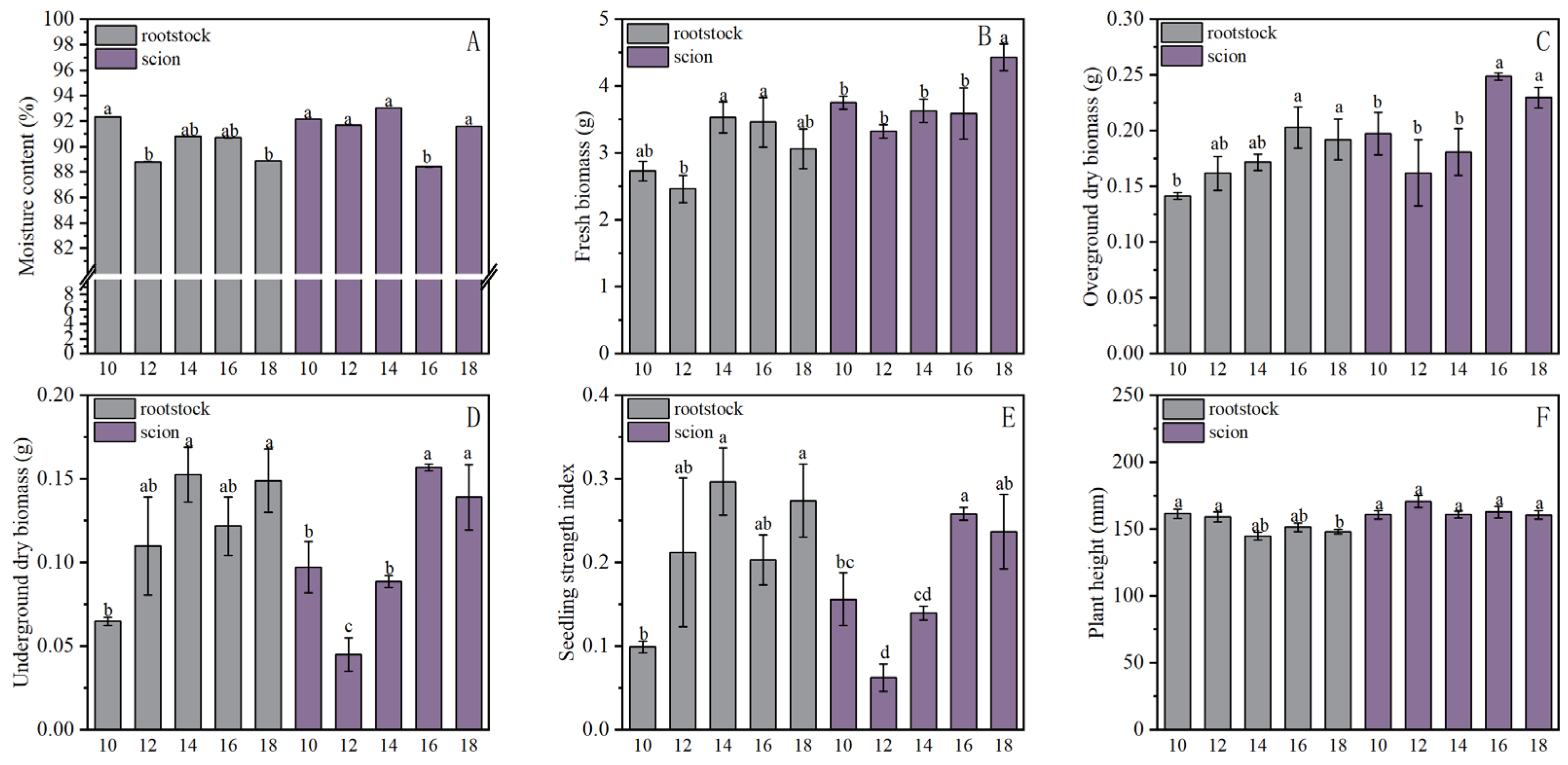
3.2.2. Effects on Morphology and Longevity
Effect of Light Formulation on Morphological Properties
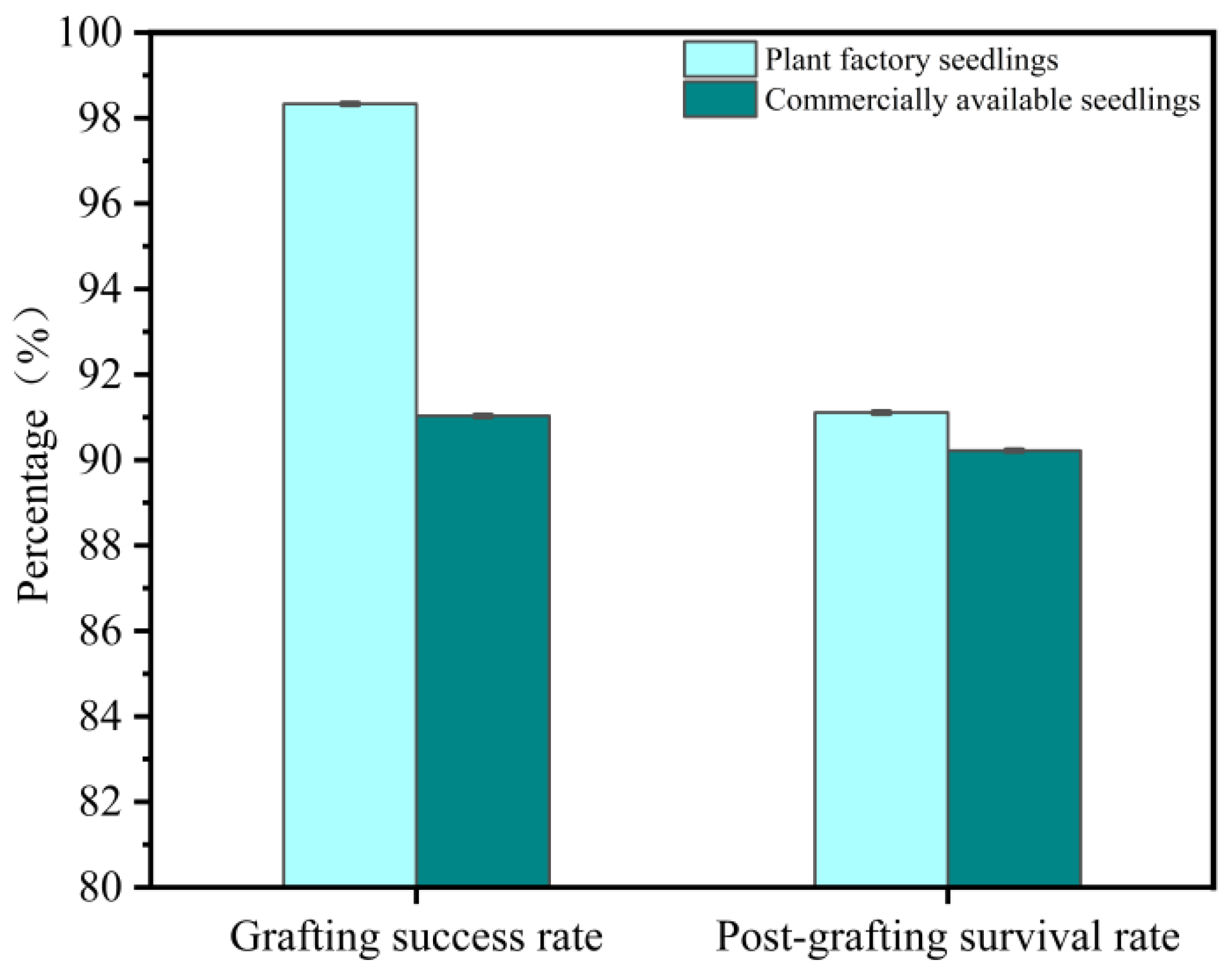
3.3. Comparative Grafting Experiment Results
3.3.1. Morphological and Mechanical Properties of Commercial Seedlings
3.3.2. Experimental Result
4. Discussion
4.1. Forces on Seedlings during Autografting
4.2. Effect of Light Intensity on Seedlings
4.3. Effect of Photoperiod on Quality
4.4. Effect of Light Formulation on Quality
4.5. Comparison of Commercial Seedling and Light-Regulated Seedlings
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, B.; Bennett, M.A.; Kleinhenz, M.D. A new method to estimate vegetable seedling vigor, piloted with tomato, for use in grafting and other contexts. HortTechnology 2016, 26, 767–775. [Google Scholar] [CrossRef]
- Lee, H.; Kim, M.-K.; Choi, H.-S.; Kang, J.-H.; Ju, H.-J.; Seo, J.-K. Efficient transmission and propagation of tomato chlorosis virus by simple single-leaflet grafting. Plant Pathol. J. 2017, 33, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.; Kubota, C.; Rivard, C.L.; Pliakoni, E.D. Evaluating ethylene sensitivity and exogenous ethylene impact on early growth of grafted and nongrafted tomato seedlings. HortTechnology 2022, 32, 129–133. [Google Scholar] [CrossRef]
- Lee, J.-M. Cultivation of grafted vegetables I. Current status, grafting methods, and benefits. HortScience 1994, 29, 235–239. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Hung, Y.-C.; Chen, W.-L.; Huang, Y.-I. Mechanism optimization of the clamping and cutting arrangement device for solanaceae scion and stock seedlings. Appl. Sci. 2023, 13, 1548. [Google Scholar] [CrossRef]
- Lee, J.-M.; Kubota, C.; Tsao, S.; Bie, Z.; Echevarria, P.H.; Morra, L.; Oda, M. Current status of vegetable grafting: Diffusion, grafting techniques, automation. Sci. Hortic. 2010, 127, 93–105. [Google Scholar] [CrossRef]
- Xie, Z.; Gu, S.; Chu, Q.; Li, B.; Fan, K.; Yang, Y.; Yang, Y.; Liu, X. Development of a high-productivity grafting robot for solanaceae. Int. J. Agric. Biol. Eng. 2020, 13, 82–90. [Google Scholar] [CrossRef]
- Yan, G.; Feng, M.; Lin, W.; Huang, Y.; Tong, R.; Cheng, Y. Review and prospect for vegetable grafting robot and relevant key technologies. Agriculture 2022, 12, 1578. [Google Scholar] [CrossRef]
- Kubota, C.; McClure, M.A.; Kokalis-Burelle, N.; Bausher, M.G.; Rosskopf, E.N. Vegetable grafting: History, use, and current technology status in north america. HortScience 2008, 43, 1664–1669. [Google Scholar] [CrossRef]
- Liang, H.; Zhu, J.; Ge, M.; Wang, D.; Liu, K.; Zhou, M.; Sun, Y.; Zhang, Q.; Jiang, K.; Shi, X. A comparative analysis of the grafting efficiency of watermelon with a grafting machine. Horticulturae 2023, 9, 600. [Google Scholar] [CrossRef]
- Fu, X.; Shi, J.; Huang, Y.; Zhu, E.; Bie, Z.; Lin, W. Design and experiment of full-tray grafting device for grafted melon seedling production. Agriculture 2022, 12, 861. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Chen, S.; Chiu, Y.-C.; Lin, L.-H.; Chang, Y.-S. Growth and union acclimation process of sweet pepper grafted by a tubing-grafting robotic system. Hortic. Environ. Biotechnol. 2012, 53, 93–101. [Google Scholar] [CrossRef]
- Kim, H.M.; Hwang, S.J. Comparison of pepper grafting efficiency by grafting robot. J. Bio-Environ. Control 2015, 24, 57–62. [Google Scholar] [CrossRef]
- Tian, S.; Ashraf, M.A.; Kondo, N.; Shiigi, T.; Momin, M.A. Optimization of machine vision for tomato grafting robot. Sens. Lett. 2013, 11, 1190–1194. [Google Scholar] [CrossRef]
- Jiang, K.; Zhang, Q.; Chen, L.; Guo, W.; Zheng, W. Design and optimization on rootstock cutting mechanism of grafting robot for cucurbit. Int. J. Agric. Biol. Eng. 2020, 13, 117–124. [Google Scholar] [CrossRef]
- Pardo-Alonso, J.-L.; Carreño-Ortega, Á.; Martínez-Gaitán, C.-C.; Golasi, I.; Gómez Galán, M. Conventional industrial robotics applied to the process of tomato grafting using the splicing technique. Agronomy 2019, 9, 880. [Google Scholar] [CrossRef]
- Meiramkulova, K.; Tanybayeva, Z.; Kydyrbekova, A.; Turbekova, A.; Aytkhozhin, S.; Zhantasov, S.; Taukenov, A. The efficiency of led irradiation for cultivating high-quality tomato seedlings. Sustainability 2021, 13, 9426. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Z.; Cheng, J.; Cai, X.; Cheng, F.; Yang, Y.; Yan, Z. Morphological and physiological traits of greenhouse-grown tomato seedlings as influenced by supplemental white plus red versus red plus blue leds. Agronomy 2022, 12, 2450. [Google Scholar] [CrossRef]
- Liu, X.Y.; Jiao, X.L.; Chang, T.T.; Guo, S.R.; Xu, Z.G. Photosynthesis and leaf development of cherry tomato seedlings under different led-based blue and red photon flux ratios. Photosynthetica 2018, 56, 1212–1217. [Google Scholar] [CrossRef]
- Kim, H.M.; Hwang, S.J. The growth and development of ‘mini chal’ tomato plug seedlings grown under monochromatic or combined red and blue light-emitting diodes. Hortic. Sci. Technol. 2019, 37, 190–205. [Google Scholar] [CrossRef]
- Kim, E.-Y.; Park, S.-A.; Park, B.-J.; Lee, Y.; Oh, M.-M. Growth and antioxidant phenolic compounds in cherry tomato seedlings grown under monochromatic light-emitting diodes. Hortic. Environ. Biotechnol. 2015, 55, 506–513. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y. Magic blue light: A versatile mediator of plant elongation. Plants 2023, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; An, S.; Lee, B.; Chun, C. Improvement of growth and morphology of vegetable seedlings with supplemental far-red enriched led lights in a plant factory. Horticulturae 2020, 6, 109. [Google Scholar] [CrossRef]
- Khoshimkhujaev, B.; Kwon, J.K.; Park, K.S.; Choi, H.G.; Lee, S.Y. Effect of monochromatic uv-a led irradiation on the growth of tomato seedlings. Hortic. Environ. Biotechnol. 2014, 55, 287–292. [Google Scholar] [CrossRef]
- Zheng, J.; Gan, P.; Ji, F.; He, D.; Yang, P. Growth and energy use efficiency of grafted tomato transplants as affected by led light quality and photon flux density. Agriculture 2021, 11, 816. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, H.; Ma, L.; Yan, L.; Mu, Z.; Mu, Y.; Gu, S. Effect of light quality on the growth and mechanical property of tomato grafted seedlings. Chiang Mai J. Sci. 2024, 51, e2024023. [Google Scholar] [CrossRef]
- Liu, X.; Shi, R.; Gao, M.; He, R.; Li, Y.; Liu, H. Effects of led light quality on the growth of pepper (Capsicum spp.) seedlings and the development after transplanting. Agronomy 2022, 12, 2269. [Google Scholar] [CrossRef]
- Zong, W.; Huang, X.; Xu, A.; Fan, X.; Huang, X. Bending and shearing characteristics of cotton seedling stem. Trans. Chin. Soc. Agric. Eng. 2012, 28, 118–124. [Google Scholar]
- Peng, Y.; Gu, S.; Chu, Q.; Zhang, Q.; Xu, X.; Li, B.; Wang, Y. Design of stock feeding device of grafting robot for solanaceae. Trans. Cin. Soc. Agric. Eng. 2016, 32, 76–82. [Google Scholar]
- Yasar, F.; Uzal, O. Oxidative stress and antioxidant enzyme activities in tomato (Solanum lycopersicum) plants grown at two different light intensities. Gesunde Pflanz. 2022, 75, 479–485. [Google Scholar] [CrossRef]
- Yasar, F.; Üzal, Ö.; Yasar, F.; Akin, S. Effects of two different light intensities on plant growth, ion uptake and distribution in tomato plants. J. Elem. 2022, 27, 663–678. [Google Scholar] [CrossRef]
- Chen, D.; Yuan, K.; Zhang, J.; Wang, Z.; Sun, Z.; Zhang, H.; Hu, J. Response analysis of fluorescence parameters of tomato seedlings oriented to vertical light environment adaptation. Plant Sci. 2022, 314, 111118. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-S.; Zhang, C.; Kang, H.-M.; Mackay, B. Control of stretching of cucumber and tomato plug seedlings using supplemental light. Hortic. Environ. Biotechnol. 2008, 49, 287–292. [Google Scholar]
- Huber, B.M.; Louws, F.J.; Hernández, R. Impact of different daily light integrals and carbon dioxide concentrations on the growth, morphology, and production efficiency of tomato seedlings. Front. Plant Sci. 2021, 12, 618853. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, Y.; Zou, J.; Lin, S.; Jin, C.; Shi, M.; Yang, B.; Yang, Y.; Jin, D.; Li, R.; et al. Effects of different light intensity on the growth of tomato seedlings in a plant factory. PLoS ONE 2023, 18, e0294876. [Google Scholar]
- García-Caparrós, P.; Sabio, F.; Barbero, F.J.; Chica, R.M.; Lao, M.T. Physiological responses of tomato and cucumber seedlings under different light–dark cycles. Agronomy 2020, 10, 945. [Google Scholar] [CrossRef]
- Cuassolo, F.; Navarro, M.B.; Balseiro, E.; Modenutti, B. Effect of light on particulate and dissolved organic matter production of native and exotic macrophyte species in patagonia. Hydrobiologia 2015, 766, 29–42. [Google Scholar] [CrossRef]
- Hwang, H.; An, S.; Pham, M.D.; Cui, M.; Chun, C. The combined conditions of photoperiod, light intensity, and air temperature control the growth and development of tomato and red pepper seedlings in a closed transplant production system. Sustainability 2020, 12, 9939. [Google Scholar] [CrossRef]
- Song, J.; Fan, Y.; Li, X.; Li, Y.; Mao, H.; Zuo, Z.; Zou, Z. Effects of daily light integral on tomato (Solanum lycopersicon L.) grafting and quality in a controlled environment. Int. J. Agric. Biol. Eng. 2022, 15, 44–50. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, Y.; Han, J.; Hu, X.; Li, X.; Zhao, H.; Bai, L.; Shi, Y.; Ahammed, G.J. Interactive effects of iron and photoperiods on tomato plant growth and fruit quality. J. Plant Growth Regul. 2022, 42, 376–389. [Google Scholar] [CrossRef]
- Cao, K.; Cui, L.; Ye, L.; Zhou, X.; Bao, E.; Zhao, H.; Zou, Z. Effects of red light night break treatment on growth and flowering of tomato plants. Front. Plant Sci. 2016, 7, 527. [Google Scholar] [CrossRef] [PubMed]
- Lanoue, J.; Thibodeau, A.; Little, C.; Zheng, J.; Grodzinski, B.; Hao, X. Light spectra and root stocks affect response of greenhouse tomatoes to long photoperiod of supplemental lighting. Plants 2021, 10, 1674. [Google Scholar] [CrossRef] [PubMed]











| Considerations Test No. | Light Quality (Red/Blue) | Light Intensity (μmol m−2 s−1) | Photoperiod (H) |
|---|---|---|---|
| 1 | 50:50 | 150 | 14 |
| 2 | 50:50 | 200 | 16 |
| 3 | 50:50 | 250 | 18 |
| 4 | 25:75 | 150 | 16 |
| 5 | 25:75 | 200 | 18 |
| 6 | 25:75 | 250 | 14 |
| 7 | 75:25 | 150 | 18 |
| 8 | 75:25 | 200 | 14 |
| 9 | 75:25 | 250 | 16 |
| Parameter Type | Hypocotyl Length (mm) | Stem Diameter (mm) | Plant Height (mm) | RCF (N) | ACF (N) | BS (MPa) | SS (MPa) |
|---|---|---|---|---|---|---|---|
| Rootstock | 32.265 ± 2.71 | 2.96 ± 0.23 | 89.89 ± 2.99 | 24.36 ± 0.44 | 1.54 ± 0.12 | 0.32 ± 0.06 | 0.35 ± 0.03 |
| Scion | 36.154 ± 1.82 | 3.05 ± 0.15 | 125.17 ± 1.83 | 21.48 ± 0.35 | 1.37 ± 0.17 | 0.24 ± 0.02 | 0.27 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Deng, H.; Li, H.; Ma, L.; He, T.; Yao, Z.; Mu, Z.; Gu, S.; Mu, Y. Growing Tomato Seedlings Suitable for Mechanical Grafting under Regulated Light Regime. Agronomy 2024, 14, 2322. https://doi.org/10.3390/agronomy14102322
Wang Y, Deng H, Li H, Ma L, He T, Yao Z, Mu Z, Gu S, Mu Y. Growing Tomato Seedlings Suitable for Mechanical Grafting under Regulated Light Regime. Agronomy. 2024; 14(10):2322. https://doi.org/10.3390/agronomy14102322
Chicago/Turabian StyleWang, Yichi, Hongxuan Deng, Huiwen Li, Lidan Ma, Tao He, Zhenquan Yao, Zeyi Mu, Song Gu, and Yinghui Mu. 2024. "Growing Tomato Seedlings Suitable for Mechanical Grafting under Regulated Light Regime" Agronomy 14, no. 10: 2322. https://doi.org/10.3390/agronomy14102322





