Cadmium (Cd) Tolerance and Phytoremediation Potential in Fiber Crops: Research Updates and Future Breeding Efforts
Abstract
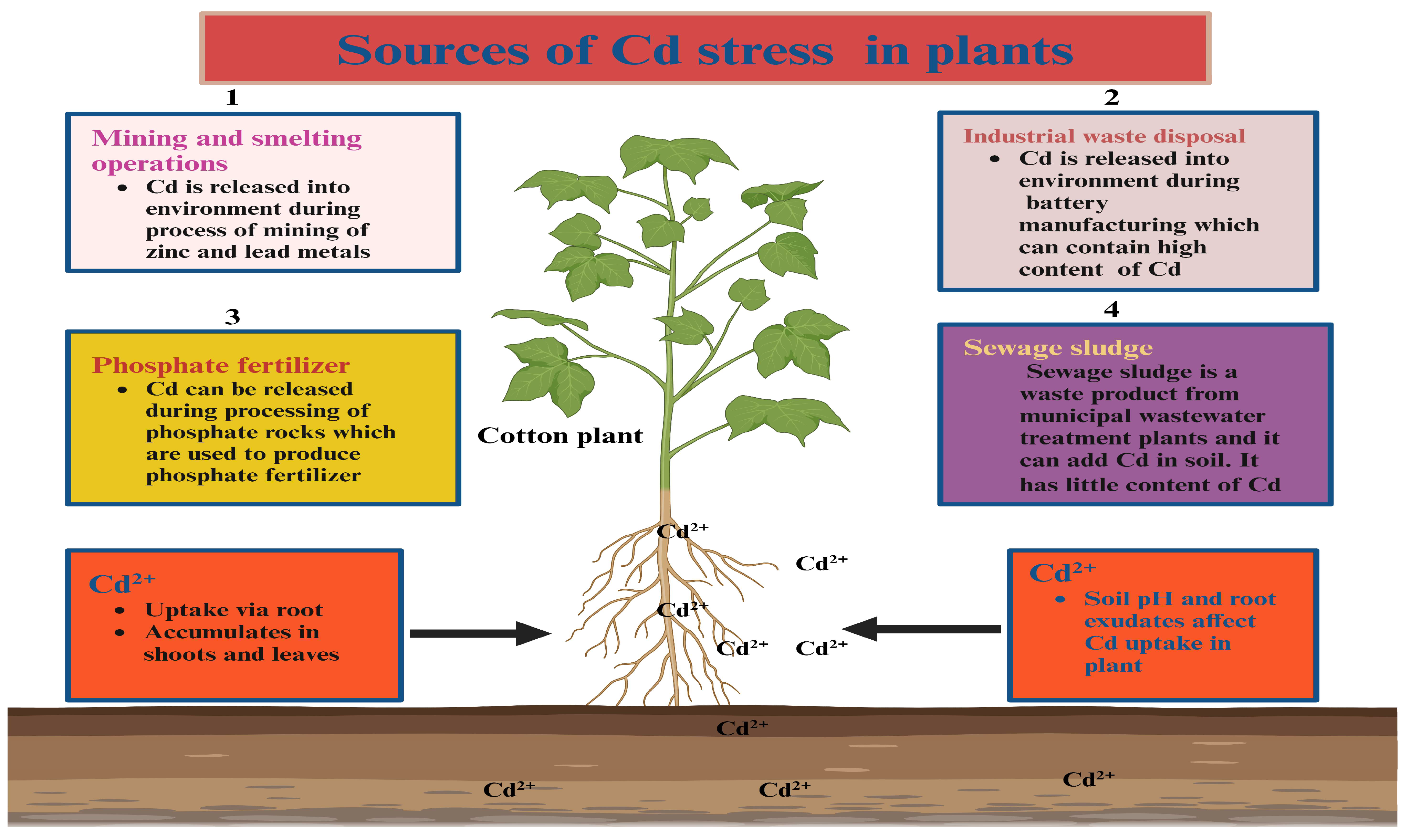
:1. Introduction
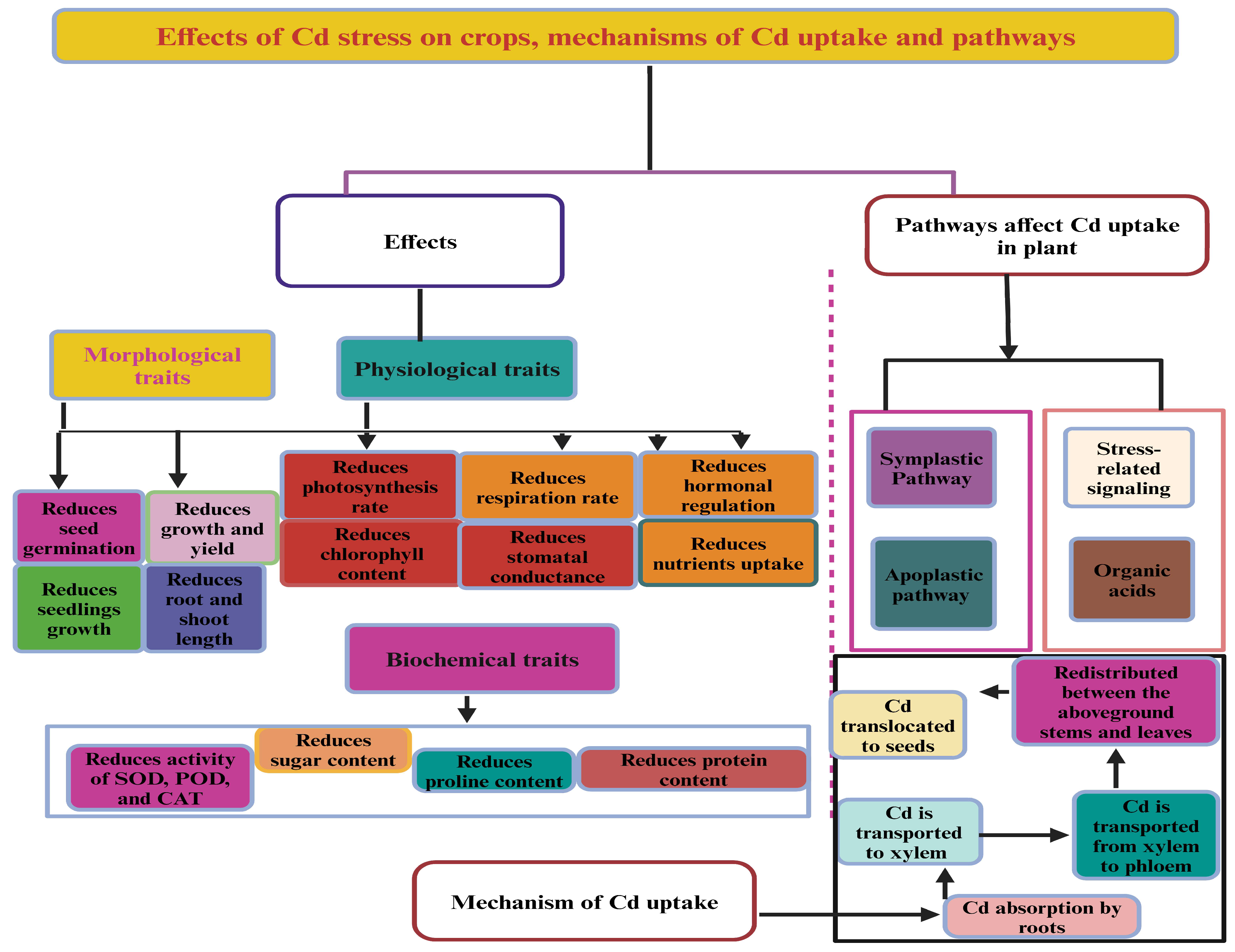
2. Effects of Cd Toxicity on Fiber Crops
2.1. Effects of Cd Stress on Kenaf
2.2. Effects of Cd Stress on Hemp, Jute, Cotton, and Flax
3. Cadmium Absorption and Accumulation in Kenaf (Hibiscus cannabinus L.)
Molecular Mechanism of Cd Tolerance in Kenaf
4. Uptake and Accumulation of Cd, and the Use of Agronomic Approaches to Increase Cd Tolerance in Cotton
Molecular Mechanism of Cd Tolerance in Cotton
5. Cadmium Tolerance in Flax, and Absorption, Accumulation, and Growth Traits
5.1. Agronomic Approaches
5.2. Role of Genetic Factors in Regulating Cd Tolerance in Flax
6. Cd Uptake, Absorption, and Accumulation in Jute
6.1. Role of Agronomic Approaches in Cd Tolerance
6.2. Molecular Factors Regulating Cd Tolerance in Jute
7. Cadmium Uptake, Accumulation, and Tolerance in Hemp (Cannabis sativa)
| Hemp Cultivars | Cd Content/Accumulation | References |
|---|---|---|
| Purple Tiger | 23.2 mg·kg−1 dw in leaves | [125] |
| KC Dora | 3 µg g−1 in seeds | [118] |
| Henola | 0.51 mg/kg in seeds | [112] |
| Apricot Auto, Alpha Explorer, Von, T1 | 1056.8, 2274.2, 512.4, 16.1 in roots. Concentration represented as mg·kg−1 dw | [122] |
| Futura 75 and Tisza | Both cultivars accumulated 0.28 and 0.21 mg/kg in roots | [127] |
| Hemp variety | Cd contents in stem and roots ranged from 0.1 to 0.4 mg/g of dry mass | [128] |
| Carmagnola | 1.7 mg/kg in fiber | [129] |
| Felina 32 | 0.39 mg/kg dry weight | [130] |
| Uso 31 | 1.80 mg kg−1 in leaves | [131] |
| Yunma No. 1 | 279.0 mg/kg | [40] |
| Santhica 27 | 5.59 mg/kg of DW in roots | [132] |
7.1. Role of Agronomic Techniques in Increasing Cd Tolerance in Hemp
7.2. Genetic Analysis of Cd Tolerance in Hemp
8. Role of CRISPR/Cas9 in Cd Tolerance in Fiber Crops
9. Conclusions and Future Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, Y.; Liu, X.; Bai, J.; Shih, K.; Zeng, E.Y.; Cheng, H. Assessing heavy metal pollution in the surface soils of a region that had undergone three decades of intense industrialization and urbanization. Environ. Sci. Pollut. Res. 2013, 20, 6150–6159. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Adeel, M.; Gulshan, A.B.; Iqbal, J.; Khan, S.; Rehman, M.; Azeem, M. Effects of organic and inorganic passivators on the immobilization of cadmium in contaminated soils: A review. Environ. Eng. Sci. 2019, 36, 986–998. [Google Scholar] [CrossRef]
- Bell, J.E.; Sherry, R.; Luo, Y. Changes in soil water dynamics due to variation in precipitation and temperature: An ecohydrological analysis in a tallgrass prairie. Water Resour. Res. 2010, 46. [Google Scholar] [CrossRef]
- Daud, M.; Quiling, H.; Lei, M.; Ali, B.; Zhu, S. Ultrastructural, metabolic and proteomic changes in leaves of upland cotton in response to cadmium stress. Chemosphere 2015, 120, 309–320. [Google Scholar] [CrossRef]
- Chai, L.; Yang, Y.; Yang, H.; Zhao, Y.; Wang, H. Transcriptome analysis of genes expressed in the earthworm Eisenia fetida in response to cadmium exposure. Chemosphere 2020, 240, 124902. [Google Scholar] [CrossRef]
- Parmar, P.; Kumari, N.; Sharma, V. Structural and functional alterations in photosynthetic apparatus of plants under cadmium stress. Bot. Stud. 2013, 54, 45. [Google Scholar] [CrossRef]
- Wani, P.; Khan, M.; Zaidi, A. Impact of heavy metal toxicity on plant growth, symbiosis, seed yield and nitrogen and metal uptake in chickpea. Aust. J. Exp. Agric. 2007, 47, 712–720. [Google Scholar] [CrossRef]
- Ci, D.; Jiang, D.; Dai, T.; Jing, Q.; Cao, W. Effects of cadmium on plant growth and physiological traits in contrast wheat recombinant inbred lines differing in cadmium tolerance. Chemosphere 2009, 77, 1620–1625. [Google Scholar] [CrossRef]
- Wang, R.; Sang, P.; Guo, Y.; Jin, P.; Cheng, Y.; Yu, H.; Xie, Y.; Yao, W.; Qian, H. Cadmium in food: Source, distribution and removal. Food Chem. 2023, 405, 134666. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Toubanaki, D.K.; Kyzas, G.Z. Detection of Arsenic, Chromium, Cadmium, Lead, and Mercury in Fish: Effects on the Sustainable and Healthy Development of Aquatic Life and Human Consumers. Sustainability 2023, 15, 16242. [Google Scholar] [CrossRef]
- Peana, M.; Pelucelli, A.; Chasapis, C.T.; Perlepes, S.P.; Bekiari, V.; Medici, S.; Zoroddu, M.A. Biological Effects of Human Exposure to Environmental Cadmium. Biomolecules 2022, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Gutsch, A.; Keunen, E.; Guerriero, G.; Renaut, J.; Cuypers, A.; Hausman, J.F.; Sergeant, K. Long-term cadmium exposure influences the abundance of proteins that impact the cell wall structure in Medicago sativa stems. Plant Biol. 2018, 20, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Yahaghi, Z.; Shirvani, M.; Nourbakhsh, F.; Pueyo, J.J. Uptake and effects of lead and zinc on alfalfa (Medicago sativa L.) seed germination and seedling growth: Role of plant growth promoting bacteria. S. Afr. J. Bot. 2019, 124, 573–582. [Google Scholar] [CrossRef]
- Ahmad, P.; Raja, V.; Ashraf, M.; Wijaya, L.; Bajguz, A.; Alyemeni, M.N. Jasmonic acid (JA) and gibberellic acid (GA3) mitigated Cd-toxicity in chickpea plants through restricted cd uptake and oxidative stress management. Sci. Rep. 2021, 11, 19768. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Azzam, M.A.; Balawi, T.A.; Raja, V.; Bhat, J.A.; Ahmad, P. Mitigation of Negative Effects of Chromium (VI) Toxicity in Faba Bean (Vicia faba) Plants through the Supplementation of Kinetin (KN) and Gibberellic Acid (GA3). Plants 2022, 11, 3302. [Google Scholar] [CrossRef]
- Mansoor, S.; Ali Wani, O.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive oxygen species in plants: From source to sink. Antioxidants 2022, 11, 225. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Suhani, I.; Sahab, S.; Srivastava, V.; Singh, R.P. Impact of cadmium pollution on food safety and human health. Curr. Opin. Toxicol. 2021, 27, 1–7. [Google Scholar] [CrossRef]
- Zhang, Q.; Cai, W.; Ji, T.-T.; Ye, L.; Lu, Y.-T.; Yuan, T.-T. WRKY13 enhances cadmium tolerance by promoting D-CYSTEINE DESULFHYDRASE and hydrogen sulfide production. Plant Physiol. 2020, 183, 345–357. [Google Scholar] [CrossRef]
- Rahman, M.F.; Ghosal, A.; Alam, M.F.; Kabir, A.H. Remediation of cadmium toxicity in field peas (Pisum sativum L.) through exogenous silicon. Ecotoxicol. Environ. Saf. 2017, 135, 165–172. [Google Scholar] [CrossRef]
- Bhargava, A.; Carmona, F.F.; Bhargava, M.; Srivastava, S. Approaches for enhanced phytoextraction of heavy metals. J. Environ. Manag. 2012, 105, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, D.; Huang, Y.; Huang, S. Physiological response to cadmium stress in kenaf (Hibiscus cannabinus L.) seedlings. Ind. Crops Prod. 2017, 107, 453–457. [Google Scholar] [CrossRef]
- Shi, G.; Liu, C.; Cui, M.; Ma, Y.; Cai, Q. Cadmium tolerance and bioaccumulation of 18 hemp accessions. Appl. Biochem. Biotechnol. 2012, 168, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Nizam, M.; Zaman, M.; Rahman, M.; Hossain, M. Cadmium remediation potentials of jute, kenaf and mesta at early growing stage. J. Agrofor. Environ. 2015, 9, 69–74. [Google Scholar]
- Chen, H.; Li, Y.; Ma, X.; Guo, L.; He, Y.; Ren, Z.; Kuang, Z.; Zhang, X.; Zhang, Z. Analysis of potential strategies for cadmium stress tolerance revealed by transcriptome analysis of upland cotton. Sci. Rep. 2019, 9, 86. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.; Kumar, V.; Rani, A.; Jain, R. Cannabis sativa: A plant suitable for phytoremediation and bioenergy production. Phytoremediation Potential Bioenergy Plants 2017, 269–285. [Google Scholar] [CrossRef]
- Yin, M.; Pan, L.; Liu, J.; Yang, X.; Tang, H.; Zhou, Y.; Huang, S.; Pan, G. Proanthocyanidins alleviate cadmium stress in industrial hemp (Cannabis sativa L.). Plants 2022, 11, 2364. [Google Scholar] [CrossRef]
- Sun, Q.; Ye, Z.H.; Wang, X.R.; Wong, M.H. Cadmium hyperaccumulation leads to an increase of glutathione rather than phytochelatins in the cadmium hyperaccumulator Sedum alfredii. J. Plant Physiol. 2007, 164, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Douchiche, O.; Soret-Morvan, O.; Chaïbi, W.; Morvan, C.; Paynel, F. Characteristics of cadmium tolerance in ‘Hermes’ flax seedlings: Contribution of cell walls. Chemosphere 2010, 81, 1430–1436. [Google Scholar] [CrossRef]
- Luo, D.; Lu, H.; Wang, C.; Mubeen, S.; Cao, S.; Yue, J.; Pan, J.; Wu, X.; Wu, Q.; Zhang, H. Physiological and DNA methylation analysis provides epigenetic insights into kenaf cadmium tolerance heterosis. Plant Sci. 2023, 331, 111663. [Google Scholar] [CrossRef]
- Yue, J.; Tang, M.; Zhang, H.; Luo, D.; Cao, S.; Hu, Y.; Huang, Z.; Wu, Q.; Wu, X.; Pan, J. The transcription factor HcERF4 confers salt and drought tolerance in kenaf (Hibiscus cannabinus L.). Plant Cell Tissue Organ Cult. 2022, 150, 207–221. [Google Scholar] [CrossRef]
- Cui, W.; Yao, P.; Pan, J.; Dai, C.; Cao, H.; Chen, Z.; Zhang, S.; Xu, S.; Shen, W. Transcriptome analysis reveals insight into molecular hydrogen-induced cadmium tolerance in alfalfa: The prominent role of sulfur and (homo) glutathione metabolism. BMC Plant Biol. 2020, 20, 58. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chen, T.; Li, Z.; Jia, R.; Luo, D.; Tang, M.; Lu, H.; Hu, Y.; Yue, J.; Huang, Z. Transcriptome analysis revealed key genes and pathways related to cadmium-stress tolerance in Kenaf (Hibiscus cannabinus L.). Ind. Crops Prod. 2020, 158, 112970. [Google Scholar] [CrossRef]
- Huang, Y.; Li, D.; Zhao, L.; Chen, A.; Li, J.; Tang, H.; Pan, G.; Chang, L.; Deng, Y.; Huang, S. Comparative transcriptome combined with physiological analyses revealed key factors for differential cadmium tolerance in two contrasting hemp (Cannabis sativa L.) cultivars. Ind. Crops Prod. 2019, 140, 111638. [Google Scholar] [CrossRef]
- Luo, D.; Wang, C.; Cao, S.; Mubeen, S.; Mackon, E.; Yue, J.; Rehman, M.; Pan, J.; Wu, X.; Wu, Q. Physiological and transcriptome analysis reveals key genes and molecular basis into heterosis of kenaf (Hibiscus cannabinus L.) under drought stress. Environ. Exp. Bot. 2023, 209, 105293. [Google Scholar] [CrossRef]
- Cleophas, F.N.; Zahari, N.Z.; Murugayah, P.; Rahim, S.A.; Mohd Yatim, A.N. Phytoremediation: A Novel Approach of Bast Fiber Plants (Hemp, Kenaf, Jute and Flax) for Heavy Metals Decontamination in Soil. Toxics 2022, 11, 5. [Google Scholar] [CrossRef]
- Kashif, M.H.; Wei, F.; Tang, D.; Tang, M.; Luo, D.; Hai, L.; Li, R.; Chen, P. iTRAQ-based comparative proteomic response analysis reveals regulatory pathways and divergent protein targets associated with salt-stress tolerance in kenaf (Hibiscus cannabinus L.). Ind. Crops Prod. 2020, 153, 112566. [Google Scholar] [CrossRef]
- Jia, R.; Ding, X.; Tang, D.; Wei, F.; Chang, M.; Li, Z.; Liang, Z.; Chen, P. Effects of seed germination and seedling growth of two homonuclear-heterocytoplasmic kenaf hybrid cultivars under cadmium stress. J. South. Agric. 2019, 50, 1688–1694. [Google Scholar]
- Arbaoui, S.; Campanella, B.; Rezgui, S.; Paul, R.; Bettaieb, T. Bioaccumulation and photosynthetic activity response of kenaf (Hibicus cannabinus L.) to cadmium and zinc. Greener J. Agric. Sci. 2014, 4, 91–100. [Google Scholar]
- Zhao, X.; Guo, Y.; Papazoglou, E.G. Screening flax, kenaf and hemp varieties for phytoremediation of trace element-contaminated soils. Ind. Crops Prod. 2022, 185, 115121. [Google Scholar] [CrossRef]
- Ajuwon, O.R.; Bada, B.S.; Olujimi, O.O. Growth and antioxidative responses to excess cadmium in kenaf (Hibiscus cannabinus L.). Fresenius Environ. Bull. 2010, 19, 2637–2643. [Google Scholar]
- Li, F.-t.; QI, J.-m.; ZHANG, G.-y.; LIN, L.-h.; FANG, P.-p.; TAO, A.-f.; XU, J.-t. Effect of cadmium stress on the growth, antioxidative enzymes and lipid peroxidation in two kenaf (Hibiscus cannabinus L.) plant seedlings. J. Integr. Agric. 2013, 12, 610–620. [Google Scholar] [CrossRef]
- Cao, S.; Wang, M.; Pan, J.; Luo, D.; Mubeen, S.; Wang, C.; Yue, J.; Wu, X.; Wu, Q.; Zhang, H. Physiological, transcriptome and gene functional analysis provide novel sights into cadmium accumulation and tolerance mechanisms in kenaf. J. Environ. Sci. 2024, 137, 500–514. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Bao, Q.; Tian, G.; Fu, K.; Cheng, H. Heavy metal cadmium tolerance on the growth characteristics of industrial hemp (Cannabis sativa L.) in China. In Proceedings of the International Conference on Advances in Energy, Environment and Chemical Engineering, Changsha, China, 26–27 September 2015; pp. 289–295. [Google Scholar]
- Sun, S.; Feng, Y.; Huang, G.; Zhao, X.; Song, F. Rhizophagus irregularis enhances tolerance to cadmium stress by altering host plant hemp (Cannabis sativa L.) photosynthetic properties. Environ. Pollut. 2022, 314, 120309. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Cai, Q.; Liu, Q.; Wu, L. Salicylic acid-mediated alleviation of cadmium toxicity in hemp plants in relation to cadmium uptake, photosynthesis, and antioxidant enzymes. Acta Physiol. Plant. 2009, 31, 969–977. [Google Scholar] [CrossRef]
- Marabesi, A.O.; Nambeesan, S.U.; van Iersel, M.W.; Lessl, J.T.; Coolong, T.W. Cadmium exposure is associated with increased transcript abundance of multiple heavy metal associated transporter genes in roots of hemp (Cannabis sativa L.). Front. Plant Sci. 2023, 14, 1183249. [Google Scholar] [CrossRef]
- Luyckx, M.; Hausman, J.-F.; Isenborghs, A.; Guerriero, G.; Lutts, S. Impact of cadmium and zinc on proteins and cell wall-related gene expression in young stems of hemp (Cannabis sativa L.) and influence of exogenous silicon. Environ. Exp. Bot. 2021, 183, 104363. [Google Scholar] [CrossRef]
- Dewanjee, S.; Gangopadhyay, M.; Sahu, R.; Karmakar, S. Cadmium induced pathophysiology: Prophylactic role of edible jute (Corchorus olitorius) leaves with special emphasis on oxidative stress and mitochondrial involvement. Food Chem. Toxicol. 2013, 60, 188–198. [Google Scholar] [CrossRef]
- Hassan, M.; Dagari, M.; Babayo, A. Effect of citric acid on cadmium ion uptake and stress response of hydroponically grown jute mallow (Corchorus olitorius). J. Environ. Anal. Toxicol. 2016, 6, 375. [Google Scholar]
- Farooq, M.; Ali, S.; Hameed, A.; Bharwana, S.; Rizwan, M.; Ishaque, W.; Farid, M.; Mahmood, K.; Iqbal, Z. Cadmium stress in cotton seedlings: Physiological, photosynthesis and oxidative damages alleviated by glycinebetaine. S. Afr. J. Bot. 2016, 104, 61–68. [Google Scholar] [CrossRef]
- Hancock, L.M.; Ernst, C.L.; Charneskie, R.; Ruane, L.G. Effects of cadmium and mycorrhizal fungi on growth, fitness, and cadmium accumulation in flax (Linum usitatissimum; Linaceae). Am. J. Bot. 2012, 99, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Wu, X.; Li, J.; Li, L. Exploring the impact of cadmium stress on morphophysiological traits and GhPCS gene expression in upland cotton seedlings. S. Afr. J. Bot. 2024, 167, 72–81. [Google Scholar] [CrossRef]
- Douchiche, O.; Driouich, A.; Morvan, C. Impact of cadmium on early stages of flax fibre differentiation: Ultrastructural aspects and pectic features of cell walls. Plant Physiol. Biochem. 2011, 49, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Arbaoui, S.; Evlard, A.; Mhamdi, M.E.W.; Campanella, B.; Paul, R.; Bettaieb, T. Potential of kenaf (Hibiscus cannabinus L.) and corn (Zea mays L.) for phytoremediation of dredging sludge contaminated by trace metals. Biodegradation 2013, 24, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, Z.; Luo, D.; Jia, R.; Lu, H.; Tang, M.; Hu, Y.; Yue, J.; Huang, Z. Comparative transcriptomic analysis reveals key genes and pathways in two different cadmium tolerance kenaf (Hibiscus cannabinus L.) cultivars. Chemosphere 2021, 263, 128211. [Google Scholar] [CrossRef]
- Guo, Y.; Xiao, Q.; Zhao, X.; Wu, Z.; Dai, Z.; Zhang, M.; Qiu, C.; Long, S.; Wang, Y. Phytoremediation with kenaf (Hibiscus cannabinus L.) for cadmium-contaminated paddy soil in southern China: Translocation, uptake, and assessment of cultivars. Environ. Sci. Pollut. Res. 2023, 30, 1244–1252. [Google Scholar] [CrossRef]
- Pan, J.; Cao, S.; Xu, G.; Rehman, M.; Li, X.; Luo, D.; Wang, C.; Fang, W.; Xiao, H.; Liao, C. Comprehensive analysis reveals the underlying mechanism of arbuscular mycorrhizal fungi in kenaf cadmium stress alleviation. Chemosphere 2023, 314, 137566. [Google Scholar] [CrossRef]
- Cao, S.; Pan, J.; Rehman, M.; Luo, D.; Wang, Q.; Jin, G.; Li, R.; Chen, T.; Chen, P. Exogenous nitric oxide alleviates cadmium toxicity in kenaf (Hibiscus cannabinus L.) through modulating cd deposition and regulating key genes and involved pathways. Ind. Crops Prod. 2024, 221, 119359. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, H.; Lv, X.; Zhang, Y.; Wang, W. Effects of biochar and biofertilizer on cadmium-contaminated cotton growth and the antioxidative defense system. Sci. Rep. 2020, 10, 20112. [Google Scholar] [CrossRef]
- Xu, N.; Zhang, N.; Yi, P.; Chen, L.; Dai, H.; Zhang, J.; Li, W.; Li, R.; Liu, A.; Zhou, Z. Integrated physio-biochemistry and RNA-seq revealed the mechanism underlying biochar-mediated alleviation of compound heavy metals (Cd, Pb, As) toxicity in cotton. Ecotoxicol. Environ. Saf. 2024, 284, 116974. [Google Scholar] [CrossRef]
- Khan, A.; Jie, Z.; Xiangjun, K.; Ullah, N.; Short, A.W.; Diao, Y.; Zhou, R.; Xiong, Y.-C. Pre treatment of melatonin rescues cotton seedlings from cadmium toxicity by regulating key physio-biochemical and molecular pathways. J. Hazard. Mater. 2023, 445, 130530. [Google Scholar] [CrossRef] [PubMed]
- An, M.; Wang, H.; Fan, H.; Ippolito, J.; Meng, C.; E, Y.; Li, Y.; Wang, K.; Wei, C. Effects of modifiers on the growth, photosynthesis, and antioxidant enzymes of cotton under cadmium toxicity. J. Plant Growth Regul. 2019, 38, 1196–1205. [Google Scholar] [CrossRef]
- An, M.; Hong, D.; Chang, D.; Zhang, C.; Fan, H.; Wang, K. Polymer amendment regulates cadmium migration in cadmium contaminated cotton field: Insights from genetic adaptation and phenotypic plasticity. Sci. Total Environ. 2022, 807, 151075. [Google Scholar] [CrossRef] [PubMed]
- An, M.; Chang, D.; Wang, X.; Wang, K. Protective effects of polymer amendment on specific metabolites in soil and cotton leaves under cadmium contamination. Ecotoxicol. Environ. Saf. 2023, 264, 115463. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, N.; Geetha, N.; Manish, T.; Sahi, S.; Venkatachalam, P. Zinc oxide nanocatalyst mediates cadmium and lead toxicity tolerance mechanism by differential regulation of photosynthetic machinery and antioxidant enzymes level in cotton seedlings. Toxicol. Rep. 2021, 8, 295–302. [Google Scholar]
- Yu, S.-Y.; Wang, N.; Zhu, L.-X.; Xu, W.-J.; Zhang, Y.-J.; Sun, H.-C.; Zhang, K.; Li, A.-C.; Bai, Z.-Y.; Liu, L.-T. Melatonin mitigates cadmium toxicity by promoting root development, delaying root senescence, and regulating cadmium transport in cotton. Ecotoxicol. Environ. Saf. 2024, 283, 116786. [Google Scholar] [CrossRef]
- Muhammad, U.; Zhang, Y.; Ali, A.; Li, H.; Han, Y.; Sun, Y.; Wang, J.; Wang, W.; Samrana, S.; Khan, S. Melatonin reduces cadmium accumulation through cell wall fraction fixation capacity in cotton seedlings. Plant Stress 2024, 12, 100444. [Google Scholar] [CrossRef]
- Farooq, M.A.; Ali, S.; Hameed, A.; Ishaque, W.; Mahmood, K.; Iqbal, Z. Alleviation of cadmium toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes; suppressed cadmium uptake and oxidative stress in cotton. Ecotoxicol. Environ. Saf. 2013, 96, 242–249. [Google Scholar] [CrossRef]
- Ahmar, S.; Gill, R.A.; Jung, K.H.; Faheem, A.; Qasim, M.U.; Mubeen, M.; Zhou, W. Conventional and Molecular Techniques from Simple Breeding to Speed Breeding in Crop Plants: Recent Advances and Future Outlook. Int. J. Mol. Sci. 2020, 21, 2590. [Google Scholar] [CrossRef]
- Rasheed, A.; Al-Huqail, A.A.; Ali, B.; Alghanem, S.M.S.; Shah, A.A.; Azeem, F.; Rizwan, M.; Al-Qthanin, R.N.; Soudy, F.A. Molecular characterization of genes involved in tolerance of cadmium in Triticum aestivum (L.) under Cd stress. J. Hazard. Mater. 2023, 464, 132955. [Google Scholar] [CrossRef]
- Han, M.; Lu, X.; Yu, J.; Chen, X.; Wang, X.; Malik, W.A.; Wang, J.; Wang, D.; Wang, S.; Guo, L. Transcriptome analysis reveals cotton (Gossypium hirsutum) genes that are differentially expressed in cadmium stress tolerance. Int. J. Mol. Sci. 2019, 20, 1479. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yan, X.; Li, J.; Wu, X.; Wang, X. Metabolome and transcriptome association analysis revealed key factors involved in melatonin mediated cadmium-stress tolerance in cotton. Front. Plant Sci. 2022, 13, 995205. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Cui, Y.; Peng, F.; Guo, L.; Cui, R.; Xu, N.; Huang, H.; Han, M.; Fan, Y.; Zhang, M. GhCYS2 governs the tolerance against cadmium stress by regulating cell viability and photosynthesis in cotton. Ecotoxicol. Environ. Saf. 2023, 263, 115386. [Google Scholar] [CrossRef] [PubMed]
- Douchiche, O.; Chaïbi, W.; Morvan, C. Cadmium tolerance and accumulation characteristics of mature flax, cv. Hermes: Contribution of the basal stem compared to the root. J. Hazard. Mater. 2012, 235, 101–107. [Google Scholar] [CrossRef]
- Smykalova, I.; Vrbova, M.; Tejklova, E.; Vetrovcova, M.; Griga, M. Large scale screening of heavy metal tolerance in flax/linseed (Linum usitatissimum L.) tested in vitro. Ind. Crops Prod. 2010, 32, 527–533. [Google Scholar] [CrossRef]
- Bjelková, M.; Genčurová, V.; Griga, M. Accumulation of cadmium by flax and linseed cultivars in field-simulated conditions: A potential for phytoremediation of Cd-contaminated soils. Ind. Crops Prod. 2011, 33, 761–774. [Google Scholar] [CrossRef]
- House, M.A.; Young, L.W.; Liu, X.; Liber, K.; Diederichsen, A.; Booker, H.M. Comparative analysis of cadmium uptake and distribution in contrasting canadian flax cultivars. BMC Res. Notes 2020, 13, 424. [Google Scholar] [CrossRef]
- Brutch, E.; Zabegaeva, O.; Nozkova, J.; Brutch, N. Cadmium tolerance and its absorption ability in fibre flax and linseed varieties. Turk. J. Agric. For. 2022, 46, 83–89. [Google Scholar]
- Guo, Y.; Qiu, C.; Long, S.; Wang, H.; Wang, Y. Cadmium accumulation, translocation, and assessment of eighteen Linum usitatissimum L. cultivars growing in heavy metal contaminated soil. Int. J. Phytoremediation 2020, 22, 490–496. [Google Scholar] [CrossRef]
- Belkadhi, A.; Hédiji, H.; Abbes, Z.; Djebali, W.; Chaïbi, W. Influence of salicylic acid pre-treatment on cadmium tolerance and its relationship with non-protein thiol production in flax root. Afr. J. Biotechnol. 2012, 11, 9788–9796. [Google Scholar] [CrossRef]
- Nazir, S.; Arif, Y.; Mir, A.R.; Siddiqui, H.; Faizan, M.; Alam, P.; Shamsul, H. Comparative and interactive response of salicylic acid, 24–epibrassinolide or sodium nitroprusside against cadmium stress in Linum usitatissimum. J. Umm Al-Qura Univ. Appl. Sci. 2014, 1–13. [Google Scholar] [CrossRef]
- Belkadhi, A.; De Haro, A.; Soengas, P.; Obregon, S.; Cartea, M.E.; Chaibi, W.; Djebali, W. Salicylic acid increases tolerance to oxidative stress induced by hydrogen peroxide accumulation in leaves of cadmium-exposed flax (Linum usitatissimum L.). J. Plant Interact. 2014, 9, 647–654. [Google Scholar] [CrossRef]
- Belkadhi, A.; De Haro, A.; Obregon, S.; Chaïbi, W.; Djebali, W. Exogenous salicylic acid protects phospholipids against cadmium stress in flax (Linum usitatissimum L.). Ecotoxicol. Environ. Saf. 2015, 120, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Ozhimkova, E.; Uschapovsky, I.; Manaenkov, O. Study of Varietal Differences in the Composition of Heteropolysaccharides of Oil Flax and Fiber Flax. Polysaccharides 2023, 4, 78–87. [Google Scholar] [CrossRef]
- Chaikivskyi, T.; Zbarzhevetska, A. Use of flax processing products in the food industry. Академічні візії 2023. Available online: https://academy-vision.org/index.php/av/article/view/388 (accessed on 5 November 2024).
- Yadav, B.; Kaur, V.; Narayan, O.P.; Yadav, S.K.; Kumar, A.; Wankhede, D.P. Integrated omics approaches for flax improvement under abiotic and biotic stress: Current status and future prospects. Front. Plant Sci. 2022, 13, 931275. [Google Scholar] [CrossRef]
- Akhmetshina, A.O.; Strygina, K.V.; Khlestkina, E.K.; Porokhovinova, E.A.; Brutch, N.B. High-throughput sequencing techniques to flax genetics and breeding. Ecol. Genet. 2020, 18, 103–124. [Google Scholar] [CrossRef]
- Zyablitsin, A.; Dmitriev, A.; Krasnov, G.; Bolsheva, N.; Rozhmina, T.; Muravenko, O.; Fedorova, M.; Snezhkina, A.; Kudryavtseva, A.; Melnikova, N. CAX3 gene is involved in flax response to high soil acidity and aluminum exposure. Mol. Biol. 2018, 52, 514–519. [Google Scholar] [CrossRef]
- Berková, V.; Berka, M.; Griga, M.; Kopecká, R.; Prokopová, M.; Luklová, M.; Horáček, J.; Smýkalová, I.; Čičmanec, P.; Novák, J. Molecular mechanisms underlying flax (Linum usitatissimum L.) tolerance to cadmium: A case study of proteome and metabolome of four different flax genotypes. Plants 2022, 11, 2931. [Google Scholar] [CrossRef]
- Hradilová, J.; Řehulka, P.; Řehulková, H.; Vrbová, M.; Griga, M.; Brzobohatý, B. Comparative analysis of proteomic changes in contrasting flax cultivars upon cadmium exposure. Electrophoresis 2010, 31, 421–431. [Google Scholar] [CrossRef]
- Khan, N.; You, F.M.; Datla, R.; Ravichandran, S.; Jia, B.; Cloutier, S. Genome-wide identification of ATP binding cassette (ABC) transporter and heavy metal associated (HMA) gene families in flax (Linum usitatissimum L.). BMC Genom. 2020, 21, 722. [Google Scholar] [CrossRef]
- Khan, N. Designing Genomic Solutions for Abiotic Traits in Flax (Linum usitatissimum L.). Ph.D. Thesis, Université d’Ottawa/University of Ottawa, Ottawa, ON, Canada, 2022. [Google Scholar]
- Saleem, M.H.; Ali, S.; Rehman, M.; Hasanuzzaman, M.; Rizwan, M.; Irshad, S.; Shafiq, F.; Iqbal, M.; Alharbi, B.M.; Alnusaire, T.S. Jute: A potential candidate for phytoremediation of metals—A review. Plants 2020, 9, 258. [Google Scholar] [CrossRef]
- Oladebeye, A.O.; Adebayo, J.O.; Fatai, A. Potential Phytoremediation of Designated Heavy Metal-contaminated Soils by Jute (Corchorus capsularisis) and Scent Leaf (Ocimum gratissimum). IOSR J. Environ. Sci. Toxicol. Food Technol. 2023, 17, 01–09. [Google Scholar]
- Guo, Y.; Qiu, C.; Long, S.; Wang, Y. Cadmium accumulation and translocation in different jute (Corchorus capsularis L.) cultivars growing in heavy metal contaminated paddy soil. J. Agro-Environ. Sci. 2019, 38, 1929–1935. [Google Scholar]
- Hou, W.; Liao, X.; Tang, X.; Zhao, Y.; Li, C. Absorption and enrichment of heavy metals cadnium and lead in different jute varieties. Southwest China J. Agric. Sci. 2020, 33, 2075–2081. [Google Scholar]
- Patrick, D.; Francis, M.; Chiroma, T.; Adams, F. The rate of absorption of cadmium in roots of jute (Corchorus Olitorius) grown on polluted soil. Savannah J. Sci. Eng. Technol. 2024, 2, 026–032. [Google Scholar]
- Abubakari, M.; Moomin, A.; Nyarko, G.; Dawuda, M. Heavy metals concentrations and risk assessment of roselle and jute mallow cultivated with three compost types. Ann. Agric. Sci. 2017, 62, 145–150. [Google Scholar] [CrossRef]
- Liu, D.; Tao, L.; Li, X.; Xiong, C.; Yang, X.; Nie, Q.; Lin, J. Selenium Application Decreases the Enrichment and Human Exposure Risk of Cadmium in the Leaf of Jute (Corchorus capsularis L.) Planted in Uncontaminated Purple Soil. Sustainability 2022, 14, 9535. [Google Scholar] [CrossRef]
- Deng, C.; Yang, Z.; Cheng, C.; Dai, Z.; Tang, Q.; Xu, Y.; Chen, X.; Su, J. Regulating the Cd tolerance of jute (Corchorus olitorius L.) with graphene oxide nanosheets and the toxicity responses. Environ. Eng. Sci. 2021, 38, 1158–1167. [Google Scholar] [CrossRef]
- Mitra, J.; Datta, S. Genomic Designing for Abiotic Stress Resistance in Jute. In Genomic Designing for Abiotic Stress Resistant Technical Crops; Springer: Berlin/Heidelberg, Germany, 2022; pp. 119–155. [Google Scholar]
- Zhang, L.; Ming, R.; Zhang, J.; Tao, A.; Fang, P.; Qi, J. De novo transcriptome sequence and identification of major bast-related genes involved in cellulose biosynthesis in jute (Corchorus capsularis L.). BMC Genom. 2015, 16, 1062. [Google Scholar] [CrossRef]
- Yang, Z.; Lu, R.; Dai, Z.; Yan, A.; Tang, Q.; Cheng, C.; Xu, Y.; Yang, W.; Su, J. Salt-Stress response mechanisms using de novo transcriptome sequencing of salt-tolerant and sensitive Corchorus spp. genotypes. Genes 2017, 8, 226. [Google Scholar] [CrossRef]
- Samanta, P.; Sadhukhan, S.; Basu, A. Identification of differentially expressed transcripts associated with bast fiber development in Corchorus capsularis by suppression subtractive hybridization. Planta 2015, 241, 371–385. [Google Scholar] [CrossRef]
- Hauqe, S.; Ferdous, A.S.; Sarker, S.K.; Islam, M.T.; Hossain, K.; Khan, H. Identification and expression profiling of microRNAs and their corresponding targets related to phytoremediation of heavy metals in jute (Corchorus olitorius var. O-9897). Bioresearch Commun. (BRC) 2016, 2, 152–157. [Google Scholar]
- Rahman, K.; Ahmed, N.; Raihan, M.R.H.; Nowroz, F.; Jannat, F.; Rahman, M.; Hasanuzzaman, M. Jute responses and tolerance to abiotic stress: Mechanisms and approaches. Plants 2021, 10, 1595. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, A.; Chen, J.; Dai, Z.; Su, J.; Deng, C.; Ye, G.; Cheng, C.; Tang, Q.; Zhang, X. Machine learning phenotyping and GWAS reveal genetic basis of cd tolerance and absorption in jute. Environ. Pollut. 2024, 362, 124918. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Li, Y.; Liu, W.; Liu, S.; Min, J.; Xiong, H.; Dong, Z.; Duan, Y.; Yu, Y.; Li, X. QTL mapping and candidate gene analysis of cadmium accumulation in polished rice by genome-wide association study. Sci. Rep. 2020, 10, 11791. [Google Scholar] [CrossRef]
- Yan, H.; Guo, H.; Xu, W.; Dai, C.; Kimani, W.; Xie, J.; Zhang, H.; Li, T.; Wang, F.; Yu, Y. GWAS-assisted genomic prediction of cadmium accumulation in maize kernel with machine learning and linear statistical methods. J. Hazard. Mater. 2023, 441, 129929. [Google Scholar] [CrossRef]
- Kumar, M.; Prusty, M.R.; Pandey, M.K.; Singh, P.K.; Bohra, A.; Guo, B.; Varshney, R.K. Application of CRISPR/Cas9-mediated gene editing for abiotic stress management in crop plants. Front. Plant Sci. 2023, 14, 1157678. [Google Scholar] [CrossRef]
- Wielgusz, K.; Praczyk, M.; Irzykowska, L.; Świerk, D. Fertilization and soil pH affect seed and biomass yield, plant morphology, and cadmium uptake in hemp (Cannabis sativa L.). Ind. Crops Prod. 2022, 175, 114245. [Google Scholar] [CrossRef]
- Visković, J.; Zheljazkov, V.D.; Sikora, V.; Noller, J.; Latković, D.; Ocamb, C.M.; Koren, A. Industrial hemp (Cannabis sativa L.) agronomy and utilization: A review. Agronomy 2023, 13, 931. [Google Scholar] [CrossRef]
- Golia, E.E.; Bethanis, J.; Ntinopoulos, N.; Kaffe, G.-G.; Komnou, A.A.; Vasilou, C. Investigating the potential of heavy metal accumulation from hemp. The use of industrial hemp (Cannabis Sativa L.) for phytoremediation of heavily and moderated polluted soils. Sustain. Chem. Pharm. 2023, 31, 100961. [Google Scholar] [CrossRef]
- Androudi, M.; Liava, V.; Tsaliki, E.; Ipsilantis, I.; Golia, E.E. Use of Cannabis sativa L. for Improving Cadmium-Contaminated Mediterranean Soils—Effect of Mycorrhizal Colonization on Phytoremediation Capacity. Soil Syst. 2024, 8, 100. [Google Scholar] [CrossRef]
- Linger, P.; Müssig, J.; Fischer, H.; Kobert, J. Industrial hemp (Cannabis sativa L.) growing on heavy metal contaminated soil: Fibre quality and phytoremediation potential. Ind. Crops Prod. 2002, 16, 33–42. [Google Scholar] [CrossRef]
- Linger, P.; Ostwald, A.; Haensler, J. Cannabis sativa L. growing on heavy metal contaminated soil: Growth, cadmium uptake and photosynthesis. Biol. Plant. 2005, 49, 567–576. [Google Scholar] [CrossRef]
- Testa, G.; Corinzia, S.A.; Cosentino, S.L.; Ciaramella, B.R. Phytoremediation of Cadmium-, Lead-, and Nickel-Polluted Soils by Industrial Hemp. Agronomy 2023, 13, 995. [Google Scholar] [CrossRef]
- Citterio, S.; Santagostino, A.; Fumagalli, P.; Prato, N.; Ranalli, P.; Sgorbati, S. Heavy metal tolerance and accumulation of Cd, Cr and Ni by Cannabis sativa L. Plant Soil 2003, 256, 243–252. [Google Scholar] [CrossRef]
- Saastamoinen, M.; Eurola, M.; Hietaniemi, V. Oil, protein, chlorophyll, cadmium and lead contents of seeds in oil and fiber flax (Linum usitatissimum L.) cultivars and in oil hemp (Cannabis sativa L.) cultivar Finola cultivated in south-western part of Finland. J. Food Chem. Nanotechnol. 2016, 2, 73–76. [Google Scholar] [CrossRef]
- Mihoc, M.; Pop, G.; Alexa, E.; Radulov, I. Nutritive quality of Romanian hemp varieties (Cannabis sativa L.) with special focus on oil and metal contents of seeds. Chem. Cent. J. 2012, 6, 122. [Google Scholar] [CrossRef]
- Marabesi, A.O.; Lessl, J.T.; Coolong, T.W. Cadmium Bioconcentration and Translocation Potential in Day Neutral and Photoperiod Sensitive Hemp Grown Hydroponically for the Medicinal Market. Water 2023, 15, 2176. [Google Scholar] [CrossRef]
- Thurston, D. Evaluation of Biochar Rate and Hemp Cultivar for the Phytoremediation of Heavy-Metal-Contaminated Soil from the Tar Creek Superfund Site. Master’s Thesis, University of Arkansas, Fayetteville, AR, USA, 2023. [Google Scholar]
- Irshad, M.; Ahmad, S.; Pervez, A.; Inoue, M. Phytoaccumulation of heavy metals in natural plants thriving on wastewater effluent at Hattar industrial estate, Pakistan. Int. J. Phytoremediation 2015, 17, 154–158. [Google Scholar] [CrossRef]
- Nash, A.O.; Joshee, N.; Sherman, S.; Lessl, J.T.; Coolong, T. Accumulation and histochemical localization of cadmium in hemp (Cannabis sativa L.) leaf and root tissue. HortScience 2024, 59, 1150–1157. [Google Scholar] [CrossRef]
- Guo, Y.; Wen, L.; Zhao, X.; Xing, C.; Huang, R. Industrial hemp (Cannabis sativa L.) can utilize and remediate soil strongly contaminated with Cu, As, Cd, and Pb by phytoattenuation. Chemosphere 2024, 358, 142199. [Google Scholar] [CrossRef]
- Flajšman, M.; Košmelj, K.; Grčman, H.; Ačko, D.K.; Zupan, M. Industrial hemp (Cannabis sativa L.)—A valuable alternative crop for growing in agricultural soils contaminated with heavy metals. Environ. Sci. Pollut. Res. 2023, 30, 115414–115429. [Google Scholar] [CrossRef] [PubMed]
- Brennecke, T.; Čechová, L.; Horáková, K.; Šimoníková, L.; Buday, J.; Prochazka, D.; Modlitbová, P.; Novotný, K.; Miziolek, A.W.; Pořízka, P. Imaging the distribution of nutrient elements and the uptake of toxic metals in industrial hemp and white mustard with laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2023, 205, 106684. [Google Scholar] [CrossRef]
- De Vos, B.; Souza, M.; Michels, E.; Meers, E. Industrial hemp (Cannabis sativa L.) in a phytoattenuation strategy: Remediation potential of a Cd, Pb and Zn contaminated soil and valorization potential of the fibers for textile production. Ind. Crops Prod. 2022, 178, 114592. [Google Scholar] [CrossRef]
- Husain, R.; Weeden, H.; Bogush, D.; Deguchi, M.; Soliman, M.; Potlakayala, S.; Katam, R.; Goldman, S.; Rudrabhatla, S. Enhanced tolerance of industrial hemp (Cannabis sativa L.) plants on abandoned mine land soil leads to overexpression of cannabinoids. PLoS ONE 2019, 14, e0221570. [Google Scholar] [CrossRef] [PubMed]
- Canu, M.; Mulè, P.; Spanu, E.; Fanni, S.; Marrone, A.; Carboni, G. Hemp Cultivation in Soils Polluted by Cd, Pb and Zn in the Mediterranean Area: Sites Characterization and Phytoremediation in Real Scale Settlement. Appl. Sci. 2022, 12, 3548. [Google Scholar] [CrossRef]
- Luyckx, M.; Hausman, J.-F.; Blanquet, M.; Guerriero, G.; Lutts, S. Silicon reduces cadmium absorption and increases root-to-shoot translocation without impacting growth in young plants of hemp (Cannabis sativa L.) on a short-term basis. Environ. Sci. Pollut. Res. 2021, 28, 37963–37977. [Google Scholar] [CrossRef]
- Sun, S.; Fan, X.; Feng, Y.; Wang, X.; Gao, H.; Song, F. Arbuscular mycorrhizal fungi influence the uptake of cadmium in industrial hemp (Cannabis sativa L.). Chemosphere 2023, 330, 138728. [Google Scholar] [CrossRef]
- Ali, N.; Hadi, F.; Ali, M.; Mehmood, A.; Seleiman, M.F.; Khan, N. Expression analysis of transcription factor (CBF/DREB) gene and biochemical response of Cannabis sativa to cadmium toxicity and foliar application of molybdenum. S. Afr. J. Bot. 2023, 162, 160–170. [Google Scholar] [CrossRef]
- Garau, M.; Cascio, M.L.; Vasileiadis, S.; Sizmur, T.; Nieddu, M.; Pinna, M.V.; Sirca, C.; Spano, D.; Roggero, P.P.; Garau, G. Using biochar for environmental recovery and boosting the yield of valuable non-food crops: The case of hemp in a soil contaminated by potentially toxic elements (PTEs). Heliyon 2024, 10, e28050. [Google Scholar] [CrossRef]
- Yin, M.; Pan, G.; Tao, J.; Doblin, M.S.; Zeng, W.; Pan, L.; Zhao, L.; Li, Z.; Jiang, H.; Chang, L. Identification of MYB genes reveals their potential functions in cadmium stress response and the regulation of cannabinoid biosynthesis in hemp. Ind. Crops Prod. 2022, 180, 114607. [Google Scholar] [CrossRef]
- Luyckx, M.; Hausman, J.-F.; Sergeant, K.; Guerriero, G.; Lutts, S. Molecular and biochemical insights into early responses of hemp to Cd and Zn exposure and the potential effect of Si on stress response. Front. Plant Sci. 2021, 12, 711853. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Tehsin, Z.; Malik, S.; Asad, S.; Shahzad, M.; Bilal, M.; Khan, S. Phytoremediation potential of hemp (Cannabis sativa L.): Identification and characterization of heavy metals responsive genes. Clean-Soil Air Water 2016, 44, 195–201. [Google Scholar]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Geng, M.; Yuan, J.; Zhan, J.; Liu, L.; Chen, Y.; Wang, Y.; Qin, W.; Duan, H.; Zhao, H. GhRCD1 promotes cotton tolerance to cadmium by regulating the GhbHLH12–GhMYB44–GhHMA1 transcriptional cascade. Plant Biotechnol. J. 2024, 22, 1777–1796. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ye, R.; Liang, Y.; Zhang, S.; Liu, X.; Sun, C.; Li, F.; Yi, J. Generation of low-cadmium rice germplasms via knockout of OsLCD using CRISPR/Cas9. J. Environ. Sci. 2023, 126, 138–152. [Google Scholar] [CrossRef]

| Cultivars | Total Genes | Role | Reference |
|---|---|---|---|
| CP032 | HcERF.C3 gene was proven to have a positive effect on Cd tolerance and Cd homeostasis via virus-induced gene-silencing analysis. | [59] | |
| GH and YJ | 2221 and 3321 | Activating the antioxidant defense system, heavy metal transport and detoxification, substance transport, plant hormone, and calcium signals. | [56] |
| FH991 | 3926 | Transport and catabolism, heavy metals’ transport, detoxification of heavy metals, antioxidant activities, carbohydrate and energy metabolism. | [33] |
| F and Z | 3439 | Phenylpropanoid biosynthesis, plant hormone signal transduction pathways, vacuolar compartmentalization of Cd, and Cd uptake and transport. | [43] |
| Zhe 367 | AMF increases the expression of critical genes (Hc.GH3.1, Hc. AKR, and Hc. PHR1), which increases Cd tolerance in kenaf. | [58] | |
| CP085, CP089 and their hybrid F1 seedlings | Expression of NPF2.7, NADP-ME, NAC71, TPP-D, LRR-RLKs, and DHX51 was altered due to cadmium stress; related to cytosine methylation regulation. | [30] |
| Cultivars | Genes | Functions | References |
|---|---|---|---|
| C184 cotton species | 1151 DEGs in roots | Binding action and catalytic activity, mainly metal iron binding, and some cellular and metabolic processes. | [25] |
| Han 242 | 4627 DEGs in the root, 3022 DEGs in the stem, and 3854 DEGs in the leaves | Heavy metal transporter-coding genes (CDF, ABC, and HMA), heat shock genes (HSP), and annexin genes | [72] |
| Cotton variety CCRI 45 | 5573, 7105, 7253, 25, 198, and 9 upregulated, and 6644, 7192, 7404, 9, and 59, downregulated, 195, 150, 150, 12, 24, 59 upregulated and 16, 11, 23, 38, 127, 66 downregulated differentially accumulated metabolites (DAMs) | These mechanisms include enhancing antioxidant capacity through regulating APX, flavonoids, and alkaloids, accumulating secondary metabolites associated with Cd chelation (like amino acids and derivatives), and controlling the transportation of cadmium ions through the activation of ABC transporters. In conclusion, this study offers new insights into how cotton responds to Cd stress through MT-mediated detoxification. | [73] |
| Cotton cultivar J-4B | LOC107894197, LOC107955631, LOC107899273 | Improvement in leaf functions and plant growth. | [62] |
| Variety | Cd Dose | Genes | Function | References |
|---|---|---|---|---|
| Purple Tiger | 0, 2.5, 10 and 25 mg L−1 | CsHMA3 CsHMA1, CsHMA4, and CsHMA5 | Regulate Cd uptake via roots; transport to shoot, leaves, and lower tissues. | [47] |
| Hemp genome | 113 MYB genes | CsMYB024-induced cannabidiol biosynthesis pathway. | [136] | |
| Santhica 27 | 10 μM for one week | 122 proteins in leaves affected by Cd stress | PCS1-1, which increases phytochelatin biosynthesis. | [137] |
| Yunnan Hemp No. 1 | CsANR and CsLAR | Proanthocyanidins’ synthesis. | [27] | |
| NA | NA | Glutathione-disulfide reductase (GSR) and phospholipase D-α (PLDα) | These genes are responsible for the accumulation and tolerance of Cd in hemp leaves. | [138] |
| Hemp | CBF/DREB | A positive correlation was found between Cd accumulation and increased proline content. | [134] | |
| Yunma No. 1 (Ym), Neimengguxiaolidama (Nx) | 22 DEGs for heavy metal detoxification process in Nx and 118 in Ym. | Results showed that peroxidase-, glutathione-, and S-transferase-regulated genes were both significantly upregulated in Nx and Ym. Cd-tolerance protein/phytochelatin synthase I thioredoxin reductase-related genes were specifically upregulated in Ym. | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasheed, A.; He, P.; Long, Z.; Gillani, S.F.A.; Wang, Z.; Morsy, K.; Hashem, M.; Jie, Y. Cadmium (Cd) Tolerance and Phytoremediation Potential in Fiber Crops: Research Updates and Future Breeding Efforts. Agronomy 2024, 14, 2713. https://doi.org/10.3390/agronomy14112713
Rasheed A, He P, Long Z, Gillani SFA, Wang Z, Morsy K, Hashem M, Jie Y. Cadmium (Cd) Tolerance and Phytoremediation Potential in Fiber Crops: Research Updates and Future Breeding Efforts. Agronomy. 2024; 14(11):2713. https://doi.org/10.3390/agronomy14112713
Chicago/Turabian StyleRasheed, Adnan, Pengliang He, Zhao Long, Syed Faheem Anjum Gillani, Ziqian Wang, Kareem Morsy, Mohamed Hashem, and Yucheng Jie. 2024. "Cadmium (Cd) Tolerance and Phytoremediation Potential in Fiber Crops: Research Updates and Future Breeding Efforts" Agronomy 14, no. 11: 2713. https://doi.org/10.3390/agronomy14112713
APA StyleRasheed, A., He, P., Long, Z., Gillani, S. F. A., Wang, Z., Morsy, K., Hashem, M., & Jie, Y. (2024). Cadmium (Cd) Tolerance and Phytoremediation Potential in Fiber Crops: Research Updates and Future Breeding Efforts. Agronomy, 14(11), 2713. https://doi.org/10.3390/agronomy14112713






