Structural Derivatives of β-Asarone from Acorus calamus Linn. as Insecticide Candidates and the Insecticidal Mechanism Against Small Brown Planthopper
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Reagents
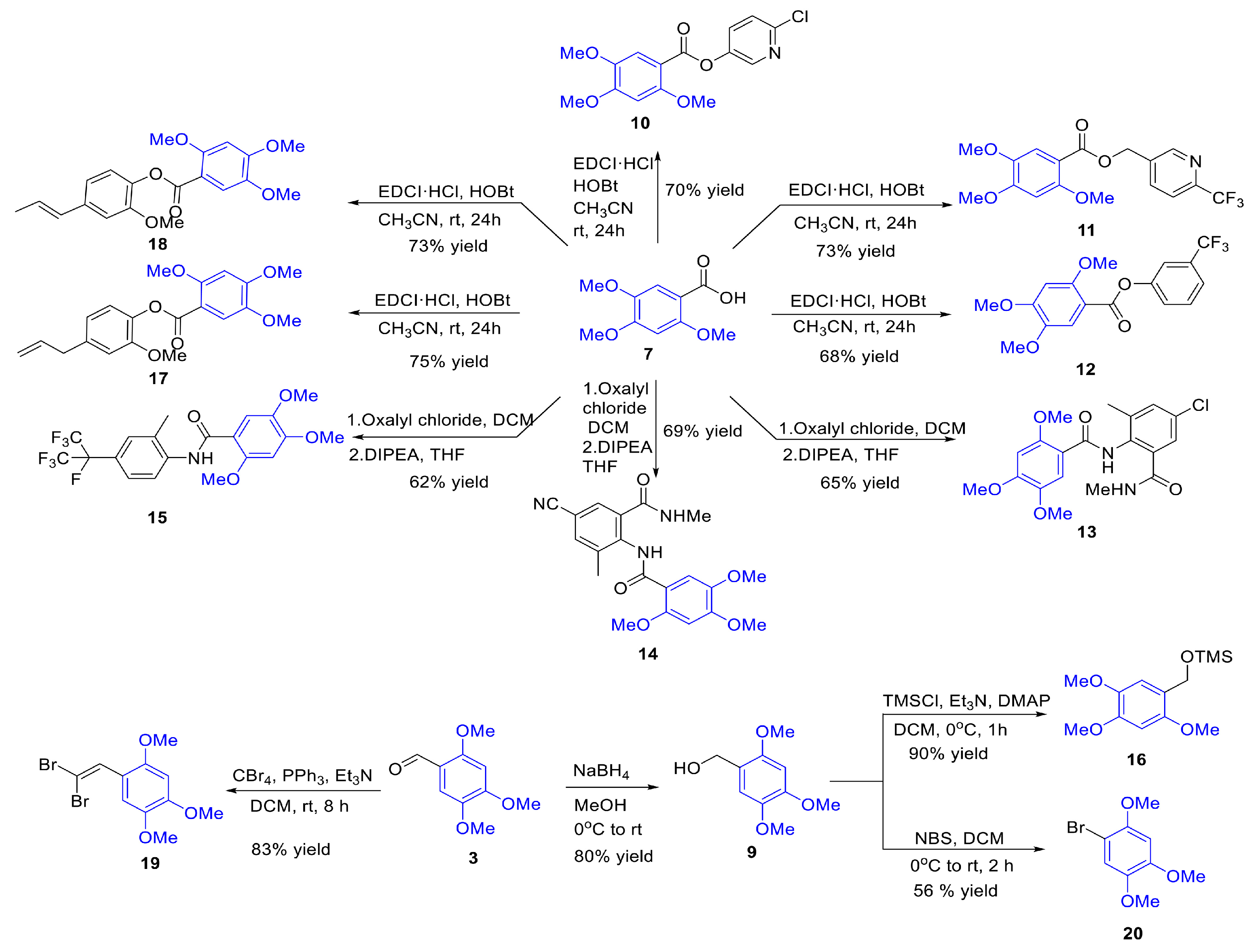
2.2. Modification and Synthesis of Active Compounds
2.3. Toxicity Bioassay
2.4. Sample Collection
2.4.1. Sample Collection for Transcriptome Sequencing and Quantitative Validation
2.4.2. Sample Collection for Instar Expression Profile and Tissue Expression Profile
2.5. RNA Isolation, Library Construction, and Sequencing
2.6. Differential Expression Analysis and Function Enrichment
2.7. Quantitative Real-Time PCR
2.8. Gene Identification and Phylogenetic Analysis
2.9. RNA Interference
2.10. Statistical Analysis
3. Results
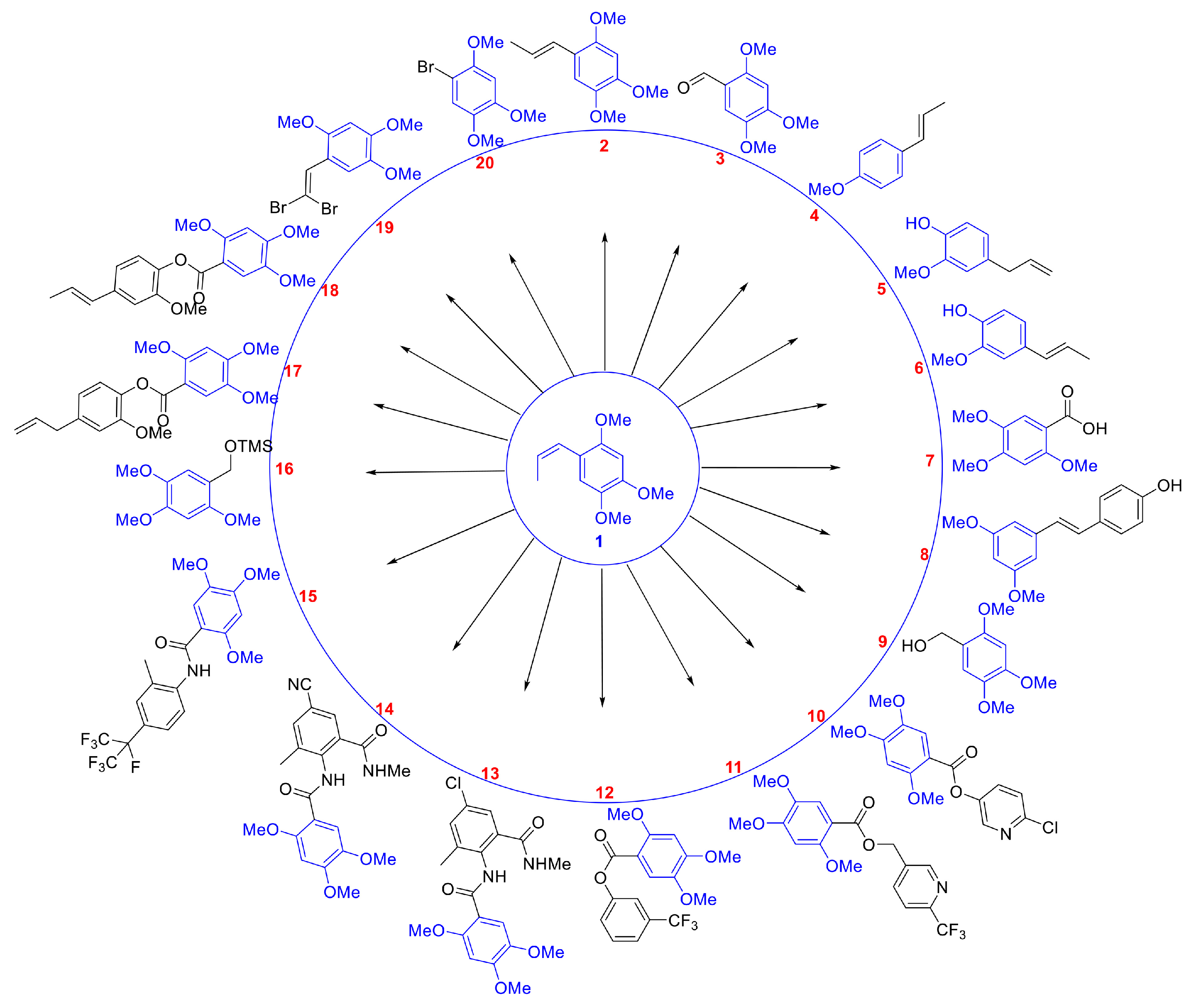
3.1. The Synthesis of Insecticidal Active Compounds
3.2. Insecticide Activity of 20 Compounds
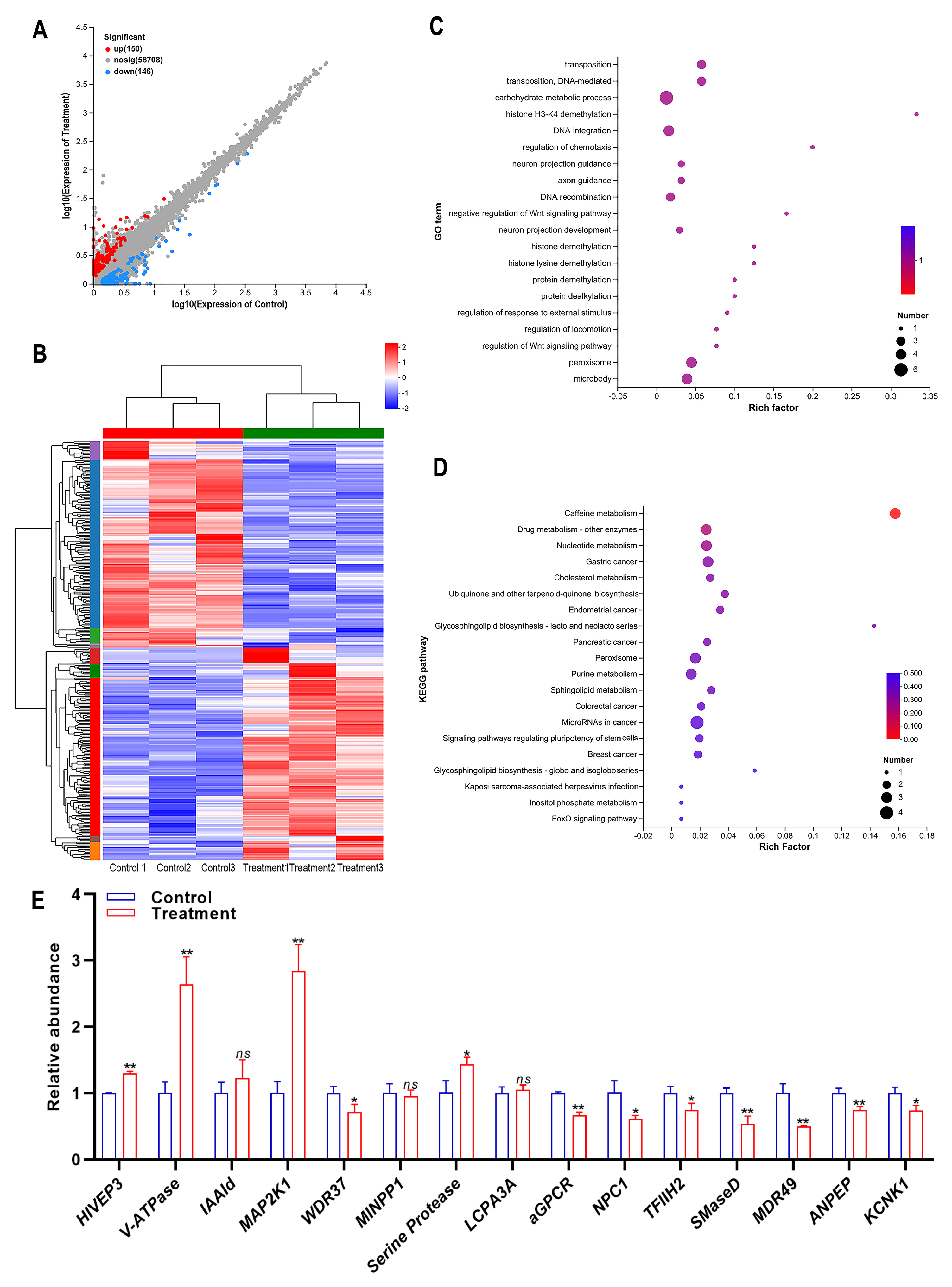
3.3. Overall Analysis of Sequencing Data
3.4. Functional Annotation and Analysis of Differentially Expressed Genes (DEGs)
3.5. Screening of DEGs Associated with the Insecticidal Activity of Compound 10
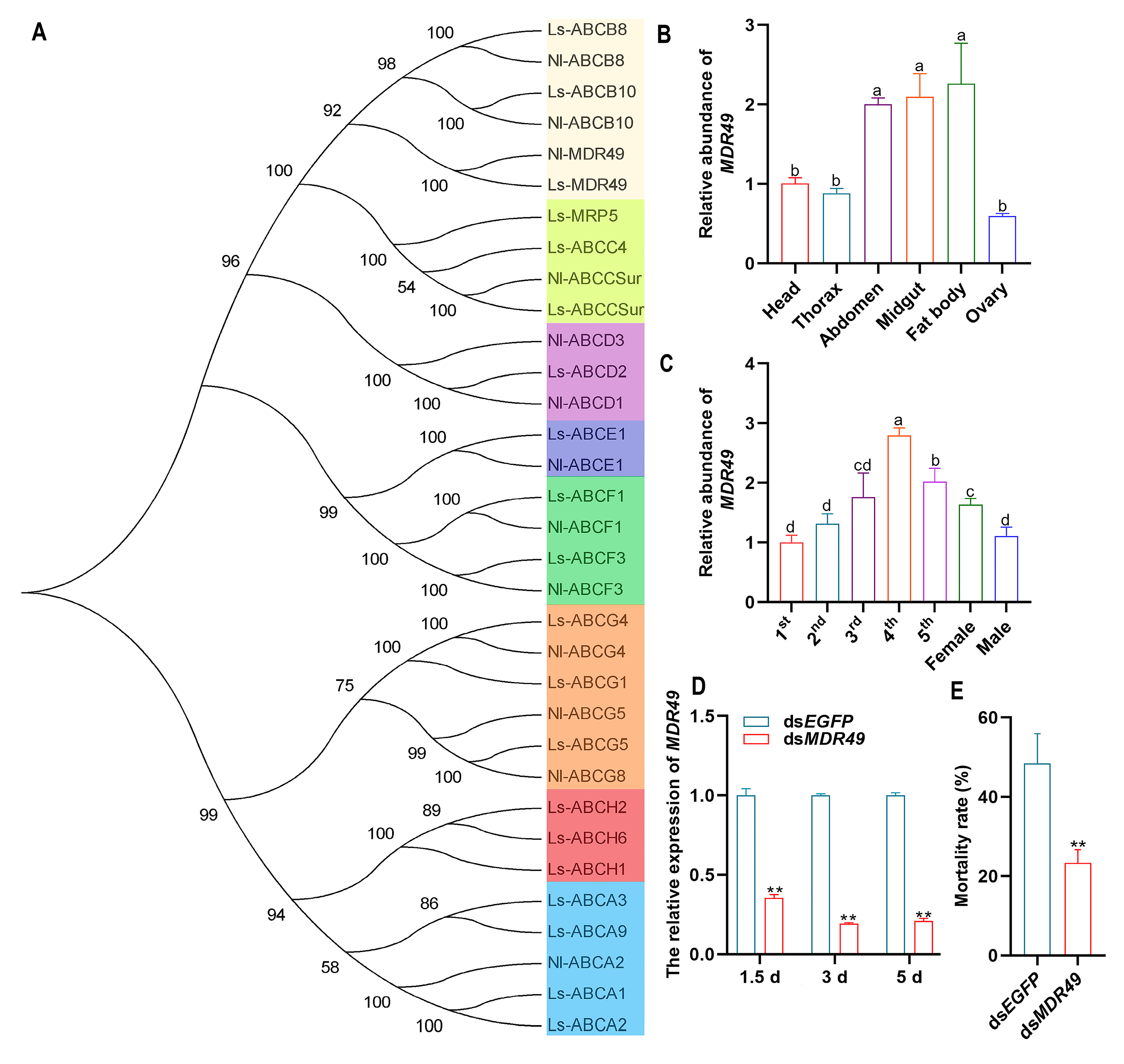
3.6. MDR49 Affects the Insecticidal Activity of Compound 10 Against SBPH
3.7. The Insecticidal Spectrum of Compound 10
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Elzaki, M.E.A.; Zhang, W.; Feng, A.; Qiou, X.; Zhao, W.; Han, Z. Constitutive overexpression of cytochrome P450 associated with imidacloprid resistance in Laodelphax striatellus (Fallen). Pest Manag. Sci. 2016, 72, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, A.; Xue, C.; Tian, H.; Zhang, Y.; Zhou, M.; Zhao, M.; Liu, Z.; Zhang, J. MicroRNA PC-5p-3991_515 mediates triflumezopyrim susceptibility in the small brown planthopper through regulating the post-transcriptional expression of P450 CYP417A2. Pest Manag. Sci. 2024, 80, 1761–1770. [Google Scholar] [CrossRef]
- Tanno, F.; Nakatsu, A.; Toriyama, S.; Kojima, M. Complete nucleotide sequence of Northern cereal mosaic virus and its genome organization. Arch. Virol. 2000, 145, 1373–1384. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, L.; Ren, Y.D.; Wang, X.F. Rice black-streaked dwarf virus: From multiparty interactions among plant–virus–vector to intermittent epidemics. Mol. Plant Pathol. 2020, 21, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Hajano, J.; Raza, A.; Zhang, L.; Liu, W.; Wang, X. Ribavirin targets sugar transporter 6 to suppress acquisition and transmission of rice stripe tenuivirus by its vector Laodelphax striatellus. Pest Manag. Sci. 2020, 76, 4086–4092. [Google Scholar] [CrossRef]
- Dively, G.P.; Kamel, A. Insecticide residues in pollen and nectar of a cucurbit crop and their potential exposure to pollinators. J. Agric. Food Chem. 2012, 60, 4449–4456. [Google Scholar] [CrossRef]
- Wu, J.; Ge, L.; Liu, F.; Song, Q.; Stanley, D. Pesticide-induced planthopper population resurgence in rice cropping systems. Annu. Rev. Entomol. 2020, 65, 409–429. [Google Scholar] [CrossRef]
- Imai, T.; Masuda, R. Insecticidal activities of methyleugenol and β-asarone, from the herbal medicines Saishin and Sekishōkon, and other alkoxy-propenyl-benzene derivatives against the cigarette beetle Lasioderma serricorne (Coleoptera: Anobiidae). Appl. Entomol. Zool. 2017, 52, 183–188. [Google Scholar] [CrossRef]
- Arasu, M.V.; Viayaraghavan, P.; Ilavenil, S.; Al-Dhabi, N.A.; Choi, K.C. Essential oil of four medicinal plants and protective properties in plum fruits against the spoilage bacteria and fungi. Ind. Crops Prod. 2019, 133, 54–62. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, W.; Yang, C.; Hua, H. Supercritical fluid CO2 extraction of Acorus calamus L. (Arales: Araceae) and its contact toxicity to Sitophilus zeamais Motschusky (Coleoptera: Curculionidae). Nat. Prod. Res. 2012, 26, 1498–1503. [Google Scholar] [CrossRef]
- Reddy, S.G.E.; Kirti Dolma, S.; Koundal, R.; Singh, B. Chemical composition and insecticidal activities of essential oils against diamondback moth, Plutella xylostella (L.) (Lepidoptera: Yponomeutidae). Nat. Prod. Res. 2016, 30, 1834–1838. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sharma, S.; Sharma, S.; Kumari, A.; Kumar, D.; Nadda, G.; Padwad, Y.; Ogra, R.K.; Kumar, N. Chemical composition, cytotoxicity and insecticidal activities of Acorus calamus accessions from the western Himalayas. Ind. Crops Prod. 2016, 94, 520–527. [Google Scholar] [CrossRef]
- Xu, X.; Li, X.; Liu, Z.; Wang, F.; Fan, L.; Wu, C.; Yao, Y. Knockdown of CYP301B1 and CYP6AX1v2 increases the susceptibility of the brown planthopper to beta-asarone, a potential plant-derived insecticide. Int. J. Biol. Macromol. 2021, 171, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Fang, Y.; Che, W.; Zhang, Q.; Wang, J.; Luo, C. The toxicity, sublethal effects, and biochemical mechanism of β-asarone, a potential plant-derived insecticide, against Bemisia tabaci. Int. J. Mol. Sci. 2022, 23, 10462. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, X.; Wang, F.; Han, K.; Liu, Z.; Fan, L.; Hua, H.; Cai, W.; Yao, Y. Candidate detoxification-related genes in brown planthopper, Nilaparvata lugens, in response to β-asarone based on transcriptomic analysis. Ecotoxicol. Environ. Saf. 2019, 185, 109735. [Google Scholar] [CrossRef]
- Wen, S.; Xue, Y.; Du, R.; Liu, C.; Wang, X.; Wang, Y.; Liu, C.; Wang, S.; Wang, J.; Xia, X. Toxicity and sublethal effects of triflumezopyrim on the development and detoxification enzymatic activities in the small brown planthopper (SBPH), Laodelphax striatellus (Fallen). Crop Prot. 2021, 150, 105813. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, A.; Zhang, Y.; Xue, C.; Zhao, M.; Zhang, J. Activating pathway of three metabolic detoxification phases via down-regulated endogenous microRNAs, modulates triflumezopyrim tolerance in the small brown planthopper, Laodelphax striatellus (Fallén). Int. J. Biol. Macromol. 2022, 222, 2439–2451. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q. Full-length transcriptome assembly from RNA-seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef]
- Smith-Unna, R.; Boursnell, C.; Patro, R.; Hibberd, J.M.; Kelly, S. TransRate: Reference-free quality assessment of de novo transcriptome assemblies. Genome Res. 2016, 26, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Manni, M.; Berkeley, M.R.; Seppey, M.; Simão, F.A.; Zdobnov, E.M. BUSCO update: Novel and streamlined workflows along with broader and deeper phylogenetic coverage for scoring of eukaryotic, prokaryotic, and viral genomes. Mol. Biol. Evol. 2021, 38, 4647–4654. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, A.; Wang, M.; Zhang, Y.; Zhang, J.; Zhao, M. ATP-binding cassette transporters ABCF2 and ABCG9 regulate rice black-streaked dwarf virus infection in its insect vector, Laodelphax striatellus (Fallén). Bull. Entomol. Res. 2022, 112, 327–334. [Google Scholar] [CrossRef]
- Gao, B.; Wu, J.; Huang, S.; Mu, L.; Han, Z. Insecticide resistance in field populations of Laodelphax striatellus (Fallén) (Homoptera: Delphacidae) in China and its possible mechanisms. Int. J. Pest Manag. 2008, 54, 13–19. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, C.; Liu, D.; Ding, W.; Li, Z.; Cao, J.; Xia, X. Sensitivity differences and biochemical characteristics of Laodelphax striatellus (Fallén) to seven insecticides in different areas of Shandong. China Insects 2022, 13, 780. [Google Scholar] [CrossRef]
- Cantrell, C.L.; Dayan, F.E.; Duke, S.O. Natural products as source for new pesticides. J. Nat. Prod. 2012, 75, 1231–1242. [Google Scholar] [CrossRef]
- Kiyota, H. Synthetic studies of biologically active natural products contributing to pesticide development. J. Pestic. Sci. 2020, 45, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; The International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Lorsbach, B.A.; Sparks, T.C.; Cicchillo, R.M.; Garizi, N.V.; Hahn, D.R.; Meyer, K.G. Natural products: A strategic lead generation approach in crop protection discovery. Pest Manag. Sci. 2019, 75, 2301–2309. [Google Scholar] [CrossRef] [PubMed]
- Marrone, P.G. Pesticidal natural products-status and future potential. Pest Manag. Sci. 2019, 75, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.C.; Wessels, F.J.; Lorsbach, B.A.; Nugent, B.M.; Watson, G.B. The new age of insecticide discovery-the crop protection industry and the impact of natural products. Pestic. Biochem. Physiol. 2019, 161, 12–22. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, Q.; Zhang, K.; Zhao, S. Synthesis, antimosquito activities, photodegradation, and toxic assessment of novel pyrethroids containing 2-chlorobiphenyl and 2-chlorophenylpyridine. Pest Manag. Sci. 2021, 77, 2773–2784. [Google Scholar] [CrossRef]
- Singh, S.; Mukherjee, A.; Jaiswal, D.K.; de Araujo Pereira, A.P.; Prasad, R.; Sharma, M.; Kuhad, R.C.; Shukla, A.C.; Verma, J.P. Advances and future prospects of pyrethroids: Toxicity and microbial degradation. Sci. Total Environ. 2022, 829, 154561. [Google Scholar] [CrossRef]
- Wiratno, S.; Trisawa, I.M. Research progress, formulation, and utilization of botanical pesticide. J. Litbang Pert. 2013, 32, 150–155. [Google Scholar]
- Wang, Y.; Shi, Z. Strain pesticide research and development progress of quasi deinsectization. Chem. Reag. 2024, 46, 50–58. [Google Scholar]
- Yang, R.; Liu, Z.; Han, M.; Cui, L.; Guo, Y. Preparation and biological evaluation of novel osthole-derived N-benzoylthioureas as insecticide candidates. J. Agric. Food Chem. 2022, 70, 15737–15746. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Z.; Xu, H.; Liu, Y.; Yang, X.; Sun, T.; Lu, X.; Shi, F.; Yang, Q.; Chen, W.; et al. Synthesis, antifungal activity, and 3D-QASR of novel 1,2,3,4-tetrahydroquinoline derivatives containing a pyrimidine ether scaffold as chitin synthase inhibitors. J. Agric. Food Chem. 2022, 70, 9262–9275. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, S.; Li, T.; Ma, S.; Wang, P.; Wang, G.; Su, S.; Ding, Y.; Yang, L.; Zhou, X.; et al. Design, synthesis and bioactivity evaluation of novel 2-(pyrazol-4-yl)-1,3,4-oxadiazoles containing an imidazole fragment as antibacterial agents. Molecules 2023, 28, 2442. [Google Scholar] [CrossRef]
- Huang, S.; Zuo, L.; Zhang, L.; Guo, X.; Cheng, C.; He, Y.; Cheng, G.; Yu, J.; Liu, Y.; Chen, R.; et al. Design, synthesis, and mode of action of thioacetamide derivatives as the algicide candidate based on active substructure splicing strategy. J. Agric. Food Chem. 2024, 72, 7021–7032. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Han, M.; Yan, X.; Cheng, W.; Tang, Z.; Cui, L.; Yang, R.; Guo, Y. Design, synthesis, and biological evaluation of novel osthole-based isoxazoline derivatives as insecticide candidates. J. Agric. Food Chem. 2022, 70, 7921–7928. [Google Scholar] [CrossRef]
- Shan, X.; Lv, M.; Wang, J.; Qin, Y.; Xu, H. Acaricidal and insecticidal efficacy of new esters derivatives of a natural coumarin osthole. Ind. Crops Prod. 2022, 182, 114855. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Shi, B.J.; Hu, Z.N. The insecticidal activity of periplocoside NW. Chin. Bull. Entomol. 2008, 45, 950–952. [Google Scholar]
- Li, Y.; Zeng, X.N.; Wang, W.Z.; Luo, C.H.; Yan, Q.; Tian, M. Chemical constituents from the roots of Periploca sepium with insecticidal activity. J. Asian Nat. Prod. Res. 2012, 14, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Jiang, W.; Li, Q.; Li, T.; Wu, W.; Bai, H.; Shi, B. Design, synthesis, and study of the insecticidal activity of novel steroidal 1,3,4-oxadiazoles. J. Agric. Food Chem. 2021, 69, 11572–11581. [Google Scholar] [CrossRef]
- Yooboon, T.; Kuramitsu, K.; Bullangpoti, V.; Kainoh, Y.; Furukawa, S. Cytotoxic effects of β-asarone on Sf9 insect cells. Arch. Insect Biochem. Physiol. 2019, 102, e21596. [Google Scholar] [CrossRef]
- Chen, H.P.; Yang, K.; Zheng, L.S.; You, C.X.; Wang, C.F. Repellant and insecticidal activities of shyobunone and isoshyobunone derived from the essential oil of Acorus calamus rhizomes. Pharmacogn. Mag. 2015, 11, 675–681. [Google Scholar]
- Liao, X.; Mao, K.; Ali, E.; Jin, R.; Li, Z.; Li, W.; Li, J.; Wan, H. Inheritance and fitness costs of sulfoxaflor resistance in Nilaparvata lugens (Stal). Pest Manag. Sci. 2019, 75, 2981–2988. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jin, M.; Wang, N.; Yu, Q.; Xue, C. Combined transcriptomic and proteomic analysis of flubendiamide resistance Plutella xylostella. Entomol. Res. 2020, 10, 50. [Google Scholar] [CrossRef]
- Umetsu, N.; Shirai, Y. Development of novel pesticides in the 21st century. J. Pestic. Sci. 2020, 45, 54–74. [Google Scholar] [CrossRef]
- Zhang, S.; Gu, F.; Du, Y.; Li, X.; Gong, C.; Pu, J.; Liu, X.; Wang, X. Risk assessment and resistance inheritance of triflumezopyrim resistance in Laodelphax striatellus. Pest Manag. Sci. 2022, 78, 2851–2859. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, X.; Guo, L.; Yin, T.; Liu, D.; Liu, S.; You, X.; Xia, X. Risk of resistance and the metabolic resistance mechanism of Laodelphax striatellus (Fallén) to cyantraniliprole. Pestic. Biochem. Physiol. 2023, 197, 105685. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, H.; Tan, Y.; Ni, R.; Shan, Y.; Li, F.; Dai, G.; Li, L.; Li, Y.; Pang, B. Sublethal effects of chlorantraniliprole on biological characteristics, detoxifying enzyme activity and gene expression profile in the Allium mongolicum Regel leaf beetle Galeruca daurica (Coleoptera: Chrysomelidae). J. Appl. Entomol. 2024, 3, 148. [Google Scholar] [CrossRef]
- Onozaki, Y.; Horikoshi, R.; Ohno, I.; Kitsuda, S.; Durkin, K.A.; Suzuki, T.; Asahara, C.; Hiroki, N.; Komabashiri, R.; Shimizu, R.; et al. Flupyrimin: A novel insecticide acting at the nicotinic acetylcholine receptors. J. Agric. Food Chem. 2017, 65, 7865–7873. [Google Scholar] [CrossRef]
- Guo, Y.; Hou, E.; Wen, T.; Yan, X.; Han, M.; Bai, L.P.; Fu, X.; Liu, J.; Qin, S. Development of membrane-active honokiol/magnolol amphiphiles as potent antibacterial agents against methicillin-resistant Staphylococcus aureus (MRSA). J. Med. Chem. 2021, 64, 12903–12916. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Pu, J.; Chen, F.; Wang, J.; Han, Z. Multiple ATP-binding cassette transporters are involved in insecticide resistance in the small brown planthopper, Laodelphax striatellus. Insect Mol. Biol. 2017, 26, 343–355. [Google Scholar] [CrossRef]
- Li, Z.; Davis, J.A.; Swale, D.R. Chemical inhibition of Kir channels reduces salivary secretions and phloem feeding of the cotton aphid, Aphis gossypii (Glover). Pest Manag. Sci. 2019, 75, 2725–2734. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y.Q.; Lai, T.; Liu, X.J.; Guo, F.Y.; Guo, T.; Ding, W. Acaricidal mechanism of scopoletin against Tetranychus cinnabarinus. Front. Physiol. 2019, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.C.; Yue, X.R.; Kuang, W.Q.; Li, S.L.; Tang, R.; Zhang, Z.F.; Kurban, A.; Saif-Ur-Rehman; Zhao, C.; Liu, T.X.; et al. NPC1b as a novel target in controlling the cotton bollworm, Helicoverpa armigera. Pest Manag. Sci. 2020, 76, 2233–2242. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Cheng, Z.; Qin, J.; Sun, D.; Wang, S.; Wu, Q.; Crickmore, N.; Zhou, X.; Bravo, A.; Soberón, M.; et al. MAPK-mediated transcription factor GATAd contributes to Cry1Ac resistance in diamondback moth by reducing PxmALP expression. PLoS Genet. 2022, 18, e1010037. [Google Scholar] [CrossRef]
- Ma, K.; Li, F.; Liang, P.; Chen, X.; Liu, Y.; Tang, Q.; Gao, X. RNA interference of Dicer-1 and Argonaute-1 increasing the sensitivity of Aphis gossypii Glover (Hemiptera: Aphididae) to plant allelochemical. Pestic. Biochem. Physiol. 2017, 138, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Guo, F.; Luo, J.; Zhang, Y.; Liu, J.; Zhang, Y.; Zheng, X.; Wan, F.; Ding, W. Functional analysis of an upregulated calmodulin gene related to the acaricidal activity of curcumin against Tetranychus cinnabarinus (Boisduval). Pest Manag. Sci. 2021, 77, 719–730. [Google Scholar] [CrossRef]
- Zhou, H.; Jian, Y.; Shao, Q.; Guo, F.; Zhang, M.; Wan, F.; Yang, L.; Liu, Y.; Yang, L.; Li, Y.; et al. Development of sustainable insecticide candidates for protecting pollinators: Insight into the bioactivities, selective mechanism of action and QSAR of natural coumarin derivatives against aphids. J. Agric. Food Chem. 2023, 71, 18359–18374. [Google Scholar] [CrossRef]
- Wan, P.J.; Guo, W.Y.; Yang, Y.; Lü, F.G.; Lu, W.P.; Li, G.Q. RNAi suppression of the ryanodine receptor gene results in decreased susceptibility to chlorantraniliprole in Colorado potato beetle Leptinotarsa decemlineata. J. Insect Physiol. 2014, 63, 48–55. [Google Scholar] [CrossRef]
- Wang, L.X.; Tao, S.; Zhang, Y.C.; Pei, X.G.; Gao, Y.; Song, X.Y.; Yu, Z.T.; Gao, C.F. Overexpression of ATP-binding cassette transporter Mdr49-like confers resistance to imidacloprid in the field populations of brown planthopper, Nilaparvata lugens. Pest Manag. Sci. 2022, 78, 579–590. [Google Scholar] [CrossRef]
- Sun, H.; Buchon, N.; Scott, J.G. Mdr65 decreases toxicity of multiple insecticides in Drosophila melanogaster. Insect Biochem. Mol. Biol. 2017, 89, 11–16. [Google Scholar] [CrossRef]
- Guo, Z.; Kang, S.; Chen, D.; Wu, Q.; Wang, S.; Xie, W.; Zhu, X.; Baxter, S.W.; Zhou, X.; Jurat-Fuentes, J.L.; et al. MAPK signaling pathway alters expression of midgut ALP and ABCC genes and causes resistance to Bacillus thuringiensis Cry1Ac toxin in diamondback moth. PLoS Genet. 2015, 11, e1005124. [Google Scholar] [CrossRef]
- Jin, M.H.; Tao, J.H.; Qi, L.I.; Cheng, Y.; Sun, X.X.; Wu, K.M.; Xiao, Y.T. Genome editing of the SfABCC2 gene confers resistance to Cry1F toxin from Bacillus thuringiensis in Spodoptera frugiperda. J. Integr. Agric. 2021, 20, 815–820. [Google Scholar] [CrossRef]
| Compound Number | n | LD50 (μg/pest) (95% CI) | Slope ± SE | χ2 (df) |
|---|---|---|---|---|
| 1 | 540 | 0.424 (0.318–0.639) | 1.102 ± 0.154 | 0.883 (3) |
| 2 | 540 | 0.297 (0.244–0.377) | 1.585 ± 0.167 | 1.675 (3) |
| 3 | 540 | 0.194 (0.075–382.569) | 1.390 ± 0.158 | 19.687 (3) |
| 4 | 540 | 0.304 (0.176–0.817) | 1.476 ± 0.159 | 8.678 (3) |
| 5 | 540 | 0.174 (0.092–0.373) | 1.574 ± 0.160 | 10.362 (3) |
| 6 | 540 | 0.158 (0.094–0.259) | 1.575 ± 0.161 | 6.658 (3) |
| 7 | 540 | 0.085 (0.061–0.108) | 1.459 ± 0.174 | 4.380 (3) |
| 8 | 540 | 0.073 (0.055–0.092) | 1.245 ± 0.154 | 0.388 (3) |
| 9 | 540 | 0.093 (0.074–0.112) | 1.610 ± 0.168 | 0.851 (3) |
| 10 | 540 | 0.051 (0.035–0.067) | 1.452 ± 0.176 | 3.172 (3) |
| 11 | 540 | 0.169 (0.112–0.263) | 1.804 ± 0.165 | 6.267 (3) |
| 12 | 540 | 0.179 (0.093–0.343) | 1.786 ± 0.166 | 11.744 (3) |
| 13 | 540 | 0.235 (0.122–0.446) | 1.681 ± 0.163 | 10.531 (3) |
| 14 | 540 | 0.194 (0.136–0.321) | 1.920 ± 0.184 | 5.508 (3) |
| 15 | Not detected | Not detected | Not detected | Not detected |
| 16 | 540 | 0.116 (0.068–0.197) | 2.244 ± 0.183 | 12.167 (3) |
| 17 | 540 | 0.124 (0.098–0.156) | 1.236 ± 0.151 | 0.372 (3) |
| 18 | 540 | 0.151 (0.125–0.183) | 1.573 ± 0.160 | 1.648 (3) |
| 19 | 540 | 0.196 (0.163–0.239) | 1.583 ± 0.161 | 3.367 (3) |
| 20 | 540 | 0.093 (0.077–0.110) | 1.684 ± 0.165 | 1.008 (3) |
| Insects | n | LD50 (μg/Pest) (95% CI) | Slope ± SE | χ2 (df) |
|---|---|---|---|---|
| Aphis craccivora | 363 | 0.057 (0.013–4.660) | 0.453 ± 0.193 | 0.216 (3) |
| Rhopalosiphum maidis | 306 | 0.051 (0.024–0.086) | 1.300 ± 0.356 | 0.570 (3) |
| Semiaphis heraclei | 354 | 0.061 (0.031–0.105) | 1.182 ± 0.319 | 1.026 (3) |
| Bemisia tabaci | 291 | 0.006 (0.001–0.014) | 0.831 ± 0.245 | 1.416 (3) |
| Tetranychus cinnabarinus | 396 | 0.010 (0.000–0.038) | 0.474 ± 0.195 | 0.715 (3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, A.; Zhou, Y.; Fu, X.; Wang, X.; Cheng, Y.; Zhang, Y.; Jia, X.; Zhu, Y.; Zhang, Y.; Xue, C.; et al. Structural Derivatives of β-Asarone from Acorus calamus Linn. as Insecticide Candidates and the Insecticidal Mechanism Against Small Brown Planthopper. Agronomy 2024, 14, 2420. https://doi.org/10.3390/agronomy14102420
Wang A, Zhou Y, Fu X, Wang X, Cheng Y, Zhang Y, Jia X, Zhu Y, Zhang Y, Xue C, et al. Structural Derivatives of β-Asarone from Acorus calamus Linn. as Insecticide Candidates and the Insecticidal Mechanism Against Small Brown Planthopper. Agronomy. 2024; 14(10):2420. https://doi.org/10.3390/agronomy14102420
Chicago/Turabian StyleWang, Aiyu, Yun Zhou, Xiaochen Fu, Xin Wang, Yinjie Cheng, Yifei Zhang, Xiuwen Jia, Yanwei Zhu, Yun Zhang, Chao Xue, and et al. 2024. "Structural Derivatives of β-Asarone from Acorus calamus Linn. as Insecticide Candidates and the Insecticidal Mechanism Against Small Brown Planthopper" Agronomy 14, no. 10: 2420. https://doi.org/10.3390/agronomy14102420
APA StyleWang, A., Zhou, Y., Fu, X., Wang, X., Cheng, Y., Zhang, Y., Jia, X., Zhu, Y., Zhang, Y., Xue, C., Shan, C., Zhao, M., Yang, Y., & Zhang, J. (2024). Structural Derivatives of β-Asarone from Acorus calamus Linn. as Insecticide Candidates and the Insecticidal Mechanism Against Small Brown Planthopper. Agronomy, 14(10), 2420. https://doi.org/10.3390/agronomy14102420







