A Critical Review on Soil Gas Analysis: Modern Technologies and Problems
Abstract
:1. Introduction
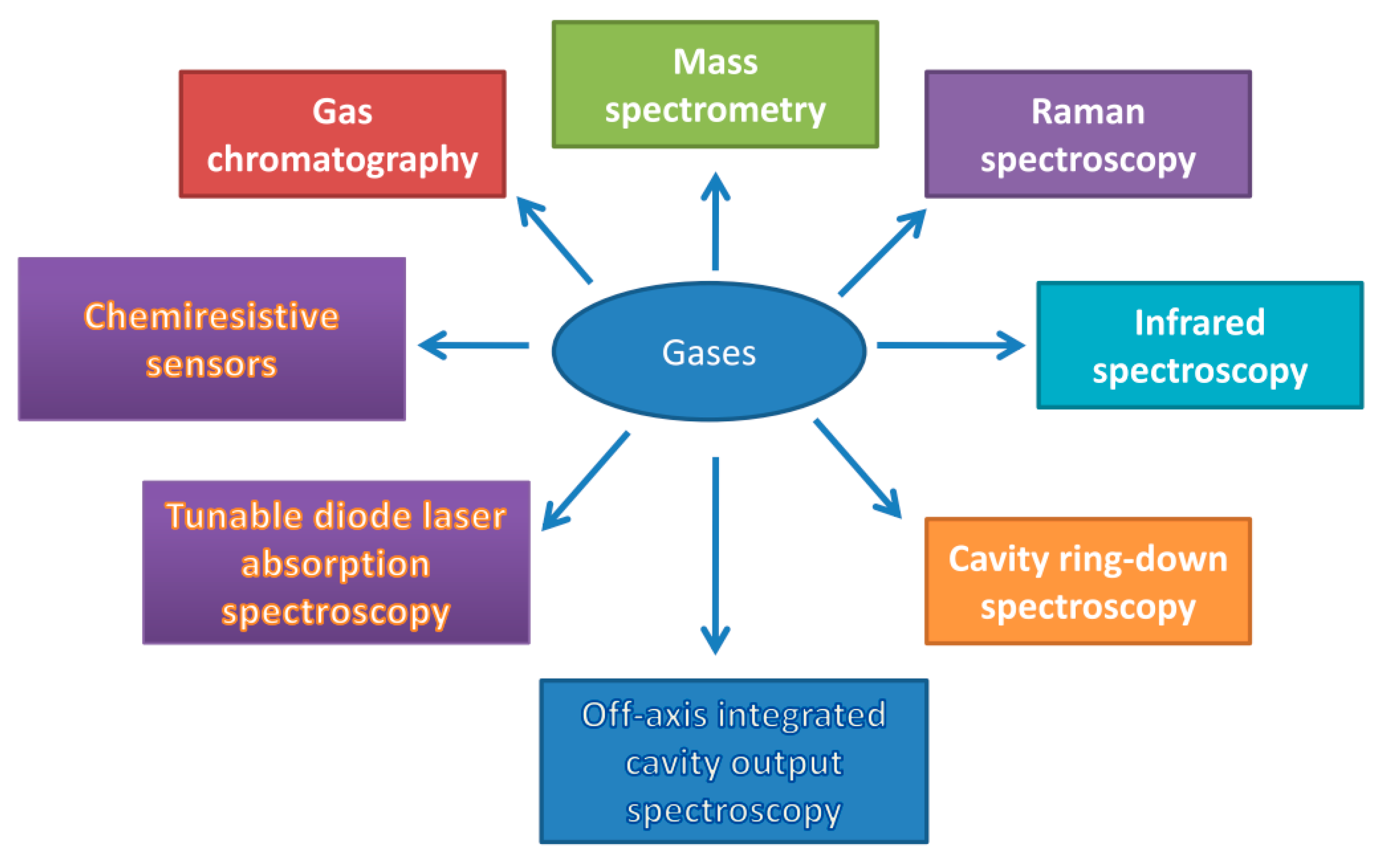
2. Optical Spectroscopy in Soil Gas Analysis
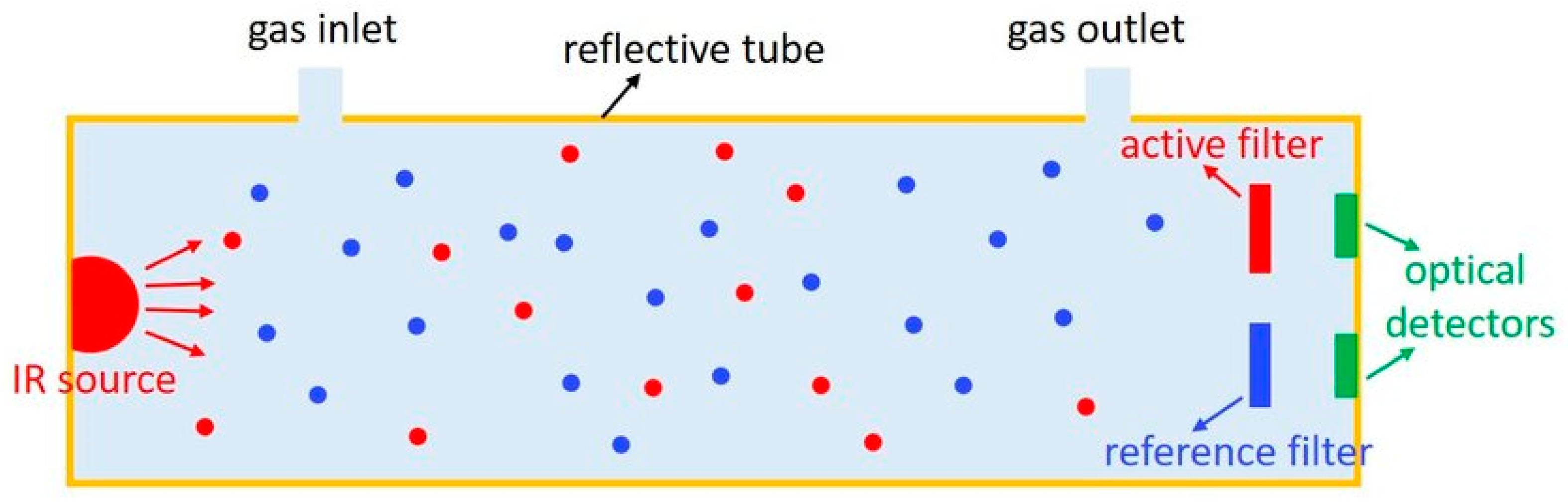
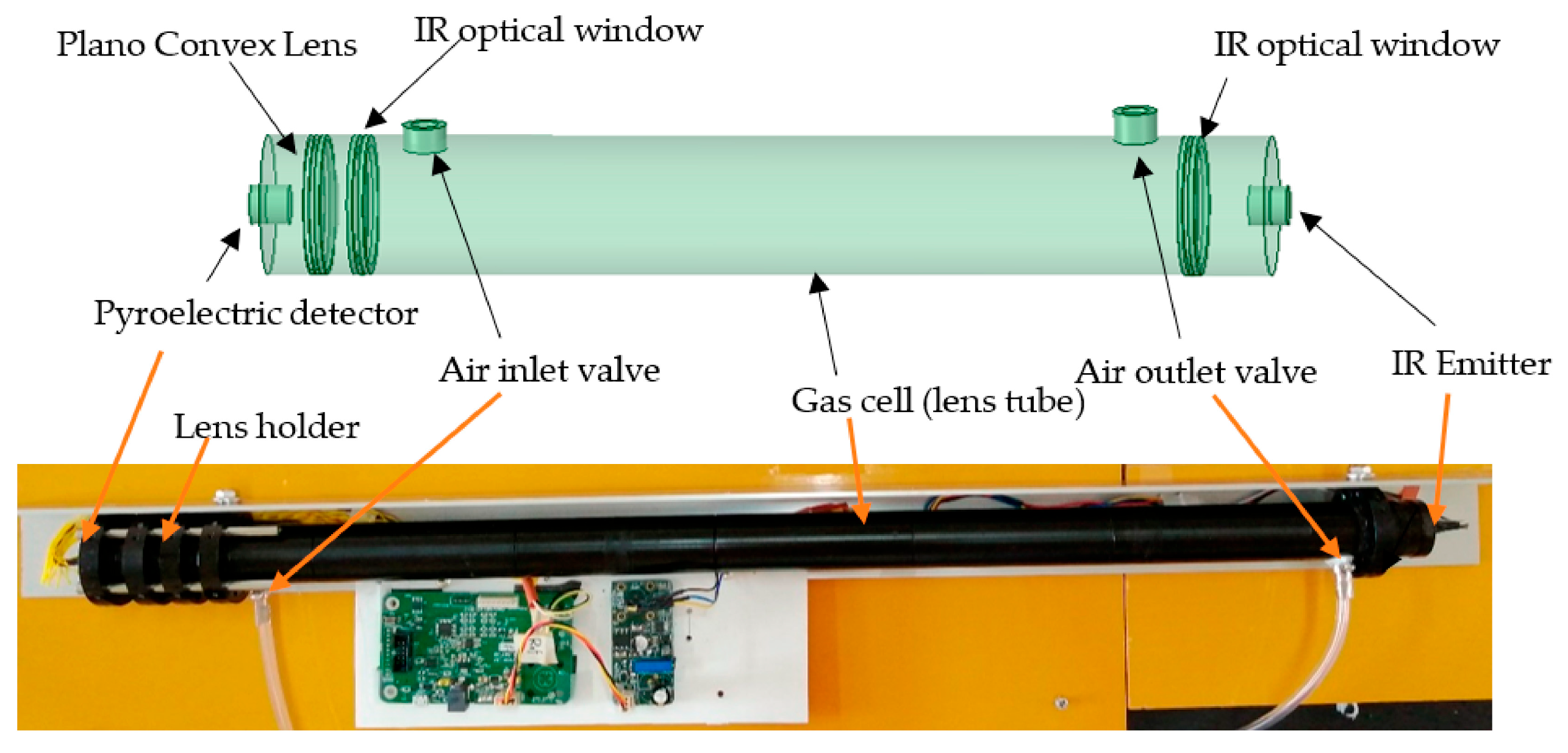
2.1. Infrared Spectroscopy
2.2. Raman Spectroscopy
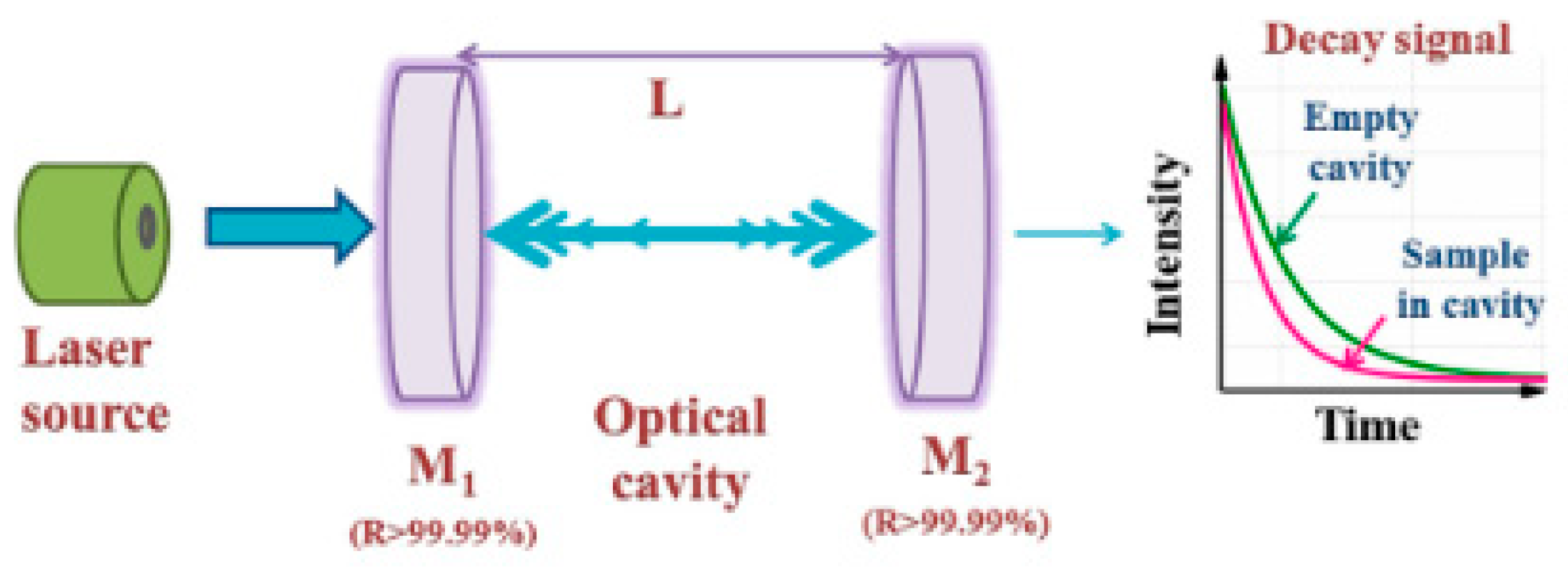
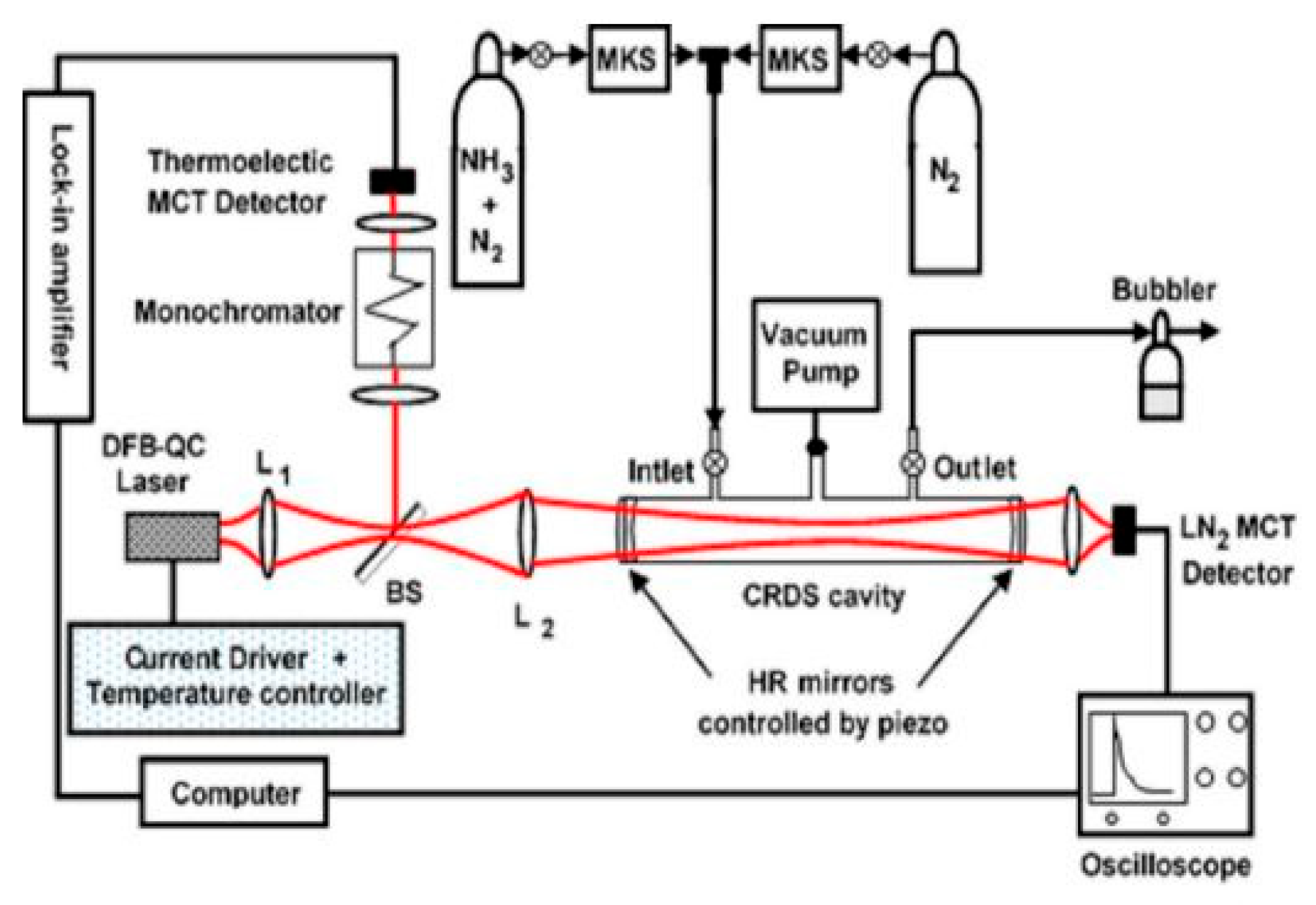
2.3. Cavity Ring-Down Spectroscopy
2.4. Off-Axis Integrated Cavity Output Spectroscopy
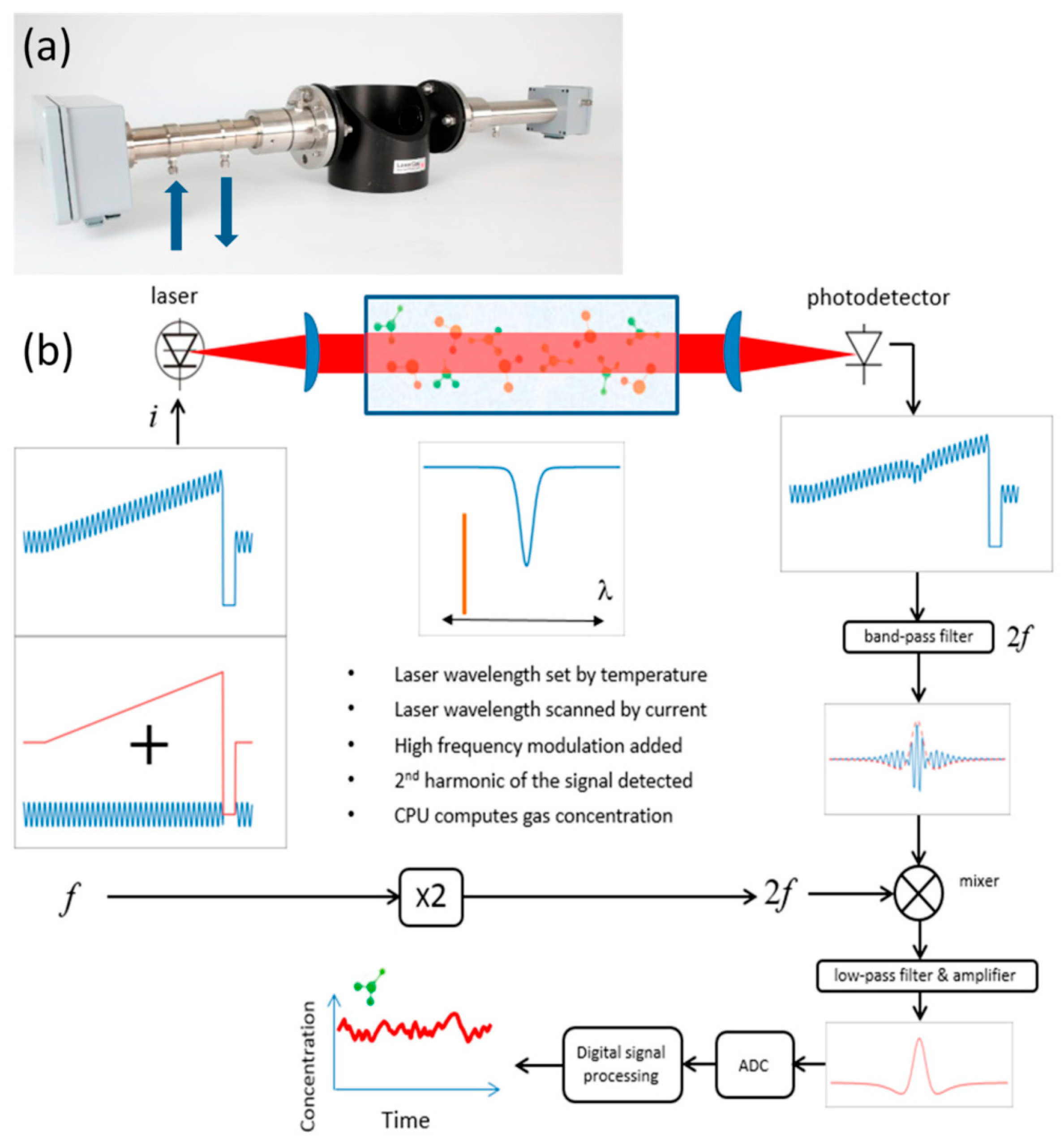
2.5. Tunable Diode Laser Absorption Spectroscopy (TDLAS)
3. Mass Spectrometry and Gas Chromatography
4. Gas Analysis Using Chemiresistive Sensors
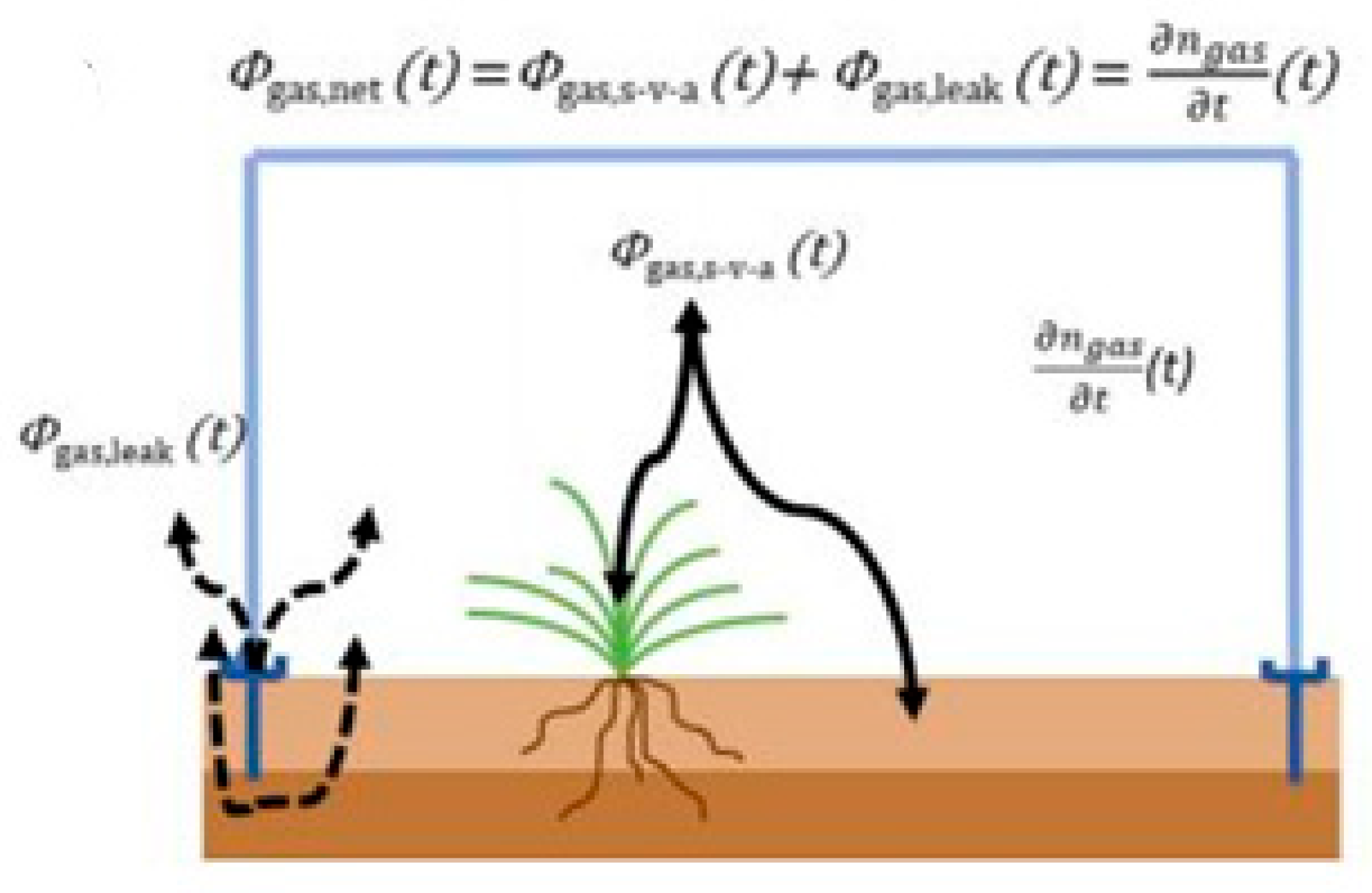
5. Methods of Sampling of Gas Emission
6. Challenges
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- de Klein, C.A.M.; Alfaro, M.A.; Giltrap, D.; Topp, C.F.E.; Simon, P.L.; Noble, A.D.L.; van der Weerden, T.J. Global Research Alliance N2O chamber methodology guidelines: Statistical considerations, emission factor calculation, and data reporting. J. Environ. Qual. 2020, 49, 1156–1167. [Google Scholar] [CrossRef] [PubMed]
- Banríon, M.; Cobelli, M.; Crowley, Q.G. Applying machine learning to model radon using topsoil geochemistry. Appl. Geochemistry 2023, 158, 105790. [Google Scholar] [CrossRef]
- Grantham, G.; Eddis, M.K.D. Contamination of Soils by Hazardous Gases: Investigation, Monitoring, Diagnosis and Treatment. In Contaminated Soil ’90, Proceedings of the Third International KfK/TNO Conference on Contaminated Soil, 10–14 December 1990, Karlsruhe, Federal Republic of Germany; Arendt, F., Hinsenveld, M., Van Den Brink, W.J., Eds.; Springer: Dordrecht, The Netherlands, 1990; pp. 681–689. ISBN 978-94-011-3270-1. [Google Scholar]
- Button, E.S.; Marshall, M.; Sánchez-Rodríguez, A.R.; Blaud, A.; Abadie, M.; Chadwick, D.R.; Jones, D.L. Greenhouse gas production, diffusion and consumption in a soil profile under maize and wheat production. Geoderma 2023, 430, 116310. [Google Scholar] [CrossRef]
- McAlary, T.; Wang, X.; Unger, A.; Groenevelt, H.; Górecki, T. Quantitative passive soil vapor sampling for VOCs-part 1: Theory. Environ. Sci. Process. Impacts 2014, 16, 482–490. [Google Scholar] [CrossRef]
- Luna, A.S. Raman Spectroscopy, Soil Analysis Applications, 3rd ed.; Lindon, J.C., Tranter, G.E., Koppenaal, D., Eds.; Academic Press: Oxford, UK, 2017; pp. 919–923. [Google Scholar]
- Zaman, M.; Heng, L.; Müller, C. Measuring Emission of Agricultural Greenhouse Gases and Developing Mitigation Options Using Nuclear and Related Techniques: Applications of Nuclear Techniques for GHGS; Springer: Berlin/Heidelberg, Germany, 2021; ISBN 9783030553968. [Google Scholar]
- Nelson, D.W.; Bremner, J.M. Factors affecting chemical transformations of nitrite in soils. Soil Biol. Biochem. 1969, 1, 229–239. [Google Scholar] [CrossRef]
- Ansari, J.; Davis, M.P.; Anderson, S.H.; Eivazi, F.; Bardhan, S. Greenhouse Gas Emissions from Row Crop, Agroforestry, and Forested Land Use Systems in Floodplain Soils. Water Air Soil Pollut. 2023, 234, 227. [Google Scholar] [CrossRef]
- Brümmer, C.; Brüggemann, N.; Butterbach-Bahl, K.; Falk, U.; Szarzynski, J.; Vielhauer, K.; Wassmann, R.; Papen, H. Soil-Atmosphere Exchange of N2O and NO in Near-Natural Savanna and Agricultural Land in Burkina Faso (W. Africa). Ecosystems 2008, 11, 582–600. [Google Scholar] [CrossRef]
- Fowler, D.; Pilegaard, K.; Sutton, M.A.; Ambus, P.; Raivonen, M.; Duyzer, J.; Simpson, D.; Fagerli, H.; Fuzzi, S.; Schjoerring, J.K.; et al. Atmospheric composition change: Ecosystems—Atmosphere interactions. Atmos. Environ. 2009, 43, 5193–5267. [Google Scholar] [CrossRef]
- Ray, R.L.; Griffin, R.W.; Fares, A.; Elhassan, A.; Awal, R.; Woldesenbet, S.; Risch, E. Soil CO2 emission in response to organic amendments, temperature, and rainfall. Sci. Rep. 2020, 10, 5849. [Google Scholar] [CrossRef]
- Chau, T.T.M.; Someya, T.; Akao, S.; Nakamura, M.; Oritate, F.; Somura, H.; Yamane, S.; Maeda, M. Emissions of CO2 and CH4 from Agricultural Soil with Kitchen Compost at Different Temperatures. J. Soil Sci. Plant Nutr. 2024, 24, 5462–5471. [Google Scholar] [CrossRef]
- Yerli, C.; Cakmakci, T.; Sahin, U. Soil CO2 Emission Linearly Increases with Organic Matter Added Using Stabilized Sewage Sludge under Recycled Wastewater Irrigation Conditions. Water Air Soil Pollut. 2023, 234, 56. [Google Scholar] [CrossRef]
- Mühlbachová, G.; Růžek, P.; Kusá, H.; Vavera, R. CO2 Emissions from Soils under Different Tillage Practices and Weather Conditions. Agronomy 2023, 13, 3084. [Google Scholar] [CrossRef]
- Müller, C. Plants affect the in situ N2O emissions of a temperate grassland ecosystem. Pflanzen beeinflussen die in situ N2O-Freisetzungen eines Grünlandökosystems in temperierten Breiten. J. Plant Nutr. Soil Sci. 2003, 166, 771–773. [Google Scholar] [CrossRef]
- Le Mer, J.; Roger, P. Production, oxidation, emission and consumption of methane by soils: A review. Eur. J. Soil Biol. 2001, 37, 25–50. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.; Zhu, Q.; Shen, Y.; Wang, X.; Wang, M.; Peng, C. A novel pathway of direct methane production and emission by eukaryotes including plants, animals and fungi: An overview. Atmos. Environ. 2015, 115, 26–35. [Google Scholar] [CrossRef]
- Bastviken, D.; Treat, C.C.; Pangala, S.R.; Gauci, V.; Enrich-Prast, A.; Karlson, M.; Gålfalk, M.; Romano, M.B.; Sawakuchi, H.O. The importance of plants for methane emission at the ecosystem scale. Aquat. Bot. 2023, 184, 103596. [Google Scholar] [CrossRef]
- Pilegaard, K. Processes regulating nitric oxide emissions from soils. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130126. [Google Scholar] [CrossRef]
- Wang, R.; Bei, N.; Wu, J.; Li, X.; Liu, S.; Yu, J.; Jiang, Q.; Tie, X.; Li, G. Cropland nitrogen dioxide emissions and effects on the ozone pollution in the North China plain. Environ. Pollut. 2022, 294, 118617. [Google Scholar] [CrossRef]
- Chataut, G.; Bhatta, B.; Joshi, D.; Subedi, K.; Kafle, K. Greenhouse gases emission from agricultural soil: A review. J. Agric. Food Res. 2023, 11, 100533. [Google Scholar] [CrossRef]
- Basheer, S.; Wang, X.; Farooque, A.A.; Nawaz, R.A.; Pang, T.; Neokye, E.O. A Review of Greenhouse Gas Emissions from Agricultural Soil. Sustainability 2024, 16, 4789. [Google Scholar] [CrossRef]
- Jaiswal, B.; Mukherjee, A.; Pandey, B.; Agrawal, M. Emission of Greenhouse Gases from Soil: An Assessment of Agricultural Management Practices. In Plant Responses to Soil Pollution; Singh, P., Singh, S.K., Prasad, S.M., Eds.; Springer: Singapore, 2020; pp. 221–248. ISBN 978-981-15-4964-9. [Google Scholar]
- Camarda, M.; De Gregorio, S.; Capasso, G.; Di Martino, R.M.R.; Gurrieri, S.; Prano, V. The monitoring of natural soil CO2 emissions: Issues and perspectives. Earth-Sci. Rev. 2019, 198, 102928. [Google Scholar] [CrossRef]
- Kibet, E.; Musafiri, C.M.; Kiboi, M.; Macharia, J.; Ng’etich, O.K.; Kosgei, D.K.; Mulianga, B.; Okoti, M.; Zeila, A.; Ngetich, F.K. Soil greenhouse gas emissions from different land utilization types in Western Kenya. Front. Soil Sci. 2022, 2, 956634. [Google Scholar] [CrossRef]
- Oertel, C.; Matschullat, J.; Zurba, K.; Zimmermann, F.; Erasmi, S. Greenhouse gas emissions from soils—A review. Geochemistry 2016, 76, 327–352. [Google Scholar] [CrossRef]
- Roscioli, J.R.; Meredith, L.K.; Shorter, J.H.; Gil-Loaiza, J.; Volkmann, T.H.M. Soil gas probes for monitoring trace gas messengers of microbial activity. Sci. Rep. 2021, 11, 8327. [Google Scholar] [CrossRef] [PubMed]
- Conrad, R. Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO). Microbiol. Rev. 1996, 60, 609–640. [Google Scholar] [CrossRef]
- Wang, J.; Zhen, J.; Hu, W.; Chen, S.; Lizaga, I.; Zeraatpisheh, M.; Yang, X. Remote sensing of soil degradation: Progress and perspective. Int. Soil Water Conserv. Res. 2023, 11, 429–454. [Google Scholar] [CrossRef]
- Zaman, M.; Kleineidam, K.; Bakken, L.; Berendt, J.; Bracken, C.; Butterbach-Bahl, K.; Cai, Z.; Chang, S.X.; Clough, T.; Dawar, K.; et al. Methodology for Measuring Greenhouse Gas Emissions from Agricultural Soils Using Non-isotopic Techniques. In Measuring Emission of Agricultural Greenhouse Gases and Developing Mitigation Options using Nuclear and Related Techniques: Applications of Nuclear Techniques for GHGs; Zaman, M., Heng, L., Müller, C., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 11–108. ISBN 978-3-030-55396-8. [Google Scholar]
- Bhowmik, A.; Fortuna, A.-M.; Cihacek, L.J.; Rahman, S.; Borhan, M.S.; Carr, P.M. Use of laboratory incubation techniques to estimate greenhouse gas footprints from conventional and no-tillage organic agroecosystems. Soil Biol. Biochem. 2017, 112, 204–215. [Google Scholar] [CrossRef]
- Cárdenas, L.M.; Hawkins, J.M.B.; Chadwick, D.; Scholefield, D. Biogenic gas emissions from soils measured using a new automated laboratory incubation system. Soil Biol. Biochem. 2003, 35, 867–870. [Google Scholar] [CrossRef]
- McGowen, E.B.; Sharma, S.; Deng, S.; Zhang, H.; Warren, J.G. An Automated Laboratory Method for Measuring CO2 Emissions from Soils. Agric. Environ. Lett. 2018, 3, 180008. [Google Scholar] [CrossRef]
- Pires, L.F.; Cássaro, F.A.M.; Bacchi, O.O.S.; Reichardt, K. Non-destructive image analysis of soil surface porosity and bulk density dynamics. Radiat. Phys. Chem. 2011, 80, 561–566. [Google Scholar] [CrossRef]
- Imadi, S.R.; Babar, M.M.; Hasan, H.; Gul, A. Soil Microflora—An Extensive Research. In Soil Science: Agricultural and Environmental Prospectives; Hakeem, K.R., Akhtar, J., Sabir, M., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 303–316. ISBN 978-3-319-34451-5. [Google Scholar]
- Merl, T.; Hu, Y.; Pedersen, J.; Zieger, S.E.; Bornø, M.L.; Tariq, A.; Sommer, S.G.; Koren, K. Optical chemical sensors for soil analysis: Possibilities and challenges of visualising NH3 concentrations as well as pH and O2 microscale heterogeneity. Environ. Sci. Adv. 2023, 2, 1210–1219. [Google Scholar] [CrossRef]
- Forster, J.C. 3-Soil sampling, handling, storage and analysis. In Methods in Applied Soil Microbiology and Biochemistry; Alef, K., Nannipieri, P.B.T.-M., Eds.; Academic Press: London, UK, 1995; pp. 49–121. ISBN 978-0-12-513840-6. [Google Scholar]
- Margenot, A.J.; Calderón, F.J.; Goyne, K.W.; Mukome, F.N.D.; Parikh, S.J. IR Spectroscopy, Soil Analysis Applications. In Encyclopedia of Spectroscopy and Spectrometry, 3rd ed.; Lindon, J.C., Tranter, G.E., Koppenaal, D., Eds.; Academic Press: Oxford, UK, 2017; pp. 448–454. ISBN 978-0-12-803224-4. [Google Scholar]
- Stripp, S.T. In Situ Infrared Spectroscopy for the Analysis of Gas-processing Metalloenzymes. ACS Catal. 2021, 11, 7845–7862. [Google Scholar] [CrossRef]
- Margenot, A.J.; Parikh, S.J.; Calderón, F.J. Fourier-transform infrared spectroscopy for soil organic matter analysis. Soil Sci. Soc. Am. J. 2023, 87, 1503–1528. [Google Scholar] [CrossRef]
- Thabit, F.N.; Negim, O.I.A.; AbdelRahman, M.A.E.; Scopa, A.; Moursy, A.R.A. Using Various Models for Predicting Soil Organic Carbon Based on DRIFT-FTIR and Chemical Analysis. Soil Syst. 2024, 8, 22. [Google Scholar] [CrossRef]
- Krivoshein, P.K.; Volkov, D.S.; Rogova, O.B.; Proskurnin, M.A. FTIR Photoacoustic and ATR Spectroscopies of Soils with Aggregate Size Fractionation by Dry Sieving. ACS Omega 2022, 7, 2177–2197. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, A.; Buurman, P.; Cozzolino, V.; Spaccini, R.; Piccolo, A. Infrared spectra of soil organic matter under a primary vegetation sequence. Chem. Biol. Technol. Agric. 2020, 7, 1–12. [Google Scholar] [CrossRef]
- Śliwińska, A.; Smolinski, A.; Kucharski, P. Simultaneous Analysis of Heavy Metal Concentration in Soil Samples. Appl. Sci. 2019, 9, 4705. [Google Scholar] [CrossRef]
- Bruckman, V.J.; Wriessnig, K. Improved soil carbonate determination by FT-IR and X-ray analysis. Environ. Chem. Lett. 2013, 11, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Materazzi, S.; Gentili, A.; Curini, R. Applications of evolved gas analysis: Part 1: EGA by infrared spectroscopy. Talanta 2006, 68, 489–496. [Google Scholar] [CrossRef]
- Jia, X.; Roels, J.; Baets, R.; Roelkens, G. On-Chip Non-Dispersive Infrared CO2 Sensor Based on an Integrating Cylinder. Sensors 2019, 19, 4260. [Google Scholar] [CrossRef]
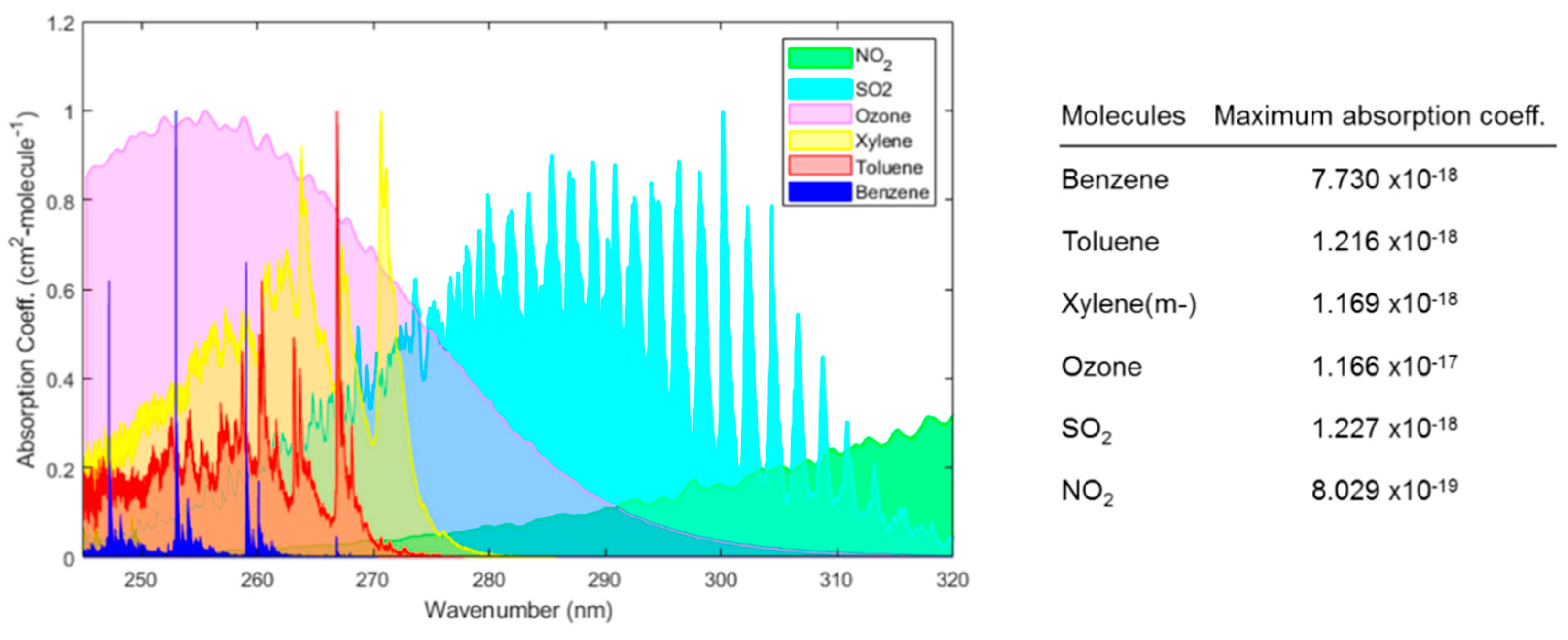
- Khan, S.; Newport, D.; Le Calvé, S. Gas Detection Using Portable Deep-UV Absorption Spectrophotometry: A Review. Sensors 2019, 19, 5210. [Google Scholar] [CrossRef] [PubMed]
- Abbas, O.; Dardenne, P.; Baeten, V. Chapter 3—Near-Infrared, Mid-Infrared, and Raman Spectroscopy. In Chemical Analysis of Food; Picó, Y., Ed.; Academic Press: Boston, MA, USA, 2012; pp. 59–89. ISBN 978-0-12-384862-8. [Google Scholar]
- Kaufmann, P.; Chrzanowski, H.M.; Vanselow, A.; Ramelow, S. Mid-IR spectroscopy with NIR grating spectrometers. Opt. Express 2022, 30, 5926. [Google Scholar] [CrossRef]
- Vicente, I.S.; Pinillos, S.C.; Bernal, J.G.; Asensio, J.S. New approaches on gas phase molecular absorption spectrometry detection in gas chromatography. Fresenius. J. Anal. Chem. 1996, 355, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Vitkin, V.; Polishchuk, A.; Chubchenko, I.; Popov, E.; Grigorenko, K.; Kharitonov, A.; Davtian, A.; Kovalev, A.; Kurikova, V.; Camy, P.; et al. Raman Laser Spectrometer: Application to 12C/13C Isotope Identification in CH4 and CO2 Greenhouse Gases. Appl. Sci. 2020, 10, 7473. [Google Scholar] [CrossRef]
- Chang, A.; Bond, T.; Sahota, S.; Arteaga, J.; Demory, B.; Delmas, W.; Tumkur, T.; Khitrov, V. CO2 Leakage Detection by Raman Spectroscopy with Hollow Core Photonic Crystal Fiber. In Proceedings of the CLEO 2023, San Jose, CA, USA, 7–12 May 2023; p. JW2A.117. [Google Scholar]
- Guo, J.; Luo, Z.; Liu, Q.; Yang, D.; Dong, H.; Huang, S.; Kong, A.; Wu, L. High-Sensitivity Raman Gas Probe for In Situ Multi-Component Gas Detection. Sensors 2021, 21, 3539. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.A.Z.; Oehlschlaeger, M.A. Deep Learning for Gas Sensing via Infrared Spectroscopy. Sensors 2024, 24, 1873. [Google Scholar] [CrossRef]
- Xing, Z.; Tian, K.; Du, C.; Li, C.; Zhou, J.; Chen, Z. Agricultural soil characterization by FTIR spectroscopy at micrometer scales: Depth profiling by photoacoustic spectroscopy. Geoderma 2019, 335, 94–103. [Google Scholar] [CrossRef]
- Lin, C.H.; Grant, R.H.; Heber, A.J.; Johnston, C.T. Application of open-path Fourier transform infrared spectroscopy (OP-FTIR) to measure greenhouse gas concentrations from agricultural fields. Atmos. Meas. Tech. 2019, 12, 3403–3415. [Google Scholar] [CrossRef]
- Schütze, C.; Lau, S.; Reiche, N.; Sauer, U.; Borsdorf, H.; Dietrich, P. Ground-based remote sensing with open-path fourier-Transform infrared (OP-FTIR) spectroscopy for large-scale monitoring of greenhouse gases. Energy Procedia 2013, 37, 4276–4282. [Google Scholar] [CrossRef]
- Seybold, C.A.; Ferguson, R.; Wysocki, D.; Bailey, S.; Anderson, J.; Nester, B.; Schoeneberger, P.; Wills, S.; Libohova, Z.; Hoover, D.; et al. Application of Mid-Infrared Spectroscopy in Soil Survey. Soil Sci. Soc. Am. J. 2019, 83, 1746–1759. [Google Scholar] [CrossRef]
- Torrent, J.; Barrón, V. Diffuse Reflectance Spectroscopy. In Methods of Soil Analysis Part 5—Mineralogical Methods; SSSA Book Series; Soil Science Society of America: Madison, WI, USA, 2008; pp. 367–385. ISBN 9780891188575. [Google Scholar]
- Griffith, D.W.T.; Jamie, I.M.; Beasley, P.A.; Denmead, O.T.; Leuning, R.; Galbally, I.E.; Meyer, C.P. FTIR in the Paddock: Trace gas soil flux measurements using FTIR spectroscopy. AIP Conf. Proc. 1998, 430, 211–214. [Google Scholar] [CrossRef]
- Dubowski, Y.; Harush, D.; Shaviv, A.; Stone, L.; Linker, R. Real Time Monitoring of N2O Emissions from Agricultural Soils using FTIR Spectroscopy. Soil Sci. Soc. Am. J. 2014, 78, 61–69. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, H.; Li, J.; Wan, H.; Guo, Q.; Zhu, H.; Liu, H.; Yi, F. Non-dispersive infrared multi-gas sensing via nanoantenna integrated narrowband detectors. Nat. Commun. 2020, 11, 5245. [Google Scholar] [CrossRef] [PubMed]
- Bandara, K.M.T.S.; Sakai, K.; Nakandakari, T.; Yuge, K. A Low-Cost NDIR-Based N2O Gas Detection Device for Agricultural Soils: Assembly, Calibration Model Validation, and Laboratory Testing. Sensors 2021, 21, 1189. [Google Scholar] [CrossRef] [PubMed]
- Welsh, H.L.; Stansbury, E.J.; Romanko, J.; Feldman, T. Raman Spectroscopy of Gases. J. Opt. Soc. Am. 1955, 45, 338–343. [Google Scholar] [CrossRef]
- Xing, Z.; Du, C.; Shen, Y.; Ma, F.; Zhou, J. A method combining FTIR-ATR and Raman spectroscopy to determine soil organic matter: Improvement of prediction accuracy using competitive adaptive reweighted sampling (CARS). Comput. Electron. Agric. 2021, 191, 106549. [Google Scholar] [CrossRef]
- Lacroix, E.; De Donato, P.; Lafortune, S.; Caumon, M.C.; Barres, O.; Liu, X.; Derrien, M.; Piedevache, M. In situ continuous monitoring of dissolved gases (N2, O2, CO2, H2) prior to H2 injection in an aquifer (Catenoy, France) by on-site Raman and infrared spectroscopies: Instrumental assessment and geochemical baseline establishment. Anal. Methods 2021, 13, 3806–3820. [Google Scholar] [CrossRef]
- Petrov, D.; Matrosov, I. Natural Gas Analysis Using Polarized Raman Spectroscopy. Anal. Chem. 2023, 95, 9409–9414. [Google Scholar] [CrossRef]
- Buldakov, M.A.; Korolkov, V.A.; Matrosov, I.I.; Petrov, D.V.; Tikhomirov, A.A.; Korolev, B. V Analyzing natural gas by spontaneous Raman scattering spectroscopy. J. Opt. Technol. 2013, 80, 426–430. [Google Scholar] [CrossRef]
- Chibirev, I.; Mazzoleni, C.; van der Voort, D.D.; Borysow, J.; Fink, M. Raman spectrometer for field determination of H2O in natural gas pipelines. J. Nat. Gas Sci. Eng. 2018, 55, 426–430. [Google Scholar] [CrossRef]
- Yin, Z.; Zheng, R.; Li, L.; Xi, S.; Luan, Z.; Sun, C.; Zhang, X. In situ Raman quantitative monitoring of methanogenesis: Culture experiments of a deep-sea cold seep methanogenic archaeon. Front. Microbiol. 2023, 14, 1128064. [Google Scholar] [CrossRef]
- Huang, B.; Zhao, Q.; Sun, C.; Zhu, L.; Zhang, H.; Zhang, Y.; Liu, C.; Li, F. Trace Analysis of Gases and Liquids with Spontaneous Raman Scattering Based on the Integrating Sphere Principle. Anal. Chem. 2022, 94, 13311–13314. [Google Scholar] [CrossRef] [PubMed]
- Numata, Y.; Shinohara, Y.; Kitayama, T.; Tanaka, H. Rapid and accurate quantitative analysis of fermentation gases by Raman spectroscopy. Process Biochem. 2013, 48, 569–574. [Google Scholar] [CrossRef]
- Alden, M.; Blomqvist, J.; Edner, H.; Lundberg, H. Raman spectroscopy in the analysis of fire gases. Fire Mater. 1983, 7, 32–37. [Google Scholar] [CrossRef]
- Niklas, C.; Wackerbarth, H.; Ctistis, G. A Short Review of Cavity-Enhanced Raman Spectroscopy for Gas Analysis. Sensors 2021, 21, 1698. [Google Scholar] [CrossRef] [PubMed]
- Petrov, D.V.; Matrosov, I.I.; Zaripov, A.R.; Tanichev, A.S. Raman Natural Gas Analyzer: Effects of Composition on Measurement Precision. Sensors 2022, 22, 3492. [Google Scholar] [CrossRef] [PubMed]
- Jurowski, K.; Noga, M.; Kobylarz, D.; Niżnik, Ł.; Krośniak, A. Multimodal Imaging Using Raman Spectroscopy and FTIR in a Single Analytical Instrument with a Microscope (Infrared Raman Microscopy AIRsight, Shimadzu): Opportunities and Applications. Int. J. Mol. Sci. 2024, 25, 6884. [Google Scholar] [CrossRef]
- Taquet, N.; Pironon, J.; De Donato, P.; Lucas, H.; Barres, O. Efficiency of combined FTIR and Raman spectrometry for online quantification of soil gases: Application to the monitoring of carbon dioxide storage sites. Int. J. Greenh. Gas Control. 2013, 12, 359–371. [Google Scholar] [CrossRef]
- Maiwald, M.; Sowoidnich, K.; Sumpf, B. Portable shifted excitation Raman difference spectroscopy for on-site soil analysis. J. Raman Spectrosc. 2022, 53, 1560–1570. [Google Scholar] [CrossRef]
- Qin, R.; Zhang, Y.; Ren, S.; Nie, P. Rapid Detection of Available Nitrogen in Soil by Surface-Enhanced Raman Spectroscopy. Int. J. Mol. Sci. 2022, 23, 10404. [Google Scholar] [CrossRef]
- Fan, X.; Zeng, Y.; Zhi, Y.-L.; Nie, T.; Xu, Y.; Wang, X. Signal-to-noise ratio enhancement for Raman spectra based on optimized Raman spectrometer and convolutional denoising autoencoder. J. Raman Spectrosc. 2021, 52, 890–900. [Google Scholar] [CrossRef]
- Bai, Y.; Luo, H.; Li, Z.; Ding, Y.; Wang, Q.; Xiong, W. Enhancement of the signal-to-noise ratio by compressing the illumination of a spatial heterodyne Raman spectrometer. Instrum. Sci. Technol. 2024, 52, 583–596. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, Z.; Fan, X.; Wang, X. Modified denoising method of Raman spectra-based deep learning for Raman semi-quantitative analysis and imaging. Microchem. J. 2023, 191, 108777. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Liu, G.Y.; Sui, Y.T.; Xu, M.; Tong, L. Denoising method for Raman spectra with low signal-to-noise ratio based on feature extraction. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 250, 119374. [Google Scholar] [CrossRef]
- Berden, G.; Peeters, R.; Meijer, G. Cavity ring-down spectroscopy: Experimental schemes and applications. Int. Rev. Phys. Chem. 2000, 19, 565–607. [Google Scholar] [CrossRef]
- Pohlman, J.W.; Casso, M.; Magen, C.; Bergeron, E. Discrete Sample Introduction Module for Quantitative and Isotopic Analysis of Methane and Other Gases by Cavity Ring-Down Spectroscopy. Environ. Sci. Technol. 2021, 55, 12066–12074. [Google Scholar] [CrossRef]
- He, Y.; Englich, F.V.; Orr, B.J. Multi-species trace gas detection by rapidly swept cavity ringdown spectroscopy. In Proceedings of the 2007 Conference on Lasers and Electro-Optics (CLEO), Baltimore, MD, USA, 6–11 May 2007; pp. 9–10. [Google Scholar]
- Martin, N.A.; Ferracci, V.; Cassidy, N.; Hoffnagle, J.A. The application of a cavity ring-down spectrometer to measurements of ambient ammonia using traceable primary standard gas mixtures. Appl. Phys. B Lasers Opt. 2016, 122, 1–11. [Google Scholar] [CrossRef]
- Persijn, S.; Harren, F.; Van Der Veen, A. Quantitative gas measurements using a versatile OPO-based cavity ringdown spectrometer and the comparison with spectroscopic databases. Appl. Phys. B Lasers Opt. 2010, 100, 383–390. [Google Scholar] [CrossRef]
- Santamaria, L.; Sarno, V.D.; Natale, P.; De Rosa, M.; De Inguscio, M.; Mosca, S.; Ricciardi, I.; Calonico, D.; Levi, F.; Maddaloni, P. Comb-assisted cavity ring-down spectroscopy of a buffer-gas-cooled molecular beam. Phys. Chem. Chem. Phys. 2016, 18, 16715–16720. [Google Scholar] [CrossRef]
- Liu, D.; Yu, Z.; Lin, J. Application of Combustion Module Coupled with Cavity Ring-Down Spectroscopy for Simultaneous Measurement of SOC and δ13C-SOC. J. Spectrosc. 2018, 2018, 6893454. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, W.; Fang, B.; Yang, N.; Cheng, F.; Hu, X.; Chen, Y.; Zhang, W.; Fittschen, C.; Chen, W. Portable cavity ring-down spectrometer for an HO2 radical measurement: Instrument’s performance and potential improvement using a narrow linewidth laser. Opt. Express 2022, 30, 37446–37456. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.B.; Dudek, J.; Lehmann, K.; Rabinowitz, P. Trace gas detection with CW cavity ring-down laser absorption spectroscopy. In Proceedings of the 2000 IEEE/SEMI Advanced Semiconductor Manufacturing Conference and Workshop, Boston, MA, USA, 12–14 September 2000; pp. 203–206. [Google Scholar] [CrossRef]
- Maity, A.; Maithani, S.; Pradhan, M. Chapter 3—Cavity ring-down spectroscopy: Recent technological advances and applications. In Molecular and Laser Spectroscopy; Gupta, V.P., Ozaki, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 83–120. ISBN 978-0-12-818870-5. [Google Scholar]
- Jongma, R.T.; Boogaarts, M.G.H.; Holleman, I.; Meijer, G. Trace gas detection with cavity ring down spectroscopy. Rev. Sci. Instrum. 1995, 66, 2821–2828. [Google Scholar] [CrossRef]
- Courtois, J.; Mohamed, A.K.; Romanini, D. High-speed off-axis Cavity Ring-Down Spectroscopy with a re-entrant configuration for spectral resolution enhancement. Opt. Express 2010, 18, 4845–4858. [Google Scholar] [CrossRef] [PubMed]
- Gadedjisso-Tossou, K.S.; Stoychev, L.I.; Mohou, M.A.; Cabrera, H.; Niemela, J.; Danailov, M.B.; Vacchi, A. Cavity Ring-Down Spectroscopy for Molecular Trace Gas Detection Using a Pulsed DFB QCL Emitting at 6.8 µm. Photonics 2020, 7, 74. [Google Scholar] [CrossRef]
- Kwaśny, M.; Bombalska, A. Optical Methods of Methane Detection. Sensors 2023, 23, 2834. [Google Scholar] [CrossRef]
- Liu, Y.; He, Y.; Hu, M.; Chen, B.; Xu, Z.; Yao, L.; Wang, X.; Kan, R. Cavity ring-down spectroscopy with a laser frequency stabilized and locked to a reference target gas absorption for drift-free accurate gas sensing measurements. Front. Phys. 2023, 11, 1238869. [Google Scholar] [CrossRef]
- McHale, L.E.; Hecobian, A.; Yalin, A.P. Open-path cavity ring-down spectroscopy for trace gas measurements in ambient air. Opt. Express 2016, 24, 5523–5535. [Google Scholar] [CrossRef]
- Hu, S.M. Trace Gas Measurements Using Cavity Ring-down Spectroscopy; Elsevier Inc.: Amsterdam, The Netherlands, 2021; Volume I, ISBN 9780128150146. [Google Scholar]
- He, Y.; Jin, C.; Kan, R.; Liu, J.; Liu, W.; Hill, J.; Jamie, I.M.; Orr, B.J. Remote open-path cavity-ringdown spectroscopic sensing of trace gases in air, based on distributed passive sensors linked by km-long optical fibers. Opt. Express 2014, 22, 13170. [Google Scholar] [CrossRef]
- Elmaleh, C.; Simon, F.; Decker, J.; Dumont, J.; Cazier, F.; Fourmentin, M.; Bocquet, R.; Cuisset, A.; Mouret, G.; Hindle, F. THz cavity ring-down quantitative gas phase spectroscopy. Talanta 2023, 253, 124097. [Google Scholar] [CrossRef]
- Ma, G.; He, Y.; Chen, B.; Deng, H.; Liu, Y.; Wang, X.; Zhao, Z.; Kan, R. Quasi-simultaneous sensitive detection of two gas species by cavity-ringdown spectroscopy with two lasers. Sensors 2021, 21, 7622. [Google Scholar] [CrossRef]
- Brown, S.S.; Stark, H.; Ravishankara, A.R. Cavity ring-down spectroscopy for atmospheric trace gas detection: Application to the nitrate radical (NO3). Appl. Phys. B Lasers Opt. 2002, 75, 173–182. [Google Scholar] [CrossRef]
- Fasci, E.; D’Agostino, V.; Khan, M.A.; Gravina, S.; Porzio, G.; Gianfrani, L.; Castrillo, A. Comb-assisted cavity ring-down spectroscopy for ultra-sensitive traceable measurements of water vapour in ultra-high purity gases. J. Phys. Conf. Ser. 2023, 2439, 012017. [Google Scholar] [CrossRef]
- Dickinson, D.; Bodé, S.; Boeckx, P. System for δ13C-CO2 and xCO2 analysis of discrete gas samples by cavity ring-down spectroscopy. Atmos. Meas. Tech. 2017, 10, 4507–4519. [Google Scholar] [CrossRef]
- Wang, C.; Surampudi, A.B. An acetone breath analyzer using cavity ringdown spectroscopy: An initial test with human subjects under various situations. Meas. Sci. Technol. 2008, 19, 105604. [Google Scholar] [CrossRef]
- Maithani, S.; Pradhan, M. Cavity ring-down spectroscopy and its applications to environmental, chemical and biomedical systems. J. Chem. Sci. 2020, 132, 1–19. [Google Scholar] [CrossRef]
- Varma, R.M.; Venables, D.S.; Ruth, A.A.; Heitmann, U.; Schlosser, E.; Dixneuf, S. Long optical cavities for open-path monitoring of atmospheric trace gases and aerosol extinction. Appl. Opt. 2009, 48, B159–B171. [Google Scholar] [CrossRef]
- Wang, D.; Hu, R.; Xie, P.; Liu, J.; Liu, W.; Qin, M.; Ling, L.; Zeng, Y.; Chen, H.; Xing, X.; et al. Diode laser cavity ring-down spectroscopy for in situ measurement of NO3 radical in ambient air. J. Quant. Spectrosc. Radiat. Transf. 2015, 166, 23–29. [Google Scholar] [CrossRef]
- Ko, K.H.; Kim, Y.; Kim, T.S.; Lee, L.; Park, H. Measurement of 14CO2 using off-axis integrated cavity output spectroscopy. Appl. Phys. B Lasers Opt. 2022, 128, 149. [Google Scholar] [CrossRef]
- Shen, G.; Chao, X.; Sun, K. Modeling the optical field in off-axis integrated-cavity-output spectroscopy using the decentered Gaussian beam model. Appl. Opt. 2018, 57, 2947. [Google Scholar] [CrossRef]
- Shao, L.; Mei, J.; Chen, J.; Tan, T.; Wang, G.; Liu, K.; Gao, X. Recent advances and applications of off-axis integrated cavity output spectroscopy. Microw. Opt. Technol. Lett. 2023, 65, 1489–1505. [Google Scholar] [CrossRef]
- Moyer, E.J.; Sayres, D.S.; Engel, G.S.; St Clair, J.M.; Keutsch, F.N.; Allen, N.T.; Kroll, J.H.; Anderson, J.G. Design considerations in high-sensitivity off-axis integrated cavity output spectroscopy. Appl. Phys. B Lasers Opt. 2008, 92, 467–474. [Google Scholar] [CrossRef]
- Nadeem, F.; Mandon, J.; Cristescu, S.M.; Harren, F.J.M. Optical re-injection in Off-Axis Integrated Cavity Output Spectroscopy, modelling and experiments. In Proceedings of the 2018 Conference on Lasers and Electro-Optics (CLEO), San Jose, CA, USA, 13–18 May 2018; pp. 1–2. [Google Scholar]
- Yuan, Z.; Huang, Y.; Lu, X.; Huang, J.; Liu, Q.; Qi, G.; Cao, Z. Measurement of CO2 by wavelength modulated reinjection off-axis integrated cavity output spectroscopy at 2 µm. Atmosphere 2021, 12, 1247. [Google Scholar] [CrossRef]
- Chen, W.; Kosterev, A.A.; Tittel, F.K.; Boucher, D. Detection of H2S based on Off-Axis Integrated Cavity Output Spectroscopy. In Proceedings of the 2007 European Conference on Lasers and Electro-Optics and the International Quantum Electronics Conference, Munich, Germany, 17–22 June 2007; p. 1. [Google Scholar]
- Arslanov, D.D.; Swinkels, K.; Cristescu, S.M.; Harren, F.J.M. Real-time, subsecond, multicomponent breath analysis by Optical Parametric Oscillator based Off-Axis Integrated Cavity Output Spectroscopy. Opt. Express 2011, 19, 24078. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Bhattacharya, S.; Maity, A.; Chaudhuri, S.; Pradhan, M. Exploring Triple-Isotopic Signatures of Water in Human Exhaled Breath, Gastric Fluid, and Drinking Water Using Integrated Cavity Output Spectroscopy. Anal. Chem. 2020, 92, 5717–5723. [Google Scholar] [CrossRef]
- Parameswaran, K.R.; Rosen, D.I.; Allen, M.G.; Ganz, A.M.; Risby, T.H. Off-axis integrated cavity output spectroscopy with a mid-infrared interband cascade laser for real-time breath ethane measurements. Appl. Opt. 2009, 48, B73–B79. [Google Scholar] [CrossRef]
- Zheng, K.; Zheng, C.; Hu, L.; Song, F.; Zhang, Y.; Wang, Y.; Tittel, F.K. Near-Infrared Fiber-Coupled Off-Axis Cavity-Enhanced Thermoelastic Spectroscopic Sensor System for In Situ Multipoint Ammonia Leak Monitoring. IEEE Trans. Instrum. Meas. 2021, 70, 1–9. [Google Scholar] [CrossRef]
- Zheng, K.; Yu, L.; Zheng, C.; Xi, Z.; Zhang, Y.; Yan, G.; Zhang, H.; Zhang, Y.; Wang, Y.; Tittel, F.K. Vehicle-Deployed off-Axis Integrated Cavity Output Spectroscopic CH4/C2H6 Sensor System for Mobile Inspection of Natural Gas Leakage. ACS Sens. 2022, 7, 1685–1697. [Google Scholar] [CrossRef]
- Wang, J.; Tian, X.; Dong, Y.; Zhu, G.; Chen, J.; Tan, T.; Liu, K.; Chen, W.; Gao, X. Enhancing off-axis integrated cavity output spectroscopy (OA-ICOS) with radio frequency white noise for gas sensing. Opt. Express 2019, 27, 30517–30529. [Google Scholar] [CrossRef]
- Wang, K.; Shao, L.; Chen, J.; Wang, G.; Liu, K.; Tan, T.; Mei, J.; Chen, W.; Gao, X. A dual-laser sensor based on off-axis integrated cavity output spectroscopy and time-division multiplexing method. Sensors 2020, 20, 6192. [Google Scholar] [CrossRef]
- He, Q.; Zheng, C.; Zheng, K.; Tittel, F.K. Off-axis integrated cavity output spectroscopy for real-time methane measurements with an integrated wavelength-tunable light source. Infrared Phys. Technol. 2021, 115, 103705. [Google Scholar] [CrossRef]
- Mahesh, P.; Sreenivas, G.; Rao, P.V.N.; Dadhwal, V.K.; Sai Krishna, S.V.S.; Mallikarjun, K. High-precision surface-level CO2 and CH4 using off-axis integrated cavity output spectroscopy (OA-ICOS) over Shadnagar, India. Int. J. Remote Sens. 2015, 36, 5754–5765. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Liu, J.; Liu, W.; Kan, R.; Wang, T.; Chen, D.; Chen, J.; Wang, X.; Xia, H.; et al. Applications of a tunable diode laser absorption spectrometer in monitoring greenhouse gases. Chin. Opt. Lett. 2006, 4, 363–365. [Google Scholar]
- Avetisov, V.; Bjoroey, O.; Wang, J.; Geiser, P.; Paulsen, K.G. Hydrogen Sensor Based on Tunable Diode Laser Absorption Spectroscopy. Sensors 2019, 19, 5313. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, Y. Tunable Diode Laser Absorption Spectroscopy Based Temperature Measurement with a Single Diode Laser Near 1.4 μm. Sensors 2022, 22, 6095. [Google Scholar] [CrossRef] [PubMed]
- Tu, R.; Gu, J.; Zeng, Y.; Zhou, X.; Yang, K.; Jing, J.; Miao, Z.; Yang, J. Development and Validation of a Tunable Diode Laser Absorption Spectroscopy System for Hot Gas Flow and Small-Scale Flame Measurement. Sensors 2022, 22, 6707. [Google Scholar] [CrossRef]
- Chaulya, S.K.; Prasad, G.M. Chapter 3—Gas Sensors for Underground Mines and Hazardous Areas. In Sensing and Monitoring Technologies for Mines and Hazardous Areas; Elsevier: Amsterdam, The Netherlands, 2016; pp. 161–212. ISBN 978-0-12-803194-0. [Google Scholar]
- Sun, J.; Chang, J.; Wang, C.; Shao, J. Tunable diode laser absorption spectroscopy for detection of multi-component gas: A review. Appl. Spectrosc. Rev. 2024, 59, 1086–1107. [Google Scholar] [CrossRef]
- Bowling, D.R.; Sargent, S.D.; Tanner, B.D.; Ehleringer, J.R. Tunable diode laser absorption spectroscopy for stable isotope studies of ecosystem-atmosphere CO2 exchange. Agric. For. Meteorol. 2003, 118, 1–19. [Google Scholar] [CrossRef]
- Famulari, D.; Fowler, D.; Hargreaves, K.; Milford, C.; Nemitz, E.; Sutton, M.A.; Weston, K. Measuring Eddy Covariance Fluxes of Ammonia Using Tunable Diode Laser Absorption Spectroscopy. Water Air Soil Pollut. Focus 2004, 4, 151–158. [Google Scholar] [CrossRef]
- Brueckner, D.; Solokhina, A.; Krähenbühl, S.; Braissant, O. A combined application of tunable diode laser absorption spectroscopy and isothermal micro-calorimetry for calorespirometric analysis. J. Microbiol. Methods 2017, 139, 210–214. [Google Scholar] [CrossRef]
- Lin, S.; Chang, J.; Sun, J.; Xu, P. Improvement of the Detection Sensitivity for Tunable Diode Laser Absorption Spectroscopy: A Review. Front. Phys. 2022, 10, 853966. [Google Scholar] [CrossRef]
- Li, C.; Ding, S.; Yang, L.; Zhu, Q.; Chen, M.; Tsang, D.C.W.; Cai, G.; Feng, C.; Wang, Y.; Zhang, C. Planar optode: A two-dimensional imaging technique for studying spatial-temporal dynamics of solutes in sediment and soil. Earth-Sci. Rev. 2019, 197, 102916. [Google Scholar] [CrossRef]
- Waich, K.; Borisov, S.; Mayr, T.; Klimant, I. Dual lifetime referenced trace ammonia sensors. Sens. Actuators B Chem. 2009, 139, 132–138. [Google Scholar] [CrossRef]
- Müller, B.J.; Steinmann, N.; Borisov, S.M.; Klimant, I. Ammonia sensing with fluoroionophores—A promising way to minimize interferences caused by volatile amines. Sens. Actuators B Chem. 2018, 255, 1897–1901. [Google Scholar] [CrossRef]
- Honeyman, A.S.; Merl, T.; Spear, J.R.; Koren, K. Optode-based chemical imaging of laboratory burned soil reveals millimeter-scale heterogeneous biogeochemical responses. Environ. Res. 2023, 224, 115469. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, M.A.; Clerici, L.; Marinone, E.; Frattini, V.; Dellavedova, P. Advances in Hydrocarbon Speciation for Soil Gas Analysis. Separations 2023, 10, 489. [Google Scholar] [CrossRef]
- Katsumi, N.; Yonebayashi, K.; Okazaki, M.; Nishiyama, S.; Nishi, T.; Hosaka, A.; Watanabe, C. Characterization of soil organic matter with different degrees of humification using evolved gas analysis-mass spectrometry. Talanta 2016, 155, 28–37. [Google Scholar] [CrossRef]
- Duff, D.; Lennard, C.; Li, Y.; Doyle, C.; Edge, K.J.; Holland, I.; Lothridge, K.; Johnstone, P.; Beylerian, P.; Spikmans, V. Portable gas chromatography–mass spectrometry method for the in-field screening of organic pollutants in soil and water at pollution incidents. Environ. Sci. Pollut. Res. 2023, 30, 93088–93102. [Google Scholar] [CrossRef]
- Maurin, N.; Sayen, S.; Guillon, E. Gas chromatography–mass spectrometry analysis of organic pollutants in French soils irrigated with agro-industrial wastewater. Front. Environ. Sci. 2023, 11, 1125487. [Google Scholar] [CrossRef]
- Schnitzer, M.; Schulten, H.-R. Analysis of Organic Matter in Soil Extracts and Whole Soils by Pyrolysis-Mass Spectrometry. In Advances in Agronomy; Sparks, D., Ed.; Academic Press: Cambridge, MA, USA, 1995; Volume 55, pp. 167–217. ISBN 0065-2113. [Google Scholar]
- Leinweber, P.; Schulten, H.-R. -R. Differential thermal analysis, thermogravimetry and in-source pyrolysis-mass spectrometry studies on the formation of soil organic matter. Thermochim. Acta 1992, 200, 151–167. [Google Scholar] [CrossRef]
- Alpers, C.N.; Dettman, D.L.; Lohmann, K.C.; Brabec, D. Stable isotopes of carbon dioxide in soil gas over massive sulfide mineralization at Crandon, Wisconsin. J. Geochem. Explor. 1990, 38, 69–86. [Google Scholar] [CrossRef]
- Zhu, X.; Di, D.; Ma, M.; Shi, W. Stable Isotopes in Greenhouse Gases from Soil: A Review of Theory and Application. Atmosphere 2019, 10, 377. [Google Scholar] [CrossRef]
- Lubbers, I.M.; van Groenigen, K.J.; Fonte, S.J.; Six, J.; Brussaard, L.; van Groenigen, J.W. Greenhouse-gas emissions from soils increased by earthworms. Nat. Clim. Chang. 2013, 3, 187–194. [Google Scholar] [CrossRef]
- Sheppard, S.K.; Lloyd, D. Direct mass spectrometric measurement of gases in soil monoliths. J. Microbiol. Methods 2002, 50, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, N.; Toma, Y.; Iwai, Y.; Furutani, H.; Hondo, T.; Hatano, R.; Toyoda, M. Mass spectrometric multiple soil-gas flux measurement system with a portable high-resolution mass spectrometer (MULTUM) coupled to an automatic chamber for continuous field observations. Atmos. Meas. Tech. 2020, 13, 6657–6673. [Google Scholar] [CrossRef]
- Christiansen, J.R.; Outhwaite, J.; Smukler, S.M. Comparison of CO2, CH4 and N2O soil-atmosphere exchange measured in static chambers with cavity ring-down spectroscopy and gas chromatography. Agric. For. Meteorol. 2015, 211–212, 48–57. [Google Scholar] [CrossRef]
- Munksgaard, N.C.; Davies, K.; Wurster, C.M.; Bass, A.M.; Bird, M.I. Field-based cavity ring-down spectrometry of δ13C in soil-respired CO2. Isot. Environ. Health Stud. 2013, 49, 232–242. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, Y.; Naidu, R.; Chadalavada, S. Environmental Technology & Innovation Application of portable gas chromatography—Mass spectrometer for rapid field based determination of TCE in soil vapour and groundwater. Environ. Technol. Innov. 2021, 21, 101274. [Google Scholar] [CrossRef]
- McDonald, M.; Lewis, K.; Gentry, T.; DeLaune, P. Soil pore space gas probes for use in agricultural research. Soil Secur. 2021, 5, 100015. [Google Scholar] [CrossRef]
- Burford, J.R.; Bremner, J.M. Gas chromatographic determination of carbon dioxide evolved from soils in closed systems. Soil Biol. Biochem. 1972, 4, 191–197. [Google Scholar] [CrossRef]
- Mondini, C.; Sinicco, T.; Cayuela, M.L.; Sanchez-Monedero, M.A. A simple automated system for measuring soil respiration by gas chromatography. Talanta 2010, 81, 849–855. [Google Scholar] [CrossRef]
- Zgonnik, V.; Beaumont, V.; Deville, E.; Larin, N.; Pillot, D.; Farrell, K.M. Evidence for natural molecular hydrogen seepage associated with Carolina bays (surficial, ovoid depressions on the Atlantic Coastal Plain, Province of the USA). Prog. Earth Planet. Sci. 2015, 2, 1–15. [Google Scholar] [CrossRef]
- Peng, G.; Tisch, U.; Adams, O.; Hakim, M.; Shehada, N.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Haick, H. Diagnosing lung cancer in exhaled breath using gold nanoparticles. Nat. Nanotechnol. 2009, 4, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Penza, M.; Rossi, R.; Alvisi, M.; Signore, M.A.; Serra, E.; Paolesse, R.; D’Amico, A.; Di Natale, C. Metalloporphyrins-modified carbon nanotubes networked films-based chemical sensors for enhanced gas sensitivity. Sens. Actuators B Chem. 2010, 144, 387–394. [Google Scholar] [CrossRef]
- Mubeen, S.; Lai, M.; Zhang, T.; Lim, J.H.; Mulchandani, A.; Deshusses, M.A.; Myung, N.V. Hybrid tin oxide-SWNT nanostructures based gas sensor. Electrochim. Acta 2013, 92, 484–490. [Google Scholar] [CrossRef]
- Sakthivel, R.; Geetha, A.; Dineshkumar, J. Design and fabrication of CoS2/graphene hybrid composite film sensor for NO2 gas-sensing performance. J. Mater. Sci. Mater. Electron. 2023, 34, 1495. [Google Scholar] [CrossRef]
- Kumbhakar, P.; Chowde Gowda, C.; Mahapatra, P.L.; Mukherjee, M.; Malviya, K.D.; Chaker, M.; Chandra, A.; Lahiri, B.; Ajayan, P.M.; Jariwala, D.; et al. Emerging 2D metal oxides and their applications. Mater. Today 2021, 45, 142–168. [Google Scholar] [CrossRef]
- Alagh, A.; Annanouch, F.E.; Sierra-Castillo, A.; Haye, E.; Colomer, J.F.; Llobet, E. Three-Dimensional Assemblies of Edge-Enriched WSe2Nanoflowers for Selectively Detecting Ammonia or Nitrogen Dioxide. ACS Appl. Mater. Interfaces 2022, 14, 54946–54960. [Google Scholar] [CrossRef]
- Kumar, R.; Goel, N.; Hojamberdiev, M.; Kumar, M. Transition metal dichalcogenides-based flexible gas sensors. Sens. Actuators A Phys. 2020, 303, 111875. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, X.; Xie, J.; Lu, G.; Zhang, J. Emerging van der Waals junctions based on TMDs materials for advanced gas sensors. Coord. Chem. Rev. 2021, 447, 214151. [Google Scholar] [CrossRef]
- Mendes, R.G.; Wr, P.S.; Bachmatiuk, A.; Sun, J.; Gemming, T.; Liu, Z.; Rümmeli, M.H.; Wróbel, P.S.; Bachmatiuk, A.; Sun, J.; et al. Carbon nanostructures as a multi-functional platform for sensing applications. Chemosensors 2018, 6, 60. [Google Scholar] [CrossRef]
- Fei, H.; Wu, G.; Cheng, W.Y.; Yan, W.; Xu, H.; Zhang, D.; Zhao, Y.; Lv, Y.; Chen, Y.; Zhang, L.; et al. Enhanced NO2 Sensing at Room Temperature with Graphene via Monodisperse Polystyrene Bead Decoration. ACS Omega 2019, 4, 3812–3819. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Babu, H.V.; Zhao, J.; Goñi-Urtiaga, A.; Sainz, R.; Ferritto, R.; Pita, M.; Wang, D.Y. Effect of Cu-doped graphene on the flammability and thermal properties of epoxy composites. Compos. Part B Eng. 2016, 89, 108–116. [Google Scholar] [CrossRef]
- Freddi, S.; Emelianov, A.V.; Bobrinetskiy, I.I.; Drera, G.; Pagliara, S.; Kopylova, D.S.; Chiesa, M.; Santini, G.; Mores, N.; Moscato, U.; et al. Development of a Sensing Array for Human Breath Analysis Based on SWCNT Layers Functionalized with Semiconductor Organic Molecules. Adv. Healthc. Mater. 2020, 9, e2000377. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Lee, M.H.; Safaeian, H.; Kim, T.-U.; Kim, J.-Y.; Kim, H.W.; Kim, S.S. Room Temperature Chemiresistive Gas Sensors Based on 2D MXenes. Sensors 2023, 23, 8829. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Tai, N.H.; Harsh; Islam, S.S. Transfer of microstructure pattern of CNTs onto flexible substrate using hot press technique for sensing applications. Mater. Res. Bull. 2013, 48, 2804–2808. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, B.; Wang, X.; Li, Y.; Gai, S.; Wu, Y.; Cheng, X. A high-sensitive room temperature gas sensor based on cobalt phthalocyanines and reduced graphene oxide nanohybrids for the ppb-levels of ammonia detection. RSC Adv. 2019, 9, 37518–37525. [Google Scholar] [CrossRef]
- Lin, Y.; Manalili, D.; Khodabakhsh, A.; Cristescu, S.M. Real-Time Measurement of CH4 in Human Breath Using a Compact CH4/CO2 Sensor. Sensors 2024, 24, 1077. [Google Scholar] [CrossRef]
- Drera, G.; Freddi, S.; Emelianov, A.V.; Bobrinetskiy, I.I.; Chiesa, M.; Zanotti, M.; Pagliara, S.; Fedorov, F.S.; Nasibulin, A.G.; Montuschi, P.; et al. Exploring the performance of a functionalized CNT-based sensor array for breathomics through clustering and classification algorithms: From gas sensing of selective biomarkers to discrimination of chronic obstructive pulmonary disease. RSC Adv. 2021, 11, 30270–30282. [Google Scholar] [CrossRef]
- Kwak, D.; Lei, Y.; Maric, R. Ammonia gas sensors: A comprehensive review. Talanta 2019, 204, 713–730. [Google Scholar] [CrossRef]
- Chérot-Kornobis, N.; Hulo, S.; Edmé, J.-L.; de Broucker, V.; Matran, R.; Sobaszek, A. Analysis of nitrogen oxides (NOx) in the exhaled breath condensate (EBC) of subjects with asthma as a complement to exhaled nitric oxide (FeNO) measurements: A cross-sectional study. BMC Res. Notes 2011, 4, 202. [Google Scholar] [CrossRef]
- Lee, J.; Jung, M.; Barthwal, S.; Lee, S.; Lim, S.-H. MEMS gas preconcentrator filled with CNT foam for exhaled VOC gas detection. BioChip J. 2015, 9, 44–49. [Google Scholar] [CrossRef]
- Mazzsatenta, A.; Pokorski, M.; Di Giulio, C. Volatile organic compounds (VOCs) in exhaled breath as a marker of hypoxia in multiple chemical sensitivity. Physiol. Rep. 2021, 9, e15034. [Google Scholar] [CrossRef]
- Lefferts, M.J.; Castell, M.R. Influence of soil type on chemiresistive detection of buried ANFO. Forensic Chem. 2022, 27, 100401. [Google Scholar] [CrossRef]
- Ho, C.K.; Hughes, R.C. In-Situ Chemiresistor Sensor Package for Real-Time Detection of Volatile Organic Compounds in Soil and Groundwater. Sensors 2002, 2, 23–34. [Google Scholar] [CrossRef]
- Shooshtari, M.; Salehi, A. An electronic nose based on carbon nanotube -titanium dioxide hybrid nanostructures for detection and discrimination of volatile organic compounds. Sens. Actuators B Chem. 2022, 357, 131418. [Google Scholar] [CrossRef]
- Su, P.-G.; Li, M.-C. Recognition of binary mixture of NO2 and NO gases using a chemiresistive sensors array combined with principal component analysis. Sens. Actuators A Phys. 2021, 331, 112980. [Google Scholar] [CrossRef]
- Hamadache, M.; Lee, D. Principal component analysis based signal-to-noise ratio improvement for inchoate faulty signals: Application to ball bearing fault detection. Int. J. Control Autom. Syst. 2017, 15, 506–517. [Google Scholar] [CrossRef]
- Morawski, R.Z.; Miękina, A. Application of principal components analysis and signal-to-noise ratio for calibration of spectrophotometric analysers of food. Measurement 2016, 79, 302–310. [Google Scholar] [CrossRef]
- Getino, J.; Arés, L.; Robla, J.I.; Horrillo, M.C.; Sayago, I.; Fernández, M.J.; Rodrigo, J.; Gutiérrez, J. Environmental applications of gas sensor arrays: Combustion atmospheres and contaminated soils. Sens. Actuators B Chem. 1999, 59, 249–254. [Google Scholar] [CrossRef]
- Huang, S.; Croy, A.; Panes-Ruiz, L.A.; Khavrus, V.; Bezugly, V.; Ibarlucea, B.; Cuniberti, G. Machine Learning-Enabled Smart Gas Sensing Platform for Identification of Industrial Gases. Adv. Intell. Syst. 2022, 4, 2200016. [Google Scholar] [CrossRef]
- Ketchanji Mougang, Y.C.; Endale Mangamba, L.-M.; Capuano, R.; Ciccacci, F.; Catini, A.; Paolesse, R.; Mbatchou Ngahane, H.B.; Palombi, L.; Di Natale, C. On-Field Test of Tuberculosis Diagnosis through Exhaled Breath Analysis with a Gas Sensor Array. Biosensors 2023, 13, 570. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, M.; Tokonami, S.; Suzuki, T.; Janik, M. Machine learning as a tool for analysing the impact of environmental parameters on the radon exhalation rate from soil. Radiat. Meas. 2020, 138, 106402. [Google Scholar] [CrossRef]
- Awais, M.; Naqvi, S.M.Z.A.; Zhang, H.; Li, L.; Zhang, W.; Awwad, F.A.; Ismail, E.A.A.; Khan, M.I.; Raghavan, V.; Hu, J. AI and machine learning for soil analysis: An assessment of sustainable agricultural practices. Bioresour. Bioprocess. 2023, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Adjuik, T.A.; Davis, S.C. Machine Learning Approach to Simulate Soil CO2 Fluxes under Cropping Systems. Agronomy 2022, 12, 197. [Google Scholar] [CrossRef]
- Murcek, R.; Hölzel, J.; Köhler, H.; Boye, A.; Hesse, M.; Mauermann, M. Development of a quartz crystal sensor system to monitor local soil removal during cleaning in closed food processing lines. Food Bioprod. Process. 2021, 127, 282–287. [Google Scholar] [CrossRef]
- Bristow, Q. An evaluation of the quartz crystal microbalance as a mercury vapour sensor for soil gases. J. Geochem. Explor. 1972, 1, 55–76. [Google Scholar] [CrossRef]
- Lim, J.-H.; Phiboolsirichit, N.; Mubeen, S.; Deshusses, M.A.; Mulchandani, A.; Myung, N.V. Electrical and gas sensing properties of polyaniline functionalized single-walled carbon nanotubes. Nanotechnology 2010, 21, 75502. [Google Scholar] [CrossRef]
- Pineda, D.M.; Pérez, J.C. SENose: An under U$50 electronic nose for the monitoring of soil gas emissions. Comput. Electron. Agric. 2017, 133, 15–21. [Google Scholar] [CrossRef]
- Romanak, K.D.; Womack, G.L.; Bomse, D.S. Field Test of in Situ Sensor Technology for Process-based Soil Gas Monitoring. Energy Procedia 2014, 63, 4027–4030. [Google Scholar] [CrossRef]
- Romanak, K.D.; Bomse, D.S. Field assessment of sensor technology for environmental monitoring using a process-based soil gas method at geologic CO2 storage sites. Int. J. Greenh. Gas Control 2020, 96, 103003. [Google Scholar] [CrossRef]
- Görres, C.M.; Kammann, C.; Ceulemans, R. Automation of soil flux chamber measurements: Potentials and pitfalls. Biogeosciences 2016, 13, 1949–1966. [Google Scholar] [CrossRef]
- Livingston, G.P.; Hutchinson, G.L.; Spartalian, K. Trace Gas Emission in Chambers. Soil Sci. Soc. Am. J. 2006, 70, 1459–1469. [Google Scholar] [CrossRef]
- Van Afferden, M.; Hansen, A.M.; Kaiser, C.; Chapelain, N. Laboratory test system to measure microbial respiration rate. Int. J. Environ. Pollut. 2006, 26, 220–233. [Google Scholar] [CrossRef]
- Hoyle, F.C.; Murphy, D.V.; Brookes, P.C. Microbial response to the addition of glucose in low-fertility soils. Biol. Fertil. Soils 2008, 44, 571–579. [Google Scholar] [CrossRef]
- Pihlatie, M.K.; Christiansen, J.R.; Aaltonen, H.; Korhonen, J.F.J.; Nordbo, A.; Rasilo, T.; Benanti, G.; Giebels, M.; Helmy, M.; Sheehy, J.; et al. Comparison of static chambers to measure CH4 emissions from soils. Agric. For. Meteorol. 2013, 171–172, 124–136. [Google Scholar] [CrossRef]
- Conen, F.; Smith, K.A. An explanation of linear increases in gas concentration under closed chambers used to measure gas exchange between soil and the atmosphere. Eur. J. Soil Sci. 2000, 51, 111–117. [Google Scholar] [CrossRef]
- Hutchinson, G.L.; Livingston, G.P. Vents and seals in non-steady-state chambers used for measuring gas exchange between soil and the atmosphere. Eur. J. Soil Sci. 2001, 52, 675–682. [Google Scholar] [CrossRef]
- Laemmel, T.; Mohr, M.; Longdoz, B.; Schack-Kirchner, H.; Lang, F.; Schindler, D.; Maier, M. From above the forest into the soil—How wind affects soil gas transport through air pressure fluctuations. Agric. For. Meteorol. 2019, 265, 424–434. [Google Scholar] [CrossRef]
- Liu, T.; Hu, C.; Wang, Q.; Li, J.; Huang, K.; Chen, Z.; Shi, T. Conversion relationship of rainfall-soil moisture-groundwater in Quaternary thick cohesive soil in Jianghan Plain, Hubei Province, China. China Geol. 2020, 3, 462–472. [Google Scholar]
- Song, B.; Niu, S.; Wan, S. Precipitation regulates plant gas exchange and its long-term response to climate change in a temperate grassland. J. Plant Ecol. 2016, 9, 531–541. [Google Scholar] [CrossRef]
- Mikhalchuk, A.; Kharanzhevskaya, Y.; Burnashova, E.; Nekhoda, E.; Gammerschmidt, I.; Akerman, E.; Kirpotin, S.; Nikitkin, V.; Khovalyg, A.; Vorobyev, S. Soil Water Regime, Air Temperature, and Precipitation as the Main Drivers of the Future Greenhouse Gas Emissions from West Siberian Peatlands. Water 2023, 15, 3056. [Google Scholar] [CrossRef]
- Sundari, S.; Hirano, T.; Yamada, H.; Kusin, K.; Limin, S. Effect of Groundwater Level on Soil Respiration in Tropical Peat Swamp Forests. J. Agric. Meteorol. 2012, 68, 121–134. [Google Scholar] [CrossRef]
- Bekin, N.; Agam, N. Rethinking the deployment of static chambers for CO2 flux measurement in dry desert soils. Biogeosciences 2023, 20, 3791–3802. [Google Scholar] [CrossRef]
- Verginelli, I.; Pecoraro, R.; Baciocchi, R. Using dynamic flux chambers to estimate the natural attenuation rates in the subsurface at petroleum contaminated sites. Sci. Total Environ. 2018, 619–620, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Argento, M.; Henderson, F.; Lewis, R.; Mallyon, D.A.; Risk, D.; Nickerson, N. Soil surface flux measurements are a reliable means for assessing fugitive gas migration across soils and seasons. Elementa 2022, 10, 00010. [Google Scholar] [CrossRef]
- Parker, D.B.; Casey, K.D.; Todd, R.W.; Waldrip, H.M.; Marek, G.W.; Auvermann, B.W.; Marek, T.H.; Webb, K.; Willis, W.M.; Pemberton, B.; et al. Improved chamber systems for rapid, real-time nitrous oxide emissions from manure and soil. Trans. ASABE 2017, 60, 1235–1258. [Google Scholar] [CrossRef]
- Reichman, R.; Rolston, D.E. Design and Performance of a Dynamic Gas Flux Chamber. J. Environ. Qual. 2002, 31, 1774–1781. [Google Scholar] [CrossRef]
- Jiang, J.; Gu, K.; Xu, J.; Li, Y.; Le, Y.; Hu, J. Effect of Barometric Pressure Fluctuations on Gas Transport over Soil Surfaces. Land 2023, 12, 161. [Google Scholar] [CrossRef]
- DeSutter, T.M.; Sauer, T.J.; Parkin, T.B. Porous tubing for use in monitoring soil CO2 concentrations. Soil Biol. Biochem. 2006, 38, 2676–2681. [Google Scholar] [CrossRef]
- Maier, M.; Weber, T.K.D.; Fiedler, J.; Fuß, R.; Glatzel, S.; Huth, V.; Jordan, S.; Jurasinski, G.; Kutzbach, L.; Schäfer, K.; et al. Introduction of a guideline for measurements of greenhouse gas fluxes from soils using non-steady-state chambers. J. Plant Nutr. Soil Sci. 2022, 185, 447–461. [Google Scholar] [CrossRef]
- Zaman, M.; Saggar, S.; Blennerhassett, J.D.; Singh, J. Effect of urease and nitrification inhibitors on N transformation, gaseous emissions of ammonia and nitrous oxide, pasture yield and N uptake in grazed pasture system. Soil Biol. Biochem. 2009, 41, 1270–1280. [Google Scholar] [CrossRef]
- Martins, M.R.; Sant’Anna, S.A.C.; Zaman, M.; Santos, R.C.; Monteiro, R.C.; Alves, B.J.R.; Jantalia, C.P.; Boddey, R.M.; Urquiaga, S. Strategies for the use of urease and nitrification inhibitors with urea: Impact on N2O and NH3 emissions, fertilizer-15N recovery and maize yield in a tropical soil. Agric. Ecosyst. Environ. 2017, 247, 54–62. [Google Scholar] [CrossRef]
- Barthel, M.; Bauters, M.; Baumgartner, S.; Drake, T.W.; Bey, N.M.; Bush, G.; Boeckx, P.; Botefa, C.I.; Dériaz, N.; Ekamba, G.L.; et al. Low N2O and variable CH4 fluxes from tropical forest soils of the Congo Basin. Nat. Commun. 2022, 13, 330. [Google Scholar] [CrossRef] [PubMed]
- Kristanti, R.A.; Khanitchaidecha, W.; Taludar, G.; Karácsony, P.; Cao, L.T.T.; Chen, T.-W.; Darwish, N.M.; AlMunqedhi, B.M. A Review on Thermal Desorption Treatment for Soil Contamination. Trop. Aquat. Soil Pollut. 2022, 2, 45–58. [Google Scholar] [CrossRef]
- Zhao, C.; Dong, Y.; Feng, Y.; Li, Y.; Dong, Y. Thermal desorption for remediation of contaminated soil: A review. Chemosphere 2019, 221, 841–855. [Google Scholar] [CrossRef]
- Kaushik, P.; Eliáš, M.; Prášek, J.; Michalička, J.; Zajíčková, L. Manipulating MWCNT/TiO2 heterostructure morphology at nanoscale and its implications to NO2 sensing properties. Mater. Chem. Phys. 2021, 271, 124901. [Google Scholar] [CrossRef]
- Polyakov, M.; Ivanova, V.; Klyamer, D.; Köksoy, B.; Demirba, E. A Hybrid Nanomaterial Based on Single Walled Carbon Nanotubes Cross-Linked via Axially Substituted Silicon (IV) Phthalocyanine for Chemiresistive Sensors. Molecules 2020, 25, 2073. [Google Scholar] [CrossRef]
- Harsányi, E.; Mirzaei, M.; Arshad, S.; Alsilibe, F.; Vad, A.; Nagy, A.; Ratonyi, T.; Gorji, M.; Al-Dalahme, M.; Mohammed, S. Assessment of Advanced Machine and Deep Learning Approaches for Predicting CO2 Emissions from Agricultural Lands: Insights Across Diverse Agroclimatic Zones. In Earth Systems and Environment; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar] [CrossRef]







| Raman spectroscopy | N2 250–400 | O2 25–45 | H2Ovapor 8–23 | - | CO2 (1285 cm−1) 4–14 | CO2 (1388 cm−1) 3–9 | - |
| FTIR spectroscopy | - | - | H2Ovapor 500–710 | H2Oliquid 500–710 | CO2 (3609 cm−1) 135–300 | CO2 (4983 cm−1) 7–20 | H2Oliquid 500–710 |
| Analyzer (Company) | Type of Measurement | Gases and Their Concentrations | Weight, g | Pressure of Pump, kPa | Country |
|---|---|---|---|---|---|
| Picarro G2508 | CRD spectroscopy | N2O: 0.3–200 ppm, CH4: 1.5 –12 ppm, CO2: 380–5000 ppm, NH3: 0–300 ppb, H2O: 0–3% | 22,600 | 40–133 | USA |
| Gasmet GT5000 | FTIR spectroscopy | N2O: oт 7 ppb, CH4: oт 40 ppb, CO2: oт 5 ppm, H2O: oт 0.01%, CO: oт 70 ppb, NH3: oт 70 ppb | 9400 | 60–110 | Finland |
| Li-Cor LI78xx | n/a | N2O: 0.4–100 ppm, H2O: 0–60,000 ppm, CO2: 50–2000 ppm, NH3: 0–30,000 ppb | 10,500 | 70–110 | USA |
| ABB GLA131/132/151 | OA-ICOS | N2O: 0.5–40 ppm, CH4: 0.9–100 ppm, CO2: 0.35–20,000 ppm, H2O: 0–30,000 ppm | 6400 | 70–110 | Sweden-Switzeland |
| Hansatech Instuments CFLUX-1 | IR spectroscopy | CO2: 1–30,000 ppm, H2O: 0.1–75% | 8500 | 80–115 | UK |
| Echo Instruments ECHO | Infrared, paramagnetic and electrochemical gas sensors | CO2: 0.35–5000 ppm, CH4: 0–10,000 ppm, O2: 0–25%, H2: 0–1000 ppm | 7500 | 200–340 | Slovenia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bannov, A.G.; Trubin, I.V.; Zakharov, I.K.; Maksimovskiy, E.A.; Kurmashov, P.B. A Critical Review on Soil Gas Analysis: Modern Technologies and Problems. Agronomy 2024, 14, 2374. https://doi.org/10.3390/agronomy14102374
Bannov AG, Trubin IV, Zakharov IK, Maksimovskiy EA, Kurmashov PB. A Critical Review on Soil Gas Analysis: Modern Technologies and Problems. Agronomy. 2024; 14(10):2374. https://doi.org/10.3390/agronomy14102374
Chicago/Turabian StyleBannov, Alexander G., Igor’ V. Trubin, Ilya K. Zakharov, Evgeny A. Maksimovskiy, and Pavel B. Kurmashov. 2024. "A Critical Review on Soil Gas Analysis: Modern Technologies and Problems" Agronomy 14, no. 10: 2374. https://doi.org/10.3390/agronomy14102374
APA StyleBannov, A. G., Trubin, I. V., Zakharov, I. K., Maksimovskiy, E. A., & Kurmashov, P. B. (2024). A Critical Review on Soil Gas Analysis: Modern Technologies and Problems. Agronomy, 14(10), 2374. https://doi.org/10.3390/agronomy14102374





