Effects of Metal Oxide Nanoparticles on the Growth and Genotoxicity of Garden Cress (Lepidium sativum L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanoparticles
2.2. Seedling Cultivation and Growth Conditions
2.3. Shoot and Root Length Measurements
2.4. Measurements of Chlorophyll a and b
2.5. DNA Isolation, Polymerase Chain Reaction, and Randomly Amplified Polymorphic DNA Analysis
2.6. Evaluation of Genomic Template Stability
2.7. Statistical Analysis
3. Results
3.1. Shoot and Root Lengths
3.2. Measurement of Chlorophyll a and b Absorption
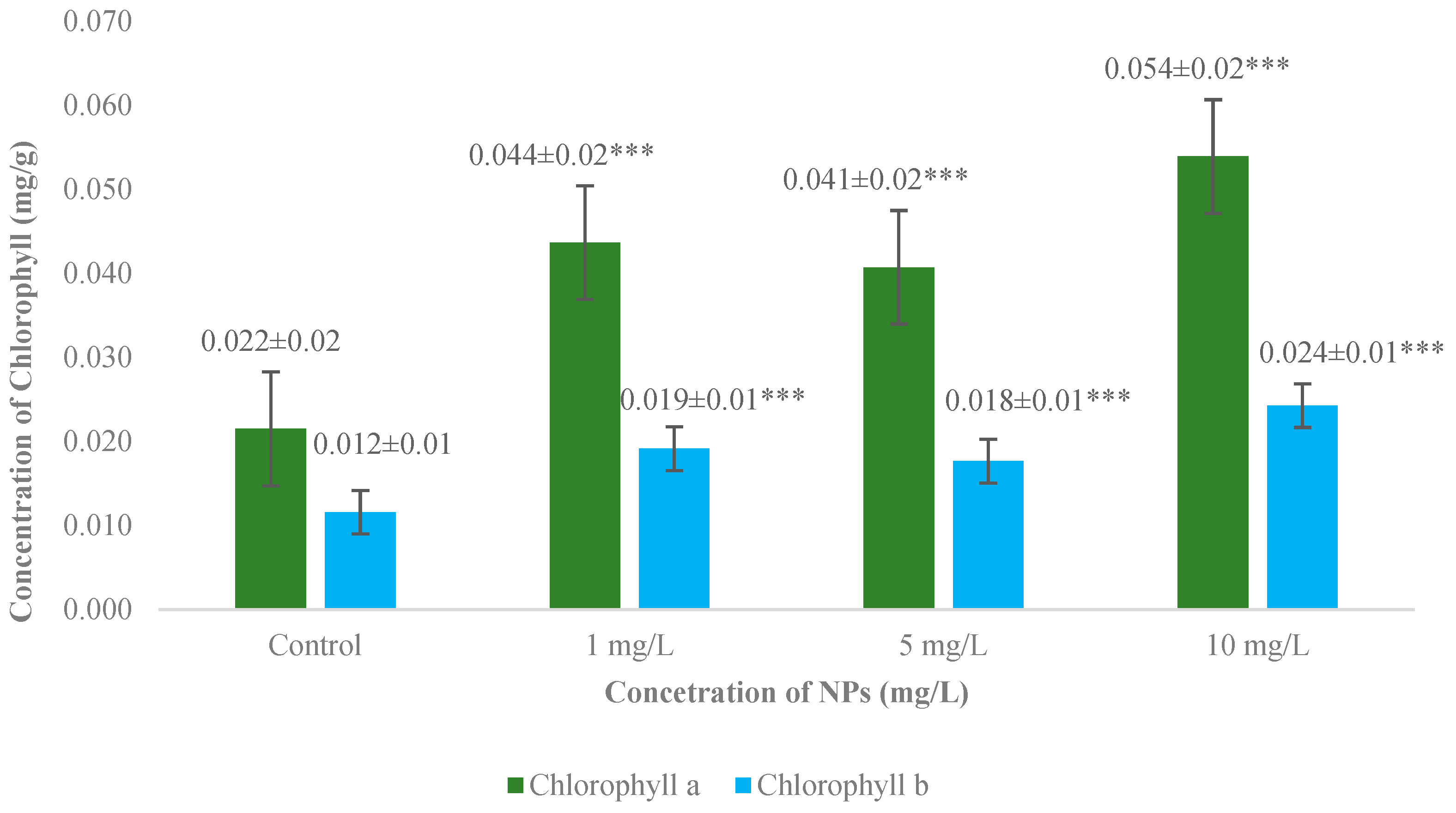
3.3. Measurement of Chlorophyll a and b Concentrations
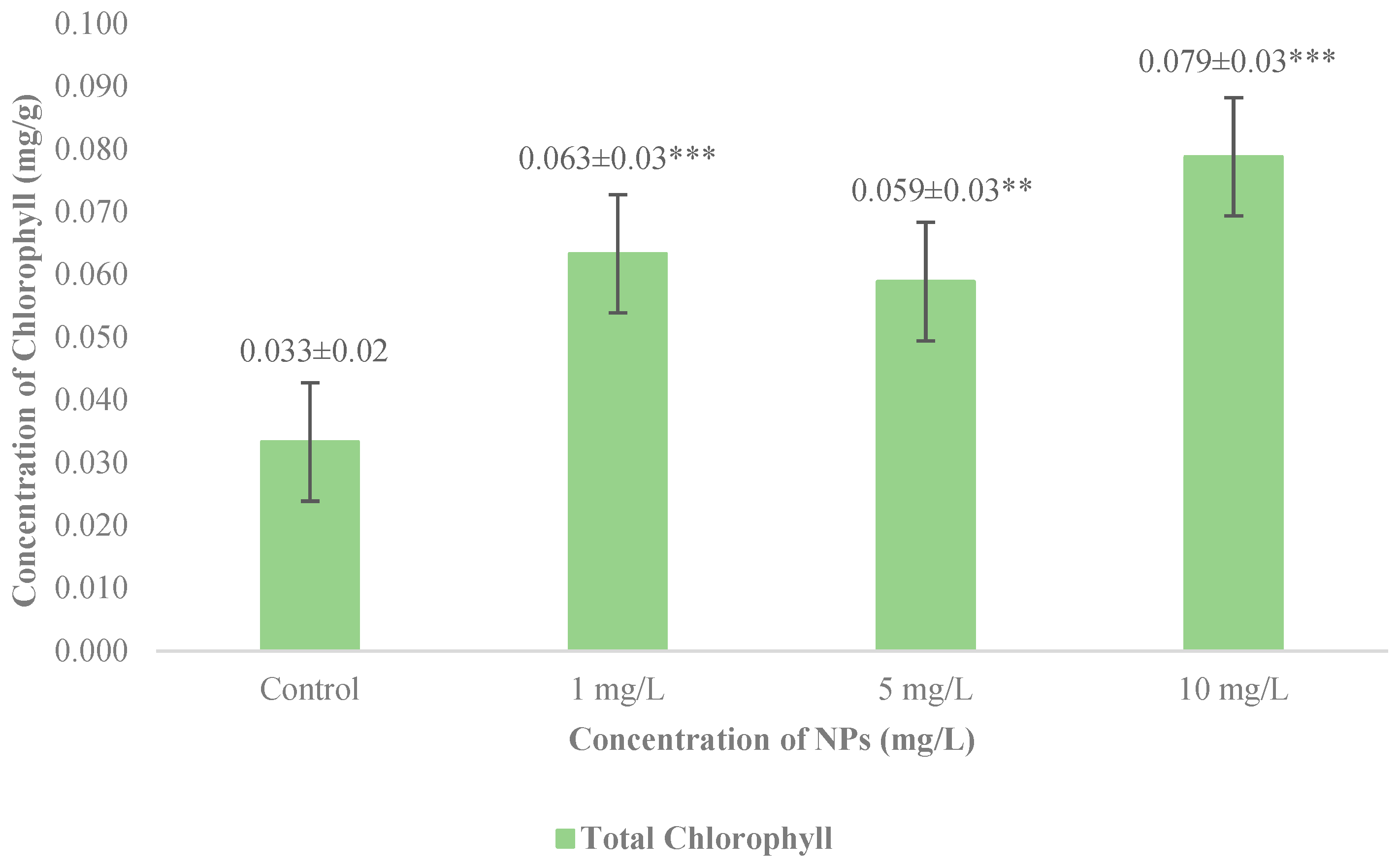
3.4. Measurements of Total Chlorophyll Concentration
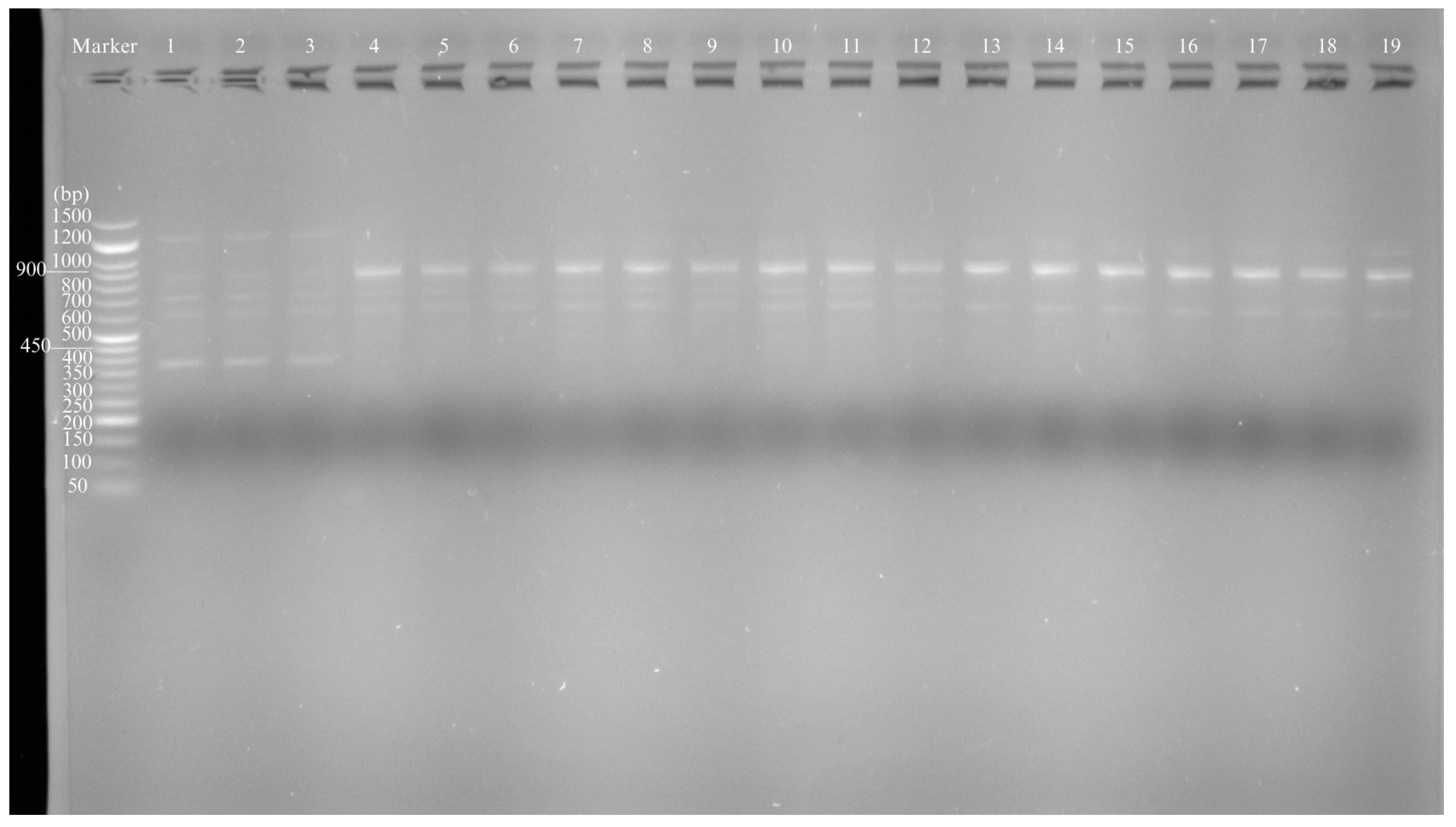
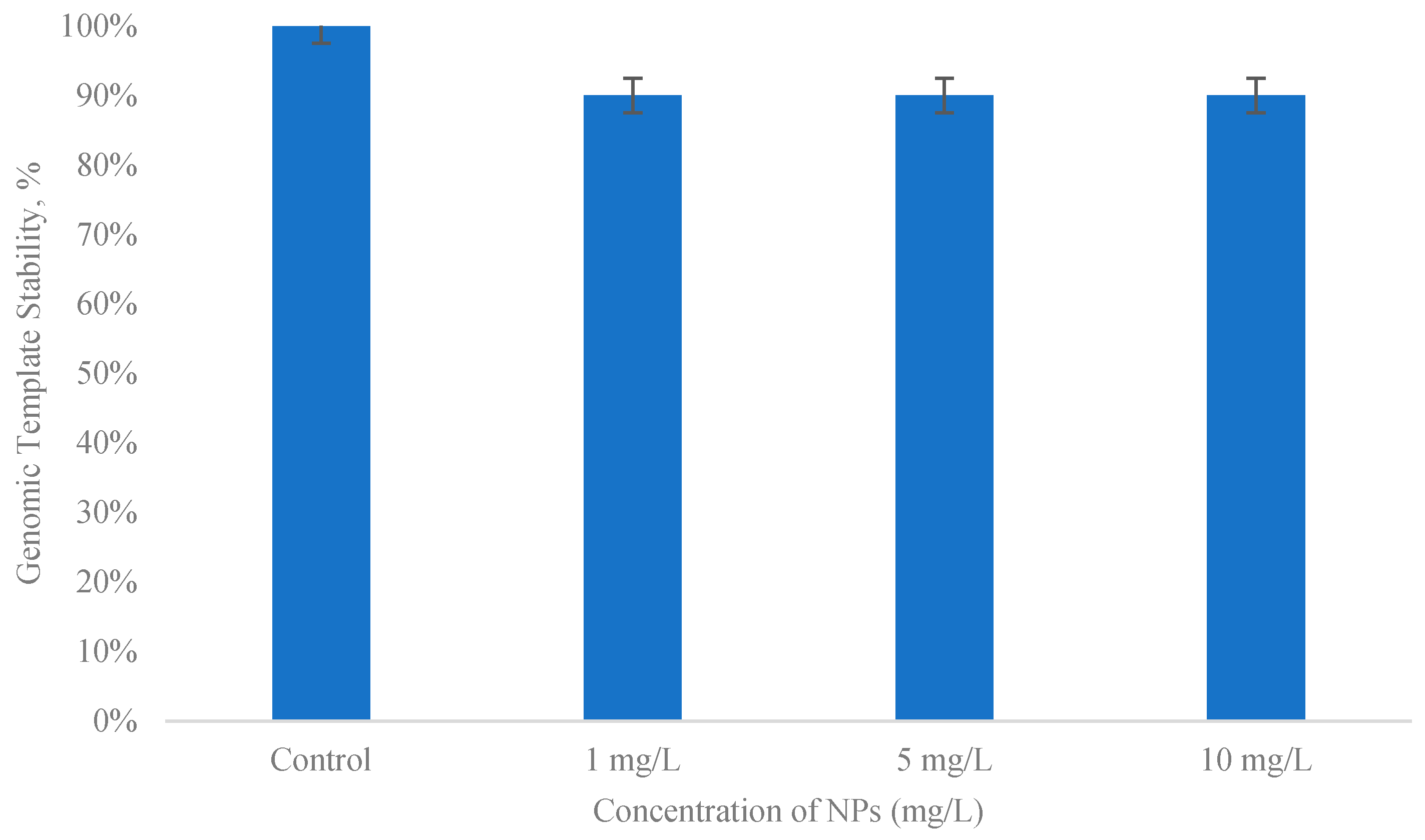
3.5. Evaluation of the Genotoxicity Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, Y.; Zhao, W.; Zhu, G.; Tan, Z.; Huang, L.; Zhang, P.; Rui, Y. Nano-pesticides and fertilizers: Solutions for global food security. Nanomaterials 2023, 14, 90. [Google Scholar] [CrossRef]
- Hasan, H.R.; Musamih, A.; Salah, K.; Jayaraman, R.; Omar, M.; Arshad, J.; Boscovic, D. Smart agriculture assurance: IoT and blockchain for trusted sustainable produce. Comput. Electron. Agric. 2024, 224, 109184. [Google Scholar] [CrossRef]
- World Health Organization. The State of Food Security and Nutrition in the World 2023: Urbanization, Agrifood Systems Transformation and Healthy Diets across the Rural–Urban Continuum; Food & Agriculture Org: Rome, Italy, 2023; Volume 2023.
- Kumar, N.; Samota, S.R.; Venkatesh, K.; Tripathi, S.C. Global trends in use of nano-fertilizers for crop production: Advantages and constraints—A review. Soil Tillage Res. 2023, 228, 105645. [Google Scholar] [CrossRef]
- Kaningini, A.G.; Nelwamondo, A.M.; Azizi, S.; Maaza, M.; Mohale, K.C. Metal nanoparticles in agriculture: A review of possible use. Coatings 2022, 12, 1586. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, K.; Abd-Elsalam, K.A. Exploring the potential of nanofertilizers for a sustainable agriculture. Plant Nano Biol. 2023, 5, 100044. [Google Scholar] [CrossRef]
- Bhaskar, M.; Kumar, A.; Rani, R. Application of nano formulations in agriculture. Biocatal. Agric. Biotechnol. 2023, 54, 102934. [Google Scholar] [CrossRef]
- Ahmed, F.; Gill, S.S.; Rao, T.N.; Arshi, N.; Kumar, S.; Prashanthi, Y. Nanofertilizers: As smart nanoformulations in the agriculture industry. In The Impact of Nanoparticles on Agriculture and Soil; Academic Press: Cambridge, MA, USA, 2023; pp. 285–299. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, M.; Luthra, G. Fundamental approaches and applications of nanotechnology: A mini review. Mater. Today Proc. 2023, in press. [CrossRef]
- Humbal, A.; Pathak, B. Application of Nanotechnology in Plant Growth and Diseases Management: Tool for Sustainable Agriculture. In Agricultural and Environmental Nanotechnology: Novel Technologies and Their Ecological Impact; Springer Nature Singapore: Singapore, 2023; pp. 145–168. [Google Scholar] [CrossRef]
- Dhankhar, N.; Kumar, J. Impact of increasing pesticides and fertilizers on human health: A review. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Ashitha, A.; Rakhimol, K.R.; Mathew, J. Fate of the conventional fertilizers in environment. In Controlled Release Fertilizers for Sustainable Agriculture; Academic Press: Cambridge, MA, USA, 2021; pp. 25–39. [Google Scholar] [CrossRef]
- Channab, B.E.; Idrissi, A.E.; Ammar, A.; Dardari, O.; Marrane, S.E.; El Gharrak, A.; Zahouily, M. Recent advances in nano-fertilizers: Synthesis, crop yield impact, and economic analysis. Nanoscale 2024, 16, 4484–4513. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Singh, M.K.; Raghuvansi, J.; Yadav, R.K.; Azim, Z. Green synthesis of nano iron oxide using Emblica officinalis L. fruit extract and its impact on growth, chlorophyll content, and metabolic activity of Solanum lycopersicum L. J. Appl. Biol. Biotechnol. 2024, 12, 173–181. [Google Scholar] [CrossRef]
- Aborisade, M.A.; Geng, H.; Oba, B.T.; Kumar, A.; Ndudi, E.A.; Battamo, A.Y.; Zhao, L. Remediation of soil polluted with Pb and Cd and alleviation of oxidative stress in Brassica rapa plant using nanoscale zerovalent iron supported with coconut-husk biochar. J. Plant Physiol. 2023, 287, 154023. [Google Scholar] [CrossRef]
- Tao, Z.; Zhou, Q.; Zheng, T.; Mo, F.; Ouyang, S. Iron oxide nanoparticles in the soil environment: Adsorption, transformation, and environmental risk. J. Hazard. Mater. 2023, 459, 132107. [Google Scholar] [CrossRef] [PubMed]
- Tombuloglu, G.; Tombuloglu, H.; Slimani, Y.; Almessiere, M.A.; Baykal, A.; Bostancioglu, S.M.; Ercan, I. Effects of foliar iron oxide nanoparticles (Fe3O4) application on photosynthetic parameters, distribution of mineral elements, magnetic behaviour, and photosynthetic genes in tomato (Solanum lycopersicum var. cerasiforme) plants. Plant Physiol. Biochem. 2024, 210, 108616. [Google Scholar] [CrossRef] [PubMed]
- Tasar, N. Genotoxic effect of iron oxide (Fe2O3) nanoparticles on Triticum aestivum (wheat). Microsc. Res. Tech. 2023, 86, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- Alhasany, A.R.; Leiby, H.R.; Noaema, A.H. Effectiveness of Spraying Nano-Fertilizers of Iron And Potassium on the Growth And Yield of Faba Bean Crop (Vicia faba L.). Int. J. Agric. Stat. Sci. 2021, 1225, 012017. [Google Scholar]
- Wang, Y.; Deng, C.; Cota-Ruiz, K.; Peralta-Videa, J.R.; Sun, Y.; Rawat, S.; Gardea-Torresdey, J.L. Improvement of nutrient elements and allicin content in green onion (Allium fistulosum) plants exposed to CuO nanoparticles. Sci. Total Environ. 2020, 725, 138387. [Google Scholar] [CrossRef]
- Cieschi, M.T.; Polyakov, A.Y.; Lebedev, V.A.; Volkov, D.S.; Pankratov, D.A.; Veligzhanin, A.A.; Lucena, J.J. Eco-friendly iron-humic nanofertilizers synthesis for the prevention of iron chlorosis in soybean (Glycine max) grown in calcareous soil. Front. Plant Sci. 2019, 10, 413. [Google Scholar] [CrossRef]
- Faizan, M.; Faraz, A.; Yusuf, M.; Khan, S.T.; Hayat, S. Zinc oxide nanoparticle-mediated changes in photosynthetic efficiency and antioxidant system of tomato plants. Photosynthetica 2018, 56, 678–686. [Google Scholar] [CrossRef]
- Upadhyay, P.K.; Dey, A.; Singh, V.K.; Dwivedi, B.S.; Singh, R.K.; Rajanna, G.A.; Shukla, G. Changes in microbial community structure and yield responses with the use of nano-fertilizers of nitrogen and zinc in wheat–maize system. Sci. Rep. 2024, 14, 1100. [Google Scholar] [CrossRef]
- Sajyan, T.K.; Alturki, S.M.; Sassine, Y.N. Nano-fertilizers and their impact on vegetables: Contribution of Nano-chelate Super Plus ZFM and Lithovit®-standard to improve salt-tolerance of pepper. Ann. Agric. Sci. 2020, 65, 200–208. [Google Scholar] [CrossRef]
- Srivastava, P.; Das, A.; Gupta, K.; Muthukumaran, M.; Kurdekar, A.K.; Sharma, U.; Zaman, M.I. Impact of Nano and Non-nano Fertilizers on Rice Quality and Productivity: A Review. Int. J. Environ. Clim. Chang. 2023, 13, 973–987. [Google Scholar] [CrossRef]
- Jośko, I.; Kusiak, M.; Różyło, K.; Baranowska-Wójcik, E.; Sierocka, M.; Sheteiwy, M.; Świeca, M. The life cycle study revealed distinct impact of foliar-applied nano-Cu on antioxidant traits of barley grain comparing with conventional agents. Food Res. Int. 2023, 164, 112303. [Google Scholar] [CrossRef] [PubMed]
- López-Luna, J.; Nopal-Hormiga, Y.; López-Sánchez, L.; Mtz-Enriquez, A.I.; Pariona, N. Effect of methods application of copper nanoparticles in the growth of avocado plants. Sci. Total Environ. 2023, 880, 163341. [Google Scholar] [CrossRef] [PubMed]
- Ghouri, F.; Sarwar, S.; Sun, L.; Riaz, M.; Haider, F.U.; Ashraf, H.; Shahid, M.Q. Silicon and iron nanoparticles protect rice against lead (Pb) stress by improving oxidative tolerance and minimizing Pb uptake. Sci. Rep. 2024, 14, 5986. [Google Scholar] [CrossRef] [PubMed]
- Shukla, G.; Singh, A.; Chaudhary, N.; Singh, S.; Basnal, N.; Gaurav, S.S. Metal nanoparticles to improve the heat resilience in wheat (Triticum aestivum L.). Nanotechnology 2024, 35, 205101. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Preparation, surface functionalization and application of Fe3O4 magnetic nanoparticles. Adv. Colloid Interface Sci. 2020, 281, 102165. [Google Scholar] [CrossRef]
- Ali, A.; Zafar, H.; Zia, M.; ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef]
- Connorton, J.M.; Balk, J.; Rodríguez-Celma, J. Iron homeostasis in plants—A brief overview. Metallomics 2017, 9, 813–823. [Google Scholar] [CrossRef]
- Morrissey, J.; Guerinot, M.L. Iron uptake and transport in plants: The good, the bad, and the ionome. Chem. Rev. 2009, 109, 4553–4567. [Google Scholar] [CrossRef]
- Li, J.; Cao, X.; Jia, X.; Liu, L.; Cao, H.; Qin, W.; Li, M. Iron deficiency leads to chlorosis through impacting chlorophyll synthesis and nitrogen metabolism in Areca catechu L. Front. Plant Sci. 2021, 12, 710093. [Google Scholar] [CrossRef]
- Kim, J.H.; Oh, Y.; Yoon, H.; Hwang, I.; Chang, Y.S. Iron nanoparticle-induced activation of plasma membrane H+-ATPase promotes stomatal opening in Arabidopsis thaliana. Environ. Sci. Technol. 2015, 49, 1113–1119. [Google Scholar] [CrossRef]
- Yoon, H.; Kang, Y.G.; Chang, Y.S.; Kim, J.H. Effects of zerovalent iron nanoparticles on photosynthesis and biochemical adaptation of soil-grown Arabidopsis thaliana. Nanomaterials 2019, 9, 1543. [Google Scholar] [CrossRef] [PubMed]
- Landa, P. Positive effects of metallic nanoparticles on plants: Overview of involved mechanisms. Plant Physiol. Biochem. 2021, 161, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Shirsat, S.; K, S. Iron oxide nanoparticles as iron micronutrient fertilizer—Opportunities and limitations. J. Plant Nutr. Soil Sci. 2023, 187, 565–588. [Google Scholar] [CrossRef]
- ul Ain, Q.; Hussain, H.A.; Zhang, Q.; Rasheed, A.; Imran, A.; Hussain, S.; Ali, K.S. Use of nano-fertilizers to improve the nutrient use efficiencies in plants. In Sustainable Plant Nutrition; Academic Press: Cambridge, MA, USA, 2023; pp. 299–321. [Google Scholar]
- Hasan, S.A.; Hayat, S.; Ahmad, A. Brassinosteroids protect photosynthetic machinery against the cadmium induced oxidative stress in two tomato cultivars. Chemosphere 2011, 84, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Akhtar, N.; Rehman, S.U.; Jamil, M. Iron Oxide Nanoparticles: Plant Response, Interaction, Phytotoxicity and Defense Mechanisms. In Nanomaterials and Nanocomposites Exposures to Plants: Response, Interaction, Phytotoxicity and Defense Mechanisms; Springer Nature Singapore: Singapore, 2023; pp. 227–245. [Google Scholar] [CrossRef]
- Rahmatizadeh, R.; Arvin, S.M.J.; Jamei, R.; Mozaffari, H.; Reza Nejhad, F. Response of tomato plants to interaction effects of magnetic (Fe3O4) nanoparticles and cadmium stress. J. Plant Interact. 2019, 14, 474–481. [Google Scholar] [CrossRef]
- Aborisade, M.A.; Oba, B.T.; Kumar, A.; Liu, J.; Chen, D.; Okimiji, O.; Zhao, L. Remediation of metal toxicity and alleviation of toxic metals-induced oxidative stress in Brassica chinensis L using biochar-iron nanocomposites. Plant Soil 2023, 493, 629–664. [Google Scholar] [CrossRef]
- Wang, H.; Wu, F.; Meng, W.; White, J.C.; Holden, P.A.; Xing, B. Engineered nanoparticles may induce genotoxicity. Environ. Sci. Technol. 2013, 47, 13212–13214. [Google Scholar] [CrossRef]
- Mohamed, S.A.K.S.; Sabita, U.; Rajendra, S.; Raman, D. Genotoxicity: Mechanisms, testing guidelines and methods. Glob. J. Pharm. Pharm. Sci. 2017, 1, 555575. [Google Scholar] [CrossRef]
- Wu, K.; Zhou, Q.; Ouyang, S. Direct and indirect genotoxicity of graphene family nanomaterials on DNA—A review. Nanomaterials 2021, 11, 2889. [Google Scholar] [CrossRef]
- Plaksenkova, I.; Jermaļonoka, M.; Bankovska, L.; Gavarāne, I.; Gerbreders, V.; Sledevskis, E.; Kokina, I. Effects of Fe3O4 nanoparticle stress on the growth and development of rocket Eruca sativa. J. Nanomater. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Abdelsalam, N.R.; Abdel-Megeed, A.; Ghareeb, R.Y.; Ali, H.M.; Salem, M.Z.; Akrami, M.; Desoky, E.S.M. Genotoxicity assessment of amino zinc nanoparticles in wheat (Triticum aestivum L.) as cytogenetical perspective. Saudi J. Biol. Sci. 2022, 29, 2306–2313. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.K.; Ghosh, S.; Kalaji, H.M.; Bosa, K.; Brestic, M.; Zivcak, M.; Hossain, Z. Nano-CuO stress induced modulation of antioxidative defense and photosynthetic performance of Syrian barley (Hordeum vulgare L.). Environ. Exp. Bot. 2014, 102, 37–47. [Google Scholar] [CrossRef]
- Kokina, I.; Plaksenkova, I.; Jermaļonoka, M.; Petrova, A. Impact of iron oxide nanoparticles on yellow medick (Medicago falcata L.). Plants. J. Plant Interact. 2020, 15, 1–7. [Google Scholar] [CrossRef]
- Jankovskis, L.; Kokina, I.; Plaksenkova, I.; Jermaļonoka, M. Impact of Different Nanoparticles on Common Wheat (Triticum aestivum L.) Plants, Course, and Intensity of Photosynthesis. Sci. World J. 2022, 2022, 3693869. [Google Scholar] [CrossRef]
- Deng, C.; Wang, Y.; Cota-Ruiz, K.; Reyes, A.; Sun, Y.; Peralta-Videa, J.; Gardea-Torresdey, J. Bok choy (Brassica rapa) grown in copper oxide nanoparticles-amended soils exhibits toxicity in a phenotype-dependent manner: Translocation, biodistribution and nutritional disturbance. J. Hazard. Mater. 2020, 398, 122978. [Google Scholar] [CrossRef]
- Palchoudhury, S.; Jungjohann, K.L.; Weerasena, L.; Arabshahi, A.; Gharge, U.; Albattah, A.; Holler, R.A. Enhanced legume root growth with pre-soaking in α-Fe2O3 nanoparticle fertilizer. RSC Adv. 2018, 8, 24075–24083. [Google Scholar] [CrossRef]
- Petrova, A.; Plaksenkova, I.; Kokina, I.; Jermaļonoka, M. Effect of Fe3O4 and CuO Nanoparticles on morphology, genotoxicity, and miRNA expression on different barley (Hordeum vulgare L.) genotypes. Sci. World J. 2021, 2021, 6644689. [Google Scholar] [CrossRef]
- Rastegaran, M.M.; Hassanpour, H.; Ziyadi, H. Synthesized Fe3O4 nanoparticles induced antioxidant activity and total phenolic and flavonoid content in Matricaria chamomilla seedlings. Iran. J. Plant Physiol. 2022, 12, 4003–4011. [Google Scholar] [CrossRef]
- Hamuda, H.E.B. Influence of engineered metal oxide nanoparticles on seed germination, seedling development and chlorophyll content. Obuda Univ. e-Bull. 2015, 5, 79. [Google Scholar]
- Hu, J.; Guo, H.; Li, J.; Gan, Q.; Wang, Y.; Xing, B. Comparative impacts of iron oxide nanoparticles and ferric ions on the growth of Citrus maxima. Environ. Pollut. 2017, 221, 199–208. [Google Scholar] [CrossRef]
- Muchkina, E.Y.; Subbotin, M.A.; Garmashova, M.K. The effect of nanoparticles of biogenic ferrihydrite on the development of Lepidium sativum L. IOP Conf. Ser. Earth Environ. Sci. 2019, 315, 042035. [Google Scholar] [CrossRef]
- Painuli, S.; Quispe, C.; Herrera-Bravo, J.; Semwal, P.; Martorell, M.; Almarhoon, Z.M.; Cho, W.C. Nutraceutical profiling, bioactive composition, and biological applications of Lepidium sativum L. Oxidative Med. Cell. Longev. 2022, 2022, 1–20. [Google Scholar] [CrossRef]
- Doke, S.; Guha, M. Garden cress (Lepidium sativum L.) seed-an important medicinal source: A Review. Cellulose 2014, 4, 69–80. [Google Scholar]
- Ju, M.; Navarreto-Lugo, M.; Wickramasinghe, S.; Milbrandt, N.B.; McWhorter, A.; Samia, A.C.S. Exploring the chelation-based plant strategy for iron oxide nanoparticle uptake in garden cress (Lepidium sativum) using magnetic particle spectrometry. Nanoscale 2019, 11, 18582–18594. [Google Scholar] [CrossRef]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Wang, X.; Xie, H.; Wang, P.; Yin, H. Nanoparticles in plants: Uptake, transport and physiological activity in leaf and root. Materials 2023, 16, 3097. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Zhou, Y.; Qin, J.G.; Yao, W.; Ma, Z. Optimization of the method for chlorophyll extraction in aquatic plants. J. Freshw. Ecol. 2010, 25, 531–538. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Pandey, H.; Kumar, S. Butylated hydroxytoluene and Butylated hydroxyanisole induced cyto-genotoxicity in root cells of Allium cepa L. Heliyon 2021, 7, e07055. [Google Scholar] [CrossRef]
- Salarizadeh, S.; Kavousi, H.R. Application of random amplified polymorphic DNA (RAPD) to detect the genotoxic effect of cadmium on tow iranian ecotypes of cumin (cuminum cyminum). J. Cell Mol. Res. 2015, 7, 38–46. [Google Scholar] [CrossRef]
- Bystrzejewska-Piotrowska, G.; Asztemborska, M.; Stęborowski, R.; Polkowska-Motrenko, H.; Danko, B.; Ryniewicz, J. Application of neutron activation for investigation of Fe3O4 nanoparticles accumulation by plants. Nukleonika 2012, 57, 427–430. [Google Scholar]
- Galaktionova, L.V.; Terehova, N.A.; Osipova, E.A.; Gusev, N.F.; Lebedev, S.V.; Gavrish, I.A. Biological effects of iron nanoparticles entering the soil. IOP Conf. Ser. Earth Environ. Sci. 2020, 579, 012087. [Google Scholar] [CrossRef]
- Kokina, I.; Plaksenkova, I.; Galek, R.; Jermaļonoka, M.; Kirilova, E.; Gerbreders, V.; Sledevskis, E. Genotoxic evaluation of Fe3O4 nanoparticles in different three barley (Hordeum vulgare L.) genotypes to explore the stress-resistant molecules. Molecules 2021, 26, 6710. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Li, P.; Zhao, X.; Ji, R.; Zhao, L. Physiological and metabolic responses of maize (Zea mays) plants to Fe3O4 nanoparticles. Sci. Total Environ. 2020, 718, 137400. [Google Scholar] [CrossRef]
- Hoffmann, N.; Tortella, G.; Hermosilla, E.; Fincheira, P.; Diez, M.C.; Lourenço, I.M.; Rubilar, O. Comparative toxicity assessment of eco-friendly synthesized superparamagnetic iron oxide nanoparticles (SPIONs) in plants and aquatic model organisms. Minerals 2022, 12, 451. [Google Scholar] [CrossRef]
- Agathokleous, E.; Feng, Z.; Peñuelas, J. Chlorophyll hormesis: Are chlorophylls major components of stress biology in higher plants? Sci. Total Environ. 2020, 726, 138637. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, R.J. Universal chlorophyll equations for estimating chlorophylls a, b, c, and d and total chlorophylls in natural assemblages of photosynthetic organisms using acetone, methanol, or ethanol solvents. Photosynthetica 2008, 46, 115–126. [Google Scholar] [CrossRef]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wu, X. Factors influencing leaf chlorophyll content in natural forests at the biome scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef]
- Muszyńska, E.; Labudda, M. Dual role of metallic trace elements in stress biology—From negative to beneficial impact on plants. Int. J. Mol. Sci. 2019, 20, 3117. [Google Scholar] [CrossRef]
- Kalisz, A.; Kornaś, A.; Skoczowski, A.; Oliwa, J.; Jurkow, R.; Gil, J.; Caruso, G. Leaf chlorophyll fluorescence and reflectance of oakleaf lettuce exposed to metal and metal (oid) oxide nanoparticles. BMC Plant Biol. 2023, 23, 329. [Google Scholar] [CrossRef]
- Syafitri, S.D.; Fevria, R. Chlorophyll Ratio of Kale (Ipomea reptans Poir.) Which Are Cultivation with Hydroponick and Non Hydroponick. Serambi Biol. 2021, 6. [Google Scholar] [CrossRef]
- Montvydienė, D.; Jagminas, A.; Jurgelėnė, Ž.; Kazlauskas, M.; Butrimienė, R.; Žukauskaitė, Z.; Kazlauskienė, N. Toxicological effects of different-sized Co–Fe (CoFe2O4) nanoparticles on Lepidium sativum L.: Towards better understanding of nanophytotoxicity. Ecotoxicology 2021, 30, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Răcuciu, M.; Tecucianu, A.; Oancea, S. Impact of magnetite nanoparticles coated with aspartic acid on the growth, antioxidant enzymes activity and chlorophyll content of maize. Antioxidants 2022, 11, 1193. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P. Genotoxicity of the nanoparticles. In The Impact of Nanoparticles on Agriculture and Soil; Academic Press: Cambridge, MA, USA, 2023; pp. 115–128. [Google Scholar]
- Kizilkaya, D.; Unal, F.; Beyzi, E.; Kulahci, M.B.; Calis Ismetoglu, G.; Yuzbasioglu, D.; Suludere, Z. Comparative investigation of iron oxide nanoparticles and microparticles using the in vitro bacterial reverse mutation and in vivo Allium chromosome aberration and comet assays. J. Nanopart. Res. 2023, 25, 173. [Google Scholar] [CrossRef]

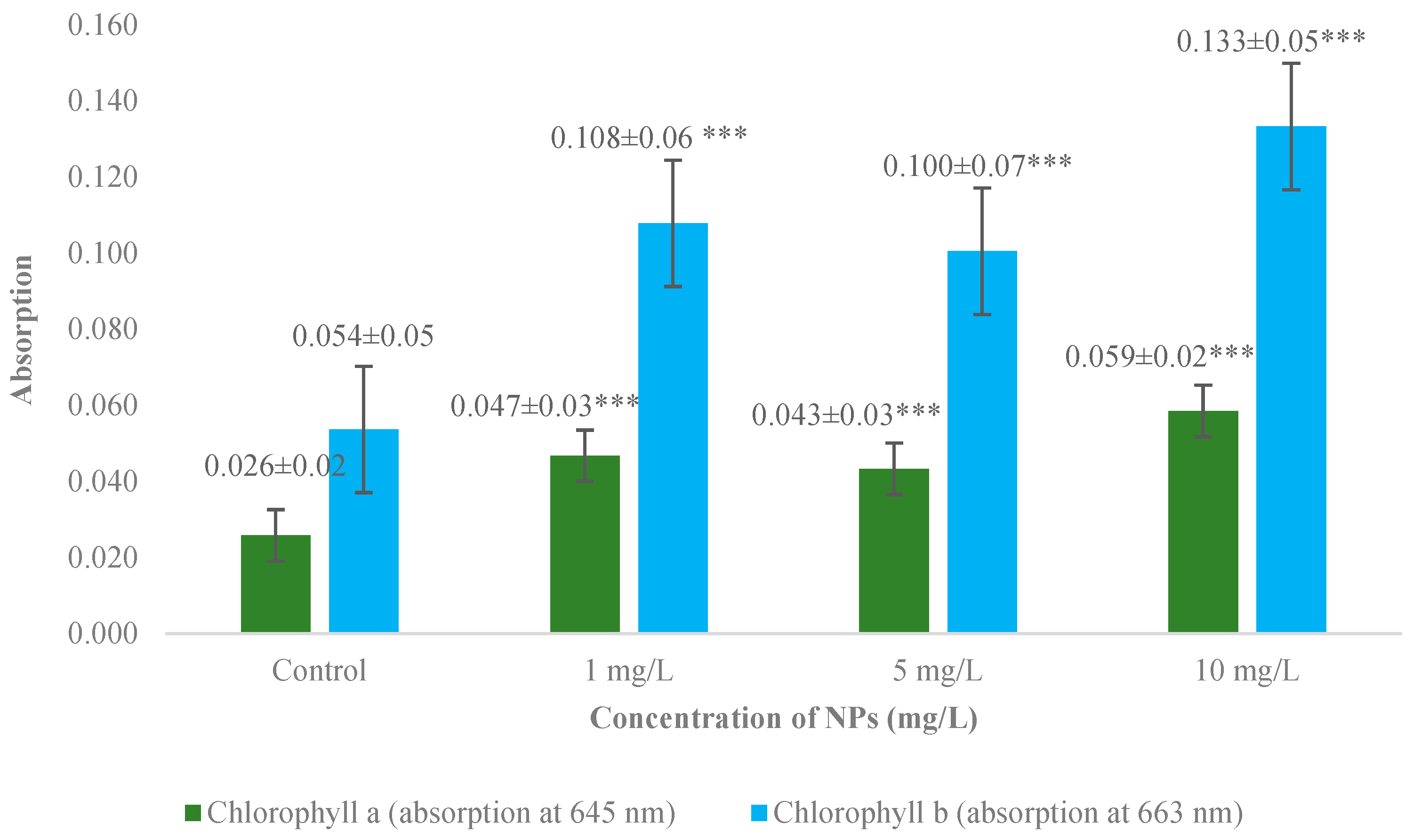
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mošenoka, A.; Kokina, I.; Plaksenkova, I.; Jermaļonoka, M.; Sledevskis, E.; Krasovska, M. Effects of Metal Oxide Nanoparticles on the Growth and Genotoxicity of Garden Cress (Lepidium sativum L.). Agronomy 2024, 14, 2324. https://doi.org/10.3390/agronomy14102324
Mošenoka A, Kokina I, Plaksenkova I, Jermaļonoka M, Sledevskis E, Krasovska M. Effects of Metal Oxide Nanoparticles on the Growth and Genotoxicity of Garden Cress (Lepidium sativum L.). Agronomy. 2024; 14(10):2324. https://doi.org/10.3390/agronomy14102324
Chicago/Turabian StyleMošenoka, Aleksandra, Inese Kokina, Ilona Plaksenkova, Marija Jermaļonoka, Eriks Sledevskis, and Marina Krasovska. 2024. "Effects of Metal Oxide Nanoparticles on the Growth and Genotoxicity of Garden Cress (Lepidium sativum L.)" Agronomy 14, no. 10: 2324. https://doi.org/10.3390/agronomy14102324







