Unravelling the Current Status of Rice Stripe Mosaic Virus: Its Geographical Spread, Biology, Epidemiology, and Management
Abstract
:1. Introduction
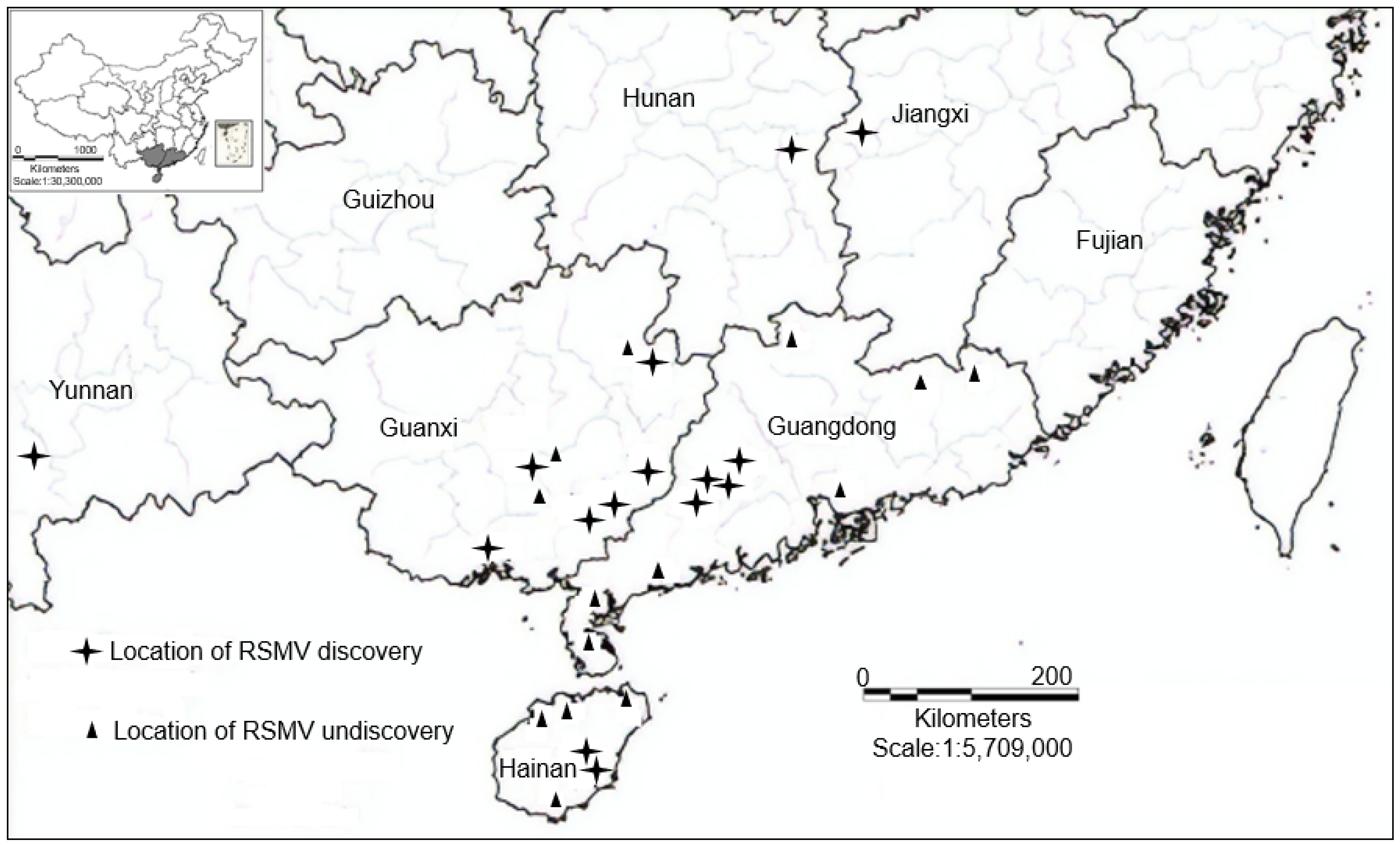
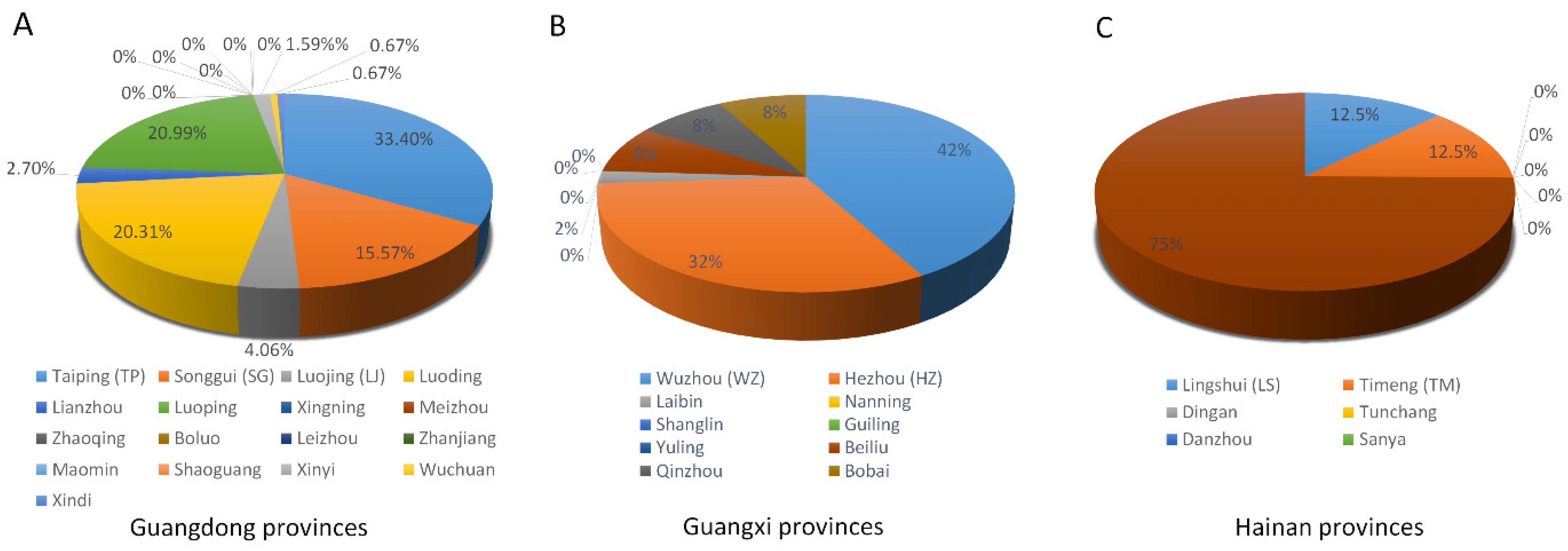
2. Geographic Distribution of RSMV Infection in China
3. Symptoms of RSMV Infection
4. Rice Yield Losses Associated with RSMV Infection
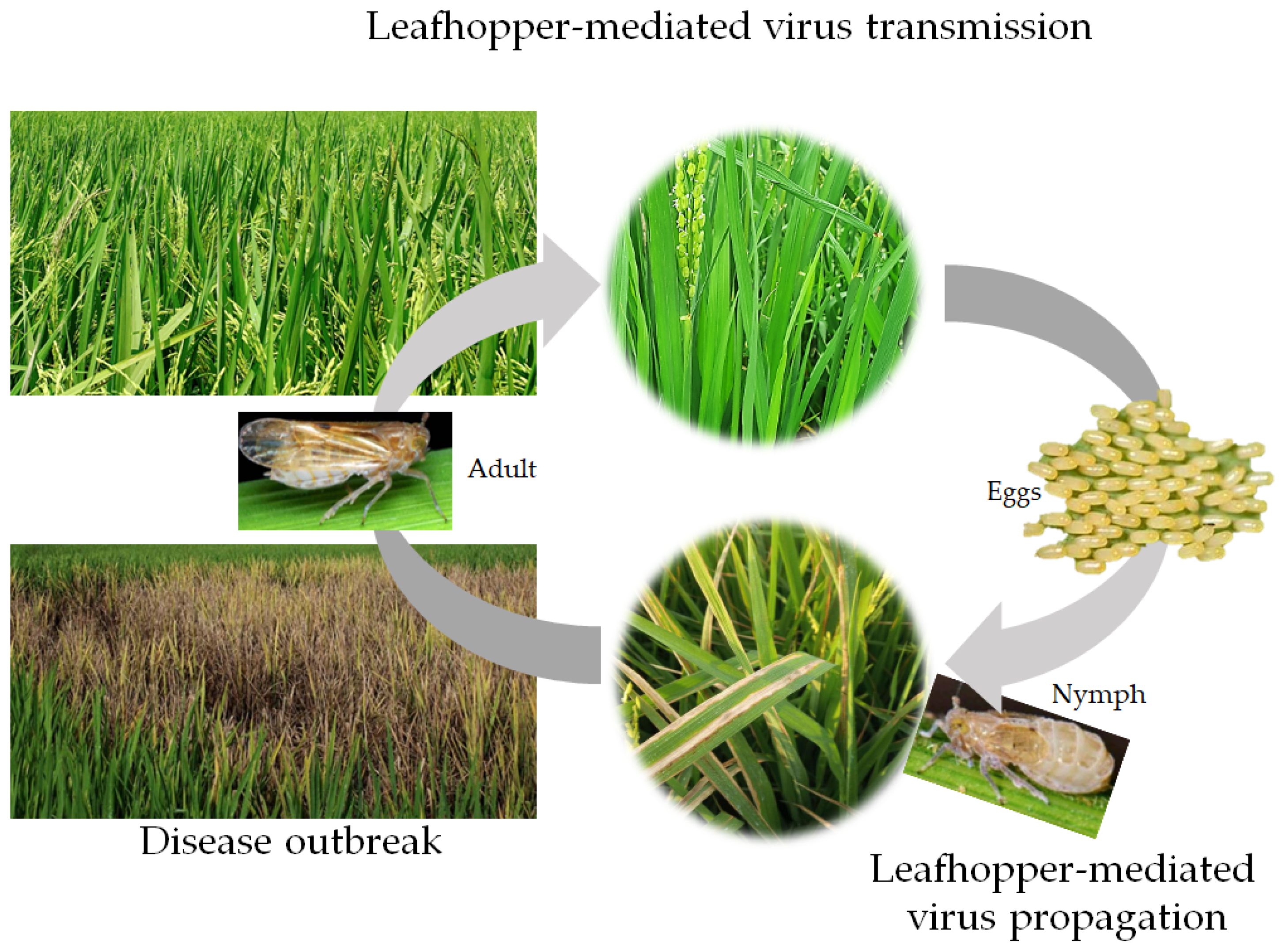
5. RSMV Infection Cycle
6. Synergistic Interaction of RSMV
7. Interplay Between Climatic Factors and Disease Dynamics
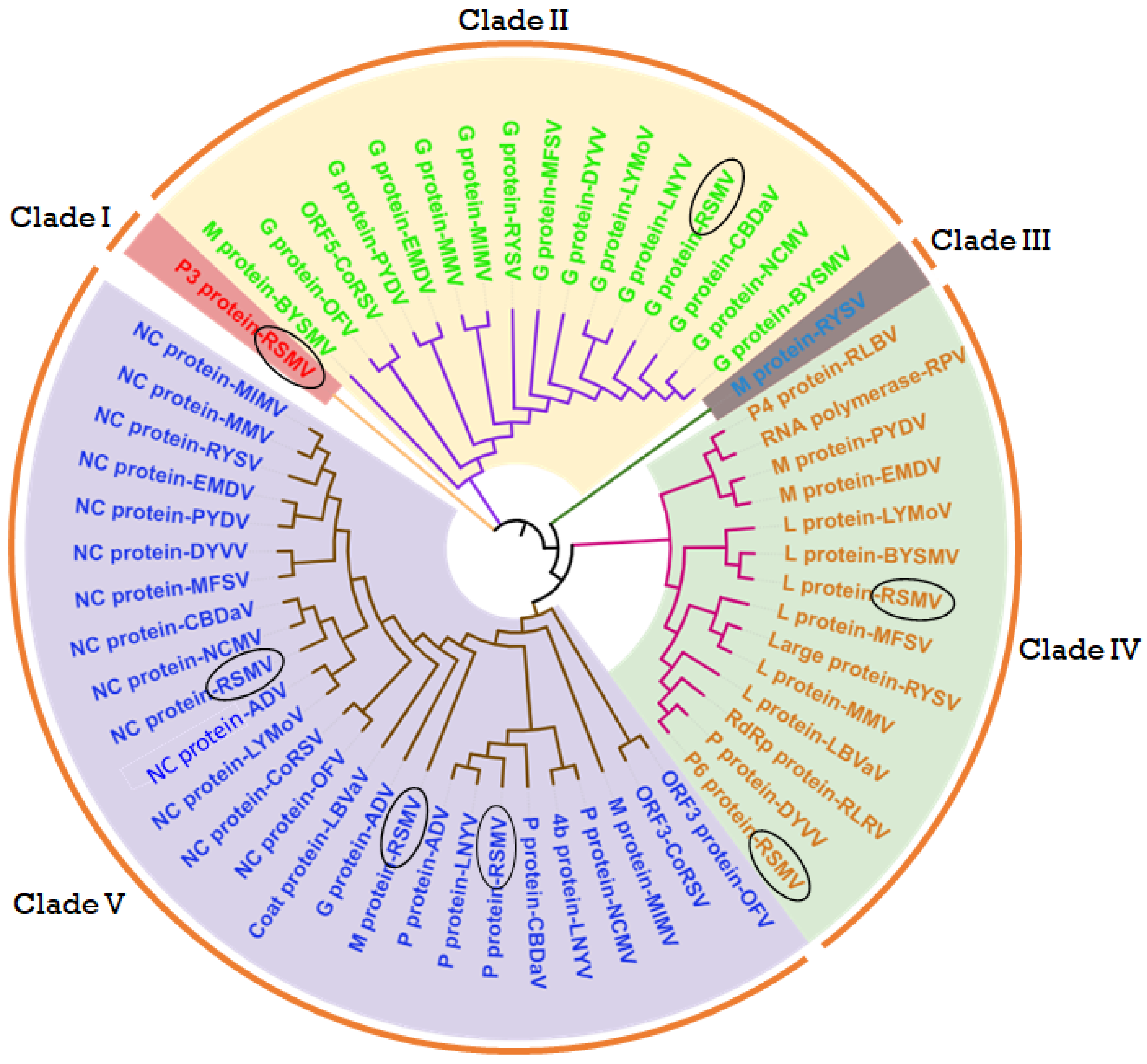
8. Protein–Protein Interaction (PPI) Network with Other Plant Viruses
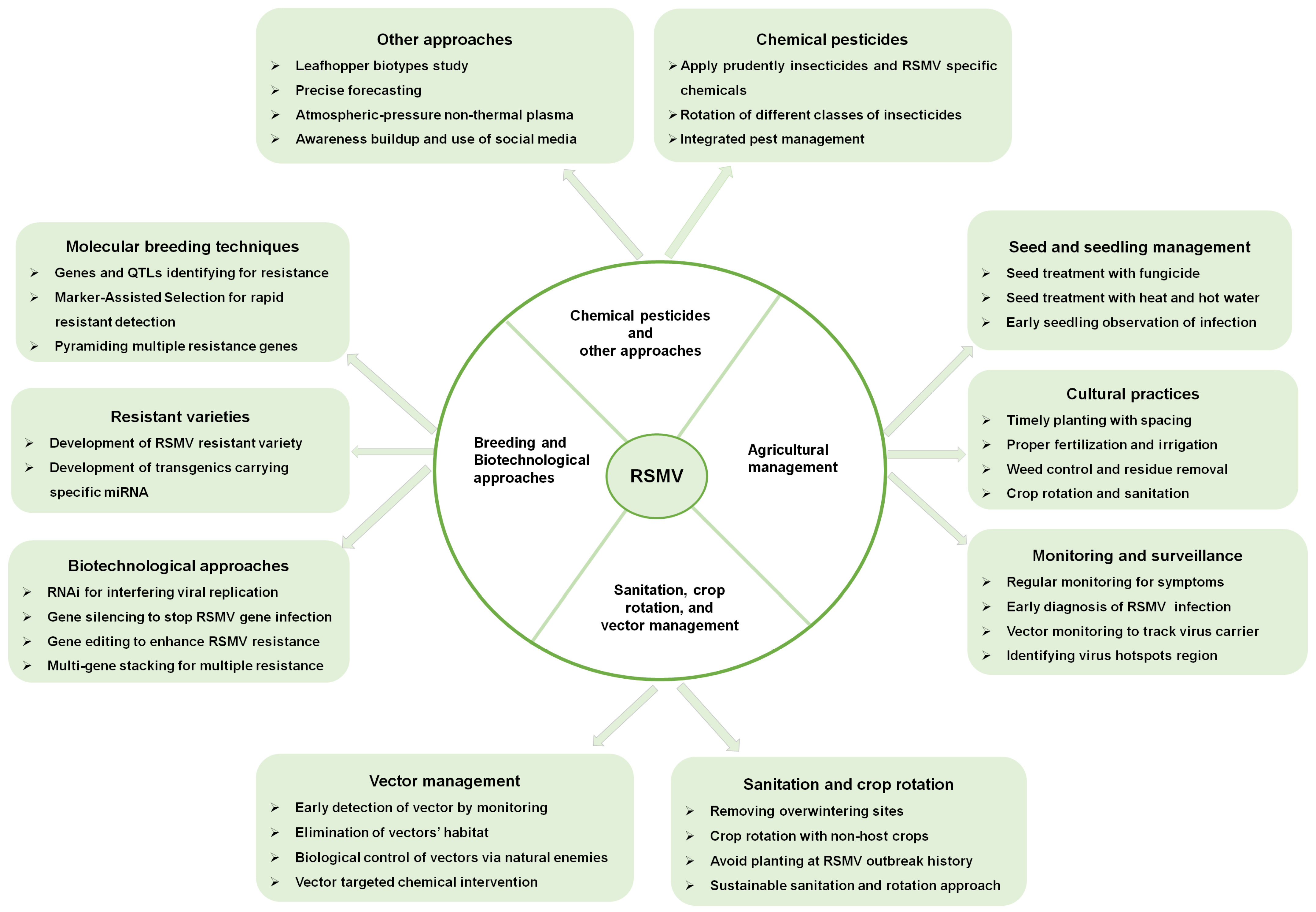
9. RSMV Disease Control Strategies
9.1. Use of Resistant Rice Varieties
9.2. Seed Treatment and Seedling Management
9.3. Monitoring and Surveillance
9.4. Sanitation and Crop Rotation
9.5. Vector Management and Cultural Practices
9.6. Molecular Breeding and Biotechnological Approaches
9.7. Chemical Pesticides
9.8. Plant Extracts and Nanotechnology
9.9. Other Approaches
10. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, X.; Zhang, T.; Chen, B.; Zhou, G. Transmission biology of rice stripe mosaic virus by an efficient insect vector Recilia dorsalis (Hemiptera: Cicadellidae). Front. Microbiol. 2017, 8, 2457. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.F.; Xie, L.; Wang, H.F.; Wang, H.D.; Chen, J.P.; Zhang, H.M. Biology of southern rice black-streaked dwarf virus: A novel fijivirus emerging in East Asia. Plant Pathol. 2017, 66, 515–521. [Google Scholar] [CrossRef]
- Guo, L.; Wu, J.; Chen, R.; Hong, J.; Zhou, X.; Wu, J. Monoclonal antibody-based serological detection of rice stripe mosaic virus infection in rice plants or leafhoppers. Virol. Sin. 2020, 35, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Huang, J.; Liu, C.; Chen, B.; Zhang, T.; Zhou, G. Rice stripe mosaic virus, a novel cytorhabdovirus infecting rice via leafhopper transmission. Front. Microbiol. 2017, 7, 2140. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Qin, Q.; Wang, Y.; Pu, Y.; Liu, L.; Wen, X.; Ji, S.; Wu, J.; Wei, C.; Ding, B. Rice dwarf virus P2 protein hijacks auxin signaling by directly targeting the rice OsIAA10 protein, enhancing viral infection and disease development. PLoS Pathog. 2016, 12, e1005847. [Google Scholar] [CrossRef] [PubMed]
- Gaafar, Y.Z.A.; Richert-Pöggeler, K.R.; Maaß, C.; Vetten, H.-J.; Ziebell, H. Characterisation of a novel nucleorhabdovirus infecting alfalfa (Medicago sativa). Virol. J. 2019, 16, 55. [Google Scholar] [CrossRef]
- Wu, J.-G.; Yang, G.-Y.; Zhao, S.-S.; Zhang, S.; Qin, B.-X.; Zhu, Y.-S.; Xie, H.-T.; Chang, Q.; Wang, L.; Hu, J.; et al. Current rice production is highly vulnerable to insect-borne viral diseases. Natl. Sci. Rev. 2022, 9, nwac131. [Google Scholar] [CrossRef]
- Gao, Q.; Xu, W.Y.; Yan, T.; Fang, X.D.; Cao, Q.; Zhang, Z.J.; Ding, Z.H.; Wang, Y.; Wang, X.B. Rescue of a plant cytorhabdovirus as versatile expression platforms for planthopper and cereal genomic studies. New Phytol. 2019, 223, 2120–2133. [Google Scholar] [CrossRef]
- Huang, Y. Discussion on the occurrence and control countermeasures of rice yellow dwarf virus. J. Guangxi Agric. 2015, 30, 15–17. [Google Scholar]
- Huang, R.; Li, Y.; Tang, G.; Hui, S.; Yang, Z.; Zhao, J.; Liu, H.; Cao, J.; Yuan, M. Dynamic phytohormone profiling of rice upon rice black-streaked dwarf virus invasion. J. Plant Physiol. 2018, 228, 92–100. [Google Scholar] [CrossRef]
- Li, S.; Hao, W.; Lu, G.; Huang, J.; Liu, C.; Zhou, G. Occurrence and identification of a new vector of rice orange leaf phytoplasma in South China. Plant Dis. 2015, 99, 1483–1487. [Google Scholar] [CrossRef] [PubMed]
- Shiba, T.; Hirae, M.; Hayano-Saito, Y.; Ohto, Y.; Uematsu, H.; Sugiyama, A.; Okuda, M. Spread and yield loss mechanisms of rice stripe disease in rice paddies. Field Crop. Res. 2018, 217, 211–217. [Google Scholar] [CrossRef]
- Sun, K.; Zhou, X.; Lin, W.; Zhou, X.; Jackson, A.O.; Li, Z. Matrix-glycoprotein interactions required for budding of a plant nucleorhabdovirus and induction of inner nuclear membrane invagination. Mol. Plant Pathol. 2018, 19, 2288–2301. [Google Scholar] [CrossRef]
- Towata, T.; Matsukura, K.; Sanada-Morimura, S.; Matsumura, M. Varietal differences in ovicidal response to the white-backed planthopper Sogatella furcifera (Hemiptera: Delphacidae) and susceptibility to Southern rice black-streaked dwarf virus in rice. Appl. Entomol. Zool. 2017, 52, 615–621. [Google Scholar] [CrossRef]
- Chen, S.; Li, W.; Huang, X.; Chen, B.; Zhang, T.; Zhou, G. Symptoms and yield loss caused by rice stripe mosaic virus. Virol. J. 2019, 16, 145. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Mo, L.; Huo, C.; Wang, Z.; Zhong, P.; Jia, D.; Zhang, X.; Chen, Q.; Chen, H. A neuron-specific antiviral mechanism modulates the persistent infection of rice rhabdoviruses in leafhopper vectors. Front. Microbiol. 2020, 11, 513. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, B.; Zhang, T.; Zhou, G.; Yang, X. Rice stripe mosaic disease: Characteristics and control strategies. Front. Microbiol. 2021, 12, 715223. [Google Scholar] [CrossRef]
- Zhao, P.; Sun, X.; Li, P.; Sun, J.; Yue, Y.; Wei, J.; Wei, T.; Jia, D. Infection characteristics of rice stripe mosaic virus in the body of the vector leafhoppers. Front. Microbiol. 2019, 9, 3258. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, D.; Yang, G.; Yu, X.; Wu, J. Rice stripe mosaic virus–encoded P4 is a weak suppressor of viral RNA silencing and is required for disease symptom development. Mol. Plant-Microbe Interact. 2020, 33, 412–422. [Google Scholar] [CrossRef]
- Yazdkhasti, E.; Hopkins, R.J.; Kvarnheden, A. Reservoirs of plant virus disease: Occurrence of wheat dwarf virus and barley/cereal yellow dwarf viruses in Sweden. Plant Pathol. 2021, 70, 1552–1561. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, K.; Zhou, X.; Jackson, A.O.; Li, Z. The matrix protein of a plant rhabdovirus mediates superinfection exclusion by inhibiting viral transcription. J. Virol. 2019, 93, e00680-19. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, H.; Chen, C.; Huang, H.; Tan, X.; Wei, Z.; Li, J.; Yan, F.; Zhang, C.; Chen, J. A class of independently evolved transcriptional repressors in plant RNA viruses facilitates viral infection and vector feeding. Proc. Natl. Acad. Sci. USA 2021, 118, e2016673118. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Fu, S.; Tao, X.; Zhou, X. Rice stripe virus: Exploring molecular weapons in the arsenal of a negative-sense RNA virus. Annu. Rev. Phytopathol. 2021, 59, 351–371. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Lin, W.; Sun, K.; Wang, S.; Zhou, X.; Jackson, A.O.; Li, Z. Specificity of plant rhabdovirus cell-to-cell movement. J. Virol. 2019, 93, e00296-19. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Liang, Q.; Chen, H.; Liu, H.; Li, G.; Zhang, X.; Chen, Q.; Wang, A.; Wei, T. Autophagy mediates a direct synergistic interaction during co-transmission of two distinct arboviruses by insect vectors. Sci. China Life Sci. 2023, 66, 1665–1681. [Google Scholar] [CrossRef]
- Jia, D.; Liu, H.; Zhang, J.; Wan, W.; Wang, Z.; Zhang, X.; Chen, Q.; Wei, T. Polyamine-metabolizing enzymes are activated to promote the proper assembly of rice stripe mosaic virus in insect vectors. Stress Biol. 2022, 2, 10. [Google Scholar] [CrossRef]
- Yang, X.; Chen, B.; Zhang, T.; Li, Z.; Xu, C.; Zhou, G. Geographic distribution and genetic diversity of rice stripe mosaic virus in southern China. Front. Microbiol. 2018, 9, 3068. [Google Scholar] [CrossRef]
- He, D.-c.; Zhan, J.-s.; Xie, L.-h. Problems, challenges and future of plant disease management: From an ecological point of view. J. Integr. Agric. 2016, 15, 705–715. [Google Scholar] [CrossRef]
- Rural Development Administration. Available online: https://www.rda.go.kr/board/board.do?boardId=farmprmninfo&prgId=day_farmprmninfoEntry&mode=updateCnt&searchOrgDeptKey=org&dataNo=100000798530 (accessed on 6 September 2024).
- Li, P.; Zhang, J.; Yue, Y.; Chen, H.; Wu, W.; Wei, T.; Jia, D. Effects of Rice stripe mosaic virus on the growth, reproduction and feeding behavior of the vector Recilia dorsalis (Hemiptera: Cicadellidae). Acta Entomol. Sin. 2020, 63, 174–180. [Google Scholar]
- Jones, R.A.C.; Sharman, M.; Trębicki, P.; Maina, S.; Congdon, B.S. Virus diseases of cereal and oilseed crops in Australia: Current position and future challenges. Viruses 2021, 13, 2051. [Google Scholar] [CrossRef]
- Adhab, M.; Al-Kuwaiti, N.; Al-Ani, R. Biodiversity and occurrence of plant viruses over four decades: Case study for Iraq. In Proceedings of the 2021 Third International Sustainability and Resilience Conference: Climate Change, Online, 15–16 November 2021; pp. 159–163. [Google Scholar]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Oakley, B. From laboratory to laptop: How science communication can bridge the gap between plant pathology and the public. Physiol. Mol. Plant Pathol. 2023, 125, 102032. [Google Scholar] [CrossRef]
- Adhab, M.; Angel, C.; Rodriguez, A.; Fereidouni, M.; Király, L.; Scheets, K.; Schoelz, J.E. Tracing the Lineage of Two Traits Associated with the Coat Protein of the Tombusviridae: Silencing Suppression and HR Elicitation in Nicotiana Species. Viruses 2019, 11, 588. [Google Scholar] [CrossRef]
- Adhab, M.; Finke, D.; Schoelz, J. Turnip aphids (Lipaphis erysimi) discriminate host plants based on the strain of Cauliflower mosaic virus infection. Emir. J. Food Agric. 2019, 31, 69–75. [Google Scholar]
- Hogenhout, S.A.; Ammar, E.-D.; Whitfield, A.E.; Redinbaugh, M.G. Insect vector interactions with persistently transmitted viruses. Annu. Rev. Phytopathol. 2008, 46, 327–359. [Google Scholar] [CrossRef]
- Zheng, L.; Mao, Q.; Xie, L.; Wei, T. Infection route of rice grassy stunt virus, a tenuivirus, in the body of its brown planthopper vector, Nilaparvata lugens (Hemiptera: Delphacidae) after ingestion of virus. Virus Res. 2014, 188, 170–173. [Google Scholar] [CrossRef]
- Wu, W.; Zheng, L.; Chen, H.; Jia, D.; Li, F.; Wei, T. Nonstructural Protein NS4 of Rice Stripe Virus Plays a Critical Role in Viral Spread in the Body of Vector Insects. PLoS ONE 2014, 9, e88636. [Google Scholar] [CrossRef]
- Syller, J. Facilitative and antagonistic interactions between plant viruses in mixed infections. Mol. Plant Pathol. 2012, 13, 204–216. [Google Scholar] [CrossRef]
- Rodrigues, N.B.; Godoy, R.S.M.; Orfano, A.S.; Chaves, B.A.; Campolina, T.B.; Costa, B.d.A.; Félix, L.d.S.; Silva, B.M.; Norris, D.E.; Pimenta, P.F.P.; et al. Brazilian Aedes aegypti as a Competent Vector for Multiple Complex Arboviral Coinfections. J. Infect. Dis. 2021, 224, 101–108. [Google Scholar] [CrossRef]
- Chávez-Calvillo, G.; Contreras-Paredes, C.A.; Mora-Macias, J.; Noa-Carrazana, J.C.; Serrano-Rubio, A.A.; Dinkova, T.D.; Carrillo-Tripp, M.; Silva-Rosales, L. Antagonism or synergism between papaya ringspot virus and papaya mosaic virus in Carica papaya is determined by their order of infection. Virology 2016, 489, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Kareem, K.T.; Taiwo, M.A. Interactions of viruses in cowpea: Effects on growth and yield parameters. Virol. J. 2007, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Tatineni, S.; Graybosch, R.A.; Hein, G.L.; Wegulo, S.N.; French, R. Wheat cultivar-specific disease synergism and alteration of virus accumulation during co-infection with Wheat streak mosaic virus and Triticum mosaic virus. Phytopathology 2010, 100, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-G.; Koo, B.-J.; Lee, E.-T.; Chang, M.-U. Allexivirus transmitted by eriophyid mites in garlic plants. J. Microbiol. Biotechnol. 2007, 17, 1833–1840. [Google Scholar]
- Xie, L.H. Research on rice virus disease in China. Trop. Agric. Res. 1986, 19, 45–50. [Google Scholar]
- Jia, D.; Luo, G.; Shi, W.; Liu, Y.; Liu, H.; Zhang, X.; Wei, T. Rice Gall Dwarf Virus Promotes the Propagation and Transmission of Rice Stripe Mosaic Virus by Co-infected Insect Vectors. Front. Microbiol. 2022, 13, 834712. [Google Scholar] [CrossRef]
- West, J.S.; Holdgate, S.; Townsend, J.A.; Edwards, S.G.; Jennings, P.; Fitt, B.D.L. Impacts of changing climate and agronomic factors on fusarium ear blight of wheat in the UK. Fungal Ecol. 2012, 5, 53–61. [Google Scholar] [CrossRef]
- Albahri, G.; Alyamani, A.A.; Badran, A.; Hijazi, A.; Nasser, M.; Maresca, M.; Baydoun, E. Enhancing essential grains yield for sustainable food security and bio-safe agriculture through latest innovative approaches. Agronomy 2023, 13, 1709. [Google Scholar] [CrossRef]
- Schoelz, J.E.; Adhab, M. Caulimoviruses (Caulimoviridae). In Encyclopedia of Virology, 4th ed.; Bamford, D.H., Zuckerman, M., Eds.; Academic Press: Oxford, UK, 2021; pp. 313–321. [Google Scholar]
- Suresh, N.T.; Ravindran, V.E.; Krishnakumar, U. A computational framework to identify cross association between complex disorders by protein-protein interaction network analysis. Curr. Bioinform. 2021, 16, 433–445. [Google Scholar] [CrossRef]
- Basar, M.A.; Hosen, M.F.; Paul, B.K.; Hasan, M.R.; Shamim, S.M.; Bhuyian, T. Identification of drug and protein-protein interaction network among stress and depression: A bioinformatics approach. Inform. Med. Unlocked 2023, 37, 101174. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Ruedas, J.B.; Perrault, J. Insertion of Enhanced Green Fluorescent Protein in a Hinge Region of Vesicular Stomatitis Virus L Polymerase Protein Creates a Temperature-Sensitive Virus That Displays No Virion-Associated Polymerase Activity In Vitro. J. Virol. 2009, 83, 12241–12252. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Green, T.J.; Zhang, X.; Tsao, J.; Qiu, S. Conserved characteristics of the rhabdovirus nucleoprotein. Virus Res. 2007, 129, 246–251. [Google Scholar] [CrossRef]
- Bejerman, N.; Giolitti, F.; De Breuil, S.; Trucco, V.; Nome, C.; Lenardon, S.; Dietzgen, R.G. Complete genome sequence and integrated protein localization and interaction map for alfalfa dwarf virus, which combines properties of both cytoplasmic and nuclear plant rhabdoviruses. Virology 2015, 483, 275–283. [Google Scholar] [CrossRef]
- Yan, T.; Zhu, J.-R.; Di, D.; Gao, Q.; Zhang, Y.; Zhang, A.; Yan, C.; Miao, H.; Wang, X.-B. Characterization of the complete genome of Barley yellow striate mosaic virus reveals a nested gene encoding a small hydrophobic protein. Virology 2015, 478, 112–122. [Google Scholar] [CrossRef]
- Jackson, A.O.; Dietzgen, R.G.; Goodin, M.M.; Bragg, J.N.; Deng, M. Biology of plant rhabdoviruses. Annu. Rev. Phytopathol. 2005, 43, 623–660. [Google Scholar] [CrossRef]
- Higgins, C.M.; Bejerman, N.; Li, M.; James, A.P.; Dietzgen, R.G.; Pearson, M.N.; Revill, P.A.; Harding, R.M. Complete genome sequence of Colocasia bobone disease-associated virus, a putative cytorhabdovirus infecting taro. Arch. Virol. 2016, 161, 745–748. [Google Scholar] [CrossRef]
- Ramalho, T.O.; Figueira, A.R.; Sotero, A.J.; Wang, R.; Duarte, P.S.G.; Farman, M.; Goodin, M.M. Characterization of Coffee ringspot virus-Lavras: A model for an emerging threat to coffee production and quality. Virology 2014, 464, 385–396. [Google Scholar] [CrossRef]
- Sasaya, T.; Kusaba, S.; Ishikawa, K.; Koganezawa, H. Nucleotide sequence of RNA2 of Lettuce big-vein virus and evidence for a possible transcription termination/initiation strategy similar to that of rhabdoviruses. J. Gen. Virol. 2004, 85, 2709–2717. [Google Scholar] [CrossRef] [PubMed]
- Dietzgen, R.G.; Innes, D.J.; Bejerman, N. Complete genome sequence and intracellular protein localization of Datura yellow vein nucleorhabdovirus. Virus Res. 2015, 205, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Miglino, R.; Sorrentino, R.; Masenga, V.; Alioto, D.; Pappu, H.R. Complete genomic characterization of eggplant mottled dwarf virus from Agapanthus sp. By deep sequencing and de novo assembly. J. Plant Pathol. 2014, 96, 593–595. [Google Scholar]
- Dietzgen, R.G.; Callaghan, B.; Wetzel, T.; Dale, J.L. Completion of the genome sequence of Lettuce necrotic yellows virus, type species of the genus Cytorhabdovirus. Virus Res. 2006, 118, 16–22. [Google Scholar] [CrossRef]
- Heim, F.; Lot, H.; Delecolle, B.; Bassler, A.; Krczal, G.; Wetzel, T. Complete nucleotide sequence of a putative new cytorhabdovirus infecting lettuce. Arch. Virol. 2008, 153, 81–92. [Google Scholar] [CrossRef]
- Tsai, C.-W.; Redinbaugh, M.G.; Willie, K.J.; Reed, S.; Goodin, M.; Hogenhout, S.A. Complete genome sequence and in planta subcellular localization of maize fine streak virus proteins. J. Virol. 2005, 79, 5304–5314. [Google Scholar] [CrossRef]
- Ghorbani, A.; Izadpanah, K.; Dietzgen, R.G. Completed sequence and corrected annotation of the genome of maize Iranian mosaic virus. Arch. Virol. 2018, 163, 767–770. [Google Scholar] [CrossRef]
- Reed, S.E.; Tsai, C.-W.; Willie, K.J.; Redinbaugh, M.G.; Hogenhout, S.A. Shotgun sequencing of the negative-sense RNA genome of the rhabdovirus Maize mosaic virus. J. Virol. Methods 2005, 129, 91–96. [Google Scholar] [CrossRef]
- Tanno, F.; Nakatsu, A.; Toriyama, S.; Kojima, M. Complete nucleotide sequence of Northern cereal mosaic virus and its genome organization. Arch. Virol. 2000, 145, 1373–1384. [Google Scholar] [CrossRef]
- Kondo, H.; Maruyama, K.; Chiba, S.; Andika, I.B.; Suzuki, N. Transcriptional mapping of the messenger and leader RNAs of orchid fleck virus, a bisegmented negative-strand RNA virus. Virology 2014, 452, 166–174. [Google Scholar] [CrossRef]
- Ghosh, D.; Brooks, R.E.; Wang, R.; Lesnaw, J.; Goodin, M.M. Cloning and subcellular localization of the phosphoprotein and nucleocapsid proteins of Potato yellow dwarf virus, type species of the genus Nucleorhabdovirus. Virus Res. 2008, 135, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, H.; Luo, Z.; Chen, X.; Fang, R.-X. Novel structure of the genome of Rice yellow stunt virus: Identification of the gene 6-encoded virion protein. J. Gen. Virol. 2003, 84, 2259–2264. [Google Scholar] [CrossRef] [PubMed]
- Chao, S.; Wang, H.; Zhang, S.; Chen, G.; Mao, C.; Hu, Y.; Yu, F.; Wang, S.; Lv, L.; Chen, L. Novel RNA Viruses Discovered in Weeds in Rice Fields. Viruses 2022, 14, 2489. [Google Scholar] [CrossRef] [PubMed]
- McGavin, W.J.; Mitchell, C.; Cock, P.J.A.; Wright, K.M.; MacFarlane, S.A. Raspberry leaf blotch virus, a putative new member of the genus Emaravirus, encodes a novel genomic RNA. J. Gen. Virol. 2012, 93, 430–437. [Google Scholar] [CrossRef]
- He, Y.; Yang, Z.; Hong, N.; Wang, G.; Ning, G.; Xu, W. Deep sequencing reveals a novel closterovirus associated with wild rose leaf rosette disease. Mol. Plant Pathol. 2015, 16, 449–458. [Google Scholar] [CrossRef]
- Hančinský, R.; Mihálik, D.; Mrkvová, M.; Candresse, T.; Glasa, M. Plant viruses infecting Solanaceae family members in the cultivated and wild environments: A review. Plants 2020, 9, 667. [Google Scholar] [CrossRef]
- Korbecka-Glinka, G.; Przybyś, M.; Feledyn-Szewczyk, B. A survey of five plant viruses in weeds and tobacco in Poland. Agronomy 2021, 11, 1667. [Google Scholar] [CrossRef]
- Makkouk, K.M.; Kumari, S.G. Epidemiology and integrated management of persistently transmitted aphid-borne viruses of legume and cereal crops in West Asia and North Africa. Virus Res. 2009, 141, 209–218. [Google Scholar] [CrossRef]
- Souza, T.A.; Macedo, M.A.; Albuquerque, L.C.; Inoue-Nagata, A.K. Host range and natural infection of tomato chlorosis virus in weeds collected in Central Brazil. Trop. Plant Pathol. 2020, 45, 84–90. [Google Scholar] [CrossRef]
- Zhou, G.; Xu, D.; Xu, D.; Zhang, M. Southern rice black-streaked dwarf virus: A white-backed planthopper-transmitted fijivirus threatening rice production in Asia. Front. Microbiol. 2013, 4, 270. [Google Scholar] [CrossRef]
- Elqdhy, M.b.; Ait Hamza, M.; Askarne, L.; Fossati-Gaschignard, O.; Lakhtar, H.; El Mousadik, A.; Ait Benoumar, A.; Msanda, F.; Boubaker, H. Biology, ecology and control of the Mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae), with special reference to biological control using entomopathogenic nematode (EPN): A review. J. Plant Dis. Prot. 2024, 131, 365–402. [Google Scholar] [CrossRef]
- Sánchez-Martín, J.; Keller, B. Contribution of recent technological advances to future resistance breeding. Theor. Appl. Genet. 2019, 132, 713–732. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yuan, Q.; Ai, X.; Chen, J.; Lu, Y.; Yan, F. Transgenic Rice Plants Expressing Artificial miRNA Targeting the Rice Stripe Virus MP Gene Are Highly Resistant to the Virus. Biology 2022, 11, 332. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, B.; Iqbal, M.S.; Batcho, A.A.; Nasir, I.A.; Rashid, B.; Husnain, T.; Henry, R.J. Target prediction of candidate miRNAs from Oryza sativa for silencing the RYMV genome. Comput. Biol. Chem. 2019, 83, 107127. [Google Scholar] [CrossRef]
- Tang, J.; Chu, C. MicroRNAs in crop improvement: Fine-tuners for complex traits. Nat. Plants 2017, 3, 17077. [Google Scholar] [CrossRef]
- Pinto, Y.M.; Kok, R.A.; Baulcombe, D.C. Resistance to rice yellow mottle virus (RYMV) in cultivated African rice varieties containing RYMV transgenes. Nat. Biotechnol. 1999, 17, 702–707. [Google Scholar] [CrossRef]
- Kisimoto, R.; Yamada, Y. A planthopper-rice virus epidemiology model: Rice stripe and small brown planthopper, Laodelphax striatellus Fallén. In Plant Virus Epidemics: Monitoring Modelling and Predicting Outbreaks; Academic Press: Sydney, Australia, 1986. [Google Scholar]
- Rees, A.R. Effect of Heat-Treatment for Virus Attenuation on Tomato Seed Viability. J. Hortic. Sci. 1970, 45, 33–40. [Google Scholar] [CrossRef]
- Koch, E.; Roberts, S.J. Non-chemical Seed Treatment in the Control of Seed-Borne Pathogens. In Global Perspectives on the Health of Seeds and Plant Propagation Material; Gullino, M.L., Munkvold, G., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 105–123. [Google Scholar]
- Ammar, E.-D.; Tsai, C.-W.; Whitfield, A.E.; Redinbaugh, M.G.; Hogenhout, S.A. Cellular and molecular aspects of rhabdovirus interactions with insect and plant hosts. Annu. Rev. Entomol. 2009, 54, 447–468. [Google Scholar] [CrossRef]
- Mrkvová, M.; Hančinský, R.; Predajňa, L.; Alaxin, P.; Achs, A.; Tomašechová, J.; Šoltys, K.; Mihálik, D.; Olmos, A.; Ruiz-García, A.B. High-throughput sequencing discloses the Cucumber mosaic virus (CMV) diversity in Slovakia and reveals new hosts of CMV from the Papaveraceae Family. Plants 2022, 11, 1665. [Google Scholar] [CrossRef]
- Sun, F.; Xu, Q.; Chen, Z.; Fan, Y.; Zhou, Y. Advances in rice black-streaked dwarf disease in China. Jiangsu J. Agric. Sci. 2013, 29, 195–201. [Google Scholar]
- Chalupniková, J.; Kundu, J.K.; Singh, K.; Bartaková, P.; Beoni, E. Wheat streak mosaic virus: Incidence in field crops, potential reservoir within grass species and uptake in winter wheat cultivars. J. Integr. Agric. 2017, 16, 523–531. [Google Scholar] [CrossRef]
- Kormelink, R.; Garcia, M.L.; Goodin, M.; Sasaya, T.; Haenni, A.-L. Negative-strand RNA viruses: The plant-infecting counterparts. Virus Res. 2011, 162, 184–202. [Google Scholar] [CrossRef] [PubMed]
- Maliano, M.R.; Macedo, M.A.; Rojas, M.R.; Gilbertson, R.L. Weed-infecting viruses in a tropical agroecosystem present different threats to crops and evolutionary histories. PLoS ONE 2021, 16, e0250066. [Google Scholar] [CrossRef]
- Miyake, T.; Haruyama, H.; Ogura, T.; Mitsui, T.; Sakurai, A. Effects of insect juvenile hormone active NC-170 on metamorphosis, oviposition and embryogenesis in leafhoppers. J. Pestic. Sci. 1991, 16, 441–448. [Google Scholar] [CrossRef]
- Ooi, A.C. Common insect pests of rice and their natural biological control. J. Agric. Sci. 2015, 1, 49–59. [Google Scholar]
- Litsinger, J.A. A farming systems approach to insect pest management for upland and lowland rice farmers in tropical Asia. In Crop Protection Strategies for Subsistence Farmers; CRC Press: Boca Raton, FL, USA, 2019; pp. 45–101. [Google Scholar]
- Rashidi, M.; Cruzado, R.K.; Hutchinson, P.J.S.; Bosque-Pérez, N.A.; Marshall, J.M.; Rashed, A. Grassy weeds and corn as potential sources of barley yellow dwarf virus spread into winter wheat. Plant Dis. 2021, 105, 444–449. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.; Zhou, G.; Zhang, T. Engineering plant virus resistance: From RNA silencing to genome editing strategies. Plant Biotechnol. J. 2020, 18, 328–336. [Google Scholar] [CrossRef]
- Chen, J.; Luo, X.; Chen, Y.; Wang, Y.; Peng, J.; Xing, Z. Recent research progress: Discovery of anti-plant virus agents based on natural scaffold. Front. Chem. 2022, 10, 926202. [Google Scholar] [CrossRef]
- Aragão, F.J.L.; Faria, J.C. First transgenic geminivirus-resistant plant in the field. Nat. Biotechnol. 2009, 27, 1086–1088. [Google Scholar] [CrossRef]
- Ganesan, U.; Suri, S.S.; Rajasubramaniam, S.; Rajam, M.V.; Dasgupta, I. Transgenic expression of coat protein gene of Rice tungro bacilliform virus in rice reduces the accumulation of viral DNA in inoculated plants. Virus Genes 2009, 39, 113–119. [Google Scholar] [CrossRef]
- Verma, V.; Sharma, S.; Devi, S.V.; Rajasubramaniam, S.; Dasgupta, I. Delay in virus accumulation and low virus transmission from transgenic rice plants expressing Rice tungro spherical virus RNA. Virus Genes 2012, 45, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Valarmathi, P.; Kumar, G.; Robin, S.; Manonmani, S.; Dasgupta, I.; Rabindran, R. Evaluation of virus resistance and agronomic performance of rice cultivar ASD 16 after transfer of transgene against Rice tungro bacilliform virus by backcross breeding. Virus Genes 2016, 52, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Jin, D.; Zhang, L.; Lu, G.; Ji, Y.; Zhou, Y.; Wang, Y.; Li, S. A rice plant expressing viral glycoprotein NSvc2-NS reduces the transmission of rice stripe virus by the small brown planthopper. Pest Manag. Sci. 2022, 78, 5325–5333. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Wang, Y.; Li, J.; Zhang, Y.; Chen, K.; Liang, Z.; Zhang, K.; Liu, J.; Xi, J.J.; Qiu, J.-L. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat. Biotechnol. 2013, 31, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Paul, N.C.; Park, S.-W.; Liu, H.; Choi, S.; Ma, J.; MacCready, J.S.; Chilvers, M.I.; Sang, H. Plant and fungal genome editing to enhance plant disease resistance using the CRISPR/Cas9 system. Front. Plant Sci. 2021, 12, 700925. [Google Scholar] [CrossRef]
- Macovei, A.; Sevilla, N.R.; Cantos, C.; Jonson, G.B.; Slamet-Loedin, I.; Čermák, T.; Voytas, D.F.; Choi, I.-R.; Chadha-Mohanty, P. Novel alleles of rice eIF4G generated by CRISPR/Cas9-targeted mutagenesis confer resistance to Rice tungro spherical virus. Plant Biotechnol. J. 2018, 16, 1918–1927. [Google Scholar] [CrossRef]
- Kis, A.; Hamar, É.; Tholt, G.; Bán, R.; Havelda, Z. Creating highly efficient resistance against wheat dwarf virus in barley by employing CRISPR/Cas9 system. Plant Biotechnol. J. 2019, 17, 1004. [Google Scholar] [CrossRef]
- Ji, X.; Zhang, H.; Zhang, Y.; Wang, Y.; Gao, C. Establishing a CRISPR–Cas-like immune system conferring DNA virus resistance in plants. Nat. Plants 2015, 1, 15144. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Y.; Ye, J.; Cao, X.; Xu, C.; Chen, B.; An, H.; Jiao, Y.; Zhang, F.; Yang, X.; et al. Establishing CRISPR/Cas13a immune system conferring RNA virus resistance in both dicot and monocot plants. Plant Biotechnol. J. 2019, 17, 1185–1187. [Google Scholar] [CrossRef]
- Muha-Ud-Din, G.; Ali, F.; Hameed, A.; Naqvi, S.A.H.; Nizamani, M.M.; Jabran, M.; Sarfraz, S.; Yong, W. CRISPR/Cas9-based genome editing: A revolutionary approach for crop improvement and global food security. Physiol. Mol. Plant Pathol. 2024, 129, 102191. [Google Scholar] [CrossRef]
- Van Esse, H.P.; Reuber, T.L.; van der Does, D. Genetic modification to improve disease resistance in crops. New Phytol. 2020, 225, 70–86. [Google Scholar] [CrossRef]
- McDonald, B.A.; Stukenbrock, E.H. Rapid emergence of pathogens in agro-ecosystems: Global threats to agricultural sustainability and food security. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20160026. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Thrall, P.H.; Papaïx, J.; Xie, L.; Burdon, J.J. Playing on a pathogen’s weakness: Using evolution to guide sustainable plant disease control strategies. Annu. Rev. Phytopathol. 2015, 53, 19–43. [Google Scholar] [CrossRef] [PubMed]
- Al-Ani, R.A.; Adhab, M.A.; El-Muadhidi, M.A.; Al-Fahad, M.A. Induced systemic resistance and promotion of wheat and barley plants growth by biotic and non-biotic agents against barley yellow dwarf virus. Afr. J. Biotechnol. 2011, 10, 12078–12084. [Google Scholar]
- Yang, J.G.; Dang, Y.G.; Li, G.Y.; Guo, L.J.; Wang, W.T.; Tan, Q.W.; Lin, Q.Y.; Wu, Z.J.; Xie, L.H. Note: Anti-viral activity of Ailanthus altissima crude extract on Rice stripe virus in rice suspension cells. Phytoparasitica 2008, 36, 405–408. [Google Scholar] [CrossRef]
- Farooq, T.; Adeel, M.; He, Z.; Umar, M.; Shakoor, N.; da Silva, W.; Elmer, W.; White, J.C.; Rui, Y. Nanotechnology and Plant Viruses: An Emerging Disease Management Approach for Resistant Pathogens. ACS Nano 2021, 15, 6030–6037. [Google Scholar] [CrossRef]
- Dutta, P.; Kumari, A.; Mahanta, M.; Biswas, K.K.; Dudkiewicz, A.; Thakuria, D.; Abdelrhim, A.S.; Singh, S.B.; Muthukrishnan, G.; Sabarinathan, K.G.; et al. Advances in Nanotechnology as a Potential Alternative for Plant Viral Disease Management. Front. Microbiol. 2022, 13, 935193. [Google Scholar] [CrossRef]
- Kora, A.J.; Mounika, J.; Jagadeeshwar, R. Rice leaf extract synthesized silver nanoparticles: An in vitro fungicidal evaluation against Rhizoctonia solani, the causative agent of sheath blight disease in rice. Fungal Biol. 2020, 124, 671–681. [Google Scholar] [CrossRef]
- Jaithon, T.; Atichakaro, T.; Phonphoem, W.; Jiraroj, T.; Sreewongchai, T.; T-Thienprasert, N.P. Potential usage of biosynthesized zinc oxide nanoparticles from mangosteen peel ethanol extract to inhibit Xanthomonas oryzae and promote rice growth. Heliyon 2024, 10, e24076. [Google Scholar] [CrossRef]
- Mankad, M.; Patil, G.; Patel, D.; Patel, P.; Patel, A. Comparative studies of sunlight mediated green synthesis of silver nanoparaticles from Azadirachta indica leaf extract and its antibacterial effect on Xanthomonas oryzae pv. oryzae. Arab. J. Chem. 2020, 13, 2865–2872. [Google Scholar] [CrossRef]
- Ito, M.; Oh, J.S.; Ohta, T.; Shiratani, M.; Hori, M. Current status and future prospects of agricultural applications using atmospheric-pressure plasma technologies. Plasma Process. Polym. 2018, 15, 1700073. [Google Scholar] [CrossRef]
- Sakudo, A.; Yagyu, Y.; Onodera, T. Disinfection and sterilization using plasma technology: Fundamentals and future perspectives for biological applications. Int. J. Mol. Sci. 2019, 20, 5216. [Google Scholar] [CrossRef] [PubMed]
- Hanbal, S.E.; Takashima, K.; Miyashita, S.; Ando, S.; Ito, K.; Elsharkawy, M.M.; Kaneko, T.; Takahashi, H. Atmospheric-pressure plasma irradiation can disrupt tobacco mosaic virus particles and RNAs to inactivate their infectivity. Arch. Virol. 2018, 163, 2835–2840. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Thrall, P.H.; Burdon, J.J. Achieving sustainable plant disease management through evolutionary principles. Trends Plant Sci. 2014, 19, 570–575. [Google Scholar] [CrossRef]

| Sr. No | Protein Name | Protein ID | Gene ID | Amino Acids | CDS (bp) | Chr. Start–End Point | Description | Putative Functions | References |
|---|---|---|---|---|---|---|---|---|---|
| 1 | L protein | YP_009553369.1 | NC_040786.1 | 2066 | 6201 | 6278–12,478 | RSMV | Large subunit of polymerase (L7), capping mRNA 5′ end, RNA binding, polyadenylation | [27,57] |
| 2 | P6 protein | APR74653.1 | NC_040786.1 | 66 | 201 | 6005–6205 | RSMV | Non-structural proteins, unknown function | [27] |
| 3 | G protein | APR74652.1 | NC_040786.1 | 536 | 1611 | 4376–5986 | RSMV | Glycoprotein (P5 protein), assemble viral particles and directly interact with M protein, promote viral budding | [13,27] |
| 4 | NC protein | YP_009553363.1 | NC_040786.1 | 491 | 1476 | 90–1565 | RSMV | Nucleocapsid, tightly bind to viral gRNA to prevent its cleavage by host cell nucleases | [27,58] |
| 5 | P protein | YP_009553364.1 | NC_040786.1 | 375 | 1128 | 1669–2796 | RSMV | Phosphoprotein, cofactor for viral polymerase, mediates correct positioning and connection of the L protein on the N-RNA template. | [19,27] |
| 6 | M protein | YP_009553366.1 | NC_040786.1 | 174 | 525 | 3730–4254 | RSMV | Silencing suppressor, matrix protein (M), affect host antiviral RNA silencing functions | [19,27] |
| 7 | P3 protein | YP_009553365.1 | NC_040786.1 | 177 | 534 | 3006–3539 | RSMV | Cell-to-cell movement, nuclear localization signals | [4,24,27] |
| 8 | G protein | KP205452.2 | NC_028237.2 | 564 | 1449 | 5529–7223 | ADV | P5 protein, assemble viral particles and directly interact with M protein | [59] |
| 9 | P protein | YP_009177016.1 | NC_028237.2 | 311 | 936 | 2060–2995 | ADV | Putative movement protein | [59] |
| 10 | NC protein | YP_009177015.2 | NC_028237.2 | 482 | 1449 | 175–1623 | ADV | Nucleocapsid | [59] |
| 11 | L protein | YP_009177231.1 | NC_028244.1 | 2056 | 6171 | 6176–12,346 | BYSMV | L protein of Cytorhabdovirus hordei | [60] |
| 12 | G protein | YP_009177229.1 | NC_028244.1 | 478 | 1437 | 4504–5940 | BYSMV | Form trans-membrane spikes | [60,61] |
| 13 | M protein | YP_009177228.1 | NC_028244.1 | 166 | 501 | 3916–4416 | BYSMV | Virus assembly, bridge between nucleocapsids and the envelope | [60] |
| 14 | NC protein | ALU34429.1 | NC_034551.1 | 422 | 1269 | 178–1446 | CBDaV | Nucleocapsid, tightly bind to viral gRNA to prevent its cleavage by host cell nucleases | [62] |
| 15 | Glycoprotein | ALU34428.1 | NC_034551.1 | 503 | 1512 | 3995–5506 | CBDaV | Glycoprotein of Colocasia bobone disease virus | [62] |
| 16 | P protein | ALU34424.1 | NC_034551.1 | 280 | 843 | 1624–2466 | CBDaV | Phosphoprotein, cofactor for viral polymerase | [62] |
| 17 | ORF5 | AHH44829.1 | NC_038756.1 | 534 | 1605 | 4739–6343 | CoRSV | [63] | |
| 18 | NC protein | AHH44825.1 | NC_038756.1 | 446 | 1341 | 105–1445 | CoRSV | Nucleocapsid, tightly bind to viral gRNA | [63] |
| 19 | ORF3 | AHH44827.1 | NC_038756.1 | 328 | 987 | 2646–3632 | CoRSV | [63] | |
| 20 | L protein | YP_002308576.1 | NC_011558.1 | 2040 | 6123 | 339–6461 | LBVaV | Large subunit of polymerase (L7), RNA binding | [64] |
| 21 | Coat protein | YP_002317202.1 | NC_011568.1 | 397 | 1194 | 4–1433 | LBVaV | [64] | |
| 22 | Glycoprotein | YP_009176976.1 | NC_028231.1 | 630 | 1893 | 4662–6554 | DYVV | Identified in Datura yellow vein nucleorhabdovirus, targeted to the endoplasmic reticulum | [65] |
| 23 | P protein | YP_009176973.1 | NC_028231.1 | 327 | 984 | 1578–2561 | DYVV | Phosphoprotein, cofactor for viral polymerase | [65] |
| 24 | NC protein | YP_009176972.1 | NC_028231.1 | 450 | 1353 | 165–1517 | DYVV | Nucleocapsid, tightly bind to viral gRNA | [65] |
| 25 | X protein | YP_009094356.1 | NC_025389.1 | 251 | 756 | 1825–2118 | EMDV | [66] | |
| 26 | G protein | YP_009094357.1 | NC_025389.1 | 615 | 1848 | 5185–7032 | EMDV | P5 protein, assemble viral particles | [66] |
| 27 | NC protein | YP_009094352.1 | NC_025389.1 | 476 | 1431 | 282–1712 | EMDV | Tightly bind to viral gRNA to prevent its cleavage | [66] |
| 28 | 4b protein | YP_425089.1 | NC_007642.1 | 302 | 909 | 2720–3765 | LNYV | [67] | |
| 29 | G protein | YP_425091.1 | NC_007642.1 | 551 | 1656 | 4412–6247 | LNYV | Assemble viral particles and interact with M protein | [67] |
| 30 | P protein | YP_425088.1 | NC_007642.1 | 300 | 903 | 1631–2712 | LNYV | Phosphoprotein, cofactor for viral polymerase | [67] |
| 31 | L protein | YP_002308376.1 | NC_011532.1 | 2068 | 6207 | 6461–12,667 | LYMoV | RNA binding, polyadenylation | [68] |
| 32 | NC protein | YP_002308371.1 | NC_011532.1 | 452 | 1359 | 164–1522 | LYMoV | Prevent cleavage by host cell nucleases | [68] |
| 33 | G protein | YP_002308375.1 | NC_011532.1 | 548 | 1647 | 4531–6177 | LYMoV | P5 protein, directly interact with M protein | [68] |
| 34 | L protein | YP_052849.1 | NC_005974.1 | 1944 | 5835 | 7657–13,633 | MFSV | Capping mRNA 5’ end, RNA binding, polyadenylation | [69] |
| 35 | NC protein | YP_052843.1 | NC_005974.1 | 462 | 1389 | 254–1642 | MFSV | Nucleocapsid, tightly bind to viral gRNA to prevent its cleavage by host cell nucleases | [69] |
| 36 | G protein | YP_052848.1 | NC_005974.1 | 596 | 1791 | 5675–7652 | MFSV | Assemble viral particles and interact with M protein | [69] |
| 37 | G protein | YP_009444712.1 | NC_036390.1 | 594 | 1785 | 4580–6364 | MIMV | Assemble viral particles and interact with M protein | [70] |
| 38 | NC protein | YP_009444708.1 | NC_036390.1 | 445 | 1338 | 210–1547 | MIMV | Nucleocapsid | [70] |
| 39 | M protein | YP_009444711.1 | NC_036390.1 | 233 | 702 | 3770–4471 | MIMV | Silencing suppressor, matrix protein (M) | [70] |
| 40 | L protein | YP_052855.1 | NC_005975.1 | 1922 | 5769 | 6244–12,039 | MMV | Large subunit of polymerase (L7), capping mRNA 5′ end, RNA binding, polyadenylation | [71] |
| 41 | G protein | YP_052854.1 | NC_005975.1 | 591 | 1776 | 4349–6240 | MMV | P5 protein, assemble viral particles and directly interact with M protein | [71] |
| 42 | NC protein | YP_052850.1 | NC_005975.1 | 447 | 1344 | 130–1681 | MMV | Nucleocapsid | [71] |
| 43 | G protein | NP_057961.1 | NC_002251.1 | 463 | 1452 | 4996–6447 | NCMV | P5 protein, assemble viral particles | [72] |
| 44 | P protein | NP_057955.1 | NC_002251.1 | 286 | 861 | 1568–2428 | NCMV | Phosphoprotein, cofactor for viral polymerase | [72] |
| 45 | NC protein | NP_057954.1 | NC_002251.1 | 265 | 1296 | 142–1437 | NCMV | Nucleocapsid, tightly bind to viral gRNA to prevent its cleavage by host cell nucleases | [72] |
| 46 | G protein | YP_001294928.1 | NC_009608.1 | 542 | 1629 | 4549–6265 | OFV | P5 protein, assemble viral particles and directly interact with M protein | [73] |
| 47 | ORF3 protein | YP_001294926.1 | NC_009608.1 | 335 | 1008 | 2597–3772 | OFV | [73] | |
| 48 | NC protein | YP_001294924.1 | NC_009608.1 | 450 | 1353 | 163–1651 | OFV | Prevent its cleavage by host cell nucleases | [73] |
| 49 | G protein | ADE45273.1 | NC_016136.1 | 607 | 1824 | 4953–6844 | PYDV | Assemble viral particles and interact with M protein | [74] |
| 50 | M protein | ADE45272.1 | NC_016136.1 | 253 | 762 | 4051–4948 | PYDV | Silencing suppressor, matrix protein (M), affect host antiviral RNA silencing functions | [74] |
| 51 | NC protein | ADE45268.1 | NC_016136.1 | 472 | 1419 | 154–1671 | PYDV | Nucleocapsid, tightly bind to viral gRNA | [74] |
| 52 | G protein | NP_620500.1 | NC_003746.1 | 669 | 2010 | 5127–7284 | RYSV | Assemble viral particles and interact with M protein | [75] |
| 53 | M protein | NP_620499.1 | NC_003746.1 | 262 | 789 | 4209–5121 | RYSV | Affect host antiviral RNA silencing functions | [75] |
| 54 | NC protein | NP_620496.1 | NC_003746.1 | 521 | 1566 | 207–1920 | RYSV | Nucleocapsid, tightly bind to viral gRNA | [75] |
| 55 | Large protein | NP_620502.1 | NC_003746.1 | 1967 | 5904 | 7860–13,847 | RYSV | [75] | |
| 56 | RNA polymerase | QKN84393.1 | - | 2137 | 6414 | - | RPV | [76] | |
| 57 | P4 protein | YP_009237267.1 | NC_029560.1 | 373 | 1122 | 473–1594 | RLBV | Phosphoprotein, cofactor for viral polymerase | [77] |
| 58 | RdRp protein | YP_009058929.1 | NC_024906.1 | 477 | 1434 | 8417–9850 | RLBV | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mas-ud, M.A.; Chowdhury, M.R.; Juthee, S.A.; Rabbee, M.F.; Matin, M.N.; Kang, S.G. Unravelling the Current Status of Rice Stripe Mosaic Virus: Its Geographical Spread, Biology, Epidemiology, and Management. Agronomy 2024, 14, 2442. https://doi.org/10.3390/agronomy14102442
Mas-ud MA, Chowdhury MR, Juthee SA, Rabbee MF, Matin MN, Kang SG. Unravelling the Current Status of Rice Stripe Mosaic Virus: Its Geographical Spread, Biology, Epidemiology, and Management. Agronomy. 2024; 14(10):2442. https://doi.org/10.3390/agronomy14102442
Chicago/Turabian StyleMas-ud, Md. Atik, Md. Rayhan Chowdhury, Sadiya Arefin Juthee, Muhammad Fazle Rabbee, Mohammad Nurul Matin, and Sang Gu Kang. 2024. "Unravelling the Current Status of Rice Stripe Mosaic Virus: Its Geographical Spread, Biology, Epidemiology, and Management" Agronomy 14, no. 10: 2442. https://doi.org/10.3390/agronomy14102442
APA StyleMas-ud, M. A., Chowdhury, M. R., Juthee, S. A., Rabbee, M. F., Matin, M. N., & Kang, S. G. (2024). Unravelling the Current Status of Rice Stripe Mosaic Virus: Its Geographical Spread, Biology, Epidemiology, and Management. Agronomy, 14(10), 2442. https://doi.org/10.3390/agronomy14102442











