Agronomic Estimation of Lupin (Lupinus pilosus L.) as a Prospective Crop
Abstract
:1. Introduction
1.1. Sustainable Food Production and Novel Protein-Rich Crops
1.2. Lupins as a Protein Crop
1.3. L. pilosus as a Potential Crop
- (1)
- Provide an initial estimation of the potential yield of seeds per cultivated area for L. pilosus in common garden net-house conditions;
- (2)
- Compare agronomic traits of different accessions aiming to link environmental factors (climatic, edaphic) to agronomic proprieties;
- (3)
- Study the effects of pollination on seed production in accessions originating from wild L. pilosus accessions;
- (4)
- Evaluate two plant growing densities, low density to simulate optimal growth conditions, and high density to mimic agricultural crop conditions.
2. Materials and Methods
2.1. Lupinus pilosus Accessions
2.2. Experimental Design and Plant Growth
2.3. Vegetative Growth Parameters
2.4. Plant Performance
2.5. Reproductive Growth Parameters
2.6. Yield Parameters
2.7. Data Analysis
2.8. Phenology
2.9. Multi-Dimension Trait Comparison
3. Results
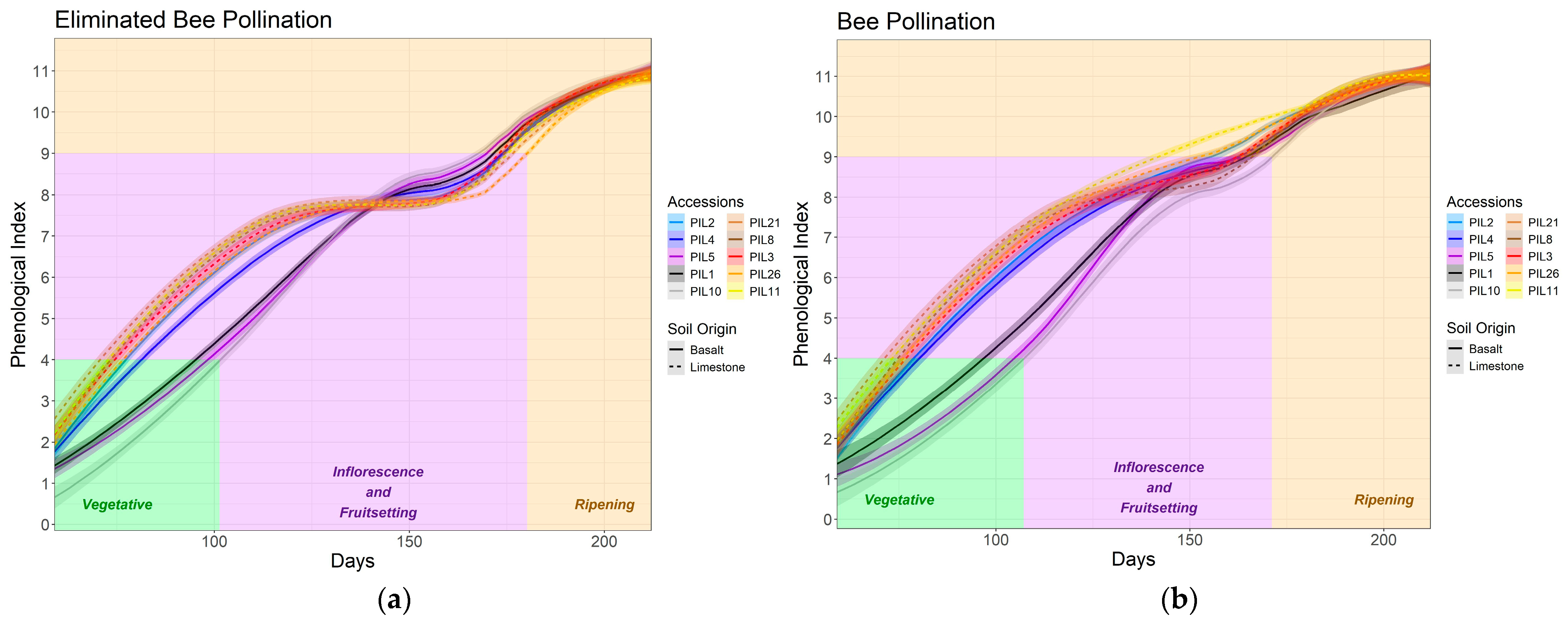
3.1. Phenology and Architecture
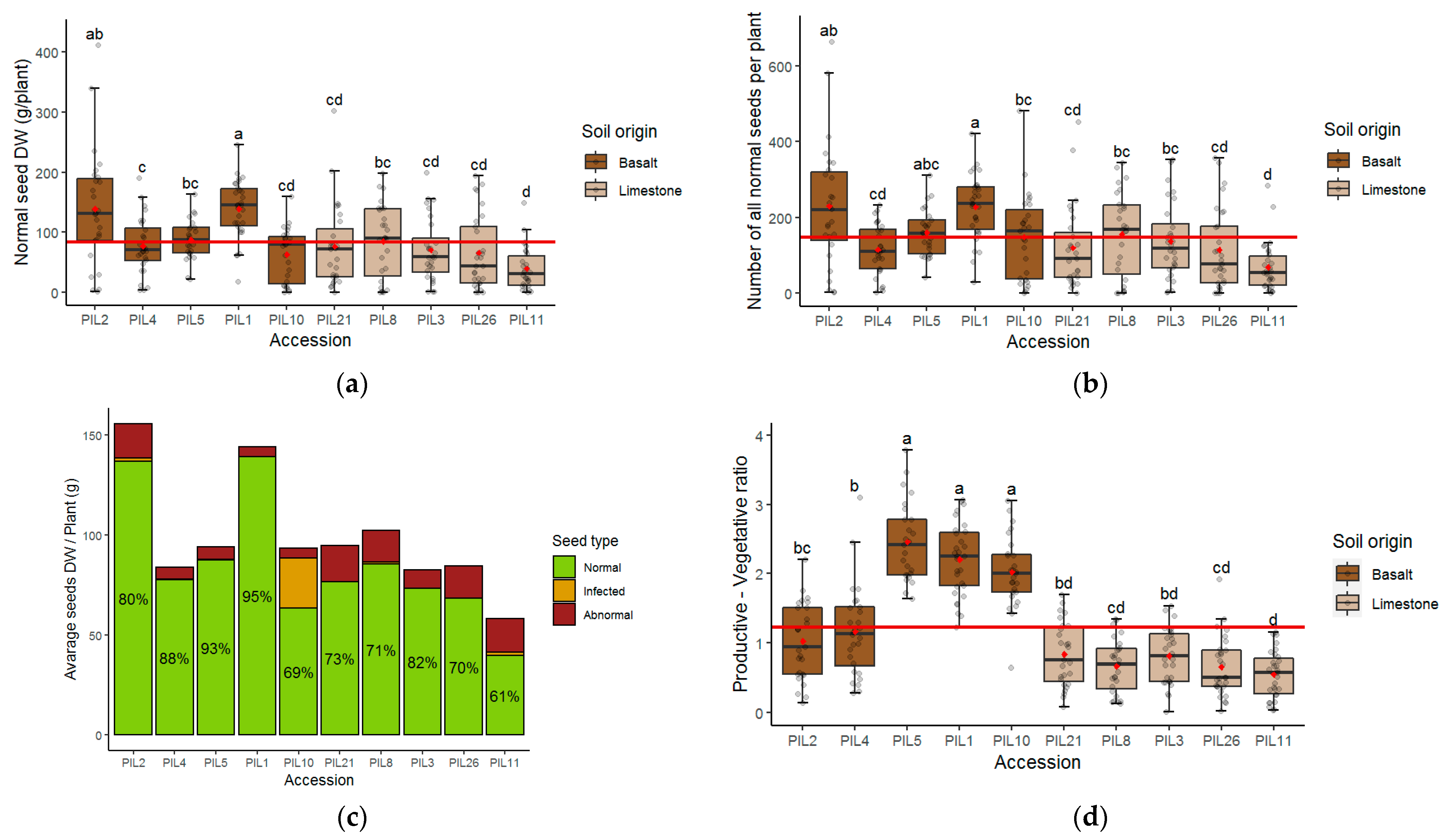
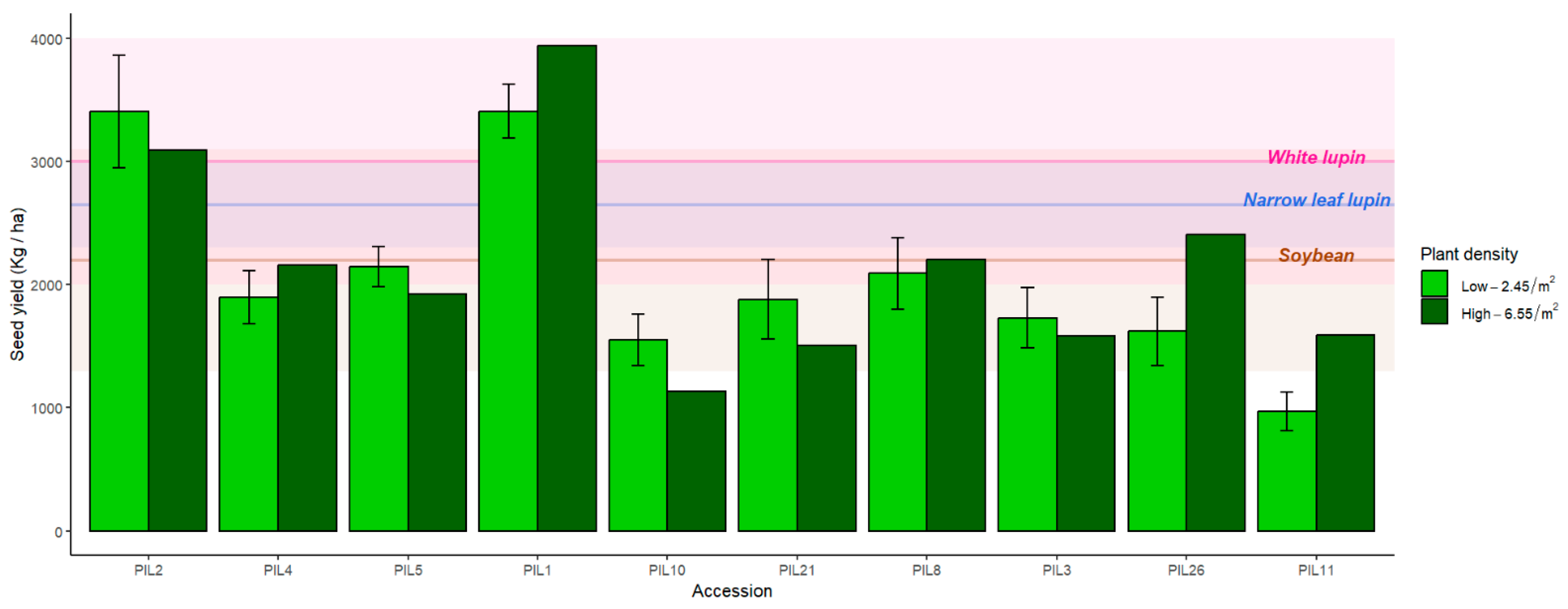
3.2. Yield of L. pilosus in Net-House Conditions
3.3. Seed Development Success Rates
3.4. Results of Multi-Dimension Trait Comparison
3.5. Effect of Honeybee Pollination on L. pilosus Success
3.6. Seed Yield in High Density
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Swallow, B.M. Optimizing expenditures for agricultural land conservation: Spatially-explicit estimation of benefits, budgets, costs and targets. Land Use Policy 2016, 59, 272–283. [Google Scholar] [CrossRef]
- Adams, M.W.; Ellingboe, A.H.; Rossman, E.C. Biological uniformity and disease epidemics. BioScience 1971, 21, 1067–1070. [Google Scholar] [CrossRef]
- Gruber, K. Agrobiodiversity: The living library. Nature 2017, 544, S8–S10. [Google Scholar] [CrossRef]
- Prost, L.; Berthet, E.T.A.; Cerf, M.; Jeuffroy, M.H.; Labatut, J.; Meynard, J.M. Innovative design for agriculture in the move towards sustainability: Scientific challenges. Res. Eng. Des. 2017, 28, 119–129. [Google Scholar] [CrossRef]
- Altieri, M.A.; Nicholls, C.I.; Henao, A.; Lana, M.A. Agroecology and the design of climate change-resilient farming systems. Agron. Sustain. Dev. 2015, 35, 869–890. [Google Scholar] [CrossRef]
- Urruty, N.; Tailliez-Lefebvre, D.; Huyghe, C. Stability, robustness, vulnerability and resilience of agricultural systems. A review. Agron. Sustain. Dev. 2016, 36, 15. [Google Scholar] [CrossRef]
- Tanksley, S.D.; McCouch, S.R. Seed banks and molecular maps: Unlocking genetic potential from the wild. Science 1997, 277, 1063–1066. [Google Scholar] [CrossRef]
- Heinemann, J.A.; Massaro, M.; Coray, D.S.; Agapito-Tenfen, S.Z.; Wen, J.D. Sustainability and innovation in staple crop production in the US Midwest. Int. J. Agric. Sustain. 2014, 12, 71–88. [Google Scholar] [CrossRef]
- Altieri, M.A. The ecological role of biodiversity in agroecosystems. Agric. Ecosyst. Environ. 1999, 74, 19–31. [Google Scholar] [CrossRef]
- Ordonez, N.; Seidl, M.F.; Waalwijk, C.; Drenth, A.; Kilian, A.; Thomma, B.P.H.J.; Ploetz, R.C.; Kema, G.H.J. Worse Comes to Worst: Bananas and Panama Disease-When Plant and Pathogen Clones Meet. PLoS Pathog. 2015, 11. [Google Scholar] [CrossRef] [PubMed]
- Koohafkan, P.; Altieri, M.A. Globally Important Agricultural Heritage Systems Concept and the Initiative. Earthscan Food Agric. 2017, 36–51. [Google Scholar] [CrossRef]
- Lincoln, N.K. Agroforestry form and ecological adaptation in ancient Hawai’i: Extent of the pakukui swidden system of Hamakua, Hawai’i Island. Agric. Syst. 2020, 181, 102808. [Google Scholar] [CrossRef]
- Harrison, J.G.; Philbin, C.S.; Gompert, Z.; Forister, G.W.; Hernandez-Espinoza, L.; Sullivan, B.W.; Wallace, I.S.; Beltran, L.; Dodson, C.D.; Francis, J.S.; et al. Deconstruction of a plant-arthropod community reveals influential plant traits with nonlinear effects on arthropod assemblages. Funct. Ecol. 2018, 32, 1317–1328. [Google Scholar] [CrossRef]
- Dabney, S.M.; Delgado, J.A.; Reeves, D.W. Using winter cover crops to improve soil and water quality. Commun. Soil. Sci. Plan. 2001, 32, 1221–1250. [Google Scholar] [CrossRef]
- Wahbi, S.; Prin, Y.; Thioulouse, J.; Sanguin, H.; Baudoin, E.; Maghraoui, T.; Oufdou, K.; Le Roux, C.; Galiana, A.; Hafidi, M.; et al. Impact of Wheat/Faba Bean Mixed Cropping or Rotation Systems on Soil Microbial Functionalities. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Ashraf, M.; Zulkifli, R.; Sanusi, R.; Tohiran, K.A.; Terhem, R.; Moslím, R.; Norhisham, A.R.; Ashton-Butt, A.; Azhar, B. Alley-cropping system can boost arthropod biodiversity and ecosystem functions in oil palm plantations. Agric. Ecosyst. Environ. 2018, 260, 19–26. [Google Scholar] [CrossRef]
- Ben-Simchon, E.; Sapir, E.; Vaknin, Y.; Shelef, O. Malvaceae spp. leaves as a novel crop for food. Int. J. Agric. For. Life Sci. 2019, 3, 279–286. [Google Scholar]
- Shelef, O.; Fernández-Bayo, J.D.; Sher, Y.; Ancona, V.; Slinn, H.; Achmon, Y. Elucidating Local Food Production to Identify the Principles and Challenges of Sustainable Agriculture. In Sustainable Food Systems from Agriculture to Industry; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 47–81. [Google Scholar]
- Shelef, O.; Guy, O.; Solowey, E.; Kam, M.; Degen, A.A.; Rachmilevitch, S. Domestication of plants for sustainable agriculture in drylands: Experience from the Negev Desert. Arid. Land. Res. Manag. 2016, 30, 209–228. [Google Scholar] [CrossRef]
- Shelef, O.; Weisberg, P.J.; Provenza, F.D. The Value of Native Plants and Local Production in an Era of Global Agriculture. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Basheer, L.; Ben-Simchon, E.; Cohen, A.; Shelef, O. From Traditional Food to Functional Food? Evaluation of Malvaceae Species as Novel Food Crops. Agronomy 2021, 11, 1294. [Google Scholar] [CrossRef]
- Ben-Simchon, E.; Grunwald, Y.; Ben-Ari, G.; Rosenfeld, A.; Shelef, O. A village a field? Agronomic evaluation of fruit trees in inhabited space—Lessons for land use policy from a case study in Israel’s Sharon Region. Land Use Policy 2022, 123. [Google Scholar] [CrossRef]
- Lo, B.; Kasapis, S.; Farahnaky, A. Lupin protein: Isolation and techno-functional properties, a review. Food Hydrocoll. 2021, 112. [Google Scholar] [CrossRef]
- Eshel, G.; Shepon, A.; Makov, T.; Milo, R. Land, irrigation water, greenhouse gas, and reactive nitrogen burdens of meat, eggs, and dairy production in the United States. Proc. Natl. Acad. Sci. USA 2014, 111, 11996–12001. [Google Scholar] [CrossRef]
- Eshel, G.; Shepon, A.; Noor, E.; Milo, R. Environmentally Optimal, Nutritionally Aware Beef Replacement Plant-Based Diets. Environ. Sci. Technol. 2016, 50, 8164–8168. [Google Scholar] [CrossRef]
- Tziva, M.; Negro, S.O.; Kalfagianni, A.; Hekkert, M.P. Understanding the protein transition: The rise of plant -based meat substitutes. Environ. Innov. Soc. Trans. 2020, 35, 217–231. [Google Scholar] [CrossRef]
- Fernie, A.R.; Yang, J.B. Domestication: An Alternative Route toward New Crops for the Future. Mol. Plant 2019, 12, 615–631. [Google Scholar] [CrossRef]
- Shrestha, S.; van’t Hag, L.; Haritos, V.S.; Dhital, S. Lupin proteins: Structure, isolation and application. Trends Food Sci. Technol. 2021, 116, 928–939. [Google Scholar] [CrossRef]
- Lucas, M.M.; Stoddard, F.L.; Annicchiarico, P.; Frias, J.; Martínez-Villaluenga, C.; Sussmann, D.; Duranti, M.; Seger, A.; Zander, P.M.; Pueyo, J.J. The future of lupin as a protein crop in Europe. Front. Plant Sci. 2015, 6, 705. [Google Scholar] [CrossRef]
- Gulisano, A.; Alves, S.; Martins, J.N.; Trindade, L.M. Genetics and Breeding of An Emerging Protein Crop. Front. Plant Sci. 2019, 10, 1385. [Google Scholar] [CrossRef]
- Guilengue, N.; Alves, S.; Talhinhas, P.; Neves-Martins, J. Genetic and Genomic Diversity in a Tarwi (Sweet) Germplasm Collection and Adaptability to Mediterranean Climate Conditions. Agronomy 2020, 10, 21. [Google Scholar] [CrossRef]
- Święcicki, W.; Kroc, M.; Kamel, K.A. Lupins. In Grain Legumes; Ron, A.M.D., Ed.; Springer: New York, NY, USA, 2015; pp. 179–218. [Google Scholar]
- Gresta, F.; Wink, M.; Prins, U.; Abberton, M.; Capraro, J.E.; Scarafoni, A.L.; Hill, G.E. Lupins in European cropping systems. In Legumes in Cropping Systems; CABI: Wallingford, UK, 2017; pp. 88–108. [Google Scholar]
- Namdar, D.; Mulder, P.P.J.; Ben-Simchon, E.; Hacham, Y.; Basheer, L.; Cohen, O.; Sternberg, M.; Shelef, O. New Analytical Approach to Quinolizidine Alkaloids and Their Assumed Biosynthesis Pathways in Lupin Seeds. Toxins 2024, 16, 163. [Google Scholar] [CrossRef] [PubMed]
- Clements, J.C.; Buirchell, B.J.; Cowling, W.A. Relationship between morphological variation and geographical origin or selection history in Lupinus pilosus. Plant Breed. 1996, 115, 16–22. [Google Scholar] [CrossRef]
- Brand, J.D.; Tang, C.; Rathjen, A.J. Screening rough-seeded lupins (Lupinus pilosus Murr. and Lupinus atlanticus Glads.) for tolerance to calcareous soils. Plant Soil 2002, 245, 261–275. [Google Scholar] [CrossRef]
- Heistinger, A.; Pistrick, K. ‘Altreier Kaffee’: Lupinus pilosus L. cultivated as coffee substitute in northern Italy (Alto Adige/Südtirol). Genet. Resour. Crop Evol. 2007, 54, 1623–1630. [Google Scholar] [CrossRef]
- Jensen, C.R.; Joernsgaard, B.; Andersen, M.N.; Christiansen, J.L.; Mogensen, V.O.; Friis, P.; Petersen, C.T. The effect of lupins as compared with peas and oats on the yield of the subsequent winter barley crop. Eur. J. Agron. 2004, 20, 405–418. [Google Scholar] [CrossRef]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The Influence of Environmental Conditions on Secondary Metabolites in Medicinal Plants: A Literature Review. Chem. Biodivers. 2021, 18, e2100345. [Google Scholar] [CrossRef]
- Frick, K.M.; Kamphuis, L.G.; Siddique, K.H.M.; Singh, K.B.; Foley, R.C. Quinolizidine Alkaloid Biosynthesis in Lupins and Prospects for Grain Quality Improvement. Front. Plant Sci. 2017, 8, 87. [Google Scholar] [CrossRef]
- Nelson, P.; Hawthorne, W.A. Development of lupins as a crop in Australia. In Linking Research and Marketing Opportunities for Pulses in the 21st Century: Proceedings of the Third International Food Legumes Research Conference; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 549–559. [Google Scholar]
- Buirchell, B.; Wallace, C. Domestication of rough seeded lupins. J. Dep. Agric. West. Aust. Ser. 4 1992, 33, 131–137. Available online: https://library.dpird.wa.gov.au/journal_agriculture4/ (accessed on 10 November 2024).
- Hufnagel, B.; Marques, A.; Soriano, A.; Marquès, L.; Divol, F.; Doumas, P.; Sallet, E.; Mancinotti, D.; Carrere, S.; Marande, W.; et al. High-quality genome sequence of white lupin provides insight into soil exploration and seed quality. Nat. Commun. 2020, 11, 492. [Google Scholar] [CrossRef]
- Horovitz, A.; Harding, J. Genetics of Lupinus. XII. The mating system of Lupinus pilosus. Bot. Gaz. 1983, 144, 276–279. [Google Scholar] [CrossRef]
- Neeman, G.; Nesher, R. Pollination Ecology and the Significance of Floral Color-Change in Lupinus-Pilosus L (Fabaceae). Isr. J. Plant Sci. 1995, 43, 135–145. [Google Scholar] [CrossRef]
- Gueguen, J.; Cerletti, P. Proteins of some legume seeds: Soybean, pea, fababean and lupin. In New and Developing Sources of Food Proteins; Hudson, B.J.F., Ed.; Springer: Boston, MA, USA, 1994; pp. 145–193. [Google Scholar]
- Gladstones, J.S.; Atkins, C.; Hamblin, J. Lupins as Crop Plants: Biology, Production and Utilization; CAB International: Wallingford, UK, 1998. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; USDA Circular No. 939; US Government Printing Office: Washington, DC, USA, 1954.
- Milfont, M.D.; Rocha, E.E.M.; Lima, A.O.N.; Freitas, B.M. Higher soybean production using honeybee and wild pollinators, a sustainable alternative to pesticides and autopollination. Environ. Chem. Lett. 2013, 11, 335–341. [Google Scholar] [CrossRef]
- Shelef, O.; Golan-Goldhirsh, A.; Gendler, T.; Rachmilevitch, S. Physiological parameters of plants as indicators of water quality in a constructed wetland. Environ. Sci. Pollut. R. 2011, 18, 1234–1242. [Google Scholar] [CrossRef]
- Shelef, O.; Summerfield, L.; Lev-Yadun, S.; Villamarin-Cortez, S.; Sadeh, R.; Herrmann, I.; Rachmilevitch, S. Thermal Benefits From White Variegation of Silybum marianum Leaves. Front. Plant Sci. 2019, 10, 688. [Google Scholar] [CrossRef] [PubMed]
- Brand, J.D.; Tang, C.; Rathjen, A.J. Adaptation of Lupinus angustifolius L. and L-pilosus Murr. to calcareous soils. Aust. J. Agric. Res. 1999, 50, 1027–1033. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2 Elegant Graphics for Data Analysis Introduction; Springer: Berlin, Germany, 2009. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots, R Package Version 0.4.0. 2020. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 15 August 2020).
- Ogle, D.H.; Wheeler, P.; Dinno, A. FSA: Fisheries Stock Analysis, R Package Version 0.8, 17, 636. 2017. Available online: https://fishr-core-team.github.io/FSA/ (accessed on 15 August 2020).
- Tang, Y.; Horikoshi, M.; Li, W.X. ggfortify: Unified Interface to Visualize Statistical Results of Popular R Packages. R J. 2016, 8, 474–485. [Google Scholar] [CrossRef]
- Arncken, C.; Klaiss, M.; Wendling, M.; Messmer, M. Cultivation of White Lupin. Legumes Translated Practice Note 4. 2020. Available online: https://swiss.legumehub.eu/ (accessed on 11 November 2024).
- Panasiewicz, K.; Faligowska, A.; Szymanska, G.; Szukala, J.; Ratajczak, K.; Sulewska, H. The Effect of Various Tillage Systems on Productivity of Narrow-Leaved Lupin-Winter Wheat-Winter Triticale-Winter Barley Rotation. Agronomy 2020, 10, 304. [Google Scholar] [CrossRef]
- FAO. FAOstat: Soybean Yield by FAO Stat 2017–2019 for—North America/EU/Australia. 2021. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 15 October 2020).
- Dong, Y.; Yang, X.; Liu, J.; Wang, B.H.; Liu, B.L.; Wang, Y.Z. Pod shattering resistance associated with domestication is mediated by a NAC gene in soybean. Nat. Commun. 2014, 5, 3352. [Google Scholar] [CrossRef] [PubMed]
- Sironi, E.; Sessa, F.; Duranti, M. A simple procedure of lupin seed protein fractionation for selective food applications. Eur. Food Res. Technol. 2005, 221, 145–150. [Google Scholar] [CrossRef]
- Hernández-López, I.; Ortiz-Solà, J.; Alamprese, C.; Barros, L.; Shelef, O.; Basheer, L.; Rivera, A.; Abadias, M.; Aguiló-Aguayo, I. Valorization of Local Legumes and Nuts as Key Components of the Mediterranean Diet. Foods 2022, 11, 3858. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.R.; Huynh, B.L.; Vinholes, P.D.; Cisse, N.; Drabo, I.; Ehlers, J.D.; Roberts, P.A.; Close, T.J. Association studies and legume synteny reveal haplotypes determining seed size in. Front. Plant Sci. 2013, 4, 95. [Google Scholar] [CrossRef] [PubMed]
- Smykal, P.; Nelson, M.N.; Berger, J.D.; von Wettberg, E.J.B. The Impact of Genetic Changes during Crop Domestication. Agronomy 2018, 8, 119. [Google Scholar] [CrossRef]
- Purugganan, M.D.; Fuller, D.Q. The nature of selection during plant domestication. Nature 2009, 457, 843–848. [Google Scholar] [CrossRef]


| Type | Trait | Value | Method |
|---|---|---|---|
| Vegetative phenology | Height growth | cm | meter |
| Width growth | cm | meter | |
| Vegetative development | Index rank | rank | |
| Architecture | Leaf number development | # | count |
| Branch number | # | count | |
| Performance | Chlorosis | Index rank | count |
| Lodging | # | count | |
| Photochemical efficiency | Fv/Fm | miniPAM | |
| Pathology | # | count | |
| Leaves DW | g | weight | |
| Stem and branch DW | g | weight | |
| Vegetative biomass DW | g | weight | |
| Reproductive phenology | Bloom development | Index rank | rank |
| Bloom structure | Index rank | rank | |
| Lateral pods development | Lateral bloom branch # | count | |
| Main stem pod development | # | count | |
| First dry pod | Day from start | observe | |
| Pod ripening time | Day from start | observe | |
| All pods opened | Day from start | observe | |
| Yield | Normal seeds number | # | count |
| Infested seeds number | # | count | |
| Abnormal seeds number | # | count | |
| Number of pods | # | count | |
| Normal seeds in closed pods | # | count | |
| Abnormal seeds in closed pods | # | count | |
| Normal seeds DW | g | weight | |
| Abnormal seeds DW | g | weight | |
| Infested seeds DW | g | weight | |
| Pod valves DW | g | weight | |
| Total ripe fruit number | # | calculated | |
| Fully developed seed number | # | calculated | |
| Fully developed seeds DW | g | weight | |
| Total seed number | # | calculated | |
| Total seed DW | g | weight | |
| Normal seeds rate (%#) | %#, %DW | calculated | |
| Abnormal seeds rate (%#) | %#, %DW | calculated | |
| Infested seeds rate (%#) | %#, %DW | calculated | |
| Developed seeds rate (%#) | %#, %DW | calculated | |
| Reproductive/Vegetative | Ratio | calculated |
| # | Collected | Site | Soil | Altitude (m) | Location | Average Seed DW (g) |
|---|---|---|---|---|---|---|
| 1 | 2017 | Golan—Mapalim | Basalt | 520 | 32°59′10.5″ N 35°45′04.1″ E | 0.498 |
| 2 | 2018 | Hula—Hamdal | Basalt | 100 | 33°05′39.3″ N 35°40′27.0″ E | 0.633 |
| 3 | 2019 | Judean Mts.—Sokho | Limestone | 330 | 31°40′52.8″ N 34°58′34.4″ E | 0.522 |
| 4 | 2018 | Golan—Ofir | Basalt | 250 | 32°48′53.6″ N 35°39′45.6″ E | 0.863 |
| 5 | 2017 | Golan—Hisfin | Basalt | 420 | 32°50′48.2″ N 35°46′46.4″ E | 0.381 |
| 8 | 2019 | Judean Mts.—Bekoa | Limestone | 150 | 31°49′21.5″ N 34°56′27.6″ E | 0.692 |
| 10 | 2017 | Golan—Hazeka | Basalt | 980 | 33°03′42.7″ N 35°50′47.1″ E | 0.442 |
| 11 | 2017 | Judean Mts.—Matta | Limestone | 620 | 31°42′58.1″ N 35°03′01.1″ E | 0.548 |
| 21 | 2019 | Carmel—Makura | Limestone | 90 | 32°38′22.2″ N 34°59′19.9″ E | 0.622 |
| 26 | 2019 | Samaria—Kedumim | Limestone | 360 | 32°12′53.9″ N 35°09′50.5″ E | 0.558 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shelef, O.; Ben-Simchon, E.; Sternberg, M.; Cohen, O. Agronomic Estimation of Lupin (Lupinus pilosus L.) as a Prospective Crop. Agronomy 2024, 14, 2804. https://doi.org/10.3390/agronomy14122804
Shelef O, Ben-Simchon E, Sternberg M, Cohen O. Agronomic Estimation of Lupin (Lupinus pilosus L.) as a Prospective Crop. Agronomy. 2024; 14(12):2804. https://doi.org/10.3390/agronomy14122804
Chicago/Turabian StyleShelef, Oren, Eyal Ben-Simchon, Marcelo Sternberg, and Ofer Cohen. 2024. "Agronomic Estimation of Lupin (Lupinus pilosus L.) as a Prospective Crop" Agronomy 14, no. 12: 2804. https://doi.org/10.3390/agronomy14122804
APA StyleShelef, O., Ben-Simchon, E., Sternberg, M., & Cohen, O. (2024). Agronomic Estimation of Lupin (Lupinus pilosus L.) as a Prospective Crop. Agronomy, 14(12), 2804. https://doi.org/10.3390/agronomy14122804







