Responses of Legumes to Rhizobia and Arbuscular Mycorrhizal Fungi Under Abiotic Stresses: A Global Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Survey and Data Compilation
2.2. Data Analysis
3. Results
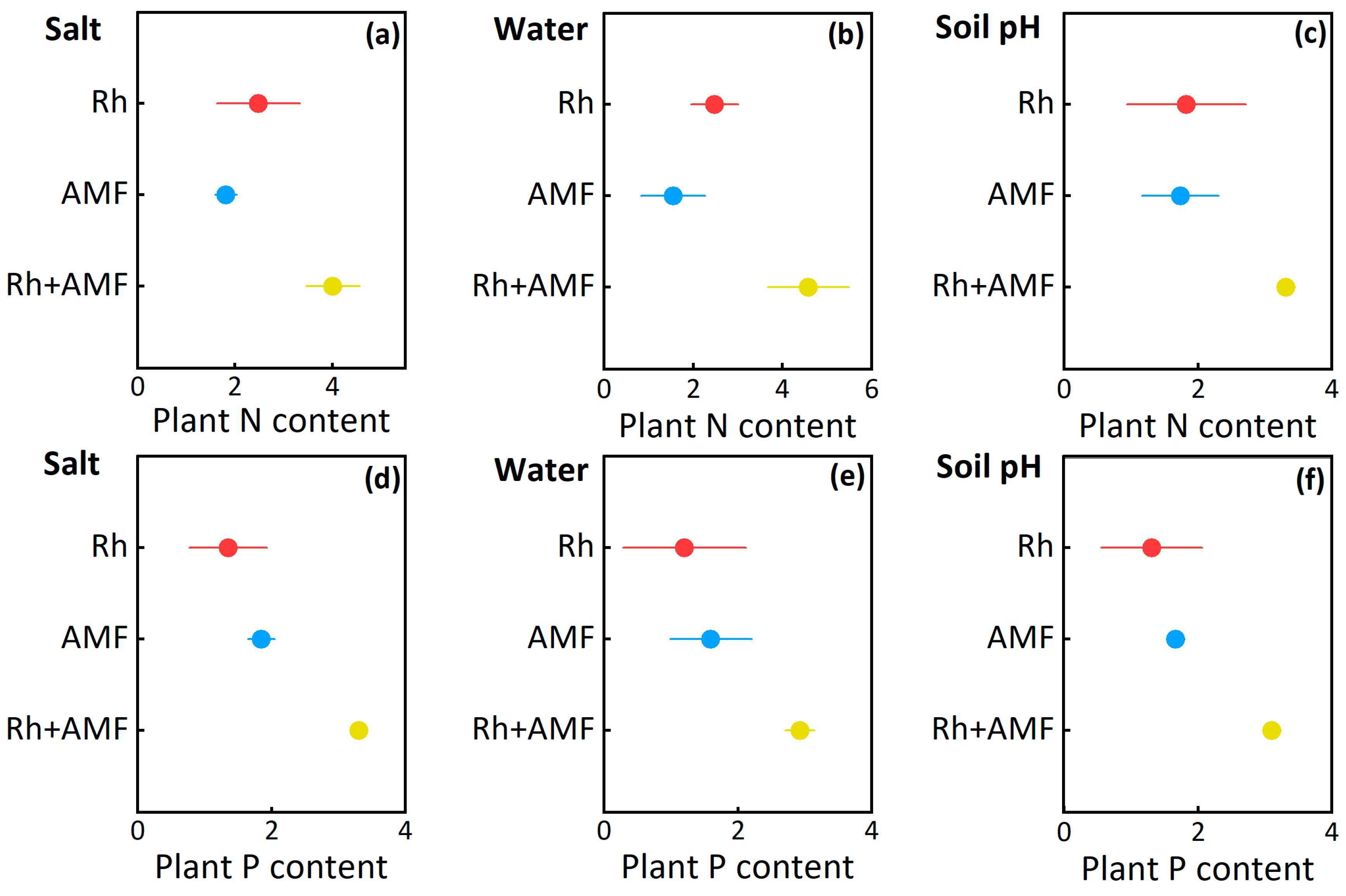
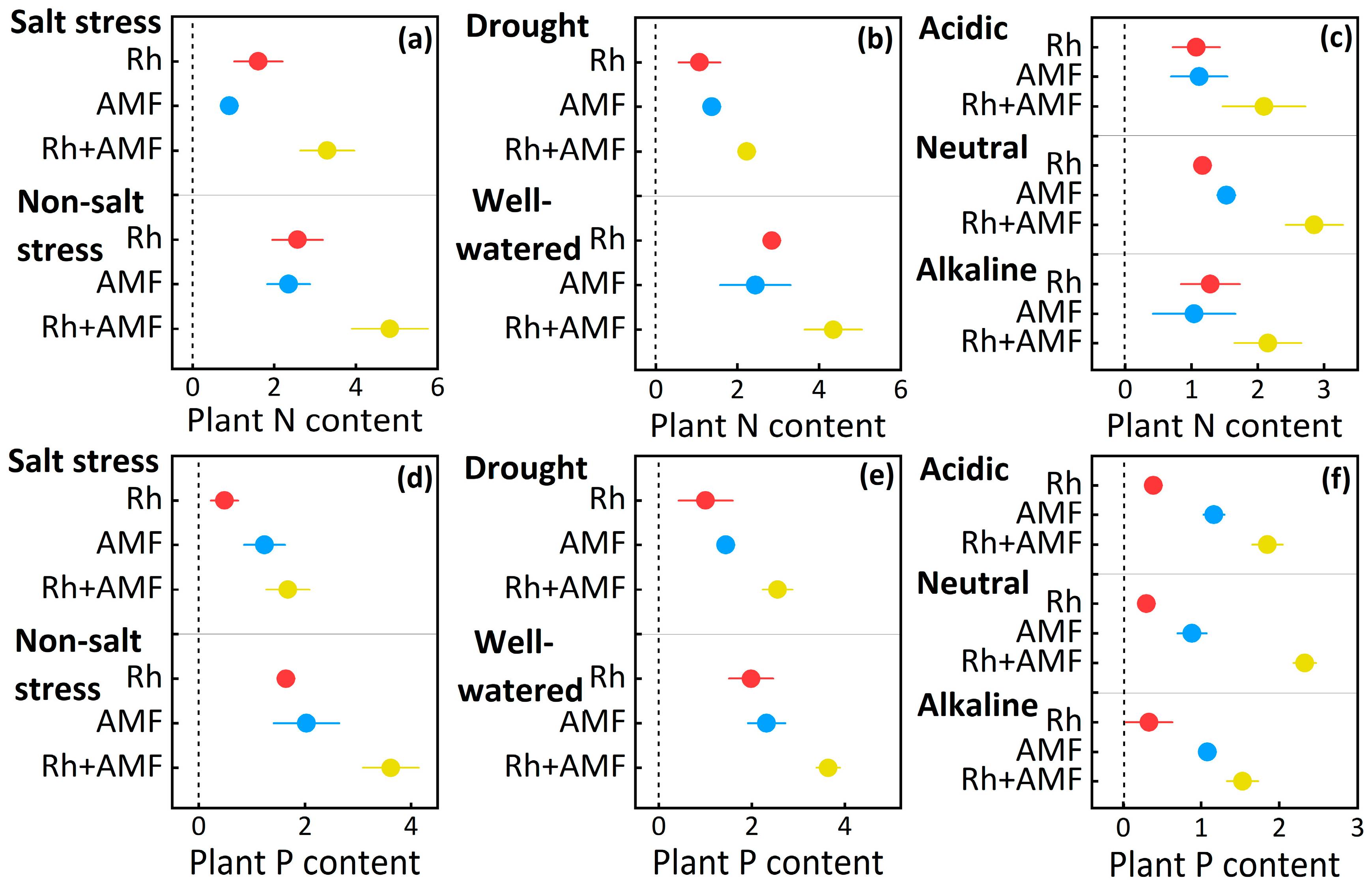
3.1. Plant Nitrogen and Phosphorus Content Responses to Symbionts
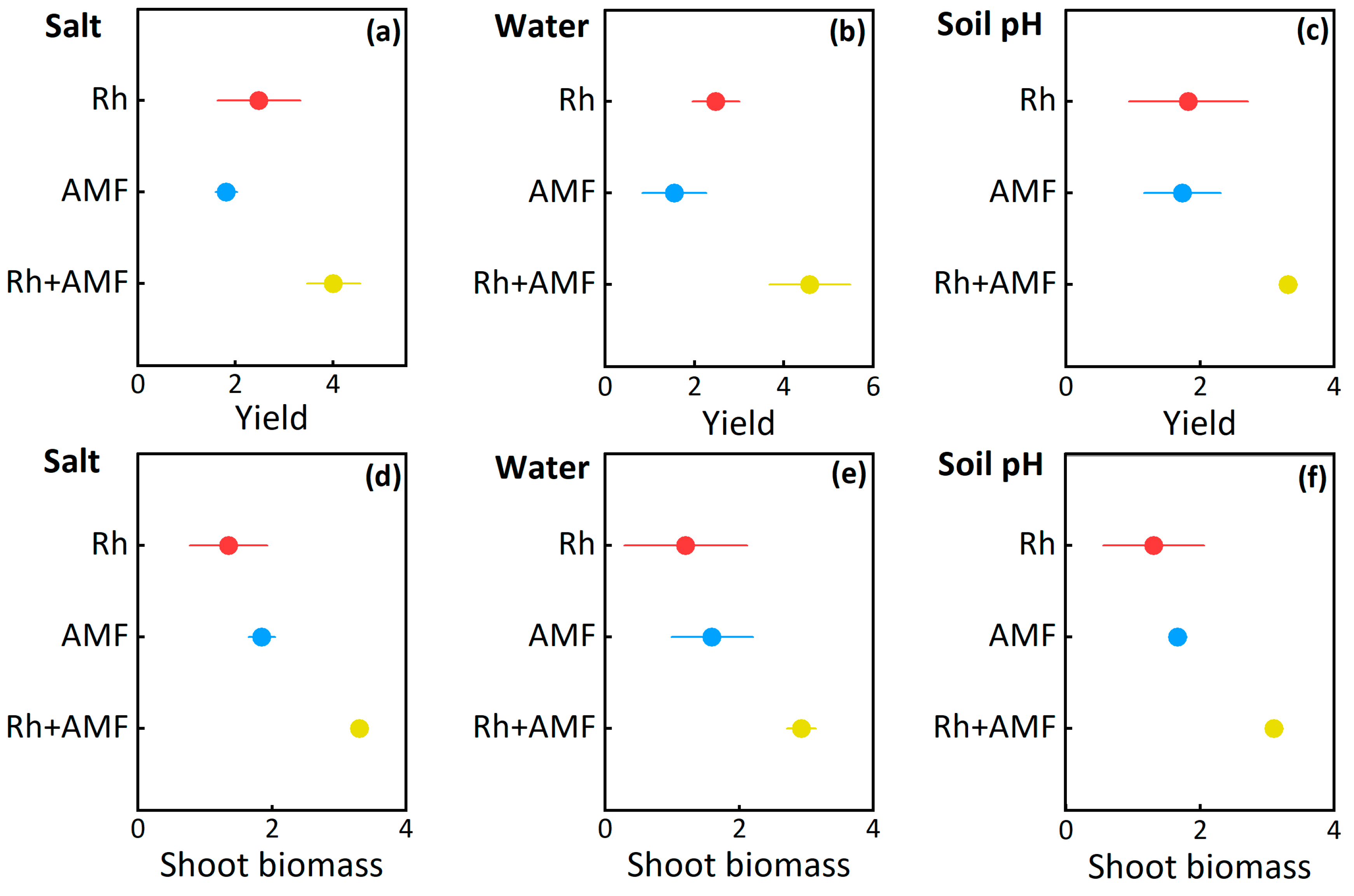
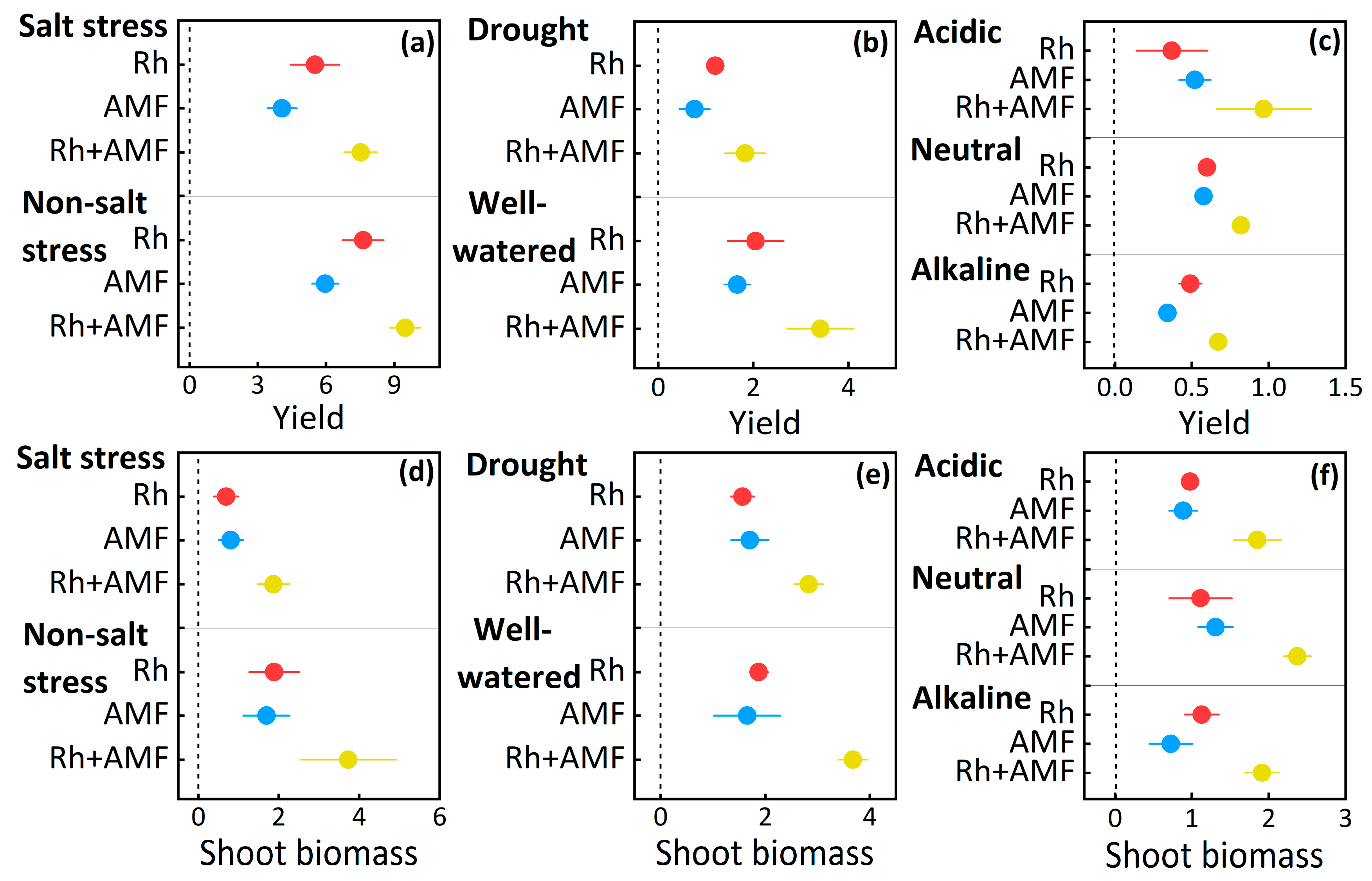
3.2. Plant Yield and Shoot Biomass Responses to Symbionts
3.3. Microbial Responses to Symbionts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hoegh-Guldberg, O.; Jacob, D.; Taylor, M.; Guillén Bolaños, T.; Bindi, M.; Brown, S.; Camilloni, I.A.; Diedhiou, A.; Djalante, R.; Ebi, K.; et al. The human imperative of stabilizing global climate change at 1.5 C. Science 2019, 365, eaaw6974. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; La, S.; Zhang, X.; Gao, L.; Tian, Y. Salt-induced recruitment of specific root-associated bacterial consortium capable of enhancing plant adaptability to salt stress. ISME J. 2021, 15, 2865–2882. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Gao, Q.; Liu, J.; Tang, J.; Hua, Z.; Sun, X. Categories of exogenous substances and their effect on alleviation of plant salt stress. Eur. J. Agron. 2023, 142, 126656. [Google Scholar] [CrossRef]
- von Storch, H. A global problem. Nature 2004, 429, 244–245. [Google Scholar] [CrossRef]
- Qiu, S.Y.; Liu, S.S.; Wei, S.J.; Cui, X.H.; Nie, M.; Huang, J.X.; He, Q.; Ju, R.T.; Li, B. Changes in multiple nvironmental factors additively enhance the dominance of an exotic plant with a novel trade-off pattern. J. Ecol. 2020, 108, 1989–1999. [Google Scholar] [CrossRef]
- Kahmen, A.; Perner, J.; Buchmann, N. Diversity-dependent productivity in semi-natural grasslands following climate perturbations. Funct. Ecol. 2015, 19, 594–601. [Google Scholar] [CrossRef]
- Xu, X.; Guo, L.; Wang, S.; Wang, X.; Ren, M.; Zhao, P.; Huang, Z.; Jia, H.; Wang, J.; Lin, A. Effective strategies for reclamation of saline-alkali soil and response mechanisms of the soil-plant system. Sci. Total Environ. 2023, 905, 167179. [Google Scholar] [CrossRef]
- Dietz, K.J.; Zörb, C.; Geilfus, C.M. Drought and crop yield. Plant Biol. 2021, 23, 881–893. [Google Scholar] [CrossRef]
- Zörb, C.; Geilfus, C.M.; Dietz, K.J. Salinity and crop yield. Plant Biol. 2019, 21, 31–38. [Google Scholar] [CrossRef]
- Melino, V.; Tester, M. Salt-Tolerant Crops: Time to Deliver. Annu. Rev. Plant Biol. 2023, 74, 671–696. [Google Scholar] [CrossRef]
- Maestre, F.T.; Delgado-Baquerizo, M.; Jeffries, T.C.; Eldridge, D.J.; Ochoa, V.; Gozalo, B.; Quero, J.L.; García-Gómez, M.; Gallardo, A.; Ulrich, W.; et al. Increasing aridity reduces soil microbial diversity and abundance in global drylands. Proc. Natl. Acad. Sci. USA 2015, 112, 15684–15689. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Q.; Meng, B.; Chai, H.; Yang, X.C.; Song, W.Z.; Li, S.X.; Lu, A.; Zhang, T.; Sun, W. Arbuscular mycorrhizal fungi alleviate drought stress in C3 (Leymus chinensis) and C4 (Hemarthria altissima) grasses via altering antioxidant enzyme activities and photosynthesis. Front. Plant Sci. 2019, 10, 499. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.L.; Chen, X.N.; Aili YL, N.E.; Ma, X.D. Effects of double nocalculation with arbuscular mycorrhizal fungi and rhizobia under different water treatments on growth and nitrogen transfer of Alhagi sparsifolia. Acta Ecol. Sin. 2022, 42, 6816–6826. [Google Scholar]
- Duan, H.X.; Luo, C.L.; Zhu, Y.; Zhao, L.; Wang, J.; Wang, W.; Xiong, Y.C. Arbuscular mycorrhizal fungus activates wheat physiology for higher reproductive allocation under drought stress in primitive and modern wheat. Eur. J. Agron. 2024, 161, 127376. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Rehman, M.M.U.; Zhu, Y.; Abrar, M.; Khan, W.; Wang, W.; Iqbal, A.; Khan, A.; Chen, Y.; Rafiq, M.; Tufail, M.A.; et al. Moisture-and period-dependent interactive effects of plant growth-promoting rhizobacteria and AM fungus on water use and yield formation in dryland wheat. Plant Soil 2024, 502, 149–165. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Paramasivan, M. Arbuscular mycorrhizal fungi and antioxidant enzymes in ameliorating drought stress: A meta-analysis. Plant Soil 2022, 480, 295–303. [Google Scholar] [CrossRef]
- Yilmaz, A.; Yildirim, E.; Yilmaz, H.; Soydemir, H.E.; Güler, E.; Ciftci, V.; Yaman, M. Use of arbuscular mycorrhizal fungi for boosting antioxidant enzyme metabolism and mitigating saline stress in Sweet Basil (Ocimum basilicum L.). Sustainability 2023, 15, 5982. [Google Scholar] [CrossRef]
- Duan, H.X.; Shi, Q.; Kang, S.P.; Gou, H.Q.; Luo, C.L.; Xiong, Y.C. Advances in research on the interactions among arbuscular mycorrhizal fungi, rhizobia, and plants. Acta Prataculturae Sin. 2024, 33, 166–182. [Google Scholar]
- Parihar, M.; Rakshit, A.; Rana, K.; Tiwari, G.; Jatav, S.S. The Effect of Arbuscular Mycorrhizal Fungi Inoculation in Mitigating Salt Stress of Pea (Pisum sativum L.). Commun. Soil Sci. Plant Anal. 2020, 51, 1545–1559. [Google Scholar] [CrossRef]
- Duan, H.X.; Luo, C.L.; Li, J.Y.; Wang, B.Z.; Naseer, M.; Xiong, Y.C. Improvement of wheat productivity and soil quality by arbuscular mycorrhizal fungi is density– and moisture–dependent. Agron. Sustain. Dev. 2021, 41, 1–12. [Google Scholar] [CrossRef]
- Abd-Alla, M.H.; El-Enany, A.W.E.; Nafady, N.A.; Khalaf, D.M.; Morsy, F.M. Synergistic interaction of Rhizobium leguminosarum bv. viciae and arbuscular mycorrhizal fungi as a plant growth promoting biofertilizers for faba bean (Vicia faba L.) in alkaline soil. Microbiol. Res. 2014, 169, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Kavadia, A.; Omirou, M.; Fasoula, D.A.; Louka, F.; Ehaliotis, C.; Ioannides, I.M. Co-inoculations with rhizobia and arbuscular mycorrhizal fungi alters mycorrhizal composition and lead to synergistic growth effects in cowpea that are fungal combination-dependent. Appl. Soil Ecol. 2021, 167, 104013. [Google Scholar] [CrossRef]
- Pereira, S.; Mucha, A.; Goncalves, B.; Bacelar, E.; Latr, A.; Ferreira, H.; Oliveira, I.; Rosa, E.; Marques, G. Improvement of some growth and yield parameters of faba bean (Vicia faba) by inoculation with Rhizobium laguerreae and arbuscular mycorrhizal fungi. Crop Pasture Sci. 2019, 70, 595–605. [Google Scholar] [CrossRef]
- Ossler, J.N.; Zielinski, C.A.; Heath, K.D. Tripartite mutualism: Facilitation or trade-offs between rhizobial and mycorrhizal symbionts of legume hosts. Am. J. Bot. 2015, 102, 1–10. [Google Scholar] [CrossRef]
- Hao, Z.P.; Xie, W.; Jiang, X.L.; Wu, Z.X.; Zhang, X.; Chen, B.D. Arbuscular mycorrhizal fungus improves rhizobium–Glycyrrhiza seedling symbiosis under drought stress. Agronomy 2019, 9, 572. [Google Scholar] [CrossRef]
- Zhou, J.; Wilson, G.W.; Cobb, A.B.; Zhang, Y.; Liu, L.; Zhang, X.; Sun, F. Mycorrhizal and rhizobial interactions influence model grassland plant community structure and productivity. Mycorrhiza 2022, 32, 15–32. [Google Scholar] [CrossRef]
- Newsham, K.K. A meta-analysis of plant responses to dark septate root endophytes. New Phytol. 2011, 190, 783–793. [Google Scholar] [CrossRef]
- Koricheva, J.; Gurevitch, J.; Mengersen, K. Handbook of Meta-Analysis in Ecology and Evolution; Princeton University Press: Princeton, NJ, USA, 2013. [Google Scholar]
- Kuppler, J.; Kotowska, M.M. A meta-analysis of responses in floral traits and flower–visitor interactions to water deficit. Glob. Chang. Biol. 2021, 27, 3095–3108. [Google Scholar] [CrossRef]
- Nakagawa, S.; Santos, E.S. Methodological issues and advances in biological meta-analysis. Evol. Ecol. 2012, 26, 1253–1274. [Google Scholar] [CrossRef]
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al. Scientists’ warning to humanity: Microorganisms and climate change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Shi, W.; Kronzucker, H.J. How Plant Root Exudates Shape the Nitrogen Cycle. Trends Plant Sci. 2017, 22, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant-microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 1, 607–621. [Google Scholar] [CrossRef] [PubMed]
- de Vries, F.T.; Griffiths, R.I.; Knight, C.G.; Nicolitch, O.; Williams, A. Harnessing rhizosphere microbiomes for drought-resilient crop production. Science 2020, 368, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.N.; Wang, W.X.; Xie, Q.J.; Liu, L.X.; Zhang, D.P. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 2017, 356, 1172–1175. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef]
- Primieri, S.; Magnoli, S.M.; Koffel, T.; Stürmer, S.L.; Bever, J.D. Perennial, but not annual legumes synergistically benefit from infection with arbuscular mycorrhizal fungi and rhizobia: A meta-analysis. New Phytol. 2022, 233, 505–514. [Google Scholar] [CrossRef]
- Bai, B.; Suri, V.K.; Kumar, A.; Choudhary, A.K. Tripartite symbiosis of Pisum-Glomus-Rhizobium leads to enhanced productivity, nitrogen and phosphorus economy, quality, and biofortification in garden pea in a Himalayan acid Alfisol. J. Plant Nutr. 2017, 40, 600–613. [Google Scholar] [CrossRef]
- Leite, R.D.; Martins, L.C.; Ferreira, L.; Barbosa, E.S.; Alves BJ, R.; Zilli, J.E.; Araujo, A.P.; Jesus, E.D. Co-inoculation of Rhizobium and Bradyrhizobium promotes growth and yield of common beans. Appl. Soil Ecol. 2022, 172, 104356. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.; De Bruin, S.; Luckerhoff, L.; Van Logtestijn, R.S.; Schlaeppi, K. A widespread plant-fungal-bacterial symbiosis promotes plant biodiversity, plant nutrition and seedling recruitment. ISME J. 2016, 10, 389–399. [Google Scholar] [CrossRef]
- Ding, X.D.; Zhang, S.R.; Wang, R.P.; Li, S.; Liao, X. AM fungi and rhizobium regulate nodule growth, phosphorous (P) uptake, and soluble sugar concentration of soybeans experiencing P deficiency. J. Plant Nutr. 2016, 39, 1915–1925. [Google Scholar] [CrossRef]
- Loo, W.T.; Chua, K.O.; Mazumdar, P.; Cheng, A.; Osman, N.; Harikrishna, J.A. Arbuscular mycorrhizal symbiosis: A strategy for mitigating the impacts of climate change on tropical legume crops. Plants 2022, 11, 2875. [Google Scholar] [CrossRef] [PubMed]
- Tajini, F.; Trabelsi, M.; Drevon, J.J. Combined inoculation with Glomus intraradices and Rhizobium tropici CIAT899 increases phosphorus use efficiency for symbiotic nitrogen fixation in common bean (Phaseolus vulgaris L.). Saudi J. Biol. Sci. 2012, 19, 157–163. [Google Scholar] [CrossRef] [PubMed]
- van Rhijn, P.; Fang, Y.; Galili, S.; Shaul, O.; Atzmon, N.; Wininger, S.; Eshed, Y.; Lum, M.; Li, Y.; To, V.; et al. Expression of early nodulin genes in alfalfa mycorrhizae indicates that signal transduction. Proc. Natl. Acad. Sci. USA 1997, 94, 5467–5472. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Ogiwara, N.; Kaji, T.; Sugimoto, Y.; Ueno, M.; Sonoda, M.; Matsui, A.; Ishida, J.; Tanaka, M.; Totoki, Y.; et al. Transcriptome analysis of soybean (Glycine max) root genes differentially expressed in rhizobial, arbuscular mycorrhizal, and dual symbiosis. J. Plant Res. 2019, 132, 541–568. [Google Scholar] [CrossRef]
- Abdel-Lateif, K.; Bogusz, D.; Hocher, V. The role of flavonoids in the establishment of plant roots endosymbioses with arbuscular mycorrhiza fungi, rhizobia and Frankia bacteria. Plant Signal. Behav. 2012, 7, 636–641. [Google Scholar] [CrossRef]
- Catford, J.G.; Staehelin, C.; Lerat, S.; Piché, Y.; Vierheilig, H. Suppression of arbuscular mycorrhizal colonization and nodulation in split-root systems of alfalfa after pre-inoculation and treatment with Nod factors. J. Exp. Bot. 2003, 386, 1481–1487. [Google Scholar] [CrossRef]
- Tsimilli-Michael, M.; Eggenberg, P.; Biro, B.; Köves-Péchy, K.; Vörös, I.; Strasser, R. Synergistic and antagonistic effects of arbuscular mycorrhizal fungi and Azospirillum and Rhizobium nitrogen-fixers on the photosynthetic activity of alfalfa, probed by the polyphasic chlorophyll a fluorescence transient O-J-I-P. Appl. Soil Ecol. 2000, 15, 169–182. [Google Scholar] [CrossRef]
- Larimer, A.L.; Clay, K.; Bever, J.D. Synergism and context dependency of interactions between arbuscular mycorrhizal fungi and rhizobia with a prairie legume. Ecology 2014, 95, 1045–1054. [Google Scholar] [CrossRef]
- Kaschuk, G.; Leffelaar, P.A.; Giller, K.E.; Alberton, O.; Hungria, M.; Kuyper, T.W. Responses of legumes to rhizobia and arbuscular mycorrhizal fungi: A meta-analysis of potential photosynthate limitation of symbioses. Soil Biol. Biochem. 2010, 42, 125–127. [Google Scholar] [CrossRef]
- Goicoechea, N.; Merino, S.; Sánchez-Díaz, M. Arbuscular mycorrhizal fungi can contribute to maintain antioxidant and carbon metabolism in nodules of Anthyllis cytisoides L. subjected to drought. J. Pant Physiol. 2005, 162, 27–35. [Google Scholar] [CrossRef]
- Erman, M.; Demir, S.; Ocak, E.; Tüfenkçi, Ş.; Oğuz, F.; Akköprü, A. Effects of Rhizobium, arbuscular mycorrhiza and whey applications on some properties in chickpea (Cicer arietinum L.) under irrigated and rainfed conditions 1-Yield, yield components, nodulation and AMF colonization. Field Crops Res. 2011, 122, 14–24. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, Y.N.; Liu, X.; Zhou, W.N.; Wang, Q.Z. Effects of inoculation with arbuscular mycoral fungi and rhizobia on growth of Medicago sativa under Saline-alkaline stress. Acta Ecol. Sin. 2018, 38, 6143–6155. [Google Scholar]
- Ashrafi, E.; Zahedi, M.; Razmjoo, J. Co-inoculations of arbuscular mycorrhizal fungi and rhizobia under salinity in alfalfa. Soil Sci. Plant Nutr. 2014, 60, 619–629. [Google Scholar] [CrossRef]
- Shafer, S.R.; Schoeneberger, M.M.; Horton, S.J.; Davey, C.B.; Miller, J.E. Effects of rhizobium, arbuscular mycorrhizal fungi and anion content of simulated rain on subterranean clover. Environ. Pollut. 1996, 92, 55–66. [Google Scholar] [CrossRef]
- Wu, H.; Yang, J.; Fu, W.; Rillig, M.C.; Cao, Z.; Zhao, A.; Hao, Z.; Zhang, X.; Chen, B.; Han, X. Identifying thresholds of nitrogen enrichment for substantial shifts in arbuscular mycorrhizal fungal community metrics in a temperate grassland of northern China. Soil Biol. Biochem. 2023, 237, 279–294. [Google Scholar] [CrossRef]
- Liu, R.J.; Chen, Y.L. Mycorrhizology; Science Press: Beijing, China, 2007; Volume 22–24. [Google Scholar]
- Oehl, F.; Laczko, E.; Bogenrieder, A.; Stahr, K.; Bösch, R.; van der Heijden, M.; Sieverding, E. Soil type and land use intensity determine the composition of arbuscular mycorrhizal fungal communities. Soil Biol. Biochem. 2010, 42, 724–738. [Google Scholar] [CrossRef]
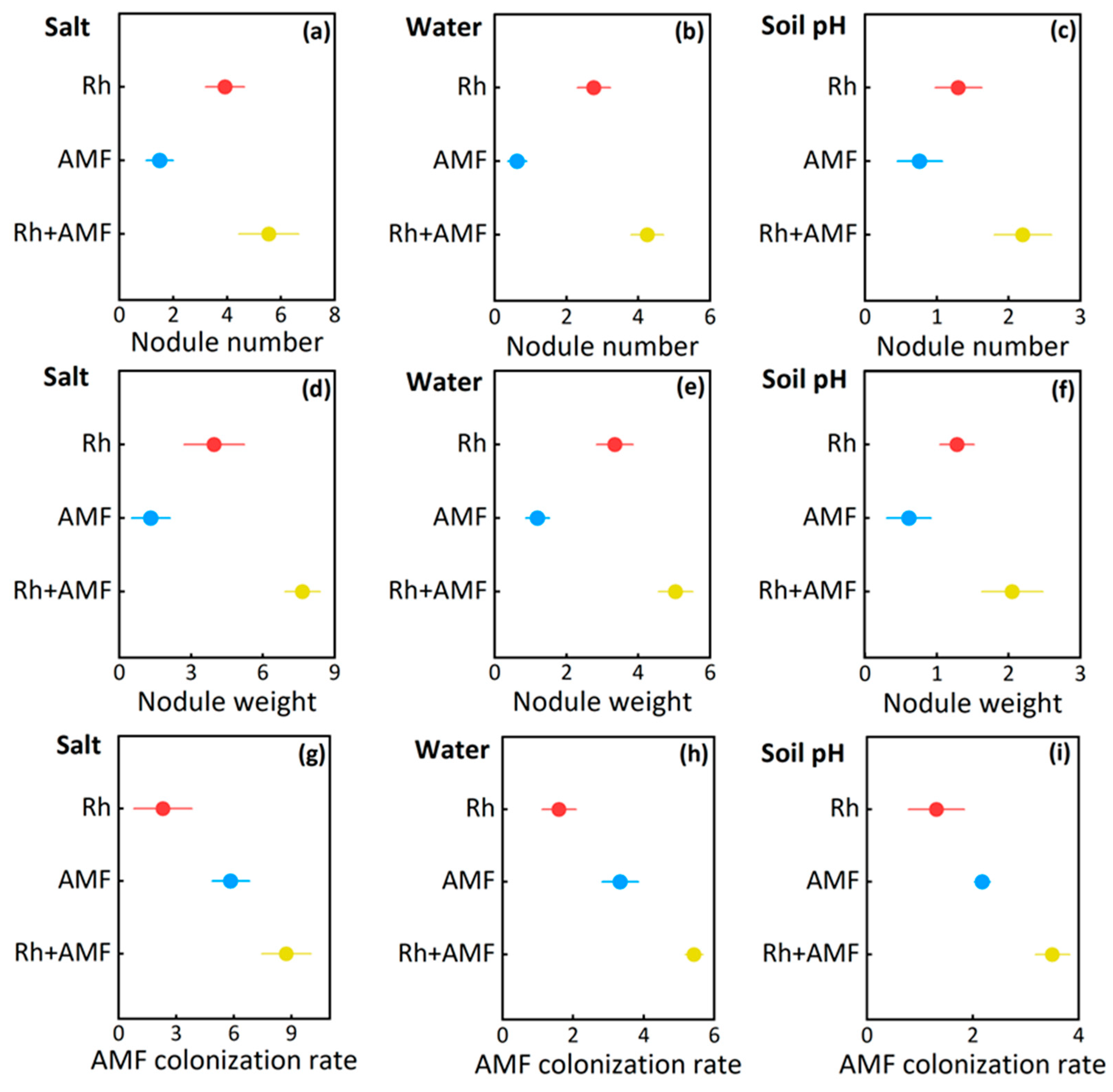
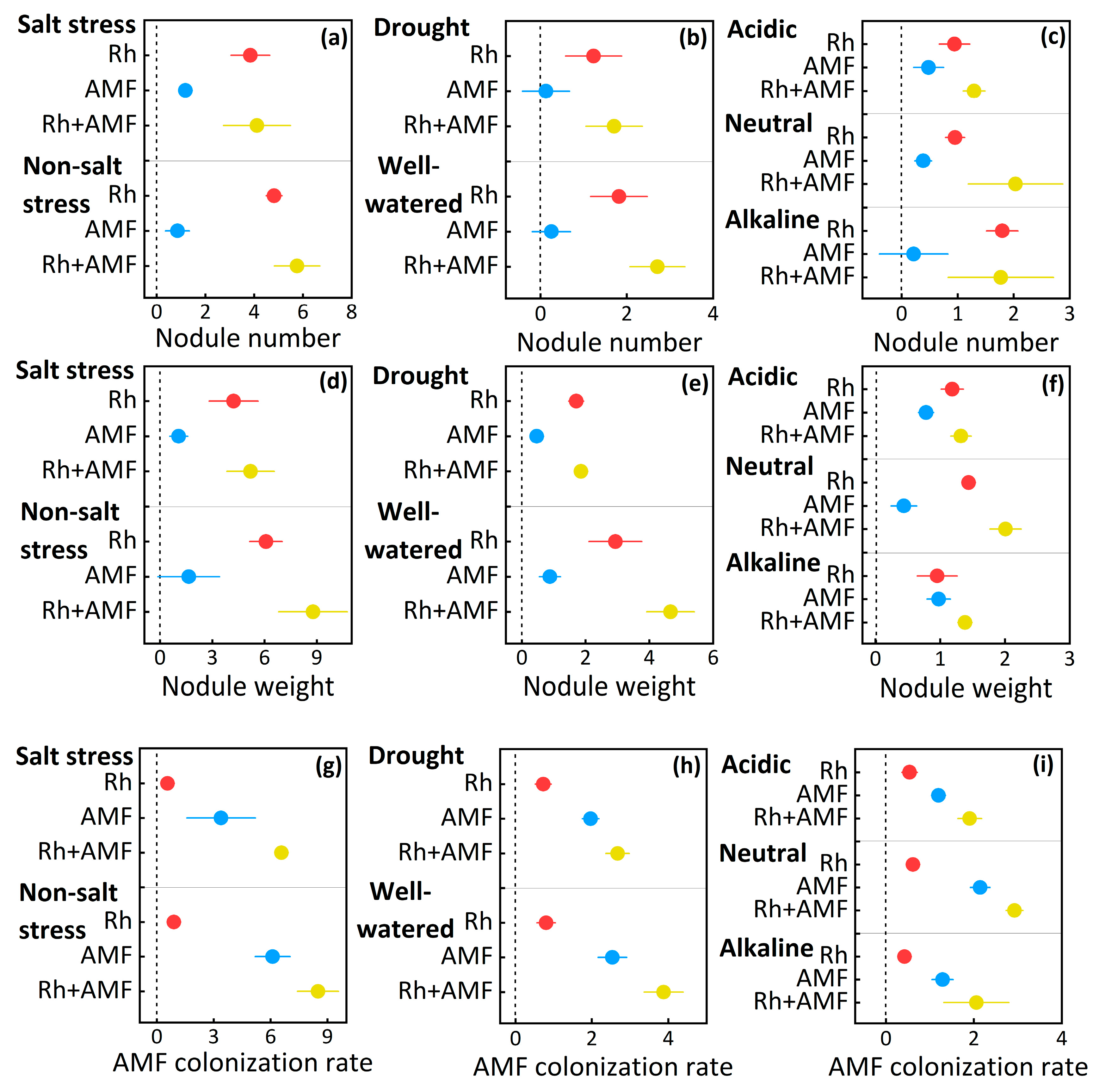
| Response Variables | Treatments | df | QM | p |
|---|---|---|---|---|
| Plant N | Inoculations | 1362 | 1125 | <0.001 |
| Plant P | Inoculations | 1298 | 527 | <0.001 |
| Yield | Inoculations | 866 | 1010 | <0.001 |
| Shoot biomass | Inoculations | 1479 | 1231 | <0.001 |
| Nodule number | Inoculations | 1164 | 420 | <0.001 |
| Nodule weight | Inoculations | 1234 | 581 | <0.001 |
| AMF colonization rate | Inoculations | 1681 | 677 | <0.001 |
| Response Variables | Moderators | Inoculations | df | QM | p |
|---|---|---|---|---|---|
| Plant N | Non-salt stress | Inoculations | 30 | 23.6 | <0.001 |
| Salt stress | Inoculations | 33 | 33.3 | 0.011 | |
| Well-watered | Inoculations | 54 | 38.7 | <0.001 | |
| Drought | Inoculations | 69 | 37.0 | <0.001 | |
| Acid soil | Inoculations | 422 | 42.0 | <0.001 | |
| Neutral soil | Inoculations | 414 | 69.4 | <0.001 | |
| Alkaline soil | Inoculations | 569 | 616 | <0.001 | |
| Plant P | Non-salt stress | Inoculations | 45 | 68.2 | <0.001 |
| Salt stress | Inoculations | 51 | 37.8 | <0.001 | |
| Well-watered | Inoculations | 43 | 50.2 | <0.001 | |
| Drought | Inoculations | 69 | 42.9 | 0.018 | |
| Acid soil | Inoculations | 409 | 36.2 | <0.001 | |
| Neutral soil | Inoculations | 411 | 521 | <0.001 | |
| Alkaline soil | Inoculations | 579 | 453 | <0.001 | |
| Shoot biomass | Non-salt stress | Inoculations | 802 | 334 | <0.001 |
| Salt stress | Inoculations | 773 | 568 | <0.001 | |
| Well-watered | Inoculations | 691 | 101 | <0.001 | |
| Drought | Inoculations | 738 | 104 | <0.001 | |
| Acid soil | Inoculations | 543 | 440 | <0.001 | |
| Neutral soil | Inoculations | 644 | 706 | <0.001 | |
| Alkaline soil | Inoculations | 688 | 742 | <0.001 | |
| Yield | Non-salt stress | Inoculations | 921 | 239 | <0.001 |
| Salt stress | Inoculations | 894 | 217 | <0.001 | |
| Well-watered | Inoculations | 799 | 246 | <0.001 | |
| Drought | Inoculations | 805 | 195 | <0.001 | |
| Acid soil | Inoculations | 718 | 207 | <0.001 | |
| Neutral soil | Inoculations | 698 | 216 | <0.001 | |
| Alkaline soil | Inoculations | 787 | 148 | <0.001 |
| Response Variables | Moderators | Inoculations | df | QM | p |
|---|---|---|---|---|---|
| Nodule number | Non-salt stress | Inoculations | 27 | 48.7 | <0.001 |
| Salt stress | Inoculations | 27 | 43.3 | <0.001 | |
| Well-watered | Inoculations | 44 | 38.7 | <0.001 | |
| Drought | Inoculations | 59 | 37.0 | <0.001 | |
| Acid soil | Inoculations | 162 | 42.0 | <0.001 | |
| Neutral soil | Inoculations | 114 | 69.4 | <0.001 | |
| Alkaline soil | Inoculations | 369 | 61.6 | <0.001 | |
| Nodule weight | Non-salt stress | Inoculations | 35 | 68.2 | <0.001 |
| Salt stress | Inoculations | 41 | 37.8 | <0.001 | |
| Well-watered | Inoculations | 53 | 50.2 | <0.001 | |
| Drought | Inoculations | 79 | 42.9 | <0.001 | |
| Acid soil | Inoculations | 209 | 62.8 | <0.001 | |
| Neutral soil | Inoculations | 111 | 52.1 | <0.001 | |
| Alkaline soil | Inoculations | 179 | 75.3 | <0.001 | |
| AMF colonization rate | Non-salt stress | Inoculations | 62 | 334 | <0.001 |
| Salt stress | Inoculations | 73 | 568 | <0.001 | |
| Well-watered | Inoculations | 91 | 101 | <0.001 | |
| Drought | Inoculations | 78 | 104 | <0.001 | |
| Acid soil | Inoculations | 87 | 440 | <0.001 | |
| Neutral soil | Inoculations | 144 | 706 | <0.001 | |
| Alkaline soil | Inoculations | 187 | 148 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, H.-X.; Luo, C.-L.; Wang, X.; Cheng, Y.-S.; Abrar, M.; Batool, A. Responses of Legumes to Rhizobia and Arbuscular Mycorrhizal Fungi Under Abiotic Stresses: A Global Meta-Analysis. Agronomy 2024, 14, 2597. https://doi.org/10.3390/agronomy14112597
Duan H-X, Luo C-L, Wang X, Cheng Y-S, Abrar M, Batool A. Responses of Legumes to Rhizobia and Arbuscular Mycorrhizal Fungi Under Abiotic Stresses: A Global Meta-Analysis. Agronomy. 2024; 14(11):2597. https://doi.org/10.3390/agronomy14112597
Chicago/Turabian StyleDuan, Hai-Xia, Chong-Liang Luo, Xia Wang, Ye-Sen Cheng, Muhammad Abrar, and Asfa Batool. 2024. "Responses of Legumes to Rhizobia and Arbuscular Mycorrhizal Fungi Under Abiotic Stresses: A Global Meta-Analysis" Agronomy 14, no. 11: 2597. https://doi.org/10.3390/agronomy14112597
APA StyleDuan, H.-X., Luo, C.-L., Wang, X., Cheng, Y.-S., Abrar, M., & Batool, A. (2024). Responses of Legumes to Rhizobia and Arbuscular Mycorrhizal Fungi Under Abiotic Stresses: A Global Meta-Analysis. Agronomy, 14(11), 2597. https://doi.org/10.3390/agronomy14112597





