Metabolome and Transcriptome Analysis Reveals Molecular Mechanisms of Soil Amendment (Volcanic Ash) Alleviating Salt–Alkali Stress in Melons (Cucumis melo L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Analysis of Physiological Changes
2.3. Transcriptome Sequencing and Data Analysis
2.4. Metabolome Analysis and Data Analysis
2.5. Integrated Transcriptomic and Metabolomic Analysis
3. Results
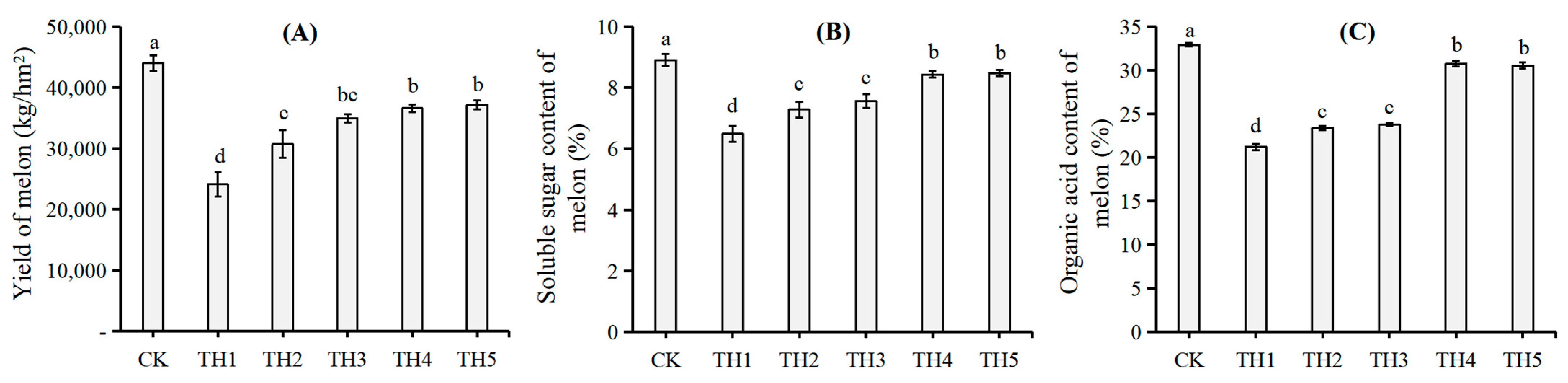
3.1. The Effect of Volcanic Ash on the Morphology and Physiological Indexes of Melons Under Salt–Alkali Stress
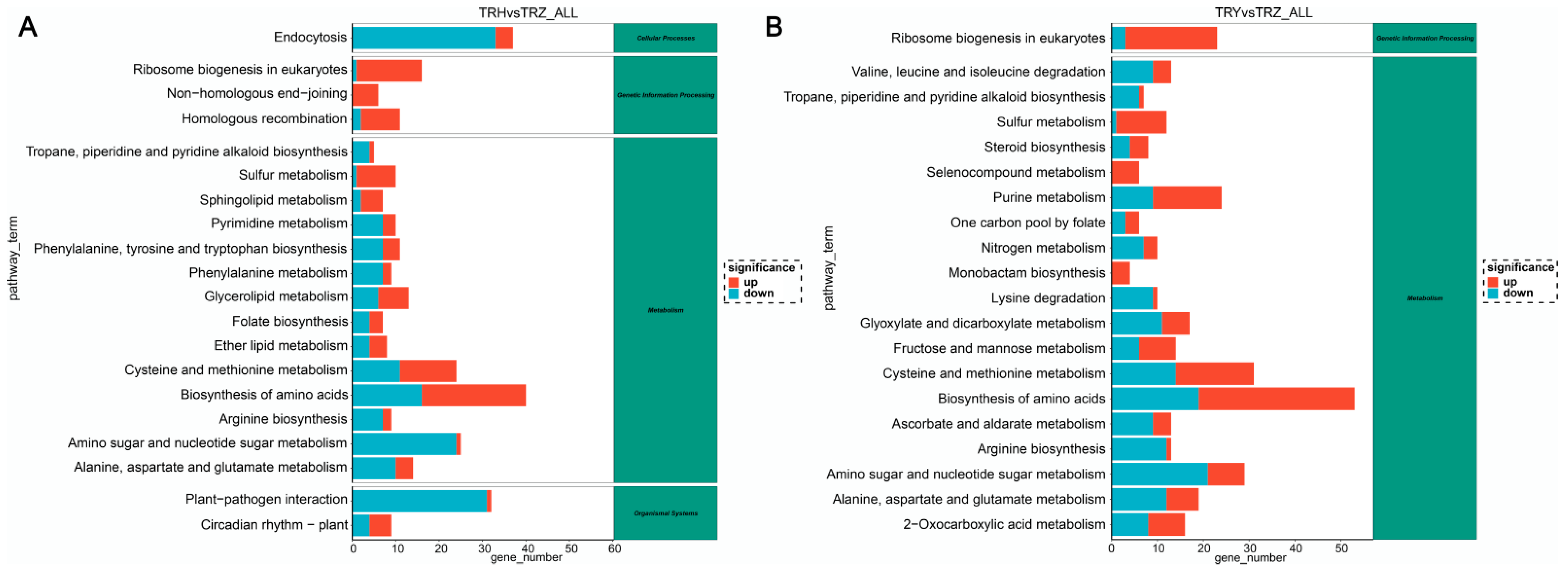
3.2. Transcriptome Characterization of Melons Under Salt–Alkali Stress
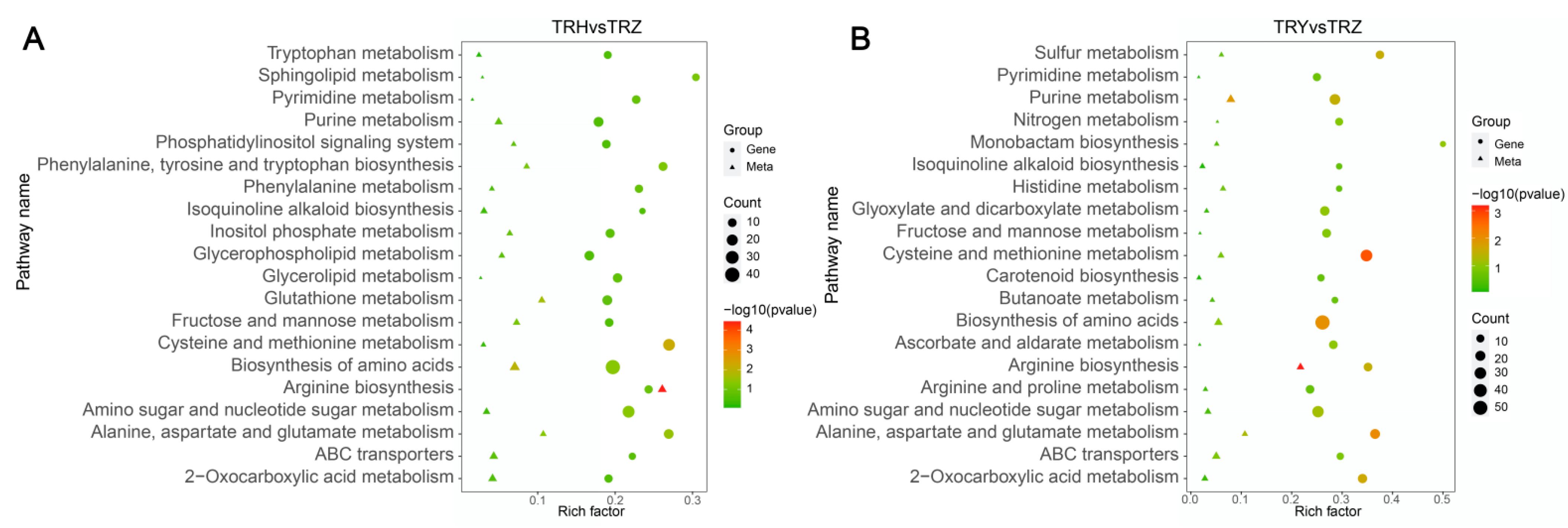
3.3. Metabolomics Profiles of Melons Under Salt–Alkali Stress
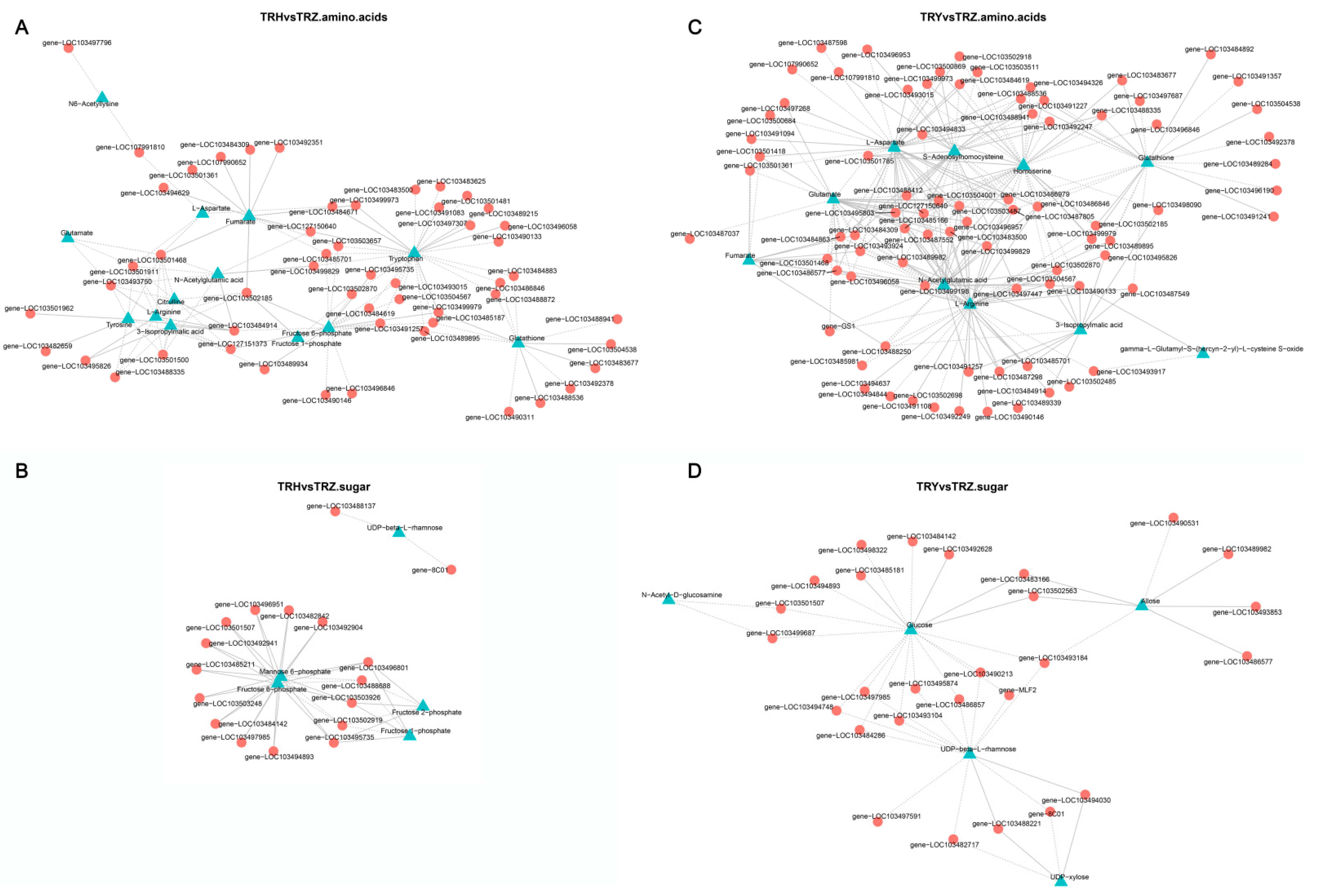
3.4. Joint Analysis of Transcriptome and Metabolome in Melons Under Salt–Alkali Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fatima, P.; Nadeem, M.; Hussain, A.; Kausar, T.; Rehman, A.; Siddique, T.; Kabir, K.; Noreen, S.; Nisar, R.; Fatima, H.; et al. Synergistic effect of microwave heating and thermosonication on the physicochemical and nutritional quality of muskmelon and sugarcane juice blend. Food Chem. 2023, 425, 136489. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Bi, Y.; Ackah, S.; Li, Z.; Li, B.; Wang, B.; Wang, Y.; Li, Y.; Prusky, D. Sodium silicate treatment accelerates biosynthesis and polymerization of suberin polyaliphatics monomers at wounds of muskmelon. Food Chem. 2023, 417, 135847. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.; Liu, S.; Zhu, X.; Xia, J. First report of muskmelon fruit rot caused by Fusarium sulawesiense in China. Plant Dis. 2023, 107, 3313. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Z. Analysis of physiological indexes and aroma-related genes of thick-skinned melon (Cucumis melo L. Hetau) under salt stress. Pak. J. Bot. 2023, 55, 1723–1728. [Google Scholar] [CrossRef]
- Fu, L.; Wang, H.; Leng, X.; Zhang, X.; Xiao, B.; Liu, H.; Xue, D.; Wang, Y.; Wu, C.; Wang, W. Genome-Wide identification and expression analysis of the CmHAK gene family in melon (Cucumis melo L.). Agronomy 2023, 9, 1138. [Google Scholar] [CrossRef]
- Zhang, Q.; Niu, W.; Du, Y.; Sun, J.; Cui, B.; Zhang, E.; Wang, Y.; Siddique, K. Effect of aerated drip irrigation and nitrogen doses on NO2 emissions, microbial activity, and yield of tomato and melonn under greenhouse conditions. Agric. Water Manag. 2023, 283, 108321. [Google Scholar] [CrossRef]
- Soltabayeva, A.; Ongaltay, A.; Omondi, J.O.; Srivastava, S. Morphological, physiological and molecular markers for salt-stressed plants. Plants 2021, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, E.; Ekinci, M.; Turan, M.; Ors, S.; Dursun, A. Physiological, morphological and biochemical responses of exogenous hydrogen sulfide in salt-stressed tomato seedlings. Sustainability 2023, 15, 1098. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt tolerant plant growth promoting rhizobacteria for enhancing crop productivity of saline soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Sui, N. Sensitivity and responses of chloroplasts to salt stress in plants. Front. Plant Sci. 2024, 15, 1374086. [Google Scholar] [CrossRef]
- Castañares, J.; Bouzo, C.A. Effect of exogenous melatonin on seed germination and seedling growth in melon (Cucumis melo L.) under salt stress. Hortic. Plant J. 2019, 5, 79–87. [Google Scholar] [CrossRef]
- Sharavdorj, K.; Byambadorj, S.; Jang, Y.; Ahn, Y.; Cho, J. Evaluating the effects of long-term salinity stress on the growth and physiology of mono and mixed crops. Agronomy 2024, 14, 287. [Google Scholar] [CrossRef]
- Lombardi, M.; Bellucci, M.; Cimini, S.; Locato, V.; Loreto, F.; Gara, L. Exploring natural variations in Arabidopsis thaliana: Plant adaptability to salt stress. Plants 2024, 13, 1069. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Hou, X.; Liang, X. Response mechanisms of plants under saline-alkali stress. Front. Plant Sci. 2021, 12, 667458. [Google Scholar] [CrossRef] [PubMed]
- Batool, A.; Taj, S.; Rashid, A.; Khalid, A.; Qadeer, S.; Saleem, A.R.; Ghufran, M. Potential of soil amendments (Biochar and Gypsum) in increasing water use efficiency of Abelmoschus esculentus L. Moench. Front. Plant Sci. 2015, 6, 733. [Google Scholar] [CrossRef]
- Ozturk, H.S.; Turkmen, C.; Erdogan, E.; Baskan, O.; Dengiz, O.; Parlak, M. Effects of a soil conditioner on some physical and biological features of soils: Results from a greenhouse study. Bioresour. Technol. 2005, 96, 1950–1954. [Google Scholar] [CrossRef]
- Lu, Y.; Jeffers, R.; Raju, A.; Kenny, T.; Ratchanniyasamu, E.; Fricke, W. Does night-time transpiration provide any benefit to wheat (Triticum aestivum L.) plants which are exposed to salt stress? Physiol. Plant. 2023, 175, e13839. [Google Scholar] [CrossRef]
- Zheng, Y.; Rao, F.; Tian, X.; Lin, S. Synergistic gel formation in geopolymers of superior mechanical strength synthesized with volcanic ash and slag. Environ. Sci. Pollut. Res. Int. 2023, 30, 26244–26255. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Z. Experimental Techniques in Plant Physiology; Jilin University Press: Changchun, China, 2008. [Google Scholar]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, 14. [Google Scholar] [CrossRef]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef]
- Bylesjö, M.; Eriksson, D.; Kusano, M.; Moritz, T.; Trygg, J. Data integration in plant biology: The O2PLS method for combined modeling of transcript and metabolite data. Plant J. 2007, 52, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Bouhaddani, S.E.; Houwing-Duistermaat, J.; Salo, P.; Perola, M.; Jongbloed, G.; Uh, H. Evaluation of O2PLS in Omics data integration. BMC Bioinform. 2016, 17 (Suppl. S2), S11. [Google Scholar] [CrossRef]
- Di, M.C.; Delfine, S.; Pizzuto, R.; Loreto, F.; Fuggi, A. Free amino acids and glycine betaine in leaf osmoregulation of spinach responding to increasing salt stress. New Phytol. 2003, 158, 455–463. [Google Scholar]
- Winter, G.; Todd, C.D.; Trovato, M.; Forlani, G.; Funck, D. Physiological implications of arginine metabolism in plants. Front. Plant Sci. 2015, 30, 534. [Google Scholar] [CrossRef]
- Jia, X.M.; Zhu, Y.F.; Hu, Y.; Zhang, R.; Cheng, L.; Zhu, Z.L.; Zhao, T.; Zhang, X.Y.; Wang, Y.X. Integrated physiologic, proteomic, and metabolomic analyses of Malus halliana adaptation to saline–alkali stress. Hortic. Res. 2019, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, D.; Zhou, R.; Wang, X.; Dossa, K.; Wang, L.; Zhang, Y.; Yu, J.; Gong, H.; Zhang, X. Transcriptome and metabolome analyses of two contrasting sesame genotypes reveal the crucial biological pathways involved in rapid adaptive response to salt stress. BMC Plant Biol. 2019, 19, 66. [Google Scholar] [CrossRef]
- Yang, D.S.; Zhang, J.; Li, M.X.; Shi, L.X. Metabolomics analysis reveals the salt-tolerant mechanism in Glycine soja. J. Plant Growth Regul. 2017, 36, 460–471. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Fang, S. Integrative analysis of metabolome and transcriptome reveals molecular regulatory mechanism of flavonoid biosynthesis in Cyclocarya paliurus under salt stress. Ind. Crops Prod. 2021, 170, 113823. [Google Scholar] [CrossRef]
- Chen, X.; Mao, X.; Huang, P. Morphological characterization of flower buds development and related gene expression profiling at bud break stage in heterodichogamous Cyclocarya paliurus (Batal.) Lljinskaja. Genes 2019, 10, 818. [Google Scholar] [CrossRef]
- Seelinger, G.; Merfort, I.; Schempp, C.M. Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Medica 2008, 74, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Oku, H.; Baba, S.; Koga, H. Lipid composition of mangrove and its relevance to salt tolerance. J. Plant Res. 2003, 116, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.; Berger, D.K.; Christensen, S.A. RNA-Seq analysis of resistant and susceptible sub-tropical maize lines reveals a role for kauralexins in tolerance to grey leaf spot Disease, caused by Cercospora Zeina. BMC Plant Biol. 2017, 17, 197. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, X.; Lu, X.; Zhao, B.; Yang, Y.; Liu, J. Integrative analysis of transcriptome and metabolome reveal mechanism of tolerance to salt stress in oat (Avena sativa L.). Plant Physiol. Biochem. 2021, 160, 315–328. [Google Scholar] [CrossRef]
- Keunen, E.; Peshev, D.; Vangronsveld, J.; Van Den Ende, W.; Cuypers, A. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef]
- Chen, X.; Xu, Z.; Zhao, B.; Yang, Y.; Mi, J.; Zhao, Z.; Liu, J. Physiological and proteomic analysis responsive mechanisms for salt stress in oat. Front. Plant Sci. 2022, 13, 891674. [Google Scholar] [CrossRef]
- Feng, G.; Xiao, P.; Wang, X.; Huang, L.; Nie, G.; Li, Z.; Peng, Y.; Li, D.; Zhang, X. Comprehensive transcriptome analysis uncovers distinct expression patterns associated with early salinity stress in annual Ryegrass (Lolium multiflorum L.). Int. J. Mol. Sci. 2022, 23, 3279. [Google Scholar] [CrossRef]
- Wan, H.; Qian, J.; Zhang, H.; Lu, H.; Li, O.; Li, R.; Yu, Y.; Wen, J.; Zhao, L.; Yi, B.; et al. Combined transcriptomics and metabolomics analysis reveals the molecular mechanism of salt tolerance of Huayouza 62, an elite cultivar in Rapeseed (Brassica napus L.). Int. J. Mol. Sci. 2022, 23, 1279. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, L.; Tian, X.; Wang, W.; Wu, C. Metabolome and Transcriptome Analysis Reveals Molecular Mechanisms of Soil Amendment (Volcanic Ash) Alleviating Salt–Alkali Stress in Melons (Cucumis melo L.). Agronomy 2024, 14, 2478. https://doi.org/10.3390/agronomy14112478
Fu L, Tian X, Wang W, Wu C. Metabolome and Transcriptome Analysis Reveals Molecular Mechanisms of Soil Amendment (Volcanic Ash) Alleviating Salt–Alkali Stress in Melons (Cucumis melo L.). Agronomy. 2024; 14(11):2478. https://doi.org/10.3390/agronomy14112478
Chicago/Turabian StyleFu, Lina, Xiaoxin Tian, Wei Wang, and Chunyan Wu. 2024. "Metabolome and Transcriptome Analysis Reveals Molecular Mechanisms of Soil Amendment (Volcanic Ash) Alleviating Salt–Alkali Stress in Melons (Cucumis melo L.)" Agronomy 14, no. 11: 2478. https://doi.org/10.3390/agronomy14112478
APA StyleFu, L., Tian, X., Wang, W., & Wu, C. (2024). Metabolome and Transcriptome Analysis Reveals Molecular Mechanisms of Soil Amendment (Volcanic Ash) Alleviating Salt–Alkali Stress in Melons (Cucumis melo L.). Agronomy, 14(11), 2478. https://doi.org/10.3390/agronomy14112478



