Enhanced Plant Growth Through Composite Inoculation of Phosphate-Solubilizing Bacteria: Insights from Plate and Soil Experiments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Soil
2.3. Screening of PSB Combinations
2.4. Plate Experiment
2.5. Soil Test
2.6. Statistical Analysis
3. Results
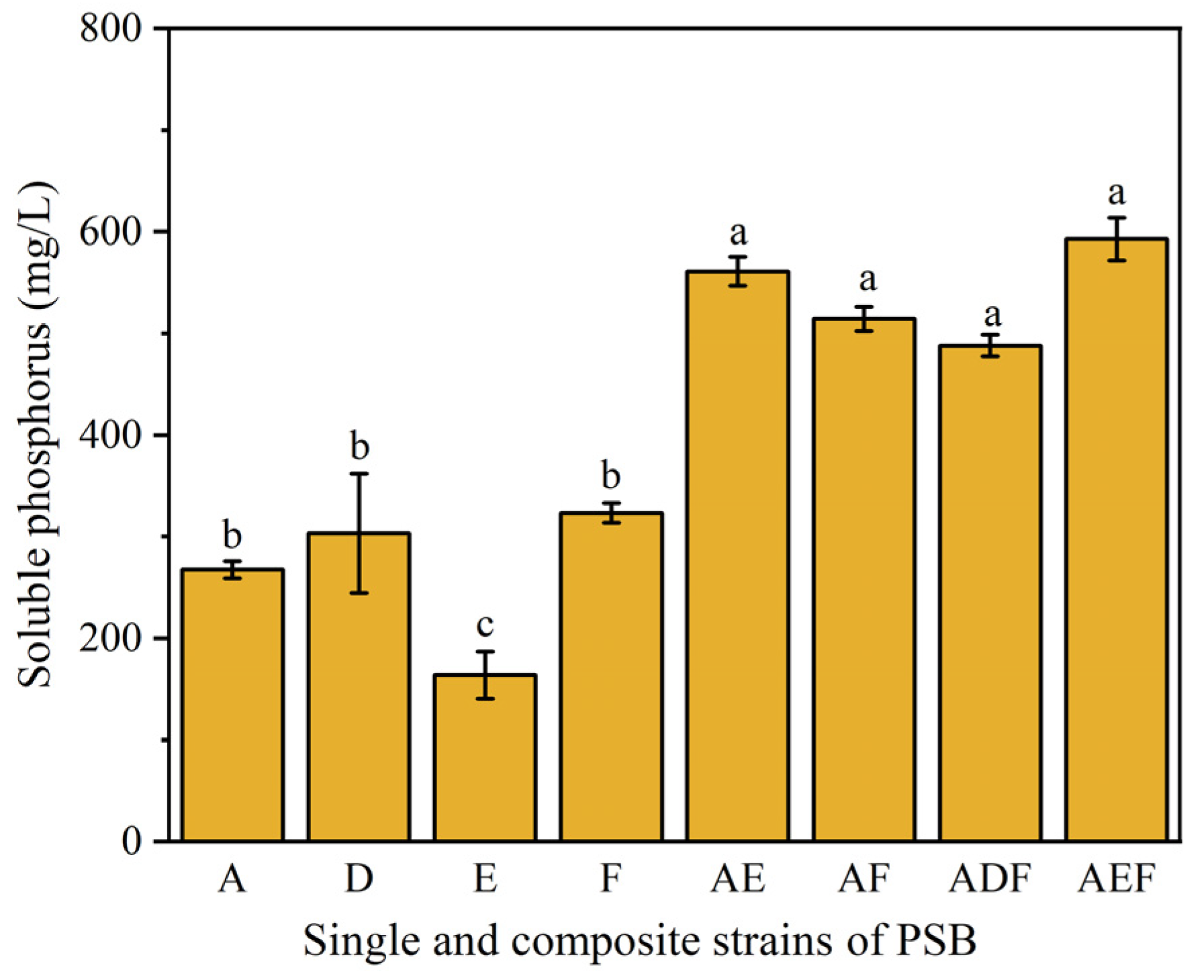
3.1. Screening of PSB Combinations and Evaluation of Their Phosphate-Solubilizing Capacities
3.2. Growth-Promoting Effects of Composite Strains in Plate Experiment
3.3. Growth-Promoting Effects of Composite Strains in Soil Test
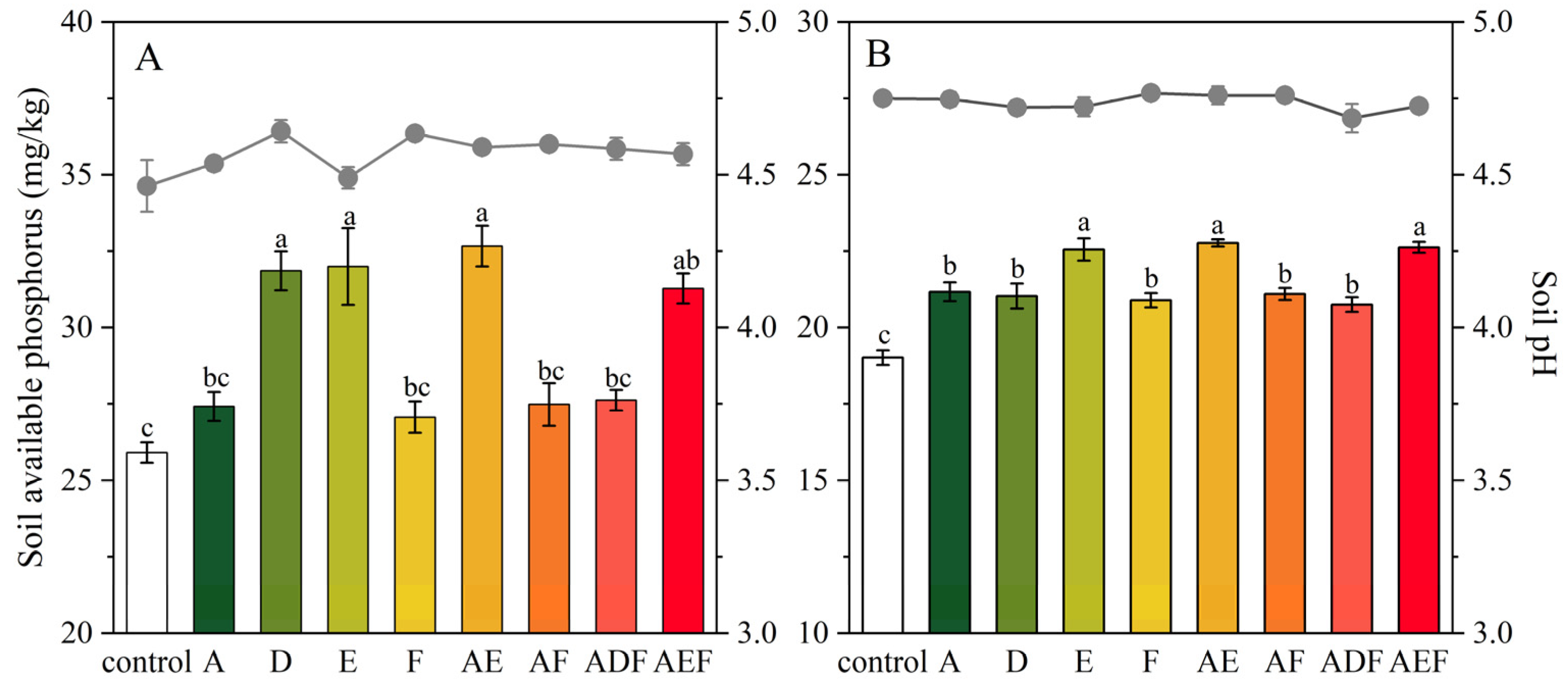
3.3.1. Soil-Available Phosphorus Content and pH
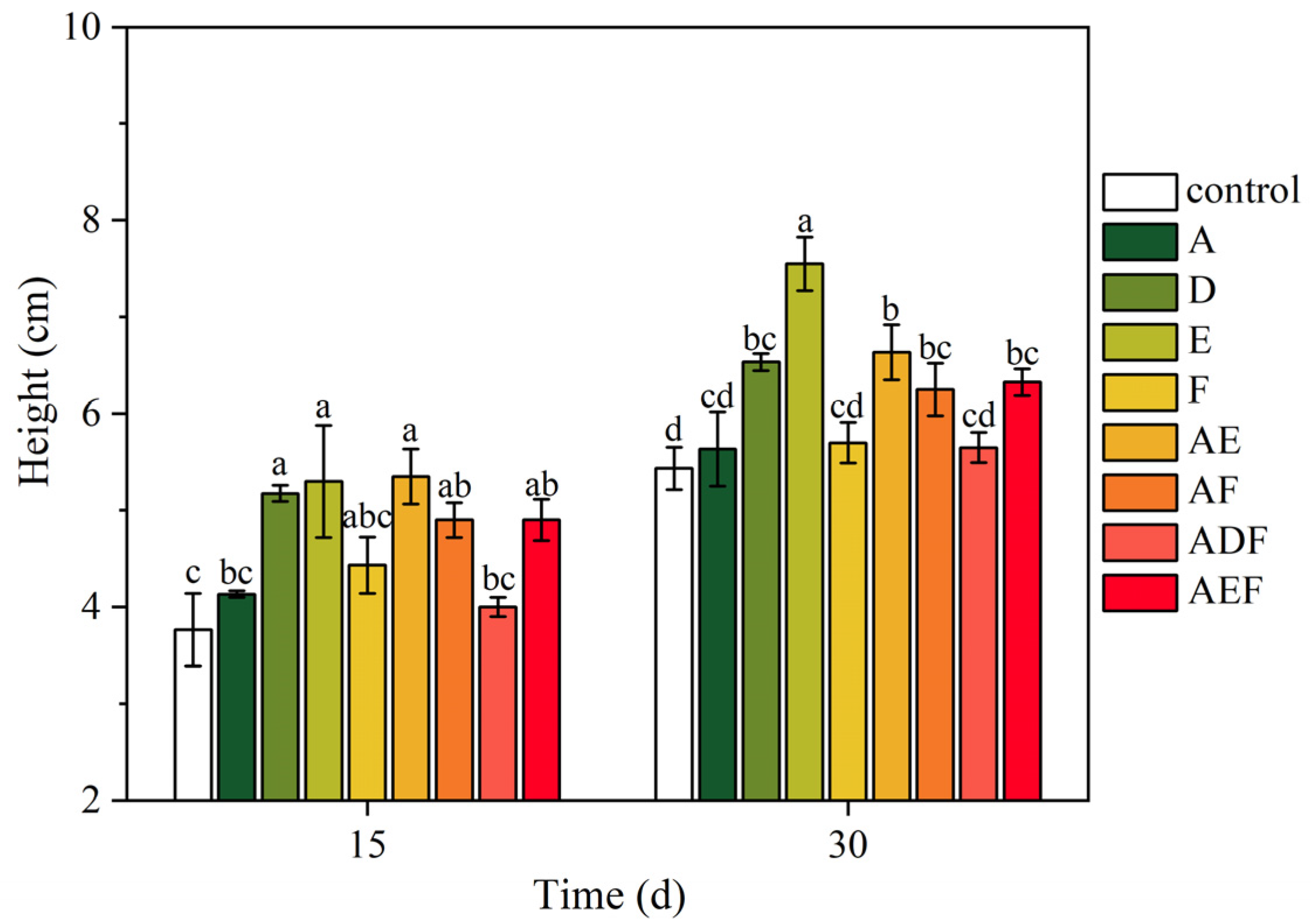
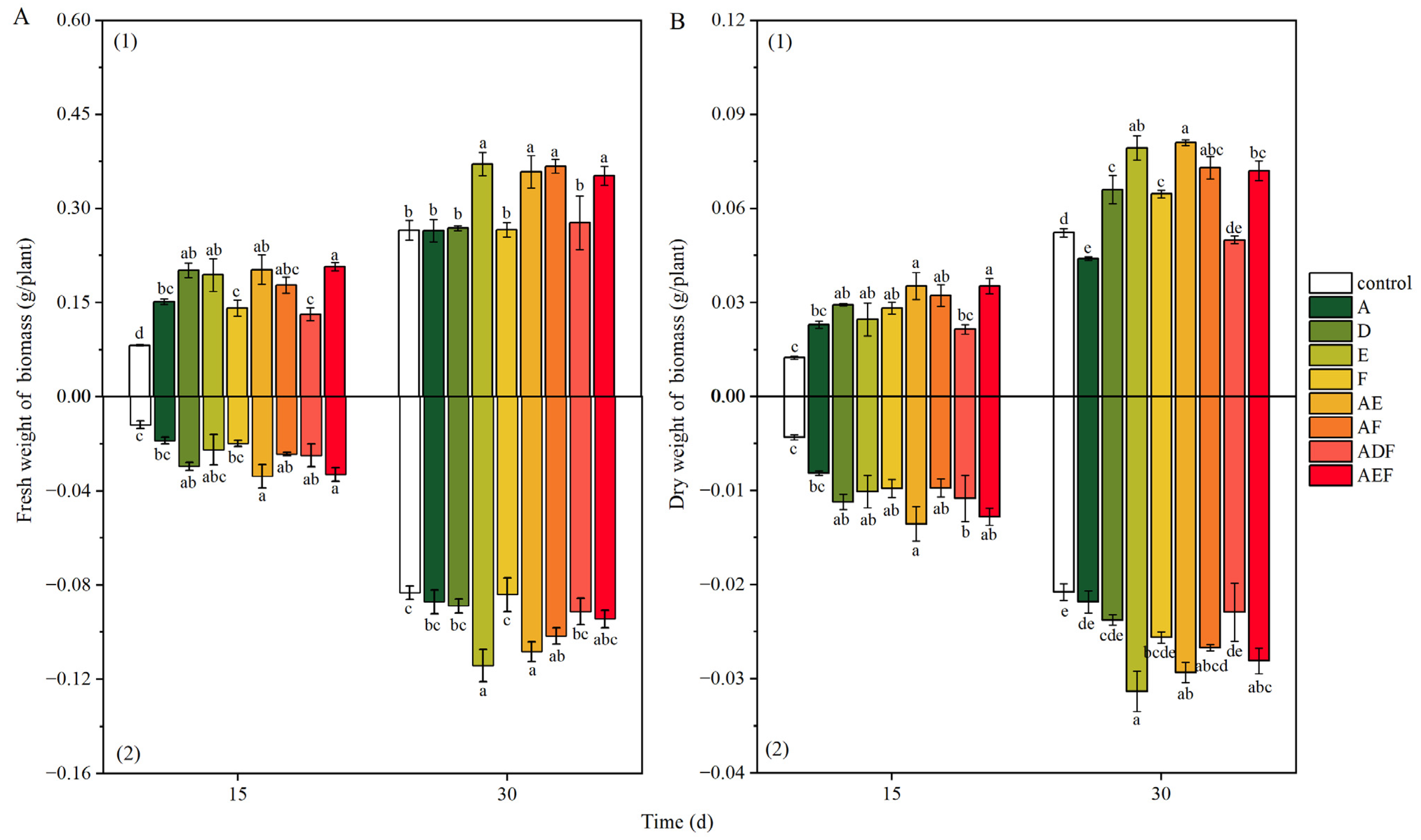
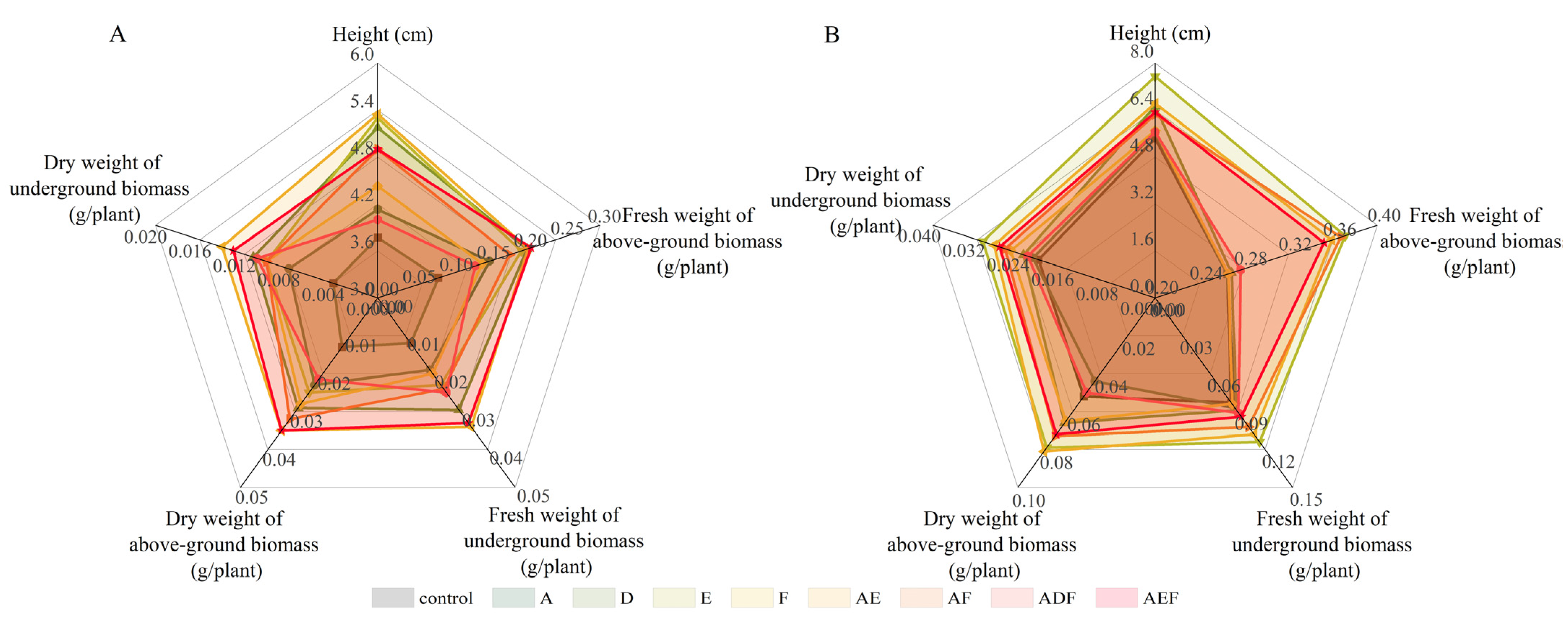
3.3.2. Growth Status of Oilseed Rape
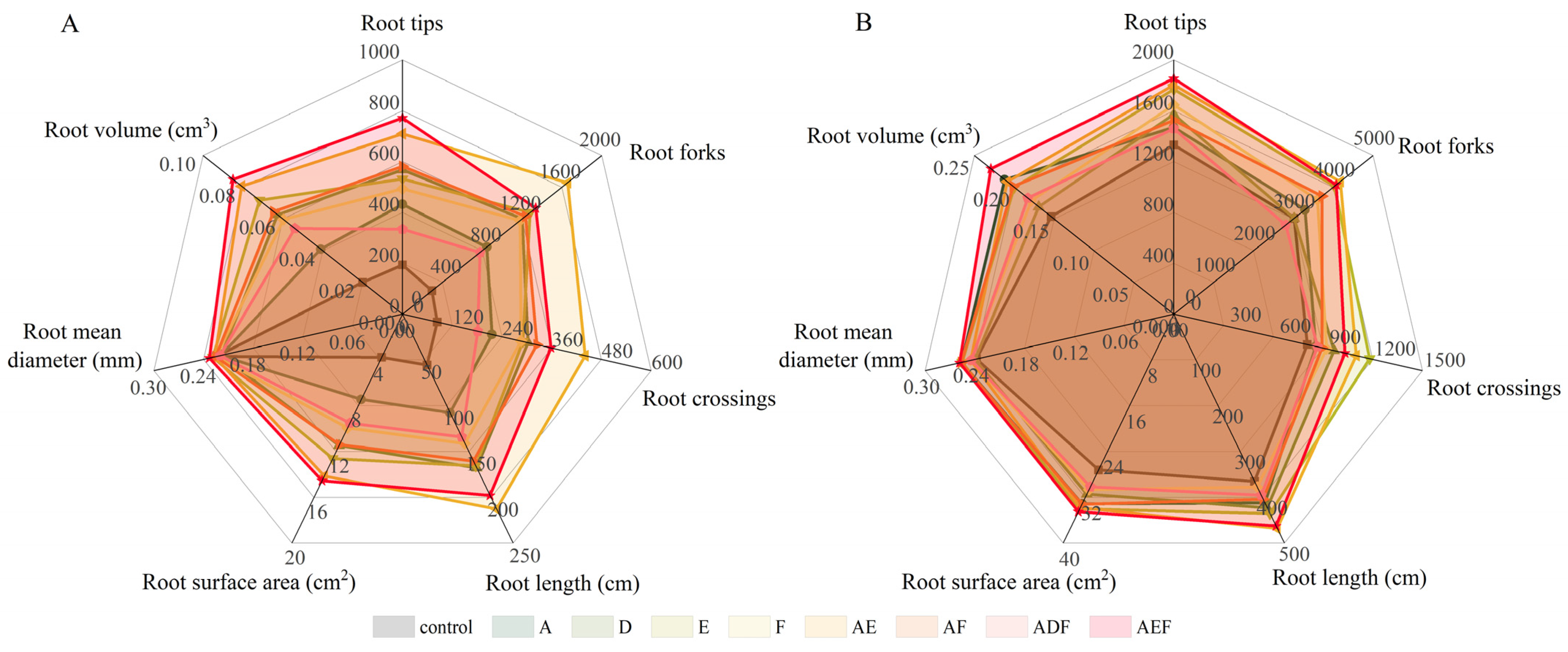
3.3.3. Morphology and Structure of Plant Roots
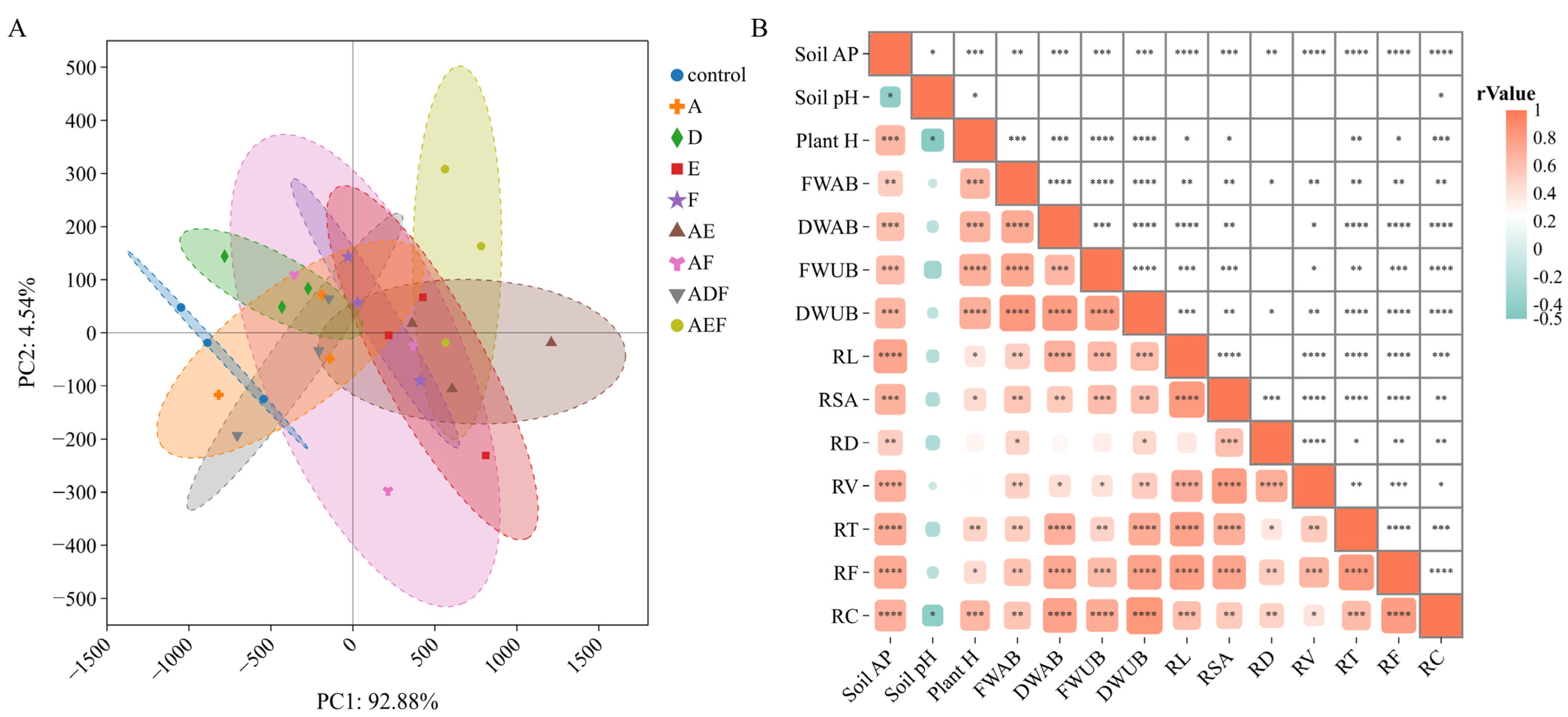
3.4. Comprehensive Analysis of the Effects and Mechanisms of Different Treatments on Oilseed Rape Cultivation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, J.; Li, M.; Whelan, M. Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: A review. Sci. Total Environ. 2018, 612, 522–537. [Google Scholar] [CrossRef] [PubMed]
- Billah, M.; Khan, M.; Bano, A.; Hassan, T.U.; Munir, A.; Gurmani, A.R. Phosphorus and phosphate solubilizing bacteria: Keys for sustainable agriculture. Geomicrobiol. J. 2019, 36, 904–916. [Google Scholar] [CrossRef]
- Divjot, K.; Rana, K.L.; Tanvir, K.; Yadav, N.; Yadav, A.N.; Kumar, M.; Kumar, V.; Dhaliwal, H.S.; Saxena, A.K. Biodiversity, current developments and potential biotechnological applications of phosphorus-solubilizing and-mobilizing microbes: A review. Pedosphere 2021, 31, 43–75. [Google Scholar]
- Balemi, T.; Negisho, K. Management of soil phosphorus and plant adaptation mechanisms to phosphorus stress for sustainable crop production: A review. J. Soil Sci. Plant Nutr. 2012, 12, 547–562. [Google Scholar] [CrossRef]
- Weber, O.; Delince, J.; Duan, Y.; Maene, L.; McDaniels, T.; Mew, M.; Schneidewind, U.; Steiner, G. Trade and finance as cross-cutting issues in the global phosphate and fertilizer market. In Sustainable Phosphorus Management; Scholz, R., Roy, A., Brand, F., Hellums, D., Ulrich, A., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 275–299. [Google Scholar]
- Chowdhury, R.B.; Moore, G.A.; Weatherley, A.J.; Arora, M. Key sustainability challenges for the global phosphorus resource, their implications for global food security, and options for mitigation. J. Clean. Prod. 2017, 140, 945–963. [Google Scholar] [CrossRef]
- Guppy, C.N.; Menzies, N.W.; Moody, P.W.; Blamey, F.P.C. Competitive sorption reactions between phosphorus and organic matter in soil: A review. Aust. J. Soil Res. 2005, 43, 189–202. [Google Scholar] [CrossRef]
- Kaminsky, L.M.; Thompson, G.L.; Trexler, R.V.; Bell, T.H.; Kao-Kniffin, J. Medicago sativa has Reduced Biomass and Nodulation When Grown with Soil Microbiomes Conditioned to High Phosphorus Inputs. Phytobiomes J. 2018, 2, 237–248. [Google Scholar] [CrossRef]
- Li, Z.R.; Sheng, Y.Q.; Yang, J.; Burton, E.D. Phosphorus release from coastal sediments: Impacts of the oxidation-reduction potential and sulfide. Mari. Pollu. Bull. 2016, 113, 176–181. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A.; Wani, P.A. Role of phosphate-solubilizing microorganisms in sustainable agriculture—A review. Agron. Sustain. Dev. 2007, 27, 29–43. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y.F.; Wu, K.B.; Hu, M.P.; Wu, H.; Chen, D.J. National estimates of environmental thresholds for upland soil phosphorus in China based on a metaanalysis. Sci. Total Environ. 2021, 780, 146677. [Google Scholar] [CrossRef]
- Chen, A.; Arai, Y.J. A review of the reactivity of phosphatase controlled by clays and clay minerals: Implications for under-standing phosphorus mineralization in soils. Clays Clay Miner. 2023, 71, 119–142. [Google Scholar] [CrossRef]
- Liu, M.; Liu, J.; Chen, X.F.; Jiang, C.Y.; Wu, M.; Li, Z.P. Shifts in bacterial and fungal diversity in a paddy soil faced with phosphorus surplus. Biol. Fertil. Soils 2018, 54, 259–267. [Google Scholar] [CrossRef]
- Ma, M.C.; Zhou, J.; Ongena, M.; Liu, W.Z.; Wei, D.; Zhao, B.S.; Guan, D.W.; Jiang, X.; Li, J. Effect of long-term fertilization strategies on bacterial community composition in a 35-year field experiment of Chinese Mollisols. AMB Express 2018, 8, 20. [Google Scholar] [CrossRef]
- Li, H.; Huang, G.; Meng, Q.; Ma, L.; Yuan, L.; Wang, F.; Zhang, W.; Cui, Z.; Shen, J.; Chen, X.; et al. Integrated soil and plant phosphorus management for crop and environment in china. a review. Plant Soil 2011, 349, 157–167. [Google Scholar] [CrossRef]
- Zhang, F.S.; Huang, C.D.; Shen, J.B.; Wei, C.Z.; Ma, W.Q.; Lv, Y.; Lu, Z.Y.; Zhu, Q.C.; Shi, X.J.; Hou, C.H.; et al. Green Intelligent Fertilizer: New Insight into Making Full Use of Mineral Nutrient Resources and Industrial Approach. Acta Petrol. Sin. 2023, 60, 1203–1212. [Google Scholar]
- Zou, T.; Zhang, X.; Davidson, E.A. Global trends of cropland phosphorus use and sustainability challenges. Nature 2022, 611, 81–87. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Yu, R.P.; Xing, Y.; Zhang, J.D.; Bao, X.G.; Lambers, H.; Li, L. Enhanced phosphorus-fertilizer-use efficiency and sustainable phosphorus management with intercropping. Agron. Sustain. Dev. 2023, 43, 57. [Google Scholar] [CrossRef]
- Liu, H.; Li, S.S.; Qiang, R.W.; Lu, E.J.; Li, C.L.; Zhang, J.J.; Gao, Q. Response of soil microbial community structure to phosphate fertilizer reduction and combinations of microbial fertilizer. Front. Environ. Sci. 2022, 10, 899727. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef]
- Yadav, A.N. Plant microbiomes for sustainable agriculture: Current research and future challenges. In Plant Microbiomes for Sustainable Agriculture; Yadav, A., Singh, J., Rastegari, A., Yadav, N., Eds.; Springer: Cham, Switzerland, 2020; Volume 25, pp. 475–482. [Google Scholar]
- Pang, F.; Li, Q.; Solanki, K.M.; Wang, Z.; Xing, Y.X.; Dong, D.F. Soil phosphorus transformation and plant uptake driven by phosphate-solubilizing microorganisms. Front. Microbiol. 2024, 15, 1383813. [Google Scholar] [CrossRef]
- Liu, J.; Qi, W.Y.; Li, Q.; Wang, S.G.; Song, C.; Yuan, X.Z. Exogenous phosphorus-solubilizing bacteria changed the rhizosphere microbial community indirectly. 3 Biotech 2020, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Aliyat, F.Z.; Maldani, M.; El Guilli, M.; Nassiri, L.; Ibijbijen, J. Phosphate solubilizing bacteria isolated from phosphate solid sludge and their ability to solubilize three inorganic phosphate forms: Calcium, iron, and aluminum phosphates. Microorganisms 2022, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.S.; Patra, J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef]
- Heydari, M.M.; Brook, R.M.; Jones, D.L. Barley Growth and Phosphorus Uptake in Response to Inoculation with Arbuscular Mycorrhizal Fungi and Phosphorus Solubilizing Bacteria. Commun. Soil Sci. Plant Anal. 2024, 55, 846–861. [Google Scholar] [CrossRef]
- Rahman, M.M.; Maqbool, N.; Ay, A.; Gülser, C.; Kizilkaya, R. Role of hazelnut husk compost and phosphate solubilizing bacteria in improving productivity and quality parameters of wheat (Triticum aestivum L.) and their effects on some soil biological properties. Commun. Soil Sci. Plant Anal. 2024, 55, 2042–2058. [Google Scholar] [CrossRef]
- Jamadar, A.M.; Kumar, B.N.A.; Potdar, M.P.; Mirajkar, K.K.; Halli, H.M.; Gurumurthy, S.; Nargund, R. Concurrent effect of phosphorus, nanoparticles and phosphorus solubilizing bacteria influences root morphology, soil enzymes and nutrients uptake in upland rice (Oryza sativa L.). J. Plant Nutr. 2024, 47, 1596–1612. [Google Scholar] [CrossRef]
- Mukherjee, S.; Sen, S.K. Exploration of novel rhizospheric yeast isolate asnfertilizing soil inoculant for improvement of maize cultivation. J. Sci. Food Agric. 2015, 95, 1491–1499. [Google Scholar] [CrossRef]
- Sundara, B.; Natarajan, V.; Hari, K. Influence of phosphorus solubilizing bacteria on the changes in soil available phosphorus and sugarcane and sugar yields. Field Crop Res. 2002, 77, 43–49. [Google Scholar] [CrossRef]
- Zhang, C.N.; Chen, H.M.; Dai, Y.; Chen, Y.; Tian, Y.X.; Huo, Z.L. Isolation and screening of phosphorus solubilizing bacteria from saline alkali soil and their potential for Pb pollution remediation. Front. Bioeng. Biotechnol. 2023, 11, 1134310. [Google Scholar] [CrossRef]
- Zineb, A.B.; Trabelsi, D.; Ayachi, I.; Barhoumi, F.; Aroca, R.; Mhamdi, R. Inoculation with elite strains of phosphate-solubilizing bacteria enhances the effectiveness of fertilization with rock phosphates. Geomicrobiol. J. 2020, 37, 22–30. [Google Scholar] [CrossRef]
- Fernández, L.A.; Zalba, P.; Gómez, M.A.; Sagardoy, M.A. Phosphate-solubilization activity of bacterial strains in soil and their effect on soybean growth under greenhouse conditions. Biol. Fertil. 2007, 43, 805–809. [Google Scholar] [CrossRef]
- Rodríguez, H.; Fraga, R.; Gonzalez, T.; Bashan, Y. Genetics of phosphate solubilization and its potential applications for improving plant growth-promoting bacteria. Plant Soil 2006, 287, 15–21. [Google Scholar] [CrossRef]
- Yu, X.; Liu, X.; Zhu, T.H.; Liu, G.H.; Mao, C. Isolation and characterization of phosphate-solubilizing bacteria from walnut and their effect on growth and phosphorus mobilization. Biol. Fertil. Soils 2011, 47, 437–446. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Wei, Z.; Xu, Y.C.; Shen, Q.R. Dissolving capacity of phosphate dissolving bacteria strains combination and their effects on corn growth. J. Plant Nutr. Fertil. 2017, 23, 262–268. [Google Scholar]
- Wang, Z.H.; Zhang, H.H.; Liu, L.; Li, S.J.; Xie, J.F.; Xue, X.; Jiang, Y. Screening of phosphate-solubilizing bacteria and their abilities of phosphorus solubilization and wheat growth promotion. BMC Microbiol. 2022, 22, 296. [Google Scholar] [CrossRef] [PubMed]
- De Zutter, N.; Ameye, M.; Bekaert, B.; Verwaeren, J.; De Gelder, L.; Audenaert, K. Uncovering new insights and misconceptions on the effectiveness of phosphate solubilizing rhizobacteria in plants: A meta-analysis. Front. Plant Sci. 2022, 13, 858804. [Google Scholar] [CrossRef]
- Elhaissoufi, W.; Ghoulam, C.; Barakat, A.; Zeroual, Y.; Bargaz, A. Phosphate bacterial solubilization: A key rhizosphere driving force enabling higher P use efficiency and crop productivity. J. Adv. Res. 2022, 38, 13–28. [Google Scholar] [CrossRef]
- Soumare, A.; Boubekri, K.; Lyamlouli, K.; Hafidi, M.; Ouhdouch, Y.; Kouisni, L. From isolation of phosphate solubilizing microbes to their formulation and use as biofertilizers: Status and needs. Front. Bioeng. Biotechnol. 2020, 7, 425. [Google Scholar] [CrossRef]
- Lu, R.K. Analytical Methods of Soil and Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 2000; pp. 1–288. [Google Scholar]
- Kowalenko, C.G.; Babuin, D. Interference problems with Phosphoantimonyl molybdenem Colorimetric Measurement of phosphorus in Soil and Plant Materials. Commun. Soil Sci. Plant Anal. 2007, 38, 1299–1316. [Google Scholar] [CrossRef]
- Yetgin, A. Exploring the dynamic nature of root plasticity and morphology in the face of changing environments. Ecol. Front. 2024, 44, 112–119. [Google Scholar] [CrossRef]
- Duque, L.O.; Villordon, A. Root Branching and Nutrient Efficiency: Status and Way Forward in Root and Tuber Crops. Front. Plant Sci. 2019, 10, 237. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Liu, W.D.; Qiu, X.Y.; Zhang, J.; Zhang, J.Y.; Bai, Y. The root microbiome: Community assembly and its contributions to plant fitness. J. Integr. Plant Biol. 2022, 64, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.W.; Teng, F.L.; Shao, X.G.; College Resources and Environment Engineering, Anshun University; College of Agronomy, Anshun University. Effects of phosphate solubilizing bacteria and their combinations on phosphorus availability in reclaimed soil. J. Henan Agric. Univ. 2019, 53, 300–306+324. [Google Scholar]
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial competition: Surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 2010, 8, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Stubbendieck, R.M.; Straight, P.D. Multifaceted Interfaces of Bacterial Competition. J. Bacteriol. 2016, 198, 2145–2155. [Google Scholar] [CrossRef]
- Zhour, H.; Bray, F.; Dandache, I.; Marti, G.; Flament, S.; Perez, A.; Lis, M.; Cabrera-Bosquet, L.; Perez, T.; Fizames, C.; et al. Wild Wheat Rhizosphere-Associated Plant Growth-Promoting Bacteria Exudates: Effect on Root Development in Modern Wheat and Composition. Int. J. Mol. Sci. 2022, 23, 15248. [Google Scholar] [CrossRef]
- Zhou, D.M.; Huang, X.F.; Chaparro, J.M.; Badri, D.V.; Manter, D.K.; Vivanco, J.M.; Guo, J.H. Root and bacterial secretions regulate the interaction between plants and PGPR leading to distinct plant growth promotion effects. Plant Soil 2016, 401, 259–272. [Google Scholar] [CrossRef]
- Xing, Y.J.; Wang, F.C.; Yu, S.R.; Zhu, Y.; Ying, Y.Q.; Shi, W.H. Enhancing Phyllostachys edulis seedling growth in phosphorus-deficient soil: Complementing the role of phosphate-solubilizing microorganisms with arbuscular mycorrhizal fungi. Plant Soil 2023, 497, 449–466. [Google Scholar] [CrossRef]
- Wu, Z.H.; Liu, J.J.; Fu, L.; Lu, D.X.; Yue, S.T.; Yang, M.Y. Effects of phosphate-solubilizing bacteria on the soil enzyme activities and microecology of soybean rhizosphere. J. China Agric. Univ. 2017, 22, 58–67. [Google Scholar]
- Zhang, T.; Xiong, J.; Tian, R.C.; Li, Y.; Zhang, Q.Y.; Li, K.; Xu, X.H.; Liang, L.M.; Zheng, Y.; Tian, B.Y. Effects of single- and mixed-bacterial inoculation on the colonization and assembly of endophytic communities in plant roots. Front. Plant Sci. 2022, 13, 928367. [Google Scholar] [CrossRef]
- Keswani, C.; Singh, S.P.; Cueto, L.; García-Estrada, C.; Mezaache-Aichour, S.; Glare, T.R.; Borriss, R.; Singh, S.P.; Blázquez, M.A.; Sansinenea, E. Auxins of microbial origin and their use in agriculture. Appl. Microbiol. Biotechnol. 2020, 104, 8549–8565. [Google Scholar] [CrossRef]
- He, A.L.; Zhao, L.Y.; Ren, W.; Li, H.R.; Paré, P.W.; Zhao, Q.; Zhang, J.L. A volatile producing Bacillus subtilis strain from the rhizosphere of Haloxylon ammodendron promotes plant root development. Plant Soil 2023, 486, 661–680. [Google Scholar] [CrossRef]
- Massucato, L.R.; Almeida, S.R.d.A.; Silva, M.B.; Mosela, M.; Zeffa, D.M.; Nogueira, A.F.; de Lima Filho, R.B.; Mian, S.; Higashi, A.Y.; Teixeira, G.M.; et al. Efficiency of Combining Strains Ag87 (Bacillus megaterium) and Ag94 (Lysinibacillus sp.) as Phosphate Solubilizers and Growth Promoters in Maize. Microorganisms 2022, 10, 1401. [Google Scholar] [CrossRef]
- Kshetri, L.; Kotoky, R.; Debnath, S.; Maheshwari, D.K.; Pandey, P. Shift in the soil rhizobacterial community for enhanced solubilization and bioavailability of phosphorus in the rhizosphere of Allium hookeri Thwaites, through bioaugmentation of phosphate-solubilizing bacteria. 3 Biotech 2024, 14, 185. [Google Scholar] [CrossRef]
- Sen, A.; Saha, N.; Sarkar, A.; Poddar, R.; Pramanik, K.; Samanta, A. Assessing the effectiveness of indigenous phosphate-solubilizing bacteria in mitigating phosphorus fixation in acid soils. 3 Biotech 2024, 14, 197. [Google Scholar] [CrossRef]
- Hu, Z.K.; Delgado-Baquerizo, M.; Fanin, N.; Chen, X.Y.; Zhou, Y.; Du, G.Z.; Hu, F.; Jiang, L.; Hu, S.J.; Liu, M.Q. Nutrient-induced acidification modulates soil biodiversity-function relationships. Nat. Commun. 2024, 15, 2858. [Google Scholar] [CrossRef]
- Sanchez-Gonzalez, M.E.; Mora-Herrera, M.E.; Wong-Villarreal, A.; De La Portilla-López, N.; Sanchez-Paz, L.; Lugo, J.; Vaca-Paulín, R.; Del Aguila, P.; Yañez-Ocampo, G. Effect of pH and Carbon Source on Phosphate Solubilization by Bacterial Strains in Pikovskaya Medium. Microorganisms 2023, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Cáceres, D.; Stokes, A.; Angeles-Alvarez, G.; Abadie, J.; Anthelme, F.; Bounous, M.; Freschet, G.T.; Roumet, C.; Weemstra, M.; Merino-Martín, L.; et al. Vegetation creates microenvironments that influence soil microbial activity and functional diversity along an elevation gradient. Soil Biol. Biochem. 2022, 165, 108485. [Google Scholar] [CrossRef]
- Solis-Hernández, A.P.; Chávez-Vergara, B.M.; Rodríguez-Tovar, A.V.; Beltrán-Paz, O.I.; Santillán, J.; Rivera-Becerril, F. Effect of the natural establishment of two plant species on microbial activity, on the composition of the fungal community, and on the mitigation of potentially toxic elements in an abandoned mine tailing. Sci. Total Environ. 2022, 802, 149788. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.D.; Wang, W.; Yi, S.J.; Zheng, F.R.; Zhang, Z.H.; Alharbi, S.A.; Filimonenko, E.; Wang, Z.L.; Kuzyakov, Y. Microbial composition in saline and alkaline soils regulates plant growth with P-solubilizing bacteria. Appl. Soil Ecol. 2024, 203, 105653. [Google Scholar] [CrossRef]
- Cheng, Y.Y.; Narayanan, M.; Shi, X.J.; Chen, X.P.; Li, Z.L.; Ma, Y. Phosphate-solubilizing bacteria: Their agroecological function and optimistic application for enhancing agro-productivity. Sci. Total Environ. 2023, 901, 166468. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Bonilla, G.A.; Durrer, A.; Cardoso, E.J. Use of compost and phosphate-solubilizing bacteria affect sugarcane mineral nutrition, phosphorus availability, and the soil bacterial community. Appl. Soil Ecol. 2021, 157, 103760. [Google Scholar] [CrossRef]
- Liang, J.L.; Liu, J.; Jia, P.; Yang, T.T.; Li, J.T. Novel phosphate-solubilizing bacteria enhance soil phosphorus cycling following ecological restoration of land degraded by mining. ISME J. 2020, 14, 1600–1613. [Google Scholar] [CrossRef]
- Guo, L.; Wang, C.; Shen, R.F. Stronger effects of maize rhizosphere than phosphorus fertilization on phosphatase activity and phosphorus-mineralizing-related bacteria in acidic soils. Rhizosphere 2022, 23, 100555. [Google Scholar] [CrossRef]
- Chen, Q.Q.; Zhao, Q.; Xie, B.X.; Lu, X.; Guo, Q.; Liu, G.X.; Zhou, M.; Tian, J.H.; Lu, W.G.; Chen, K.; et al. Soybean (Glycine max) rhizosphere organic phosphorus recycling relies on acid phosphatase activity and specific phosphorus-mineralizing-related bacteria in phosphate deficient acidic soils. J. Integr. Agric. 2024, 23, 1685–1702. [Google Scholar] [CrossRef]
- Shaw, A.N.; Cleveland, C.C. The effects of temperature on soil phosphorus availability and phosphatase enzyme activities: A cross-ecosystem study from the tropics to the Arctic. Biogeochemistry 2020, 151, 113–125. [Google Scholar] [CrossRef]
- Li, C.; Chen, X.L.; Jia, Z.H.; Zhai, L.; Zhang, B.; Grüters, U.; Ma, S.L.; Qian, J.; Liu, X.; Zhang, J.C.; et al. Meta-analysis reveals the effects of microbial inoculants on the biomass and diversity of soil microbial communities. Nat. Ecol. Evol. 2024, 8, 1270–1284. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef]
- Clémence, T.M.; Romain, S.; Franck, Z.; Julien, M.; Manuel, B.; Samuel, J. Plant mediates soil water content effects on soil microbiota independently of its water uptake. Rhizosphere 2023, 27, 100769. [Google Scholar]
- Qian, Z.Z.; Zhuang, S.Y.; Gao, J.S.; Tang, L.Z.; Jean, D.H.; Wang, F. Aeration increases soil bacterial diversity and nutrient transformation under mulching-induced hypoxic conditions. Sci. Total Environ. 2022, 817, 153017. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Malik, A.A.; Puissant, J.; Buckeridge, K.M.; Gooddall, T.; Jehmlich, N.; Chowdhury, S.; Gweon, H.S.; Peyton, J.M.; Mason, K.E.; Agtmall, M.V.; et al. Land use driven change in soil pH affects microbial carbon cycling processes. Nat. Commun. 2018, 9, 3591. [Google Scholar] [CrossRef]
- Ontman, R.; Groffman, P.M.; Driscoll, C.T.; Cheng, Z.Q. Surprising relationships between soil pH and microbial biomass and activity in a northern hardwood forest. Biogeochemistry 2023, 163, 265–277. [Google Scholar] [CrossRef]
- Wahid, F.; Fahad, S.; Danish, S.; Adnan, M.; Yue, Z.; Saud, S.; Siddiqui, M.H.; Brtnicky, M.; Hammerschmiedt, T.; Datta, R. Sustainable management with mycorrhizae and phosphate solubilizing bacteria for enhanced phosphorus uptake in calcareous soils. Agriculture 2020, 10, 334. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Li, X.; Xue, D.; Bao, C.; Zhang, K.; Chen, L.; Li, Q.; Guo, R. Enhanced Plant Growth Through Composite Inoculation of Phosphate-Solubilizing Bacteria: Insights from Plate and Soil Experiments. Agronomy 2024, 14, 2461. https://doi.org/10.3390/agronomy14112461
Li M, Li X, Xue D, Bao C, Zhang K, Chen L, Li Q, Guo R. Enhanced Plant Growth Through Composite Inoculation of Phosphate-Solubilizing Bacteria: Insights from Plate and Soil Experiments. Agronomy. 2024; 14(11):2461. https://doi.org/10.3390/agronomy14112461
Chicago/Turabian StyleLi, Mengsha, Xinjing Li, Daosheng Xue, Chengjiang Bao, Keying Zhang, Lili Chen, Qiuping Li, and Rui Guo. 2024. "Enhanced Plant Growth Through Composite Inoculation of Phosphate-Solubilizing Bacteria: Insights from Plate and Soil Experiments" Agronomy 14, no. 11: 2461. https://doi.org/10.3390/agronomy14112461
APA StyleLi, M., Li, X., Xue, D., Bao, C., Zhang, K., Chen, L., Li, Q., & Guo, R. (2024). Enhanced Plant Growth Through Composite Inoculation of Phosphate-Solubilizing Bacteria: Insights from Plate and Soil Experiments. Agronomy, 14(11), 2461. https://doi.org/10.3390/agronomy14112461





