Bactrocera oleae Control and Smart Farming Technologies for Olive Orchards in the Context of Optimal Olive Oil Quality: A Review
Abstract
:1. Introduction
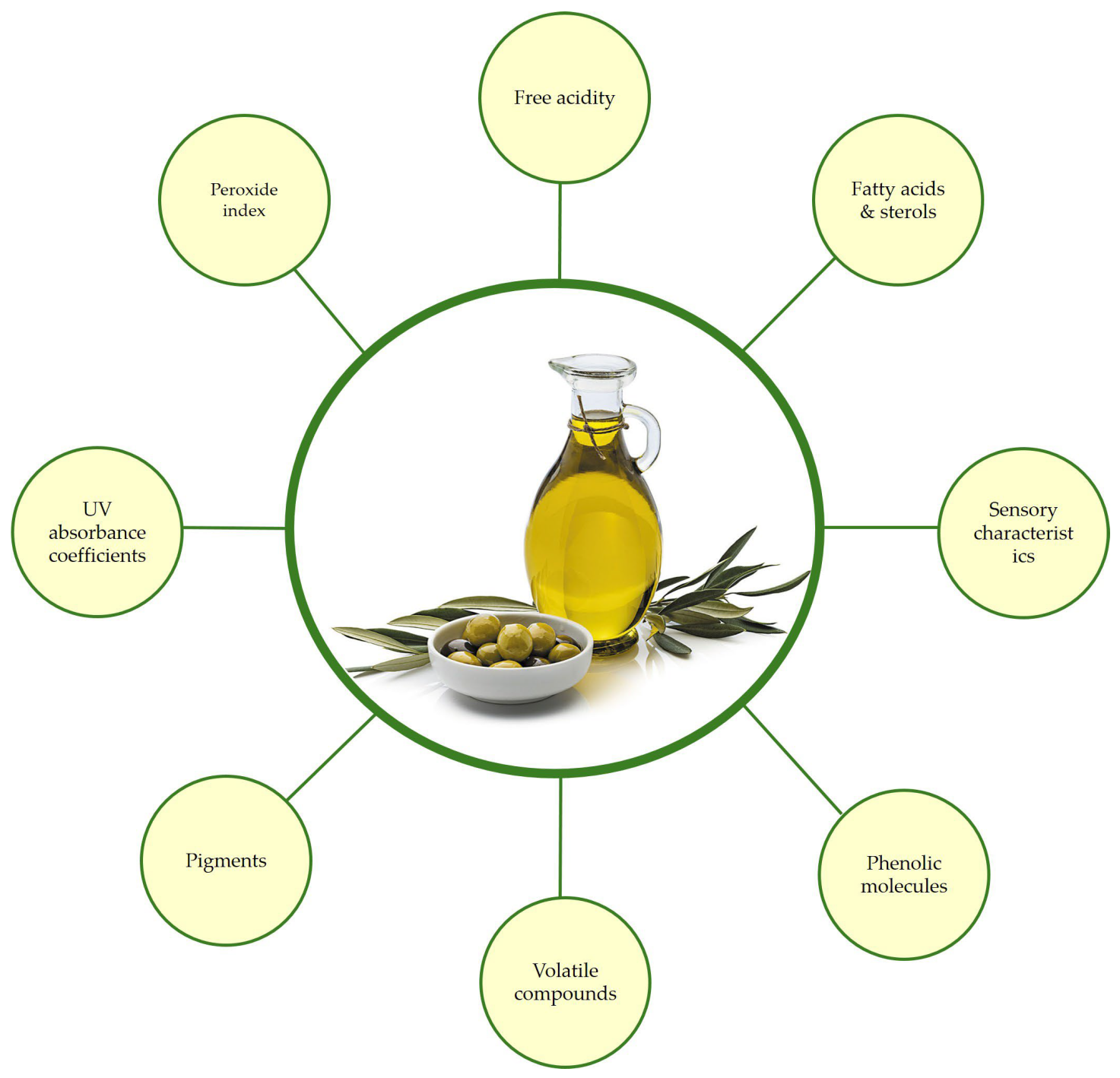
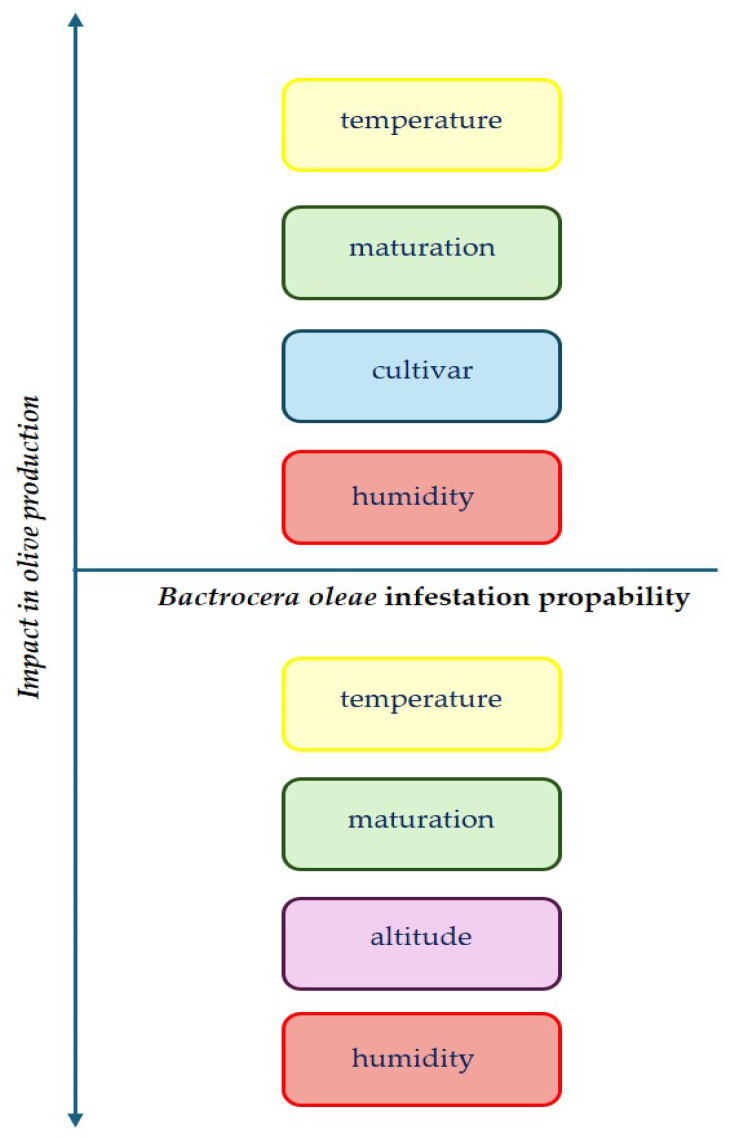
2. Impact of Bactrocera oleae Infestation on Oil Qualitative Characteristics
3. Smart Farming Technologies and Practices for Detecting Bactrocera oleae
4. Discussion
5. Future Research
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boskou, D. History and characteristics of the olive tree. In Olive Oil: Chemistry and Technology; AOCS Press: New York, NY, USA, 1996. [Google Scholar]
- Ozturk, M.; Altay, V.; Gönenç, T.M.; Unal, B.T.; Efe, R.; Akçiçek, E.; Bukhari, A. An overview of olive cultivation in Turkey: Botanical features, eco-physiology and phytochemical aspects. Agronomy 2021, 11, 295. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, I.; Iglesias-Otero, M.A.; Esteki, M.; Moldes, O.A.; Mejuto, J.C.; Simal-Gandara, J. A critical review on the use of artificial neural networks in olive oil production, characterization and authentication. Crit. Rev. Food Sci. Nutr. 2019, 59, 1913–1926. [Google Scholar] [CrossRef] [PubMed]
- Serra-Majem, L.; Tomaino, L.; Dernini, S.; Berry, E.M.; Lairon, D.; de la Cruz, J.N.; Bach-Faig, A.; Donini, L.M.; Medina, F.X.; Belahsen, R.; et al. Updating the mediterranean diet pyramid towards sustainability: Focus on environmental concerns. Int. J. Environ. Res. Public Health 2020, 17, 8758. [Google Scholar] [CrossRef] [PubMed]
- International Olive Council International Olive Council. Available online: https://www.internationaloliveoil.org/ (accessed on 30 June 2024).
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.H.; Saari, N. Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.)-A review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef]
- Soler-Rivas, C.; Espiń, J.C.; Wichers, H.J. Oleuropein and related compounds. J. Sci. Food Agric. 2000, 80, 1013–1023. [Google Scholar] [CrossRef]
- Ribarova, F.; Zanev, R.; Shishkov, S.; Rizov, N. α-Tocopherol, fatty acids and their correlations in Bulgarian foodstuffs. J. Food Compos. Anal. 2003, 16, 659–667. [Google Scholar] [CrossRef]
- Vinha, A.F.; Ferreres, F.; Silva, B.M.; Valentão, P.; Gonçalves, A.; Pereira, J.A.; Oliveira, M.B.; Seabra, R.M.; Andrade, P.B. Phenolic profiles of Portuguese olive fruits (Olea europaea L.): Influences of cultivar and geographical origin. Food Chem. 2005, 89, 561–568. [Google Scholar] [CrossRef]
- Knoops, K.T.B.; De Groot, L.C.P.G.M.; Kromhout, D.; Perrin, A.E.; Moreiras-Varela, O.; Menotti, A.; Van Staveren, W.A. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: The HALE project. JAMA 2004, 292, 1433–1439. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean Diet and Survival in a Greek Population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef]
- Covas, M.I. Bioactive effects of olive oil phenolic compounds in humans: Reduction of heart disease factors and oxidative damage. Inflammopharmacology 2008, 16, 216–218. [Google Scholar] [CrossRef]
- Daskalaki, D.; Kefi, G.; Kotsiou, K.; Tasioula-Margari, M. Evaluation of phenolic compounds degradation in virgin olive oil during storage and heating. J. Food Nutr. Res. 2009, 48, 31–41. [Google Scholar]
- Morales, M.T.; Przybylski, R. Olive Oil Oxidation BT—Handbook of Olive Oil: Analysis and Properties; Aparicio, R., Harwood, J., Eds.; Springer: Boston, MA, USA, 2013; pp. 479–522. ISBN 978-1-4614-7777-8. [Google Scholar]
- Polari, J.J.; Garcí-Aguirre, D.; Olmo-García, L.; Carrasco-Pancorbo, A.; Wang, S.C. Impact of industrial hammer mill rotor speed on extraction efficiency and quality of extra virgin olive oil. Food Chem. 2018, 242, 362–368. [Google Scholar] [CrossRef]
- López-Yerena, A.; Lozano-Castellón, J.; Olmo-Cunillera, A.; Tresserra-Rimbau, A.; Quifer-Rada, P.; Jiménez, B.; Pérez, M.; Vallverdú-Queralt, A. Effects of organic and conventional growing systems on the phenolic profile of extra-virgin olive Oil. Molecules 2019, 24, 1986. [Google Scholar] [CrossRef] [PubMed]
- López-Yerena, A.; Ninot, A.; Lozano-Castellón, J.; Escribano-Ferrer, E.; Romero-Aroca, A.J.; Belaj, A.; Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Conservation of native wild ivory-white olives from the MEDES islands natural reserve to maintain virgin olive oil diversity. Antioxidants 2020, 9, 1009. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Queralt, A.; Regueiro, J.; Rinaldi De Alvarenga, J.F.; Torrado, X.; Lamuela-Raventos, R.M. Home cooking and phenolics: Effect of thermal treatment and addition of extra virgin olive oil on the phenolic profile of tomato sauces. J. Agric. Food Chem. 2014, 62, 3314–3320. [Google Scholar] [CrossRef]
- Lozano-Castellón, J.; López-Yerena, A.; Rinaldi de Alvarenga, J.F.; Romero del Castillo-Alba, J.; Vallverdú-Queralt, A.; Escribano-Ferrer, E.; Lamuela-Raventós, R.M. Health-promoting properties of oleocanthal and oleacein: Two secoiridoids from extra-virgin olive oil. Crit. Rev. Food Sci. Nutr. 2020, 60, 2532–2548. [Google Scholar] [CrossRef]
- Fletcher, B.S. The biology of dacine fruit flies. Annu. Rev. Entomol. 1987, 32, 115–144. [Google Scholar] [CrossRef]
- Neuenschwander, P.; Michelakis, S. The infestation of Dacus oleae (Gmel.) (Diptera, Tephritidae) at harvest time and its influence on yield and quality of olive oil in Crete. Z. Für Angew. Entomol. 1978, 86, 420–433. [Google Scholar] [CrossRef]
- Tzanakakis, M. Seasonal development and dormancy of insects and mites feeding on olive: A review. Neth. J. Zool. 2002, 52, 87–224. [Google Scholar] [CrossRef]
- Malheiro, R.; Casal, S.; Baptista, P.; Pereira, J.A. A review of Bactrocera oleae (Rossi) impact in olive products: From the tree to the table. Trends Food Sci. Technol. 2015, 44, 226–242. [Google Scholar] [CrossRef]
- Kitchen, N.R. Emerging technologies for real-time and integrated agriculture decisions. Comput. Electron. Agric. 2008, 61, 1–3. [Google Scholar] [CrossRef]
- Cisternas, I.; Velásquez, I.; Caro, A.; Rodríguez, A. Systematic literature review of implementations of precision agriculture. Comput. Electron. Agric. 2020, 176, 105626. [Google Scholar] [CrossRef]
- Bochtis, D.; Sørensen, C.; Busato, P.; Hameed, I.; Rodias, E.; Green, O.; Papadakis, G. Tramline establishment in controlled traffic farming based on operational machinery cost. Biosyst. Eng. 2010, 107, 221–231. [Google Scholar] [CrossRef]
- Dias, N.P.; Zotti, M.J.; Montoya, P.; Carvalho, I.R.; Nava, D.E. Fruit fly management research: A systematic review of monitoring and control tactics in the world. Crop. Prot. 2018, 112, 187–200. [Google Scholar] [CrossRef]
- Tsipi, D.; Botitsi, H.; Economou, A. Mass Spectrometry for Analysis of Pesticide Residues and Their Metabolites; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- McKinion, J.; Jenkins, J.; Willers, J.; Zumanis, A. Spatially variable insecticide applications for early season control of cotton insect pests. Comput. Electron. Agric. 2009, 67, 71–79. [Google Scholar] [CrossRef]
- Haff, R.P.; Saranwong, S.; Thanapase, W.; Janhiran, A.; Kasemsumran, S.; Kawano, S. Automatic image analysis and spot classification for detection of fruit fly infestation in hyperspectral images of mangoes. Postharvest Biol. Technol. 2013, 86, 23–28. [Google Scholar] [CrossRef]
- Cohen, Y.; Cohen, A.; Hetzroni, A.; Alchanatis, V.; Broday, D.; Gazit, Y.; Timar, D. Spatial decision support system for Medfly control in citrus. Comput. Electron. Agric. 2008, 62, 107–117. [Google Scholar] [CrossRef]
- Grasswitz, T.R. Integrated pest management (IPM) for small-scale farms in developed economies: Challenges and opportunities. Insects 2019, 10, 179. [Google Scholar] [CrossRef]
- Partel, V.; Nunes, L.; Stansly, P.; Ampatzidis, Y. Automated vision-based system for monitoring Asian citrus psyllid in orchards utilizing artificial intelligence. Comput. Electron. Agric. 2019, 162, 328–336. [Google Scholar] [CrossRef]
- Ding, W.; Taylor, G. Automatic moth detection from trap images for pest management. Comput. Electron. Agric. 2016, 123, 17–28. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, C.; Dong, D.; Wang, K. Real-time monitoring of insects based on laser remote sensing. Ecol. Indic. 2023, 151, 110302. [Google Scholar] [CrossRef]
- Noskov, A.; Bendix, J.; Friess, N. A review of insect monitoring approaches with special reference to radar techniques. Sensors 2021, 21, 1474. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Castellón, J.; López-Yerena, A.; Domínguez-López, I.; Siscart-Serra, A.; Fraga, N.; Sámano, S.; López-Sabater, C.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A.; Pérez, M. Extra virgin olive oil: A comprehensive review of efforts to ensure its authenticity, traceability, and safety. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2639–2664. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive compounds and quality of extra virgin olive oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef]
- Conte, L.; Bendini, A.; Valli, E.; Lucci, P.; Moret, S.; Maquet, A.; Lacoste, F.; Brereton, P.; Garcia-Gonzalez, D.L.; Moreda, W.; et al. Olive oil quality and authenticity: A review of current EU legislation, standards, relevant methods of analyses, their drawbacks and recommendations for the future. Trends Food Sci. Technol. 2020, 105, 483–493. [Google Scholar] [CrossRef]
- Azizian, H.; Mossoba, M.M.; Fardin-Kia, A.R.; Delmonte, P.; Karunathilaka, S.R.; Kramer, J.K.G. Novel, rapid identification, and quantification of adulterants in extra virgin olive oil using near-infrared spectroscopy and chemometrics. Lipids 2015, 50, 705–718. [Google Scholar] [CrossRef]
- Mikrou, T.; Pantelidou, E.; Parasyri, N.; Papaioannou, A.; Kapsokefalou, M.; Gardeli, C.; Mallouchos, A. Varietal and Geographical Discrimination of Greek Monovarietal Extra Virgin Olive Oils Based on Squalene, Tocopherol, and Fatty Acid Composition. Molecules 2020, 25, 3818. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Aalizadeh, R.; Dasenaki, M.E.; Thomaidis, N.S. Application of High Resolution Mass Spectrometric methods coupled with chemometric techniques in olive oil authenticity studies—A review. Anal. Chim. Acta 2020, 1134, 150–173. [Google Scholar] [CrossRef]
- Martakos, I.; Kostakis, M.; Dasenaki, M.; Pentogennis, M.; Thomaidis, N. Simultaneous Determination of pigments, tocopherols, and squalene in greek olive oils: A study of the influence of cultivation and oil-production parameters. Foods 2019, 9, 31. [Google Scholar] [CrossRef]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Lercker, G.; Simal-Gandara, J. Phenolic molecules in virgin olive oils: A survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade alessandra. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef]
- Carluccio, M.A.; Siculella, L.; Ancora, M.A.; Massaro, M.; Scoditti, E.; Storelli, C.; Visioli, F.; Distante, A.; De Caterina, R. Olive oil and red wine antioxidant polyphenols inhibit endothelial activation: Antiatherogenic properties of Mediterranean diet phytochemicals. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.J. Effect of olive oil minor components on oxidative stress and arachidonic acid mobilization and metabolism by macrophages RAW 264.7. Free. Radic. Biol. Med. 2003, 35, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Basta, G.; Lazzerini, G.; Carluccio, M.A.; Bosetti, F.; Solaini, G.; Visioli, F.; Paolicchi, A.; De Caterina, R. Quenching of intracellular ROS generation as a mechanism for oleate-induced reduction of endothelial activation and early atherogenesis. Thromb. Haemost. 2002, 88, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Petroni, A.; Blasevich, M.; Salami, M.; Papini, N.; Montedoro, G.F.; Galli, C. Inhibition of platelet aggregation and eicosanoid production by phenolic components of olive oil. Thromb. Res. 1995, 78, 151–160. [Google Scholar] [CrossRef]
- Hashim, Y.Z.H.Y.; Gill, C.I.R.; McGlynn, H.; Rowland, I.R. Components of olive oil and chemoprevention of Colorectal Cancer. Nutr. Rev. 2005, 63, 374–386. [Google Scholar] [CrossRef]
- Stavric, B. Role of chemopreventers in human diet. Clin. Biochem. 1994, 27, 319–332. [Google Scholar] [CrossRef]
- Capriotti, A.L.; Cavaliere, C.; Crescenzi, C.; Foglia, P.; Nescatelli, R.; Samperi, R.; Laganà, A. Comparison of extraction methods for the identification and quantification of polyphenols in virgin olive oil by ultra-HPLC-QToF mass spectrometry. Food Chem. 2014, 158, 392–400. [Google Scholar] [CrossRef]
- Lerma-García, M.J.; Lantano, C.; Chiavaro, E.; Cerretani, L.; Herrero-Martínez, J.M.; Simó-Alfonso, E.F. Classification of extra virgin olive oils according to their geographical origin using phenolic compound profiles obtained by capillary electrochromatography. Food Res. Int. 2009, 42, 1446–1452. [Google Scholar] [CrossRef]
- Rotondi, A.; Bendini, A.; Cerretani, L.; Mari, M.; Lercker, G.; Gallina-Toschi, G.T. Effect of olive ripening degree on the oxidative stability and organoleptic properties of cv. nostrana di brisighella extra virgin olive oil. J. Agric. Food Chem. 2004, 52, 3649–3654. [Google Scholar] [CrossRef]
- Tasioula-Margari, M.; Tsabolatidou, E. Extraction, separation, and identification of phenolic compounds in virgin olive oil by HPLC-DAD and HPLC-MS. Antioxidants 2015, 4, 548–562. [Google Scholar] [CrossRef]
- Fu, S.; Segura-Carreteru, A.; Arráez-Román, D.; Menéndez, J.A.; De La Torre, A.; Fernández-Gutiérrez, A. Tentative characterization of novel phenolic compounds in extra virgin olive oils by rapid-resolution liquid chromatography coupled with mass spectrometry. J. Agric. Food Chem. 2009, 57, 11140–11147. [Google Scholar] [CrossRef] [PubMed]
- Bendini, A.; Cerretani, L.; Vecchi, S.; Carrasco-Pancorbo, A.; Lercker, G.; Simal-Gandara, J. Protective effects of extra virgin olive oil phenolics on oxidative stability in the presence or absence of copper ions. J. Agric. Food Chem. 2006, 54, 4880–4887. [Google Scholar] [CrossRef] [PubMed]
- Capote, F.P.; Jiménez, J.R.; De Castro, M.D.L. Sequential (step-by-step) detection, identification and quantitation of extra virgin olive oil adulteration by chemometric treatment of chromatographic profiles. Anal. Bioanal. Chem. 2007, 388, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Mildner-Szkudlarz, S.; Jeleń, H.H. Detection of olive oil adulteration with rapeseed and sunflower oils using mos electronic nose and smpe-ms. J. Food Qual. 2010, 33, 21–41. [Google Scholar] [CrossRef]
- Di Stefano, V.; Avellone, G.; Bongiorno, D.; Cunsolo, V.; Muccilli, V.; Sforza, S.; Dossena, A.; Drahos, L.; Vékey, K. Applications of liquid chromatography–mass spectrometry for food analysis. J. Chromatogr. A 2012, 1259, 74–85. [Google Scholar] [CrossRef]
- Martakos, I.; Katsianou, P.; Koulis, G.; Efstratiou, E.; Nastou, E.; Nikas, S.; Dasenaki, M.; Pentogennis, M.; Thomaidis, N. Development of analytical strategies for the determination of olive fruit bioactive compounds using UPLC-HRMS and HPLC-DAD. Chemical characterization of kolovi lesvos variety as a case study. Molecules 2021, 26, 7182. [Google Scholar] [CrossRef]
- Fanali, C.; Della Posta, S.; Vilmercati, A.; Dugo, L.; Russo, M.; Petitti, T.; Mondello, L.; de Gara, L. Extraction, analysis, and antioxidant activity evaluation of phenolic compounds in different Italian extra-virgin olive oils. Molecules 2018, 23, 3249. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Evaluation of olive oil quality with electrochemical sensors and biosensors: A review. Int. J. Mol. Sci. 2021, 22, 12708. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Classification and Antioxidant Activity Evaluation of Edible Oils by Using Nanomaterial-Based Electrochemical Sensors. Int. J. Mol. Sci. 2023, 24, 3010. [Google Scholar] [CrossRef]
- Medjkouh, L.; Tamendjari, A.; Alves, R.C.; Laribi, R.; Oliveira, M.B.P.P. Phenolic profiles of eight olive cultivars from Algeria: Effect of: Bactrocera oleae attack. Food Funct. 2018, 9, 890–897. [Google Scholar] [CrossRef]
- Helvaci, M.; Aktaş, M.; Özden, Ö. Occurrence, damage, and population dynamics of the olive fruit fly (Bactrocera oleae Gmelin) in the Turkish Republic of Northern Cyprus. Turkish J. Agric. For. 2018, 42, 453–458. [Google Scholar] [CrossRef]
- Abacıgil, T.; Kıralan, M.; Ramadan, M.F. Quality parameters of olive oils at different ripening periods as affected by olive fruit fly infestation and olive anthracnose. Rendiconti Lince- Sci. Fis. Nat. 2023, 34, 595–603. [Google Scholar] [CrossRef]
- Notario, A.; Sánchez, R.; Luaces, P.; Sanz, C.; Pérez, A.G. The Infestation of Olive Fruits by Bactrocera oleae (Rossi) Modifies the Expression of Key Genes in the Biosynthesis of Volatile and Phenolic Compounds and Alters the Composition of Virgin Olive Oil. Molecules 2022, 27, 1650. [Google Scholar] [CrossRef]
- Malheiro, R.; Casal, S.; Pinheiro, L.; Baptista, P.; Pereira, J. Olive cultivar and maturation process on the oviposition preference ofBactrocera oleae (Rossi) (Diptera: Tephritidae). Bull. Èntomol. Res. 2019, 109, 43–53. [Google Scholar] [CrossRef]
- Kokkari, A.I.; Pliakou, O.D.; Floros, G.D.; Kouloussis, N.A.; Koveos, D.S. Effect of fruit volatiles and light intensity on the re-production of Bactrocera (Dacus) oleae. J. Appl. Entomol. 2017, 141, 841–847. [Google Scholar] [CrossRef]
- Fountas, S.; Carli, G.; Sørensen, C.; Tsiropoulos, Z.; Cavalaris, C.; Vatsanidou, A.; Liakos, B.; Canavari, M.; Wiebensohn, J.; Tisserye, B. Farm management information systems: Current situation and future perspectives. Comput. Electron. Agric. 2015, 115, 40–50. [Google Scholar] [CrossRef]
- Wu, C.; Chen, Z.; Wang, D.; Song, B.; Liang, Y.; Yang, L.; Bochtis, D.D. A cloud-based in-field fleet coordination system for multiple operations. Energies 2020, 13, 775. [Google Scholar] [CrossRef]
- Fountas, S.; Mylonas, N.; Malounas, I.; Rodias, E.; Santos, C.H.; Pekkeriet, E. Agricultural robotics for field operations. Sensors 2020, 20, 2672. [Google Scholar] [CrossRef]
- Mahmud, M.S.A.; Abidin, M.S.Z.; Emmanuel, A.A.; Hasan, H.S. Robotics and Automation in Agriculture: Present and Future Applications. Appl. Model. Simul. 2020, 4, 130–140. [Google Scholar]
- Oliveira, L.F.P.; Moreira, A.P.; Silva, M.F. Advances in agriculture robotics: A state-of-the-art review and challenges ahead. Robotics 2021, 10, 52. [Google Scholar] [CrossRef]
- Goap, A.; Sharma, D.; Shukla, A.K.; Krishna, C.R. An IoT based smart irrigation management system using Machine learning and open source technologies. Comput. Electron. Agric. 2018, 155, 41–49. [Google Scholar] [CrossRef]
- Popescu, D.; Dinca, A.; Ichim, L.; Angelescu, N. New trends in detection of harmful insects and pests in modern agriculture using artificial neural networks. a review. Front. Plant Sci. 2023, 14, 1268167. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, S.; Niedbała, G. Artificial neural networks in agriculture. Agriculture 2021, 11, 497. [Google Scholar] [CrossRef]
- Bohnenkamp, D.; Behmann, J.; Mahlein, A.-K. In-field detection of Yellow Rust in Wheat on the Ground Canopy and UAV Scale. Remote Sens. 2019, 11, 2495. [Google Scholar] [CrossRef]
- Behmann, J.; Acebron, K.; Emin, D.; Bennertz, S.; Matsubara, S.; Thomas, S.; Bohnenkamp, D.; Kuska, M.T.; Jussila, J.; Salo, H.; et al. Specim IQ: Evaluation of a new, miniaturized handheld hyperspectral camera and its application for plant phenotyping and disease detection. Sensors 2018, 18, 441. [Google Scholar] [CrossRef]
- Castrignanò, A.; Buttafuoco, G.; Quarto, R.; Vitti, C.; Langella, G.; Terribile, F.; Venezia, A. A combined approach of sensor data fusion and multivariate geostatistics for delineation of homogeneous zones in an agricultural field. Sensors 2017, 17, 2794. [Google Scholar] [CrossRef]
- Barbedo, J.G.A. Data Fusion in Agriculture: Resolving Ambiguities and Closing Data Gaps. Sensors 2022, 22, 2285. [Google Scholar] [CrossRef]
- Torres, A.B.B.; da Rocha, A.R.; Coelho da Silva, T.L.; de Souza, J.N.; Gondim, R.S. Multilevel data fusion for the internet of things in smart agriculture. Comput. Electron. Agric. 2020, 171, 105309. [Google Scholar] [CrossRef]
- Marques, P.; Pádua, L.; Sousa, J.J.; Fernandes-Silva, A. Advancements in Remote Sensing Imagery Applications for Precision Management in Olive Growing: A Systematic Review. Remote Sens. 2024, 16, 1324. [Google Scholar] [CrossRef]
- Blekos, K.; Tsakas, A.; Xouris, C.; Evdokidis, I.; Alexandropoulos, D.; Alexakos, C.; Katakis, S.; Makedonas, A.; Theoharatos, C.; Lalos, A. Analysis, modeling and multi-spectral sensing for the predictive management of verticillium wilt in olive groves. J. Sens. Actuator Netw. 2021, 10, 15. [Google Scholar] [CrossRef]
- Cano Marchal, P.; Martínez Gila, D.; Illana Rico, S.; Gómez Ortega, J.; Gámez García, J. Assessment of the nutritional state for olive trees using uavs. In CONTROLO 2020: Proceedings of the 14th APCA International Conference on Automatic Control and Soft Computing, July 1–3, 2020, Bragança, Portugal; Springer: Cham, Switzerland, 2021; Volume 695, p. 695. [Google Scholar]
- Delogu, E.; Olioso, A.; Alliès, A.; Demarty, J.; Boulet, G. Evaluation of multiple methods for the production of continuous evapotranspiration estimates from tir remote sensing. Remote Sens. 2021, 13, 1086. [Google Scholar] [CrossRef]
- Noguera, M.; Aquino, A.; Ponce, J.M.; Cordeiro, A.; Silvestre, J.; Arias-Calderón, R.; Marcelo, M.d.E.; Jordão, P.; Andújar, J.M. Nutritional status assessment of olive crops by means of the analysis and modelling of multispectral images taken with UAVs. Biosyst. Eng. 2021, 211, 1–18. [Google Scholar] [CrossRef]
- Hornero, A.; Hernández-Clemente, R.; North, P.; Beck, P.; Boscia, D.; Navas-Cortes, J.; Zarco-Tejada, P. Monitoring the incidence of Xylella fastidiosa infection in olive orchards using ground-based evaluations, airborne imaging spectroscopy and Sentinel-2 time series through 3-D radiative transfer modelling. Remote Sens. Environ. 2020, 236, 111480. [Google Scholar] [CrossRef]
- Jurado, J.M.; Ortega, L.; Cubillas, J.J.; Feito, F.R. Multispectral mapping on 3D models and multi-temporal monitoring for in-dividual characterization of olive trees. Remote Sens. 2020, 12, 1106. [Google Scholar] [CrossRef]
- Stateras, D.; Kalivas, D. Assessment of olive tree canopy characteristics and yield forecast model using high resolution uav imagery. Agriculture 2020, 10, 385. [Google Scholar] [CrossRef]
- Jorge, J.; Vallbé, M.; Soler, J.A. Detection of irrigation inhomogeneities in an olive grove using the NDRE vegetation index obtained from UAV images. Eur. J. Remote Sens. 2019, 52, 169–177. [Google Scholar] [CrossRef]
- Rey, B.; Aleixos, N.; Cubero, S.; Blasco, J. XF-ROVIM. A field robot to detect olive trees infected by Xylella fastidiosa using proximal sensing. Remote Sens. 2019, 11, 221. [Google Scholar] [CrossRef]
- Solano, F.; Di Fazio, S.; Modica, G. A methodology based on GEOBIA and WorldView-3 imagery to derive vegetation indices at tree crown detail in olive orchards. Int. J. Appl. Earth Obs. Geoinformation 2019, 83, 101912. [Google Scholar] [CrossRef]
- Egea, G.; Padilla-Díaz, C.M.; Martinez-Guanter, J.; Fernández, J.E.; Pérez-Ruiz, M. Assessing a crop water stress index derived from aerial thermal imaging and infrared thermometry in super-high density olive orchards. Agric. Water Manag. 2017, 187, 210–221. [Google Scholar] [CrossRef]
- Estornell, J.; Ruiz, L.; Velázquez-Martí, B.; López-Cortés, I.; Salazar, D.; Fernández-Sarría, A. Estimation of pruning biomass of olive trees using airborne discrete-return LiDAR data. Biomass Bioenergy 2015, 81, 315–321. [Google Scholar] [CrossRef]
- López-Granados, F.; Gómez-Casero, M.T.; Peña-Barragán, J.M.; Jurado-Expósito, M.; García-Torres, L. Classifying irrigated crops as affected by phenological stage using discriminant analysis and neural networks. J. Am. Soc. Hortic. Sci. 2010, 135, 465–473. [Google Scholar] [CrossRef]
- Mamdouh, N.; Wael, M.; Khattab, A. Artificial intelligence-based detection and counting of olive fruit flies: A comprehensive survey. In Deep Learning for Sustainable Agriculture; Academic Press: Cambridge, MA, USA, 2022; pp. 357–380. [Google Scholar]
- Amr, A.; Sadder, M.; Sakarneh, N. Review Article Olive Fruit Fly Bacterocera Oleae Infestation of Olives: Effect on Quality and Detection in Olive Oil. Jordan J. Agric. Sci. 2023, 19, 56–69. [Google Scholar] [CrossRef]
- Dhonju, H.K.; Walsh, K.B.; Bhattarai, T. Management Information Systems for Tree Fruit—1: A Review. Horticulturae 2024, 10, 108. [Google Scholar] [CrossRef]
- Hallouti, A.; Ben El Caid, M.; Boubaker, H. Mediterranean fruit fly Ceratitis capitata (Wiedemann) management strategies and recent advances: A review. Int. J. Pest Manag. 2024, 1–13. [Google Scholar] [CrossRef]
- Rodias, E.; Evangelou, E.; Lampridi, M.; Bochtis, D. A Decision Support System for Green Crop Fertilization Planning. In Information and Communication Technologies for Agriculture—Theme III: Decision; Springer: Cham, Switzerland, 2022; Volume 184, pp. 265–278. [Google Scholar]
- Zhai, Z.; Martínez, J.F.; Beltran, V.; Martínez, N.L. Decision support systems for agriculture 4.0: Survey and challenges. Comput. Electron. Agric. 2020, 170, 105256. [Google Scholar] [CrossRef]
- Miranda, M.; Barceló, C.; Valdés, F.; Feliu, J.F.; Nestel, D.; Papadopoulos, N.; Sciarretta, A.; Ruiz, M.; Alorda, B. Developing and implementation of decision support system (dss) for the control of olive fruit fly, Bactrocera oleae, in mediterranean olive orchards. Agronomy 2019, 9, 620. [Google Scholar] [CrossRef]
- Pontikakos, C.M.; Tsiligiridis, T.A.; Yialouris, C.P.; Kontodimas, D.C. Pest management control of olive fruit fly (Bactrocera oleae) based on a location-aware agro-environmental system. Comput. Electron. Agric. 2012, 87, 39–50. [Google Scholar] [CrossRef]
- Murali, N.; Schneider, J.; Levine, J.; Taylor, G. Classification and re-identification of fruit fly individuals across days with convolutional neural networks. In Proceedings of the 2019 IEEE Winter Conference on Applications of Computer Vision (WACV), Waikoloa, HI, USA, 7–11 January 2019; pp. 570–578. [Google Scholar]
- Mamdouh, N.; Khattab, A. YOLO-Based Deep Learning Framework for Olive Fruit Fly Detection and Counting. IEEE Access 2021, 9, 84252–84262. [Google Scholar] [CrossRef]
- Moitra, P.; Bhagat, D.; Kamble, V.B.; Umarji, A.M.; Pratap, R.; Bhattacharya, S. First example of engineered β-cyclodextrinylated MEMS devices for volatile pheromone sensing of olive fruit pests. Biosens. Bioelectron. 2021, 173, 112728. [Google Scholar] [CrossRef]
- Doitsidis, L.; Fouskitakis, G.N.; Varikou, K.N.; Rigakis, I.I.; Chatzichristofis, S.A.; Papafilippaki, A.K.; Birouraki, A.E. Remote monitoring of the Bactrocera oleae (Gmelin) (Diptera: Tephritidae) population using an automated McPhail trap. Comput. Electron. Agric. 2017, 137, 69–78. [Google Scholar] [CrossRef]
- Potamitis, I.; Rigakis, I.; Fysarakis, K. The electronic mcphail trap. Sensors 2014, 14, 22285–22299. [Google Scholar] [CrossRef] [PubMed]
- Kalamatianos, R.; Karydis, I.; Doukakis, D.; Avlonitis, M. DiRT: The DACUS image recognition toolkit. J. Imaging 2018, 4, 129. [Google Scholar] [CrossRef]
- Tannous, M.; Stefanini, C.; Romano, D. A Deep-Learning-Based Detection Approach for the Identification of Insect Species of Economic Importance. Insects 2023, 14, 148. [Google Scholar] [CrossRef] [PubMed]
- Moscetti, R.; Haff, R.P.; Stella, E.; Contini, M.; Monarca, D.; Cecchini, M.; Massantini, R. Feasibility of NIR spectroscopy to detect olive fruit infested by Bactrocera oleae. Postharvest Biol. Technol. 2015, 99, 58–62. [Google Scholar] [CrossRef]
- Mraicha, F.; Ksantini, M.; Zouch, O.; Ayadi, M.; Sayadi, S.; Bouaziz, M. Effect of olive fruit fly infestation on the quality of olive oil from Chemlali cultivar during ripening. Food Chem. Toxicol. 2010, 48, 3235–3241. [Google Scholar] [CrossRef]
- Kalamatianos, R.; Karydis, I.; Avlonitis, M. Methods for the identification of microclimates for olive fruit fly. Agronomy 2019, 9, 337. [Google Scholar] [CrossRef]
- Lello, F.; Dida, M.; Mkiramweni, M.; Matiko, J.; Akol, R.; Nsabagwa, M.; Katumba, A. Fruit fly automatic detection and monitoring techniques: A review. Smart Agric. Technol. 2023, 5, 100294. [Google Scholar] [CrossRef]
- Coulibaly, S.; Kamsu-Foguem, B.; Kamissoko, D.; Traore, D. Deep learning for precision agriculture: A bibliometric analysis. Intell. Syst. Appl. 2022, 16, 200102. [Google Scholar] [CrossRef]
- Morrone, L.; Neri, L.; Facini, O.; Galamini, G.; Ferretti, G.; Rotondi, A. Influence of Chabazite Zeolite Foliar Applications Used for Olive Fruit Fly Control on Volatile Organic Compound Emission, Photosynthesis, and Quality of Extra Virgin Olive Oil. Plants 2024, 13, 698. [Google Scholar] [CrossRef]
- Tzerakis, K.; Psarras, G.; Kourgialas, N.N. Developing an Open-Source IoT Platform for Optimal Irrigation Scheduling and Decision-Making: Implementation at Olive Grove Parcels. Water 2023, 15, 1739. [Google Scholar] [CrossRef]
| Field Operation/Crop Focus Stage | Platform Means | Country | Reference |
|---|---|---|---|
| Disease control, tree identification, vigor | UAV | Greece | [84] |
| Fertilization, vigor | UAV | Spain | [85] |
| Irrigation | Satellite | Tunisia | [86] |
| Fertilization, vigor | UAV | Spain | [87] |
| Disease control | Satellite, manned flight | Italy | [88] |
| Vigor, tree identification | UAV | Spain | [89] |
| Yield prediction, vigor | UAV | Greece | [90] |
| Irrigation, vigor | UAV | Spain | [91] |
| Disease control, vigor | Unmanned ground vehicle | Italy | [92] |
| Vigor, tree/row identification | Satellite | Italy | [93] |
| Vigor, irrigation | UAV | Spain | [94] |
| Pruning biomass assessment | Manned flight | Spain | [95] |
| Variety/phenology, vigor | Proximal measurements | Spain | [96] |
| Type of Control 1 | Methodological Approach | Results | Reference |
|---|---|---|---|
| T | DSS demonstration (software and hardware) | 37% insecticide reduction, 42% reduction of spray duration | [103] |
| T | GIS-based LAS system | Reduced spray solution (5%), increased spray effectiveness (6%) | [104] |
| T | DL-based approach for olive flies’ recognition | >90% identification accuracy | [105] |
| T | DL framework for detection and counting of flies | Average detection precision ~97% | [106] |
| P/T | Female B. oleae pheromone detection by using MEMS device | Detection limit ~ 0.297 ppq | [107] |
| T | Remote monitoring by McPhail trap | Accuracy counting ~75% | [108] |
| T | McPhail trap demonstration (hardware) | App. 7.5% false alarms | [109] |
| T | Image recognition toolkit demonstration (hardware) | Accuracy app. 92% | [110] |
| P/T | DL-based detection approach | Precision rate 93% | [111] |
| T | NIR spectroscopy for hidden B. oleae damage detection | Classification accuracy ~94% | [112] |
| T | Olive oil quality based on infestation level and maturity | Early ripening stage min. damage by B. oleae | [113] |
| T | NN-based classification for microclimate identification methods for olive fly detection | Algorithm performance app. 66% | [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arvaniti, O.S.; Rodias, E.; Terpou, A.; Afratis, N.; Athanasiou, G.; Zahariadis, T. Bactrocera oleae Control and Smart Farming Technologies for Olive Orchards in the Context of Optimal Olive Oil Quality: A Review. Agronomy 2024, 14, 2586. https://doi.org/10.3390/agronomy14112586
Arvaniti OS, Rodias E, Terpou A, Afratis N, Athanasiou G, Zahariadis T. Bactrocera oleae Control and Smart Farming Technologies for Olive Orchards in the Context of Optimal Olive Oil Quality: A Review. Agronomy. 2024; 14(11):2586. https://doi.org/10.3390/agronomy14112586
Chicago/Turabian StyleArvaniti, Olga S., Efthymios Rodias, Antonia Terpou, Nikolaos Afratis, Gina Athanasiou, and Theodore Zahariadis. 2024. "Bactrocera oleae Control and Smart Farming Technologies for Olive Orchards in the Context of Optimal Olive Oil Quality: A Review" Agronomy 14, no. 11: 2586. https://doi.org/10.3390/agronomy14112586
APA StyleArvaniti, O. S., Rodias, E., Terpou, A., Afratis, N., Athanasiou, G., & Zahariadis, T. (2024). Bactrocera oleae Control and Smart Farming Technologies for Olive Orchards in the Context of Optimal Olive Oil Quality: A Review. Agronomy, 14(11), 2586. https://doi.org/10.3390/agronomy14112586









