Spatio-Temporal Distribution of Stored Product Insects in a Feed Mill in Greece
Abstract
1. Introduction
2. Materials and Methods
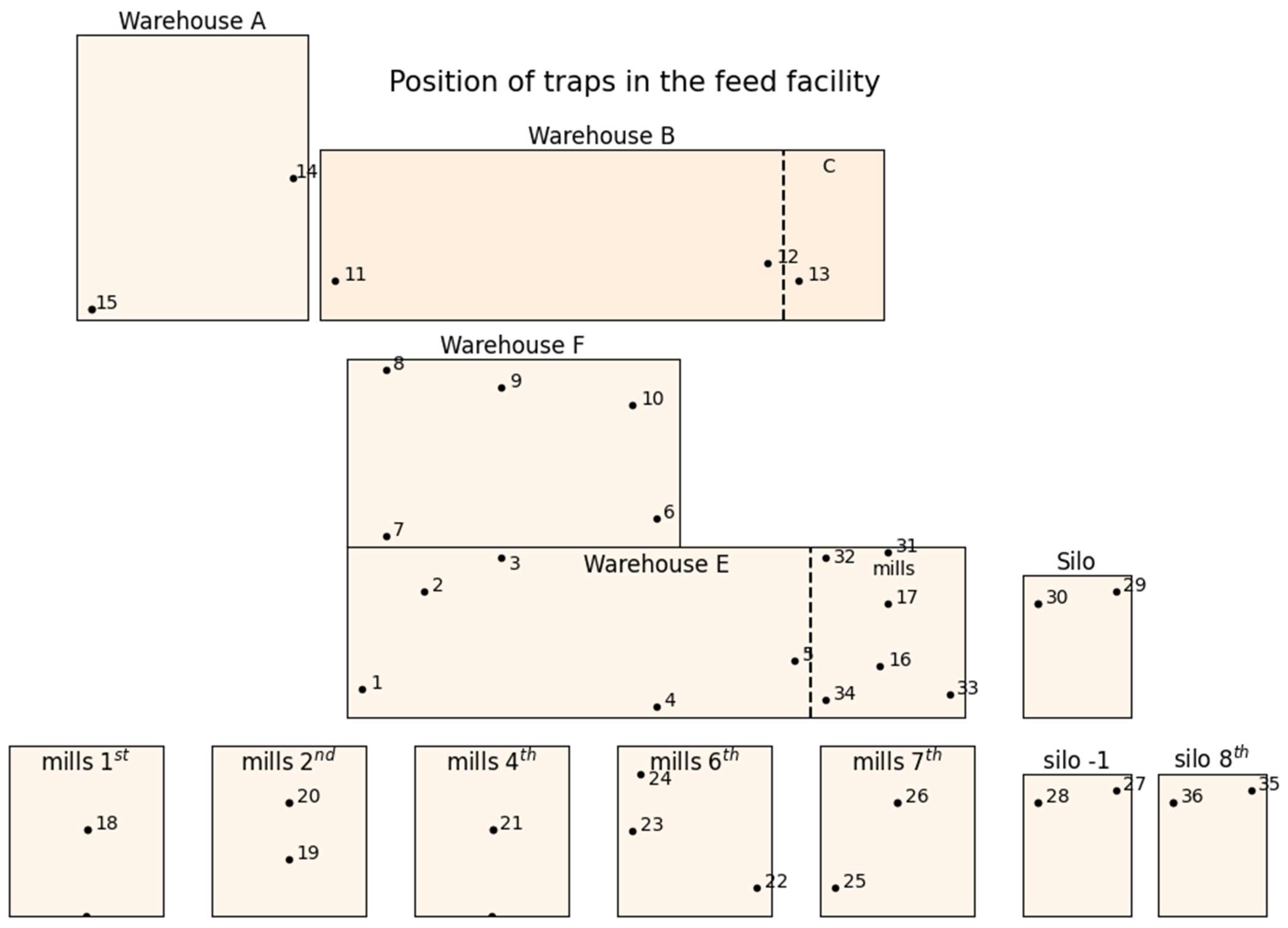
2.1. Facility Description
2.2. Experimental Design
2.3. Data Analysis
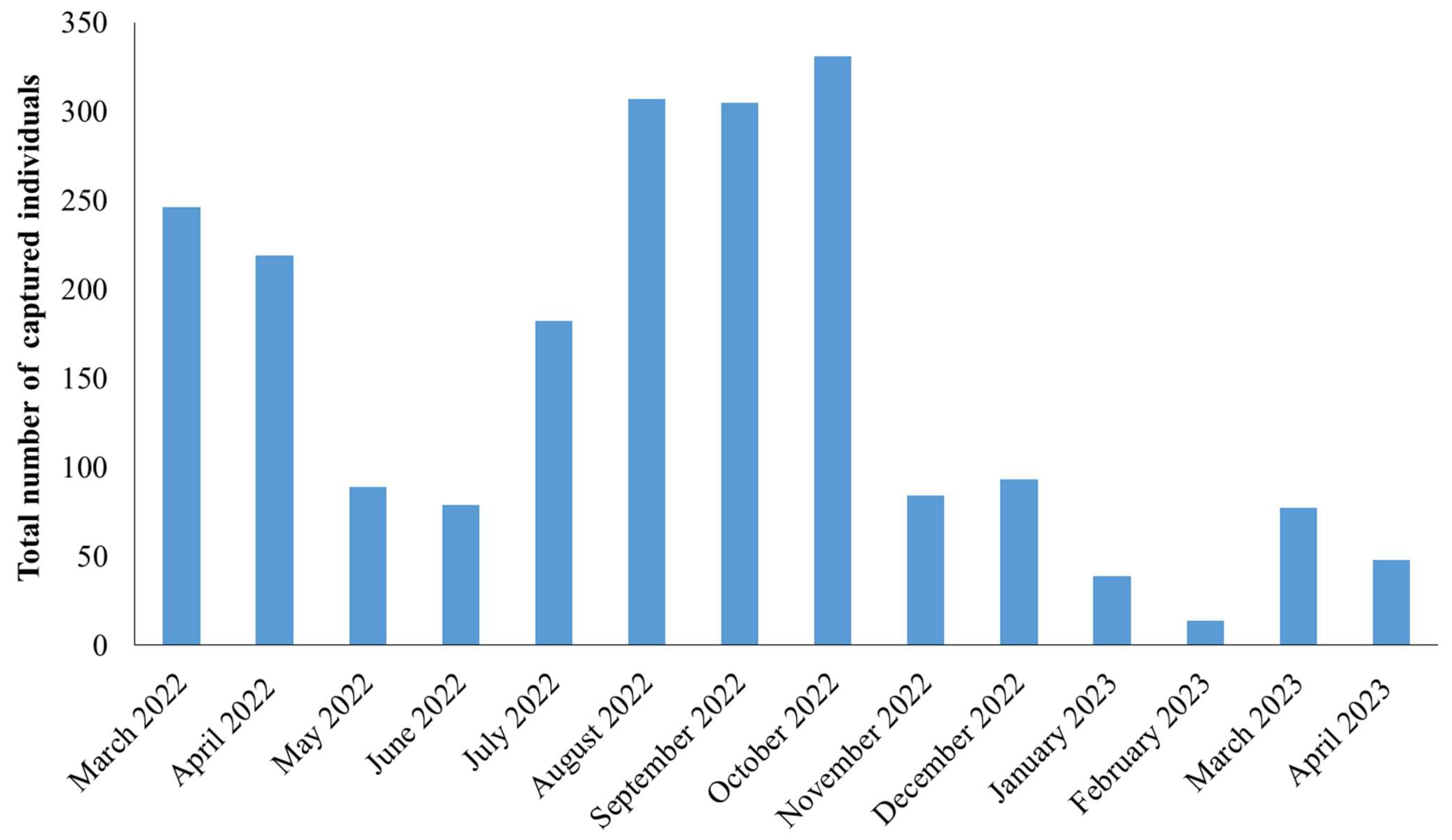
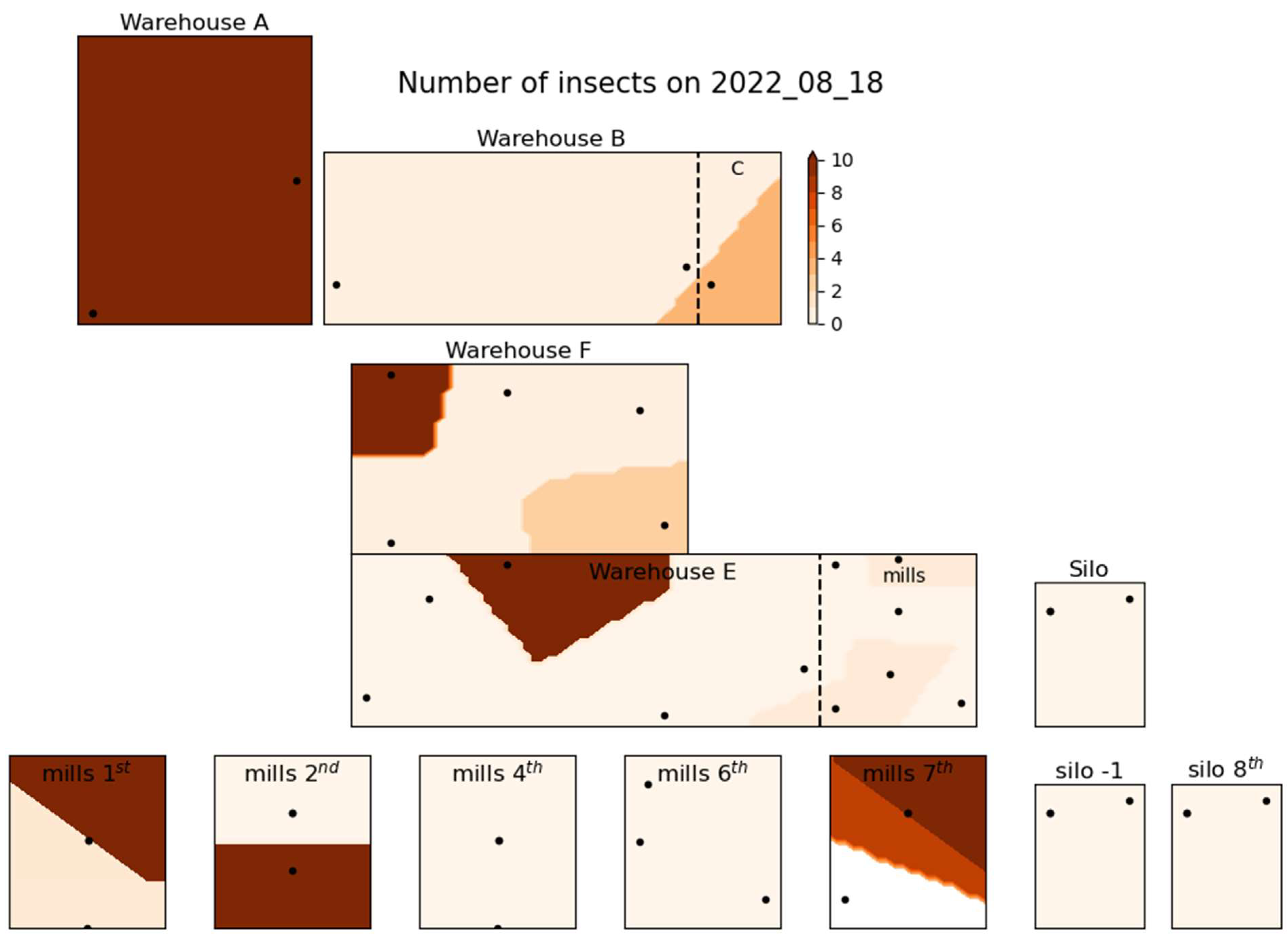
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Odum, E.P. Ecology and Our Endangered Life-Support Systems; Sinauer: Sunderland, UK, 1989; Volume 1, p. 283. ISBN 0-87893-653-1. [Google Scholar]
- Dunkel, F.V. The stored grain ecosystem: A global perspective. J. Stored Prod. Res. 1992, 28, 73–87. [Google Scholar] [CrossRef]
- Hagstrum, D.W. Seasonal variation of stored wheat environment and insect populations. Environ. Entomol. 1987, 16, 77–83. [Google Scholar] [CrossRef]
- Hagstrum, D.W. Infestation by Cryptolestes ferrugineus of newly harvested wheat stored on three Kansas farms. J. Econ. Entomol. 1989, 82, 655–659. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.G.; Palyvos, P.E.; Buchelos, C.T. Three-dimensional distribution and sampling indices of insects and mites in horizontally-stored wheat. App. Entomol. Zoolog. 2003, 38, 413–426. [Google Scholar] [CrossRef]
- Andreadis, S.; Athanassiou, C.G. A review of insect cold hardiness and its potential in stored product insect control. Crop. Prot. 2016, 91, 93–99. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Arthur, F.H. Cool down-Warm up: Gradual temperature changes do not affect the efficacy of cold treatment against stored product insects. Insects 2020, 11, 158. [Google Scholar] [CrossRef]
- Dowdy, A.K.; McGaughey, W.H. Seasonal activity of stored-product insects in and around farm-stored wheat. J. Econ. Entomol. 1994, 93, 1842–1847. [Google Scholar] [CrossRef]
- Campbell, J.F.; Toews, M.D.; Arthur, F.H.; Arbogast, R.T. Long-term monitoring of Tribolium castaneum populations in two flour mills: Seasonal patterns and impact of fumigation. J. Econ. Entomol. 2010, 103, 991–1001. [Google Scholar] [CrossRef]
- Campbell, J.F.; Toews, M.D.; Arthur, F.H.; Arbogast, R.T. Long-term monitoring of Tribolium castaneum populations in two flour mills: Rebound after fumigation. J. Econ. Entomol. 2010, 103, 1002–1011. [Google Scholar] [CrossRef]
- Semeao, A.r.A.; Campbell, J.F.; Hutchinson, J.M.S.; Whitworth, R.J.; Sloderbeck, P.E. Spatio-temporal distribution of stored-product insects around food processing and storage facilities. Agric. Ecosyst. Environ. 2013, 65, 51–162. [Google Scholar] [CrossRef]
- William, R.M.; Agrafioti, P.; Domingue, M.J.; Scheff, D.S.; Lampiri, E.; Gourgouta, M.; Baliota, G.V.; Sakka, S.; Myers, S.W.; Athanassiou, C.G. Comparison of different traps and attractants in 3 food processing facilities in Greece on the capture of stored product insects. J. Econ. Entomol. 2023, 116, 1432–1446. [Google Scholar] [CrossRef]
- Trematerra, P.; Sciarretta, A. Spatial distribution of some beetles infesting a feed mill with spatio-temporal dynamics of Oryzaephilus surinamensis, Tribolium castaneum and Tribolium confusum. J. Stored Prod. Res. 2004, 40, 363–377. [Google Scholar] [CrossRef]
- Trematerra, P.; Gentile, A.; Brunetti, L.; Collins, J. Chambers Spatio-temporal analysis of trap catches of Tribolium confusum J. du Val in a semolina mill, with a comparison of female and male distribution. J. Stored Prod. Res. 2007, 43, 315–322. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Buchelos, C.T. Detection of stored-wheat beetle species and estimation of population density using unbaited probe traps and grain trier samples. Entomol. Exp. Appl. 2001, 98, 67–78. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Buchelos, C.T. Grain properties and insect distribution trends in silos of wheat. J. Stored Prod. Res. 2020, 88, 101632. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.G.; Palyvos, N.E.; Sciarretta, A.; Trematerra, P. Spatiotemporal distribution of insects and mites in horizontally stored wheat. J. Econ. Entomol. 2005, 98, 1058–1069. [Google Scholar] [CrossRef]
- Weston, P.A.; Barney, R.J. Comparison of three trap types for monitoring of insect populations in stored grain. J. Econ. Entomol. 1998, 91, 1449–1457. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.G.; Campbell, J.F. Capture of Tribolium castaneum and Tribolium confusum (Coleoptera: Tenebrionidae) in floor traps: The effect of previous captures. J. Econ. Entomol. 2016, 109, 461–466. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.G.; Campbell, J.F. Effect of the presence of live or dead insects on subsequent captures of six stored product beetle species: The relative species matters. J. Econ. Entomol. 2017, 110, 770–775. [Google Scholar] [CrossRef]
- Bousquet, Y. Beetles Associated with Stored Products in Canada: An Identification Guide; Research Branch, Agriculture Canada: Ottawa, ON, Canada, 1990. [Google Scholar]
- Gorham, J.R. Insect and Mite Pests in Food: An Illustrated Key; U.S. Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 1991. [Google Scholar]
- Peacock, E.R. Adults and larvae of hide, larger and carpet beetles and their relatives (Coleoptera: Dermestidae) and of derodontid beetles (Coleoptera: Derodontidae). In Handbook for the Identification of British Insects; Royal Entomological Society of London: London, UK, 1993. [Google Scholar]
- Curry, J.P. The arthropods associated with the decomposition of some common grass and weed species in the soil. Soil Biol. Biochem. 1973, 5, 645–657. [Google Scholar] [CrossRef]
- Buchelos, C.T.; Athanassiou, C.G. Dominance and frequency of Coleoptera found on stored cereals and cereal products in central Greece. Entomol. Hell. 1993, 11, 17–22. [Google Scholar] [CrossRef]
- Crombie, A.C. On competition between different species of graminivorous insects. Proc. R. Soc. Lond. B Biol. Sci. 1945, 132, 362–395. [Google Scholar]
- Birch, L.C. The influence of temperature on the development of the different stages of Calandra oryzae L. and Rhyzopertha dominica Fab. (Coleoptera). Aust. J. Exp. Biol. Med. Sci. 1945, 23, 29–35. [Google Scholar] [CrossRef]
- Birch, L.C. The mortality of the immature stages of Calandra oryzae (L.) (small strain) and Rhyzopertha dominica Fab. in wheat of different moisture contents. Aust. J. Exp. Biol. Med. Sci. 1945, 23, 141–145. [Google Scholar] [CrossRef]
- Birch, L.C. A contribution to the ecology of Calandra oryzae (L.) and Rhyzopertha dominica Fab. (Coleoptera) in stored wheat. Trans. R. Soc. S. Aust. 1945, 69, 140–149. [Google Scholar]
- Giga, D.P.; Canhao, S.J. Competition between Prostephanus truncatus (Horn) and Sitophilus zeamais (Motsch.) in maize at two temperatures. J. Stored Prod. Res. 1993, 29, 63–70. [Google Scholar] [CrossRef]
- Trematerra, P.; Sciarretta, A.; Tamasi, E. Behavioural responses of Oryzaephilus surinamensis, Tribolium castaneum and Tribolium confusum to naturally and artificially damaged durum wheat kernels. Entom. Exp. Appl. 2000, 94, 195–200. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.G.; Campbell, J.F. Competition of three species of Sitophilus on rice and maize. PLoS ONE 2017, 12, e0173377. [Google Scholar] [CrossRef]
- Scheff, D.S.; Campbell, J.F.; Arthur, F.H. Seasonal, landscape, and attractant effects on lesser grain borer, Rhyzopertha dominica (F.), captures in northeast Kansas. Agronomy 2022, 12, 99. [Google Scholar] [CrossRef]
- Aitken, A.D. Insect Travelers, I: Coleoptera. In Technical Bulletin 31; Her Majesty’s Stationery Office: London, UK, 1975. [Google Scholar]
- Nansen, C.; Phillips, T.W.; Palmer, M.W. Analysis of the insect community in a stored-maize facility. Ecol. Res. 2004, 19, 197–207. [Google Scholar] [CrossRef]
- Trematerra, P.; Throne, J. Insect and mites pests of durum wheat. In American Associate of Cereal Chemists International, 2nd ed.; Sissons, M., Abecassis, J., Marchylo, B., Carcea, M., Eds.; AACC International Press: St. Paul, MN, USA, 2012; pp. 73–83. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Athanassiou, C.G.; Arthur, F.H.; Throne, J.E. Lesser grain borers, Rhyzopertha dominica, select rough rice kernels with cracked hulls for reproduction. J. Insect Sci. 2012, 12, 38. [Google Scholar] [CrossRef] [PubMed]



| Family/Taxa | Species | % of the Total Number of Adults | Dominance | % of the Total Number of Samples | Frequency |
|---|---|---|---|---|---|
| Tenebrionidae | Tribolium confusum | 19.21 | Dominant | 82.76 | Constant |
| Tribolium castaneum | 15.09 | Dominant | 65.51 | Constant | |
| Latheticus oryzae | 3.02 | Influent | 48.27 | Accessory | |
| Others | 0.28 | Recedent | 13.79 | Accidental | |
| Silvanidae | Oryzaephilus surinamensis | 3.73 | Influent | 68.96 | Constant |
| Anobiidae | Lasioderma serricorne | 3.97 | Influent | 44.82 | Accessory |
| Stegobium paniceum | 0.28 | Recedent | 17.24 | Accidental | |
| Bostrychidae | Rhyzopertha dominica | 0.70 | Recedent | 31.03 | Accessory |
| Curculionidae | Sitophilus oryzae | 11.16 | Dominant | 31.03 | Accessory |
| Sitophilus granarius | 19.40 | Dominant | 82.75 | Constant | |
| Sitophilus zeamais | 0.66 | Recedent | 6.89 | Accidental | |
| Laemophloeidae | Cryptolestes ferrugineus | 0.04 | Recedent | 11.11 | Accidental |
| Cryptolestes sp. | 0.14 | Recedent | 10.34 | Accidental | |
| Nititulidae | Carpophilus sp. | 3.73 | Influent | 34.48 | Accessory |
| Dermestidae | Trogoderma sp. | 0.91 | Recedent | 41.37 | Accessory |
| Ptinidae | Ptinus sp. | 0.18 | Recedent | 13.79 | Accidental |
| Mycetophagidae | Typhaea sp. | 0.37 | Recedent | 13.79 | Accidental |
| Lepidoptera | 6.29 | Dominant | 65.51 | Constant | |
| Formicidae | 1.65 | Recedent | 13.79 | Accidental | |
| Staphilinidae | 1.37 | Recedent | 31.03 | Accessory | |
| Diptera | 7.52 | Dominant | 68.96 | Constant | |
| Hemiptera | 0.14 | Recedent | 3.44 | Accidental |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agrafioti, P.; Lampiri, E.; Kaloudis, E.; Gourgouta, M.; Vassilakos, T.N.; Ioannidis, P.M.; Athanassiou, C.G. Spatio-Temporal Distribution of Stored Product Insects in a Feed Mill in Greece. Agronomy 2024, 14, 2812. https://doi.org/10.3390/agronomy14122812
Agrafioti P, Lampiri E, Kaloudis E, Gourgouta M, Vassilakos TN, Ioannidis PM, Athanassiou CG. Spatio-Temporal Distribution of Stored Product Insects in a Feed Mill in Greece. Agronomy. 2024; 14(12):2812. https://doi.org/10.3390/agronomy14122812
Chicago/Turabian StyleAgrafioti, Paraskevi, Evagelia Lampiri, Efstathios Kaloudis, Marina Gourgouta, Thomas N. Vassilakos, Philippos M. Ioannidis, and Christos G. Athanassiou. 2024. "Spatio-Temporal Distribution of Stored Product Insects in a Feed Mill in Greece" Agronomy 14, no. 12: 2812. https://doi.org/10.3390/agronomy14122812
APA StyleAgrafioti, P., Lampiri, E., Kaloudis, E., Gourgouta, M., Vassilakos, T. N., Ioannidis, P. M., & Athanassiou, C. G. (2024). Spatio-Temporal Distribution of Stored Product Insects in a Feed Mill in Greece. Agronomy, 14(12), 2812. https://doi.org/10.3390/agronomy14122812










