Key Microorganisms Influencing Mineral-Protected Organic Carbon Formation in Soils with Exogenous Carbon Addition
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Sample Collection
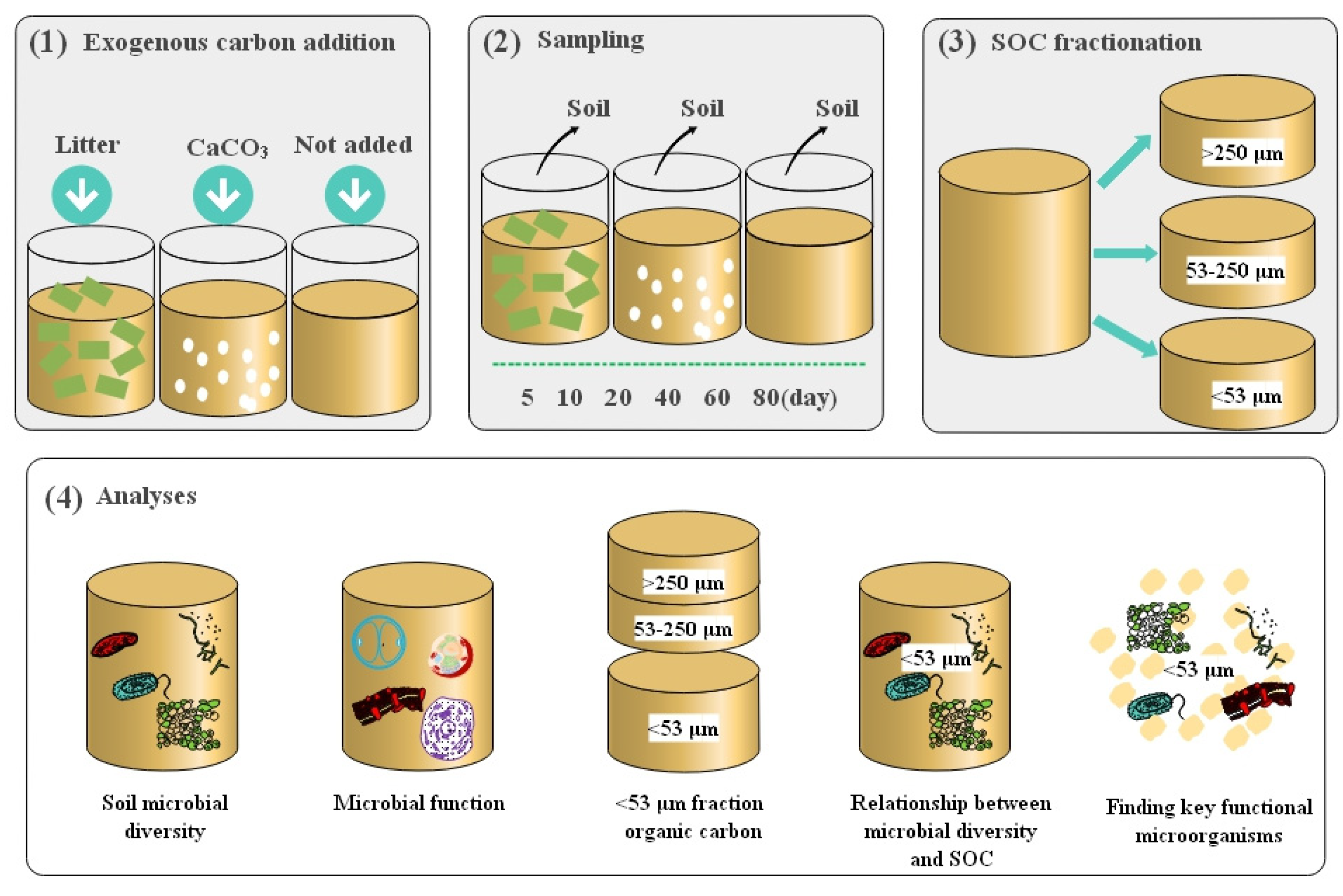
2.2. Design of Experimental Treatments
2.3. Preparation of Litter and CaCO3 Addition
2.4. Incubation Experiment
2.5. SOC Fractionation
2.6. Soil Physicochemical Measurement
2.7. Soil Microbial Determination and Treatment
2.7.1. DNA Extraction and PCR Amplification
2.7.2. Sequence Data Processing
2.7.3. Prediction of Soil’s Microbial and Ecological Functions
2.8. Statistical Analysis
3. Results
3.1. Effect of Exogenous C Addition on MPOC
3.2. Effect of Exogenous C Addition on Soil Microorganisms
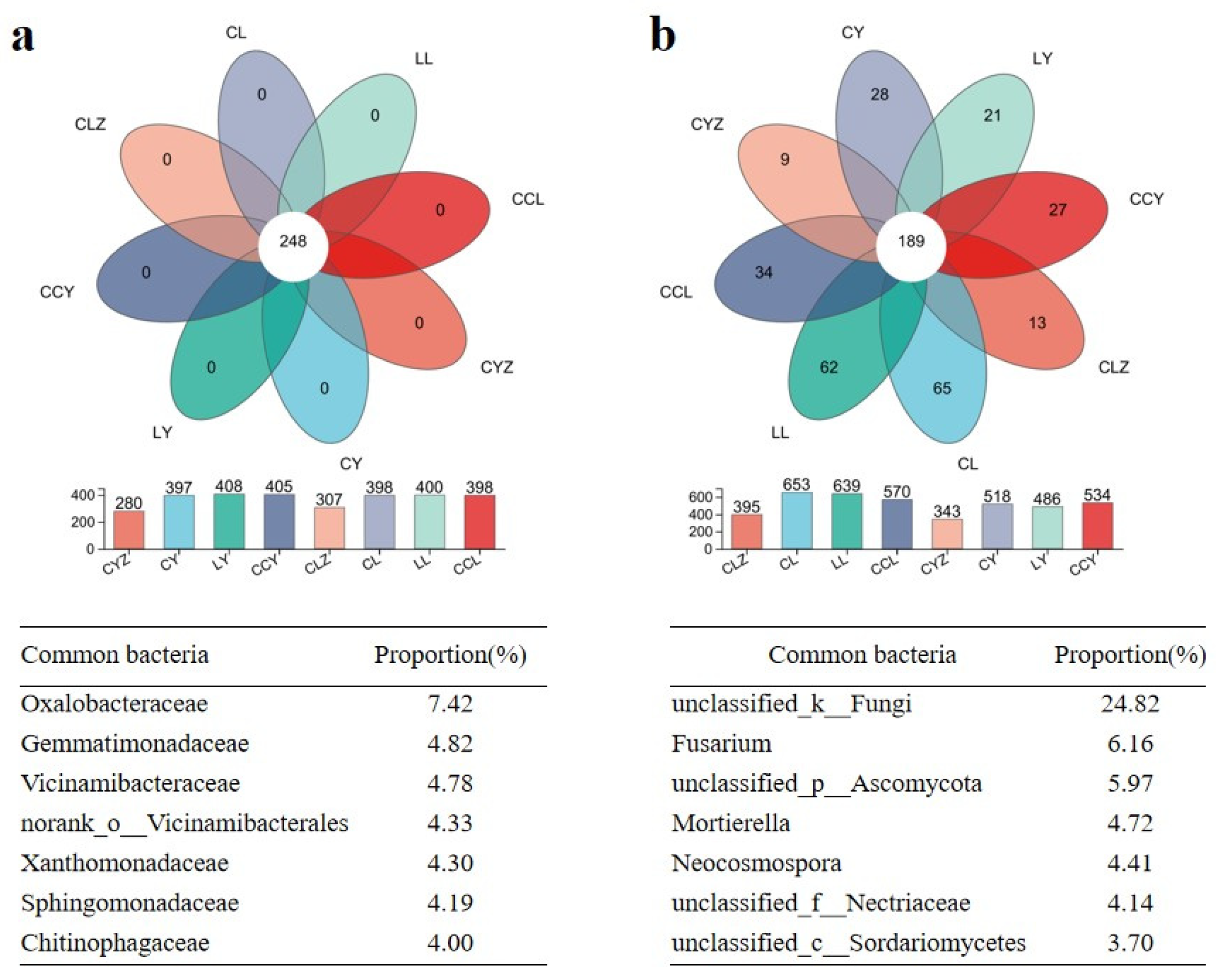
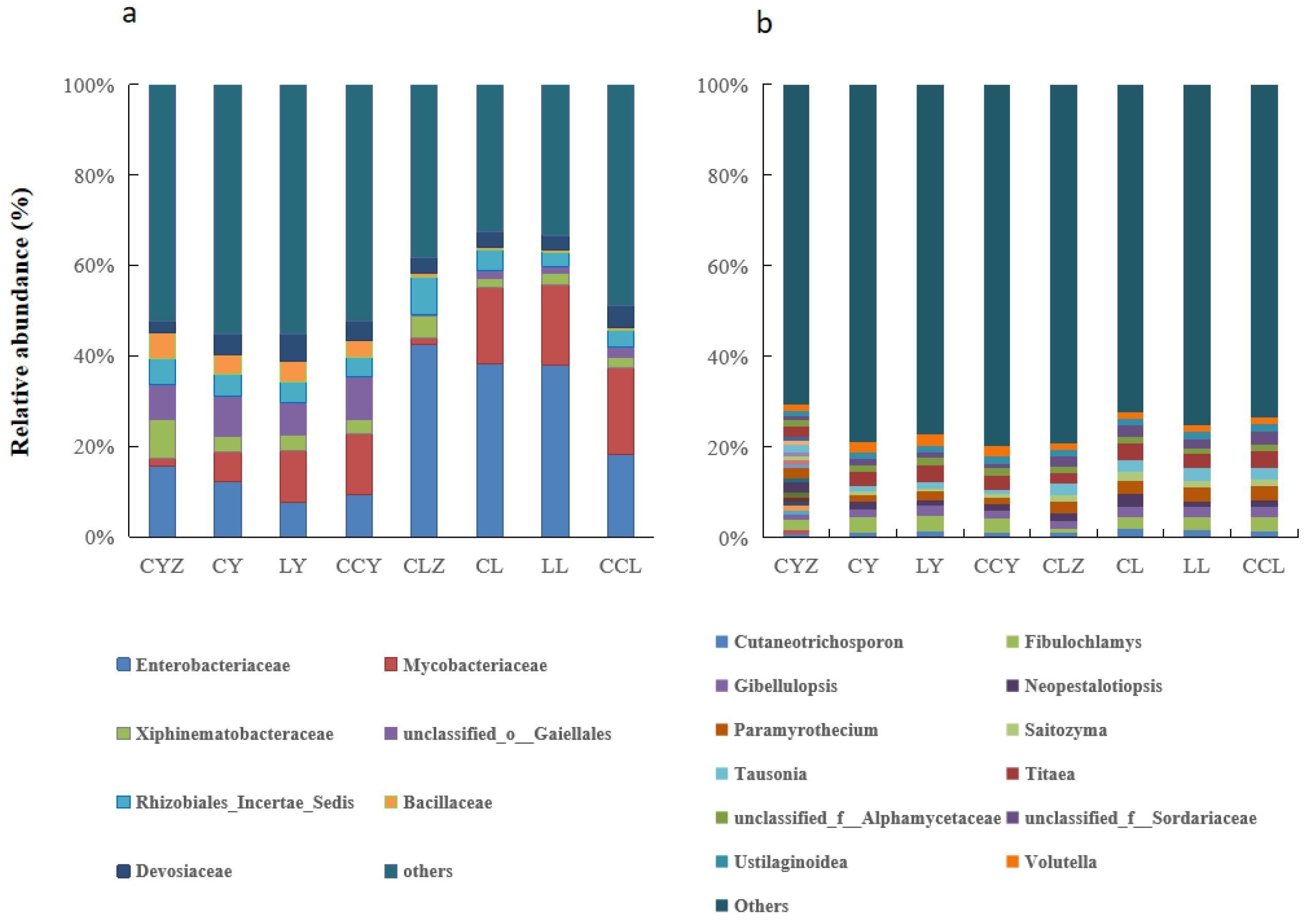
3.2.1. Impact of Exogenous C Addition on Soil’s Microbial Diversity and Community Composition
3.2.2. Composition and Functions of the Common Microbial Communities
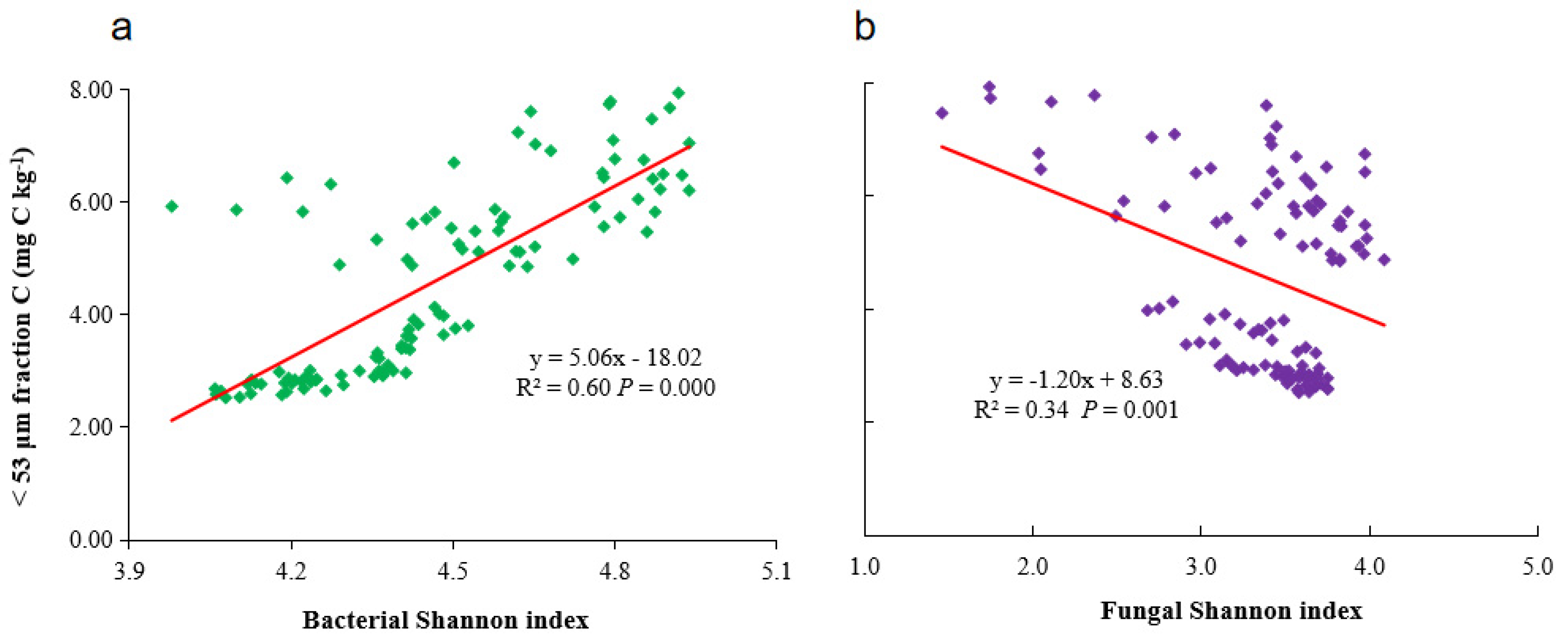
3.3. Effect of Soil Microbial Communities on MPOC
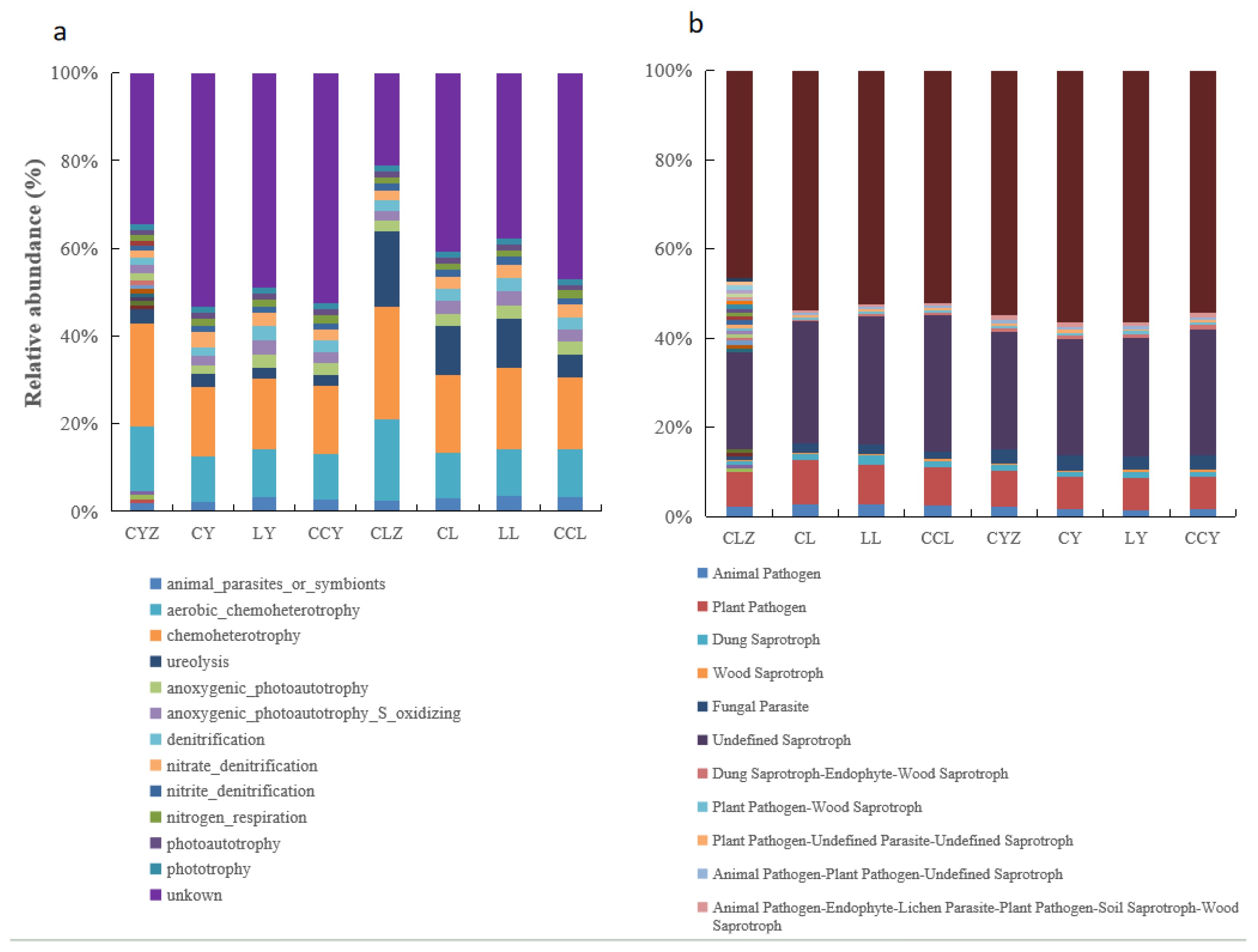
3.4. Ecological Functions of Major Microorganisms Affecting MPOC
4. Discussion
4.1. Changes in MPOC under Exogenous C Addition
4.2. Effects of Exogenous C Addition on Soil Microbial Communities
4.3. Main Microorganisms Affecting MPOC
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Angst, G.; Mueller, K.E.; Castellano, M.J.; Vogel, C.; Wiesmeier, M.; Mueller, C.W. Unlocking complex soil systems as carbon sinks: Multi-pool management as the key. Nat. Commun. 2023, 14, 2679. [Google Scholar] [CrossRef] [PubMed]
- Hicks, P.C.E.; Castanha, C.; Porras, R.C. The whole soils carbon flux in response to warming. Science 2017, 355, 1420–1423. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.J.; Li, X.Y.; Jiao, L.; Yang, Y.; Dou, Y.X.; Wang, B.R.; Huang, Q.; Liu, C.H.; Qu, T.T.; Zhou, Z.C.; et al. Advance in the formation and stabilization mechanisms of soil mineral-associated organic carbon. J. Soil Water Conserv. 2023, 37, 12–23. [Google Scholar]
- Basile, D.I.; Balesdent, J.; Pellerin, S. Reviews and syntheses: The mechanisms underlying carbon storage in soil. Biogeosciences 2020, 17, 5223–5242. [Google Scholar] [CrossRef]
- Yu, G.R.; Zhang, L.; He, H.L.; Yang, M. Process-based model and simulation system of dynamic change and spatial variation in large-scale terrestrial ecosystems. J. Appl. Ecol. 2021, 32, 2653–2665. [Google Scholar]
- Zhang, L.M.; Xu, M.G.; Lou, Y.L.; Wang, X.L.; Li, Z.F. Soil organic carbon fractionation methods. Soil Fertil. Sci. China 2014, 4, 1–6. [Google Scholar]
- Sokol, N.W.; Sanderman, J.; Bradford, M.A. Pathways of mineral-associated soil organic matter formation: Integrating the role of plant carbon source, chemistry, and point of entry. Glob. Chang. Biol. 2019, 25, 12–24. [Google Scholar] [CrossRef]
- Jilling, A.; Keiluweit, M.; Gutknecht, J.L.M. Priming mechanisms providing plants and microbes access to mineral-associated organic matter. Soil Biol. Boichem. 2021, 158, e108265. [Google Scholar] [CrossRef]
- Bramble, D.S.E.; Ulrich, S.; Schöning, I.; Mikutta, R.; Brandt, L.; Poll, C.; Kandeler, E.; Mikutta, C.; Konrad, A.; Siemens, J.; et al. Formation of mineral-associated organic matter in temperate soils is primarily controlled by mineral type and modified by land use and management intensity. Glob. Chang. Biol. 2024, 30, e17024. [Google Scholar] [CrossRef]
- Siebielec, S.; Siebielec, G.; Woźniak, M. Potential of using digestate to regenerate soil and stimulate its biological life. Pol. J. Agron. 2024, 52, 157–170. [Google Scholar]
- Różewicz, M.; Wyzińska, M.; Grabiński, J. Effect Application of Apple Pomace on Yield of Spring Wheat in Potting Experiment. Agronomy 2023, 13, 1526. [Google Scholar] [CrossRef]
- Ukalska-Jaruga, A.; Siebielec, G.; Siebielec, S.; Pecio, M. The Impact of Exogenous Organic Matter on Wheat Growth and Mineral Nitrogen Availability in Soil. Agronomy 2020, 10, 1314. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Liu, A.X.J.; Finley, B.K.; Mau, R.L.; Schwartz, E.; Dijkstra, P.; Bowker, M.A.; Hungate, B.A. The soil priming effect: Consistent across ecosystems, elusive mechanisms. Soil Biol. Biochem. 2020, 140, 1–12. [Google Scholar] [CrossRef]
- Liu, L.; Gundersen, P.; Zhang, T.; Mo, J. Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China. Soil Biol. Biochem. 2012, 44, 31–38. [Google Scholar] [CrossRef]
- Klink, S.; Keller, A.B.; Wild, A.J.; Baumert, V.L.; Gube, M.; Lehndorff, E.; Meyer, N.; Mueller, C.W.; Phillips, R.P.; Pausch, J. Stable isotopes reveal that fungal residues contribute more to mineral-associated organic matter pools than plant residues. Soil Biol. Biochem. 2022, 168, 108634. [Google Scholar] [CrossRef]
- Nonthijun, P.; Mills, N.; Mills, N.; Yongsawas, R.; Sansupa, C.; Suwannarach, N.; Jaikang, C.; Motanated, K.; Chayakdee, P.; Jongjitngam, S.; et al. Seasonal Variations in Fungal Communities on the Surfaces of Lan Na Sandstone Sculptures and Their Biodeterioration Capacities. J. Fungi 2023, 9, 833. [Google Scholar] [CrossRef]
- Arunrat, N.; Kongsurakan, P.; Solomon, L.W.; Sereenonchai, S. Fire Impacts on Soil Properties and Implications for Sustainability in Rotational Shifting Cultivation: A Review. Agriculture 2024, 14, 1660. [Google Scholar] [CrossRef]
- Andrew, T.N.; Emanuel, G.; Erland, B. Soil carbon and microbes in the warming tropics. Funct. Ecol. 2022, 10, 1365–1406. [Google Scholar]
- Cotrufo, M.F.; Haddix, M.L.; Kroeger, M.E.; Stewart, C.E. The role of plant input physical-chemical properties, and microbial and soil chemical diversity on the formation of particulate and mineral-associated organic matter. Soil Biol. Biochem. 2022, 168, 108648. [Google Scholar] [CrossRef]
- Song, Y.Y.; Liu, C.; Song, C.C.; Wang, X.W.; Ma, X.Y.; Gao, J.L.; Gao, S.Q.; Wang, L.L. Linking soil organic carbon mineralization with soil microbial and substrate properties under warming in permafrost peatlands of Northeastern China. Catena 2021, 203, 105348. [Google Scholar] [CrossRef]
- Witzgall, K.; Vidal, A.; Schubert, D.I.; Schweizer, S.A.; Buegger, F.; Pouteau, V.; Chenu, C.; Mueller, C.W. Particulate organic matter as a functional soil component for persistent soil organic carbon. Nat. Commun. 2021, 12, 4115. [Google Scholar] [CrossRef] [PubMed]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.H. Organic Carbon Mineralization and Microbial in Typical Karst Soil as a Response to the Exogenous Addition of Calcium Carbonate and Organic Substrate. Master’s Thesis, Hunan Agricultural University, Changsha, China, 2012. [Google Scholar]
- Six, J.; Conant, R.T.; Paustian, K.; Paul, E.A. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agrochemical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2001; pp. 3–109. [Google Scholar]
- Xu, N.; Tan, G.; Wang, H.; Gai, X. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil Biol. 2016, 74, 1–8. [Google Scholar] [CrossRef]
- Adams, R.I.; Miletto, M.; Taylor, J.W.; Bruns, T. D Dispersal in microbes: Fungi in indoor air are dominated by outdoor air and show dispersal limitation at short distances. ISME J. 2013, 7, 1262–1273. [Google Scholar] [CrossRef]
- Yergeau, E.; Bokhorst, S.; Huiskes, A.H.L.; Boschker, H.T.S.; Aerts, R.; Kowalchuk, G.A. Size and structure of bacterial, fungal and nematode communities along an Antarctic environmental gradient. FEMS Microbiol. Ecol. 2007, 59, 436–451. [Google Scholar] [CrossRef]
- Li, Q.R.; Tian, Y.Q.; Zhang, X.Y.; Xu, X.L.; Wang, H.M.; Yakov, K. Labile carbon and nitrogen additions affect soil organic matter decomposition more strongly than temperature. Appl. Soil Ecol. 2017, 114, 152–160. [Google Scholar] [CrossRef]
- Zhang, M.; Dong, L.G.; Fei, S.X.; Zhang, J.W.; Jiang, X.M.; Wang, Y.; Yu, X. Responses of Soil Organic Carbon Mineralization and Microbial Communities to Leaf Litter Addition under Different Soil Layers. Forests 2021, 12, 170–185. [Google Scholar] [CrossRef]
- Zheng, H.P.; Yang, T.J.; Bao, Y.Z.; He, P.P.; Yang, K.M.; Mei, X.L.; Wei, Z.; Xu, Y.C.; Shen, Q.R.; Banerjee, S. Network analysis and subsequent culturing reveal keystone taxa involved in microbial litter decomposition dynamics. Soil Biol. Biochem. 2021, 157, 108230. [Google Scholar] [CrossRef]
- Joanna, R.; Jennifer, K.; Ember, M.; Hayden, S.; Edward, B. Roots selectively decompose litter to mine nitrogen and build new soil carbon. Ecol. Lett. 2023, 27, e14331. [Google Scholar]
- Duan, P.P.; Wang, K.L.; Li, D.J. Nitrogen addition effects on soil mineral-associated carbon differ between the valley and slope in a subtropical karst forest. Geoderma 2023, 430, 116357. [Google Scholar] [CrossRef]
- Hu, L.N.; Su, Y.R.; He, X.Y.; Wu, J.S.; Zheng, H.; Li, Y.; Wang, A.H. Response of soil organic carbon mineralization in typical Karst soils following the addition of 14C-labeled rice straw and CaCO3. J. Sci. Food Agric. 2012, 92, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.W.; Deng, L.; Gunina, A.; Alharbi, S.; Wang, K.B.; Li, J.W.; Liu, Y.L.; Shangguan, Z.P.; Kuzyakov, Y. Carbon stabilization pathways in soil aggregates during long-term forest succession: Implications from δ13C signatures. Soil Biol. Biochem. 2023, 180, 108988. [Google Scholar] [CrossRef]
- Fei, C.; Zhang, S.R.; Zhang, L.; Ding, X.D. Straw is more effective than biochar in mobilizing soil organic phosphorus mineralization in saline-alkali paddy soil. Appl. Soil Ecol. 2023, 186, 104848. [Google Scholar] [CrossRef]
- Stegarescu, G.; Escuer, G.J.; Soosaar, K.; Kauer, K.; Astover, A.; Reintam, E. Effect of crop residue decomposition on soil aggregate stability. Agriculture 2020, 10, 527–539. [Google Scholar] [CrossRef]
- Kallenbach, C.M.; Frey, S.D.; Grandy, A. Stuart Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat. Commun. 2016, 7, 13630. [Google Scholar] [CrossRef]
- Zheng, T.T.; Xie, H.T.; Grant, L.T.; Bao, X.L.; Deng, F.B.; Yan, E.R.; Zhou, X.H.; Liang, C. Shifts in microbial metabolic pathway for soil carbon accumulation along subtropical forest succession. Soil Biol. Biochem. 2021, 160, 108335. [Google Scholar] [CrossRef]
- Shabtai, I.A.; Wilhelm, R.C.; Schweizer, S.A.; Buckley, D.H.; Lehmann, J. Calcium promotes persistent soil organic matter by altering microbial transformation of plant litter. Nat. Commun. 2023, 14, 6609. [Google Scholar] [CrossRef]
- Hu, X.; Yin, P.; Nong, X.; Liao, J.H. Effect of exogenous carbon addition and the freeze-thaw cycle on soil microbes and mineral nitrogen pools1. IOP Conf. Ser. Earth Environ. Sci. 2018, 108, 032046. [Google Scholar] [CrossRef]
- Ling, J.K.; Mao, X.F.; Wei, X.Y.; Tan, W.J.; Wu, Y.; Zhang, Y.C.; Bao, X.X.; Tong, L.L. The indication of soil microorganism to wetland restoration in Three River Source Area. IOP Conf. Ser. Earth Environ. Sci. 2020, 601, 012051. [Google Scholar] [CrossRef]
- Robert, W.B.; Davey, L.J. Plasticity of microbial substrate carbon use efficiency in response to changes in plant carbon input and soil organic matter status. Soil Biol. Biochem. 2024, 188, 109230. [Google Scholar]
- Keyvan, E.S.; Bahram, M.; Ghanbari, M.S.; Gohar, D.; Tohidfar, M.; Eremeev, V.; Talgre, L.; Khaleghdoust, B.; Mirmajlessi, S.M.; Luik, A.; et al. Cropping systems with higher organic carbon promote soil microbial diversity. Agric. Ecosyst. Environ. 2021, 319, 107521. [Google Scholar]
- Camenzind, T.; Mason, J.K.; Mansour, I.; Rillig, M.C.; Lehmann, J. Formation of necromass-derived soil organic carbon determined by microbial death pathways. Nat. Geosci. 2023, 16, 115–122. [Google Scholar] [CrossRef]
- Lucas, J.M.; McBride, S.G.; Strickland, M.S. Trophic level mediates soil microbial community composition and function. Soil Biol. Biochem. 2020, 143, 107756. [Google Scholar] [CrossRef]
- Zhu, M.T.; Liu, X.X.; Wang, J.M.; Liu, Z.W.; Zheng, J.F.; Bian, R.J.; Wang, G.M.; Zhang, X.H.; Li, L.Q.; Pan, G.X. Effects of biochar application on soil microbial diversity in soil aggregates from paddy soil. Acta Ecol. Sin. 2020, 40, 1–12. [Google Scholar]
- Wang, C.; Liu, D.W.; Bai, E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol. Biochem. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Cao, M.M.; Zheng, X.; Cui, L.N.; Wu, F.; Gao, H.D.; Jiang, J. Soil bacterial communities are more sensitive to short-term nitrogen deposition than fungal communities in subtropical Chinese fir forests. Forest Ecol. Manag. 2023, 549, 121490. [Google Scholar] [CrossRef]
- Zhang, L.M.; Wang, Y.; Chen, J.; Feng, L.; Li, F.B.; Yu, L.F. Characteristics and Drivers of Soil Organic Carbon Saturation Deficit in Karst Forests of China. Diversity 2022, 14, 62. [Google Scholar] [CrossRef]
- Cobos, M.; Estela, S.L.; Rodrguez, H.N.; Castro, C.G.; Grandez, M.; Castro, J.C. Soil microbial diversity and functional profiling of a tropical rainforest of a highly dissected low hill from the upper Itaya river basin revealed by analysis of shotgun metagenomics sequencing data. Data Brief 2022, 42, 108205. [Google Scholar] [CrossRef]
- Zhang, L.M.; Wang, Y.; Chen, J.; Zhang, C.F.; Cao, Y.; Cai, G.J.; Yu, L.F. Exogenous carbon addition reduces soil organic carbon: The effects of fungi on soil carbon priming exceed those of bacteria on soil carbon sequestration. Forests 2023, 14, 1268. [Google Scholar] [CrossRef]
- Hu, P.L.; Zhang, W.; Chen, H.S.; Xu, L.; Xiao, J.; Luo, Y.Q.; Wang, K.L. Lithologic control of microbial-derived carbon in forest soils. Soil Biol. Biochem. 2022, 167, 108600. [Google Scholar] [CrossRef]
- Lu, X.L. A Review of Karst Environment andBiodiversity in China. Adv. Environ. Prot. 2024, 14, 348–353. [Google Scholar] [CrossRef]
- Sheng, Y.Q.; Zhu, L.Z. 2Biochar alters microbial community and carbon sequestration potential across different soil pH. Sci. Total Environ. 2018, 622, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Gai, X.; Zhong, Z.K.; Zhang, X.P.; Bian, F.Y.; Yang, C.B. Effects of chicken farming on soil organic carbon fractions and fungal communities in a Lei bamboo (Phyllostachys praecox) forest in subtropical China. Forest Ecol. Manag. 2021, 479, 118603. [Google Scholar] [CrossRef]
- He, H.R.; Xu, M.Z.; Li, W.T.; Chen, L.; Chen, Y.N.; Moorhead, D.L.; Albert, B.C.; Liu, J.; Cui, Y.X.; Zeng, Y.; et al. Linking soil depth to aridity effects on soil microbial community composition, diversity and resource limitation. Catena 2023, 232, 107393. [Google Scholar] [CrossRef]
- Jenkins, J.R.; Viger, M.; Arnold, E.C.; Harris, Z.M.; Ventura, M.; Miglietta, F.; Girardin, C.; Edwards, R.J.; Rumpel, C.; Fornasier, F. Biochar alters the soil microbiome and soil function: Results of next-generation amplicon sequencing across Europe. Gcb. Bioenergy 2017, 9, 485–661. [Google Scholar] [CrossRef]
- Menzel, A.; Hempel, S.; Klotz, S. Mycorrhizal status helps explain invasion success of alien plant species. Ecology 2017, 98, 92–102. [Google Scholar] [CrossRef]
- Chakraborty, K.; Roy, D.A.; Saha, A.K.; Das, P. A culture based diversity of saprobic fungi associated with leaf litter of Hevea brasiliensis along a chronosequence of plantations in Tripura, Northeast India. Trop. Ecol. 2020, 61, 468–474. [Google Scholar] [CrossRef]
- Kang, P.; Pan, Y.Q.; Ran, Y.C.; Li, W.N.; Shao, M.X.; Zhang, Y.Q.; Ji, Q.B.; Ding, X.D. Soil saprophytic fungi could be used as an important ecological indicator for land management in desert steppe. Ecol. Indic. 2023, 150, 110224. [Google Scholar] [CrossRef]
- Hu, M.H.; Zhao, J.Q.; Wang, X.; Xiong, X.; Zhang, H.L.; Zhu, G.W.; Meng, Z.; Zhang, D.Q. Effects of natural warming on soil microorganisms communities and functional genes of soil organic carbon metabolism in subtropical forests. Acta Ecol. Sin. 2022, 42, 359–369. [Google Scholar]
- Yang, N.; Zhang, Y.; Li, J.J.; Xiong, X.; Zhang, H.L.; Zhu, G.W.; Meng, Z.; Zhang, D.Q. Interaction among soil nutrients, plant diversity and hypogeal fungal trophic guild modifies root-associated fungal diversity in coniferous forests of Chinese Southern Himalayas. Plant Soil 2022, 481, 395–408. [Google Scholar] [CrossRef]
| Item | pH | BD (g cm−3) | SOC (g kg−1) | TN (g kg−1) | TP (g kg−1) | Bacterial Diversity | Fungal Diversity | <53 μm C |
|---|---|---|---|---|---|---|---|---|
| Lime soil (CLZ) | 6.55 ± 0.04 | 1.18 ± 0.03 | 29.23 ± 3.05 | 2.33 ± 0.17 | 0.37 ± 0.01 | 3.84 ± 0.18 | 3.80 ± 0.09 | 4.51 ± 0.17 |
| Yellow soil (CYZ) | 6.13 ± 0.02 | 1.16 ± 0.01 | 13.31 ± 2.35 | 1.24 ± 0.11 | 0.68 ± 0.05 | 3.90 ± 0.06 | 3.62 ± 0.07 | 2.32 ± 0.05 |
| Treatments | 5 | 10 | 20 | 40 | 60 | 80 |
|---|---|---|---|---|---|---|
| CY | 2.59 ± 0.03 b | 2.75 ± 0.14 ab | 2.80 ± 0.18 ab | 2.85 ± 0.04 a | 2.96 ± 0.06 a | 3.03 ± 0.07 a |
| LY | 2.64 ± 0.08 d | 2.84 ± 0.12 c | 2.97 ± 0.16 c | 3.40 ± 0.07 b | 3.73 ± 0.07 b | 4.04 ± 0.08 a |
| CCY | 2.59 ± 0.08 d | 2.80 ± 0.09 c | 2.95 ± 0.14 c | 3.25 ± 0.03 b | 3.55 ± 0.05 a | 3.82 ± 0.05 a |
| CL | 4.91 ± 0.15 c | 4.98 ± 0.05 c | 5.23 ± 0.19 b | 5.55 ± 0.16 b | 5.70 ± 0.37 a | 5.87 ± 0.24 a |
| LL | 5.55 ± 0.23 d | 6.15 ± 0.10 c | 6.38 ± 0.23 c | 6.82 ± 0.32 b | 7.28 ± 0.22 a | 7.61 ± 0.41 a |
| CCL | 5.30 ± 0.26 d | 5.27 ± 0.10 d | 5.75 ± 0.18 c | 6.15 ± 0.29 b | 6.55 ± 0.29 a | 6.68 ± 0.27 a |
| Name | Treatments | 5 | 10 | 20 | 40 | 60 | 80 |
|---|---|---|---|---|---|---|---|
| Bacteria | CY | 4.18 ± 0.05 b | 4.18 ± 0.07 b | 4.24 ± 0.08 ab | 4.23 ± 0.11 ab | 4.37 ± 0.03 a | 4.38 ± 0.05 a |
| LY | 4.12 ± 0.06 c | 4.21 ± 0.02 b | 4.23 ± 0.07 b | 4.41 ± 0.11 a | 4.50 ± 0.09 a | 4.47 ± 0.04 a | |
| CCY | 4.07 ± 0.07 b | 4.13 ± 0.09 b | 4.36 ± 0.05 a | 4.36 ± 0.02 a | 4.41 ± 0.08 a | 4.43 ± 0.09 a | |
| CL | 4.44 ± 0.16 a | 4.63 ± 0.09 a | 4.51 ± 0.13 a | 4.50 ± 0.19 a | 4.54 ± 0.09 a | 4.60 ± 0.14 a | |
| LL | 4.08 ± 0.11 c | 4.47 ± 0.26 b | 4.64 ± 0.15 a | 4.74 ± 0.16 a | 4.76 ± 0.11 a | 4.87 ± 0.06 a | |
| CCL | 4.39 ± 0.23 c | 4.67 ± 0.09 b | 4.83 ± 0.03 a | 4.87 ± 0.06 a | 4.85 ± 0.07 a | 4.82 ± 0.05 a | |
| Fungi | CY | 3.61 ± 0.02 a | 3.40 ± 0.10 a | 3.39 ± 0.12 a | 3.73 ± 0.13 a | 3.61 ± 0.03 a | 3.45 ± 0.10 a |
| LY | 3.71 ± 0.02 a | 3.64 ± 0.10 a | 2.99 ± 0.15 b | 3.38 ± 0.24 a | 3.52 ± 0.15 a | 2.75 ± 0.12 b | |
| CCY | 3.67 ± 0.06 a | 3.58 ± 0.11 a | 3.61 ± 0.04 a | 3.62 ± 0.09 a | 3.57 ± 0.13 a | 3.54 ± 0.12 a | |
| CL | 3.85 ± 0.11 a | 3.73 ± 0.06 a | 3.46 ± 0.03 b | 3.40 ± 0.07 b | 3.24 ± 0.15 b | 2.88 ± 0.20 c | |
| LL | 3.79 ± 0.06 a | 3.63 ± 0.12 a | 3.57 ± 0.05 a | 3.55 ± 0.08 a | 2.20 ± 0.11 b | 2.07 ± 0.16 b | |
| CCL | 3.96 ± 0.10 a | 3.89 ± 0.06 a | 3.70 ± 0.22 a | 3.32 ± 0.14 b | 3.15 ± 0.09 b | 2.64 ± 0.07 c |
| Family | Ecological Functions |
|---|---|
| Beijerinckiaceae, Chitinophagaceae, Microbacteriaceae, Mycobacteriaceae, norank_o__Vicinamibacterales, Hymenobacteraceae, Sphingobacteriaceae, Streptomycetaceae, Pseudomonadaceae, Reyranellaceae, Solirubrobacteraceae, Thermomonosporaceae, norank_o__norank_c__Subgroup_22 | aerobic chemoheterotrophy, chemoheterotrophy |
| Oxalobacteraceae | ureolysis |
| Planococcaceae | manganese oxidation |
| Polyangiaceae | Cellulolysis, chemoheterotrophy |
| Acetobacteraceae | aerobic chemoheterotrophy, animal parasites or symbionts, chemoheterotrophy, human-associated functions, human pathogens all, ureolysis |
| A21b | animal parasites or symbionts, human-associated functions, human pathogens all, human pathogens pneumonia |
| Nocardiaceae | aromatic compound degradation, aliphatic non-methane hydrocarbon degradation, aromatic hydrocarbon degradation, chemoheterotrophy, hydrocarbon degradation, ureolysis |
| Xanthobacteraceae | aerobic chemoheterotrophy, anoxygenic photoautotrophy, S-oxidizing anoxygenic photoautotrophy, chemoheterotrophy, denitrification, nitrate denitrification, nitrate reduction, nitrite denitrification, nitrogen respiration, photoautotrophy, phototrophy |
| Xiphinematobacteraceae | animal parasites or symbionts |
| Sutterellaceae | animal parasites or symbionts, human-associated functions, human pathogens all, human pathogens pneumonia |
| Devosiaceae | aerobic chemoheterotrophy, chemoheterotrophy, ureolysis |
| Rhodanobacteraceae | aerobic chemoheterotrophy, chemoheterotrophy, nitrate reduction |
| Genus | Ecological Functions |
|---|---|
| Cladophialophora, Clavaria, Arthrobotrys, Conocybe, Coprinellus, Creosphaeria, Dactylella, Emericellopsis, Geminibasidium, Gonytrichum, Hamigera, Leohumicola, Leptodiscella, Lophiotrema, Lophotrichus, Monocillium, Myrmecridium, Neobulgaria, Nemania, Subplenodomus, Subulicystidium, Talaromyces, Tetracladium, Titaea, Trichaleurina, Trichocladium, Phaeosphaeriopsis, Pleurotheciella, Pseudeurotium, Sarocladium, Scolecobasidium, Scutellinia, Sporormiella, Stephanonectria, Paramicrothyrium, Paraphaeosphaeria, Nigrospora, Nodulisporium, Paraconiothyrium, Neocosmospora, Preussia | Undefined Saprotroph |
| Clonostachys, Neonectria, Cylindrocarpon, Ilyonectria, Dendryphion, Gibberella, Gibellulopsis, Olpidium | Plant Pathogen |
| Acaulopage, Simplicillium, Metarhizium | Animal Pathogen |
| Lactarius, Tomentella, Pulvinula | Ectomycorrhizal |
| Schizothecium, Cercophora, Zopfiella | Dung Saprotroph |
| Diversispora, Entrophospora | Arbuscular Mycorrhizal |
| Psathyrella, Trechispora | Wood Saprotroph |
| Purpureocillium, Syncephalis | Fungal Parasite |
| Cystofilobasidium | Leaf Saprotroph |
| Lecythophora | Endophyte |
| Serendipita | Orchid Mycorrhizal |
| Cyphellophora | Animal Pathogen–Undefined Saprotroph |
| Ascobolus | Dung Saprotroph–Wood Saprotroph |
| Kernia | Dung Saprotroph–Undefined Saprotroph |
| Stilbella | Dung Saprotroph–Endophyte–Wood Saprotroph |
| Myxotrichum | Dung Saprotroph–Ericoid Mycorrhizal–Lichenized |
| Ganoderma | Plant Pathogen–Wood Saprotroph |
| Leucosporidium | Soil Saprotroph–Undefined Saprotroph |
| Periconia | Endophyte–Plant Pathogen–Wood Saprotroph |
| Pyrenochaetopsis | Endophyte–Lichen Parasite–Undefined Saprotroph |
| Spizellomyces | Plant Pathogen–Undefined Parasite–Undefined Saprotroph |
| Pluteus | Bryophyte Parasite–Litter Saprotroph–Wood Saprotroph |
| Podospora | Dung Saprotroph–Endophyte–Litter Saprotroph–Undefined Saprotroph |
| Alternaria | Animal Pathogen–Endophyte–Plant Pathogen–Wood Saprotroph |
| Coniochaeta | Animal Pathogen–Dung Saprotroph–Endophyte–Lichen Parasite–Plant Pathogen–Undefined Saprotroph |
| Didymella | Animal Pathogen–Plant Pathogen–Undefined Saprotroph |
| Entoloma | Ectomycorrhizal–Fungal Parasite–Soil Saprotroph–Undefined Saprotroph |
| Fusarium | Animal Pathogen–Endophyte–Lichen Parasite–Plant Pathogen–Soil Saprotroph–Wood Saprotroph |
| Mortierella | Endophyte–Litter Saprotroph–Soil Saprotroph–Undefined Saprotroph |
| Trichoderma | Animal Pathogen–Endophyte–Epiphyte–Fungal Parasite–Plant Pathogen–Wood Saprotroph |
| Acremonium | Animal Pathogen–Endophyte–Fungal Parasite–Plant Pathogen–Wood Saprotroph |
| Family | Ecological Functions |
|---|---|
| A21b | animal parasites or symbionts, human-associated functions, human pathogens all, human pathogens pneumonia |
| Acetobacteraceae | aerobic chemoheterotrophy, animal parasites or symbionts, chemoheterotrophy, human-associated functions, human pathogens all, ureolysis |
| Microbacteriaceae | aerobic chemoheterotrophy, chemoheterotrophy |
| Mycobacteriaceae | aerobic chemoheterotrophy, chemoheterotrophy |
| Nocardiaceae | aromatic compound degradation, aliphatic non-methane hydrocarbon degradation, aromatic hydrocarbon degradation, chemoheterotrophy, hydrocarbon degradation, ureolysis |
| norank_o__Vicinamibacterales | Aerobic chemoheterotrophy, chemoheterotrophy |
| Oxalobacteraceae | ureolysis |
| Polyangiaceae | Cellulolysis, chemoheterotrophy |
| Pseudomonadaceae | aerobic chemoheterotrophy, chemoheterotrophy |
| Reyranellaceae | aerobic chemoheterotrophy, chemoheterotrophy |
| Streptomycetaceae | aerobic chemoheterotrophy, chemoheterotrophy |
| Thermomonosporaceae | aerobic chemoheterotrophy, chemoheterotrophy |
| Xanthobacteraceae | aerobic chemoheterotrophy, anoxygenic photoautotrophy, S-oxidizing anoxygenic photoautotrophy, chemoheterotrophy, denitrification, nitrate denitrification, nitrate reduction, nitrite denitrification, nitrogen respiration, photoautotrophy, phototrophy |
| Sutterellaceae | animal parasites or symbionts, human-associated functions, human pathogens all, human pathogens pneumonia |
| Rhodanobacteraceae | aerobic chemoheterotrophy, chemoheterotrophy, nitrate reduction |
| norank_o__norank_c__Subgroup_22 | aerobic chemoheterotrophy, chemoheterotrophy |
| Genus | Ecological Functions | Genus | Ecological Functions |
|---|---|---|---|
| Lecythophora | Endophyte | Metarhizium | Animal Pathogen |
| Tomentella | Ectomycorrhizal | Lophotrichus | Undefined Saprotroph |
| Purpureocillium | Fungal Parasite | Monocillium | Undefined Saprotroph |
| Schizothecium | Dung Saprotroph | Nemania | Undefined Saprotroph |
| Clonostachys | Plant Pathogen | Neobulgaria | Undefined Saprotroph |
| Gibberella | Plant Pathogen | Neocosmospora | Undefined Saprotroph |
| Neonectria | Plant Pathogen | Pseudeurotium | Undefined Saprotroph |
| Cylindrocarpon | Plant Pathogen | Nigrospora | Undefined Saprotroph |
| Olpidium | Plant Pathogen | Nodulisporium | Undefined Saprotroph |
| Dendryphion | Plant Pathogen | Arthrobotrys | Undefined Saprotroph |
| Coprinellus | Undefined Saprotroph | Ascobolus | Dung Saprotroph–Wood Saprotroph |
| Scolecobasidium | Undefined Saprotroph | Kernia | Dung Saprotroph–Undefined Saprotroph |
| Stephanonectria | Undefined Saprotroph | Stilbella | Dung Saprotroph–Endophyte–Wood Saprotroph |
| Subplenodomus | Undefined Saprotroph | Periconia | Endophyte–Plant Pathogen–Wood Saprotroph |
| Tetracladium | Undefined Saprotroph | Pyrenochaetopsis | Endophyte–Lichen Parasite–Undefined Saprotroph |
| Trichocladium | Undefined Saprotroph | Entoloma | Ectomycorrhizal–Fungal Parasite–Soil Saprotroph–Undefined Saprotroph |
| Gonytrichum | Undefined Saprotroph | Acremonium | Animal Pathogen–Endophyte–Fungal Parasite–Plant Pathogen–Wood Saprotroph |
| Lophiotrema | Undefined Saprotroph | Trichoderma | Animal Pathogen–Endophyte–Epiphyte-Fungal Parasite–Plant Pathogen–Wood Saprotroph |
| Clavaria | Undefined Saprotroph | Fusarium | Animal Pathogen–Endophyte–Lichen Parasite–Plant Pathogen–Soil Saprotroph–Wood Saprotroph |
| Leohumicola | Undefined Saprotroph | Coniochaeta | Animal Pathogen–Dung Saprotroph–Endophyte–Lichen Parasite–Plant Pathogen–Undefined Saprotroph |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Luo, Y.; Wang, Y.; Zhang, C.; Cai, G.; Su, W.; Yu, L. Key Microorganisms Influencing Mineral-Protected Organic Carbon Formation in Soils with Exogenous Carbon Addition. Agronomy 2024, 14, 2333. https://doi.org/10.3390/agronomy14102333
Zhang L, Luo Y, Wang Y, Zhang C, Cai G, Su W, Yu L. Key Microorganisms Influencing Mineral-Protected Organic Carbon Formation in Soils with Exogenous Carbon Addition. Agronomy. 2024; 14(10):2333. https://doi.org/10.3390/agronomy14102333
Chicago/Turabian StyleZhang, Limin, Yuanhong Luo, Yang Wang, Chengfu Zhang, Guojun Cai, Weici Su, and Lifei Yu. 2024. "Key Microorganisms Influencing Mineral-Protected Organic Carbon Formation in Soils with Exogenous Carbon Addition" Agronomy 14, no. 10: 2333. https://doi.org/10.3390/agronomy14102333
APA StyleZhang, L., Luo, Y., Wang, Y., Zhang, C., Cai, G., Su, W., & Yu, L. (2024). Key Microorganisms Influencing Mineral-Protected Organic Carbon Formation in Soils with Exogenous Carbon Addition. Agronomy, 14(10), 2333. https://doi.org/10.3390/agronomy14102333






