The Sources of Nutrients for the Growing Ear of Winter Wheat in the Critical Cereal Window
Abstract
:1. Introduction
- Does ear biomass depend solely on N supply?
- What are the sources of nutrients for the growing ear?
- Which source has the greatest effect on the N mass in the ear?
- Are the vegetative organs of winter wheat an effective sink for nutrients?
- Under what conditions does this phenomenon manifest itself?
2. Materials and Methods
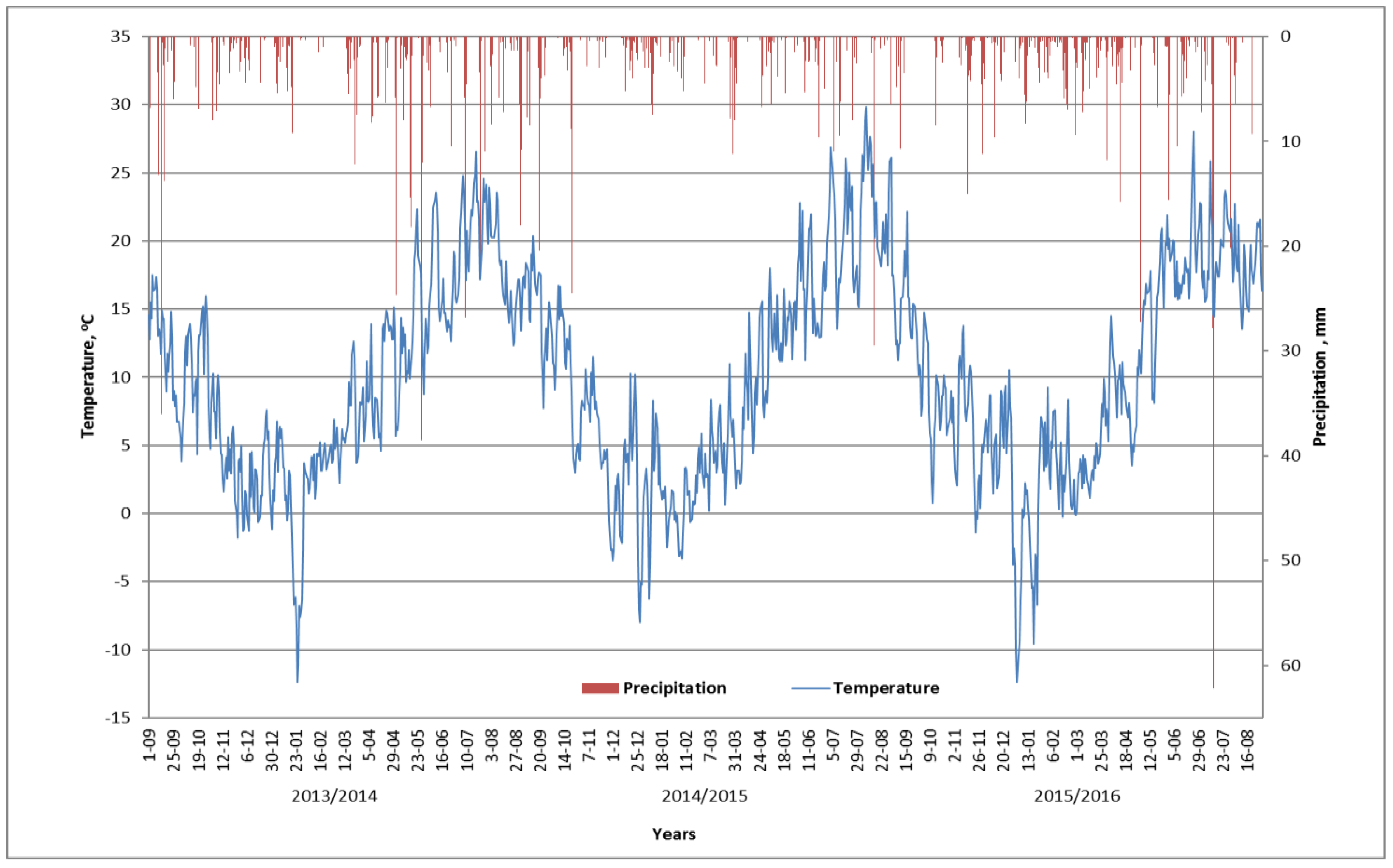
2.1. Experimental Site
2.2. Experimental Design
- (1)
- The first N doses of 40 and 80 kg N ha−1 were applied at the end of winter, just before the spring beginning of winter wheat vegetation.
- (2)
- The N dose was supplemented to 160 kg N ha−1 at the end of tillering/the beginning of shoot elongation (BBCH 29/30).
- (3)
- The N dose was supplemented to 240 kg N ha−1 when the flag leaf became visible (BBCH 39).
2.3. Plant Sampling
2.4. Calculation of Nutrient Balance During the Critical Cereal Window
2.5. Statistical Analysis
3. Results
3.1. The Mass of Nutrients in the Ears in the Full Flowering Phase
3.2. Nutrient Mass Balance in Leaves in the Critical Cereal Window
3.3. Nutrient Mass Balance in Stems in the Critical Cereal Window
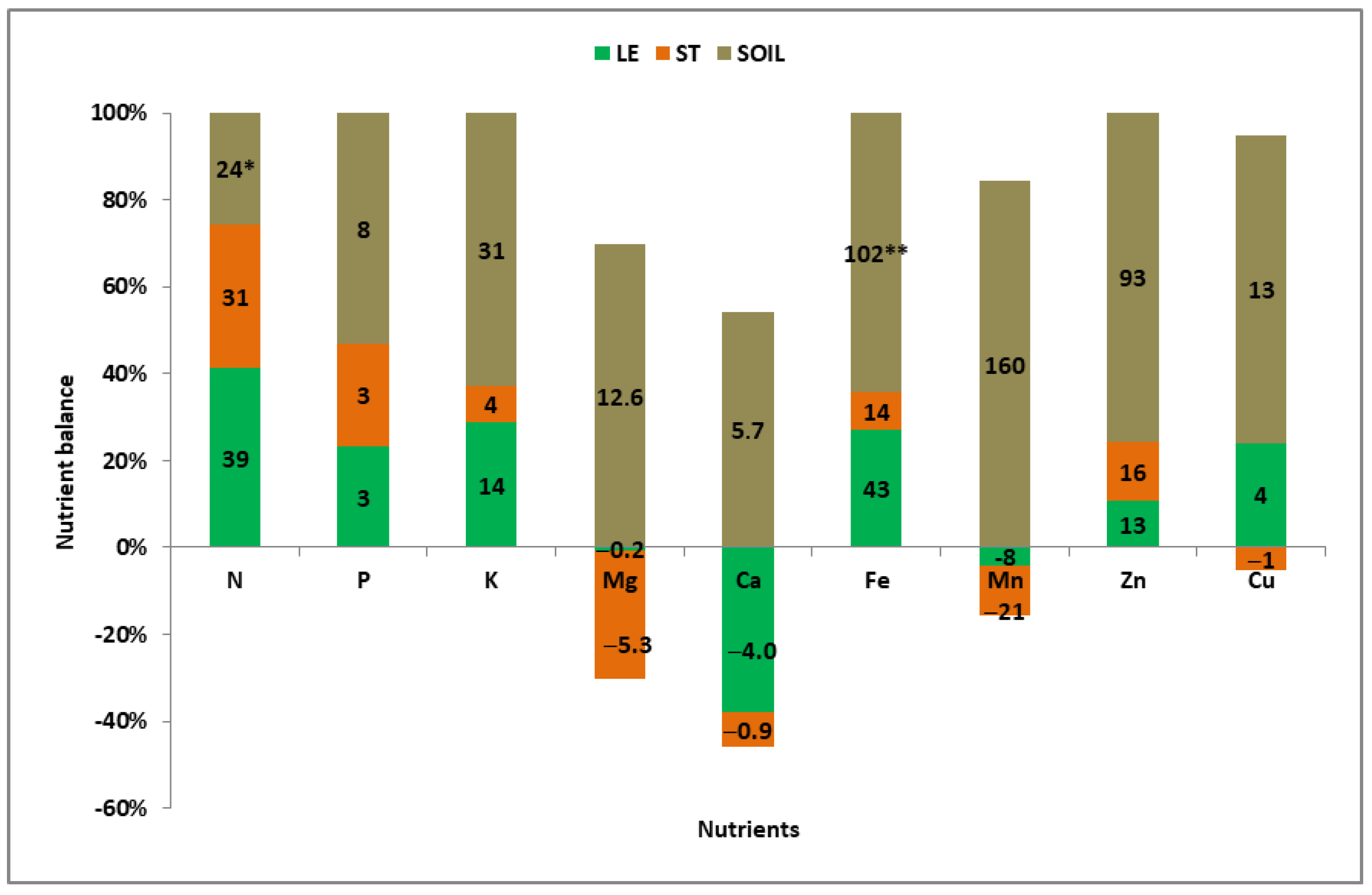
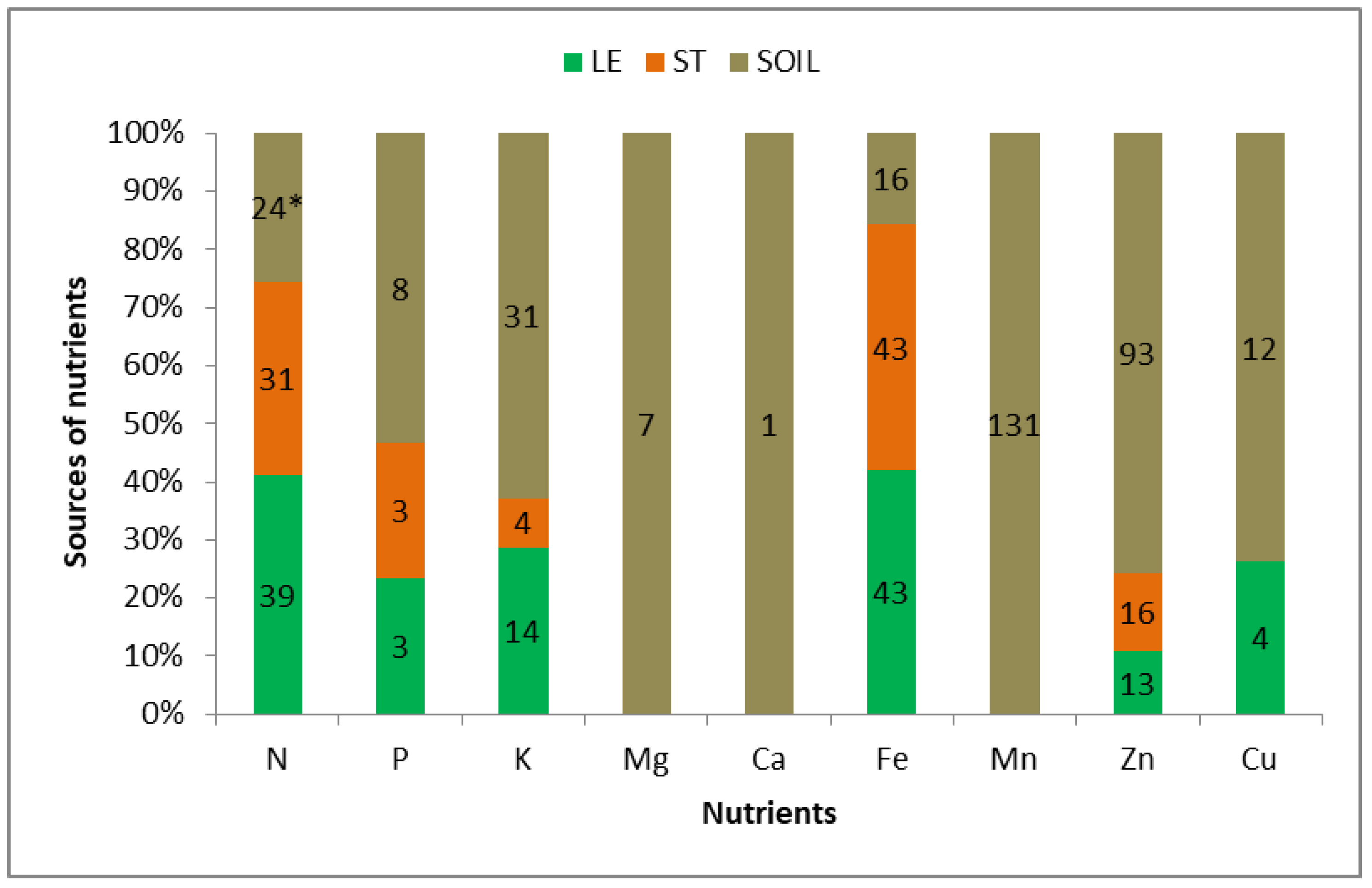
3.4. Soil Nutrient Uptake by Winter Wheat During the Critical Cereal Window
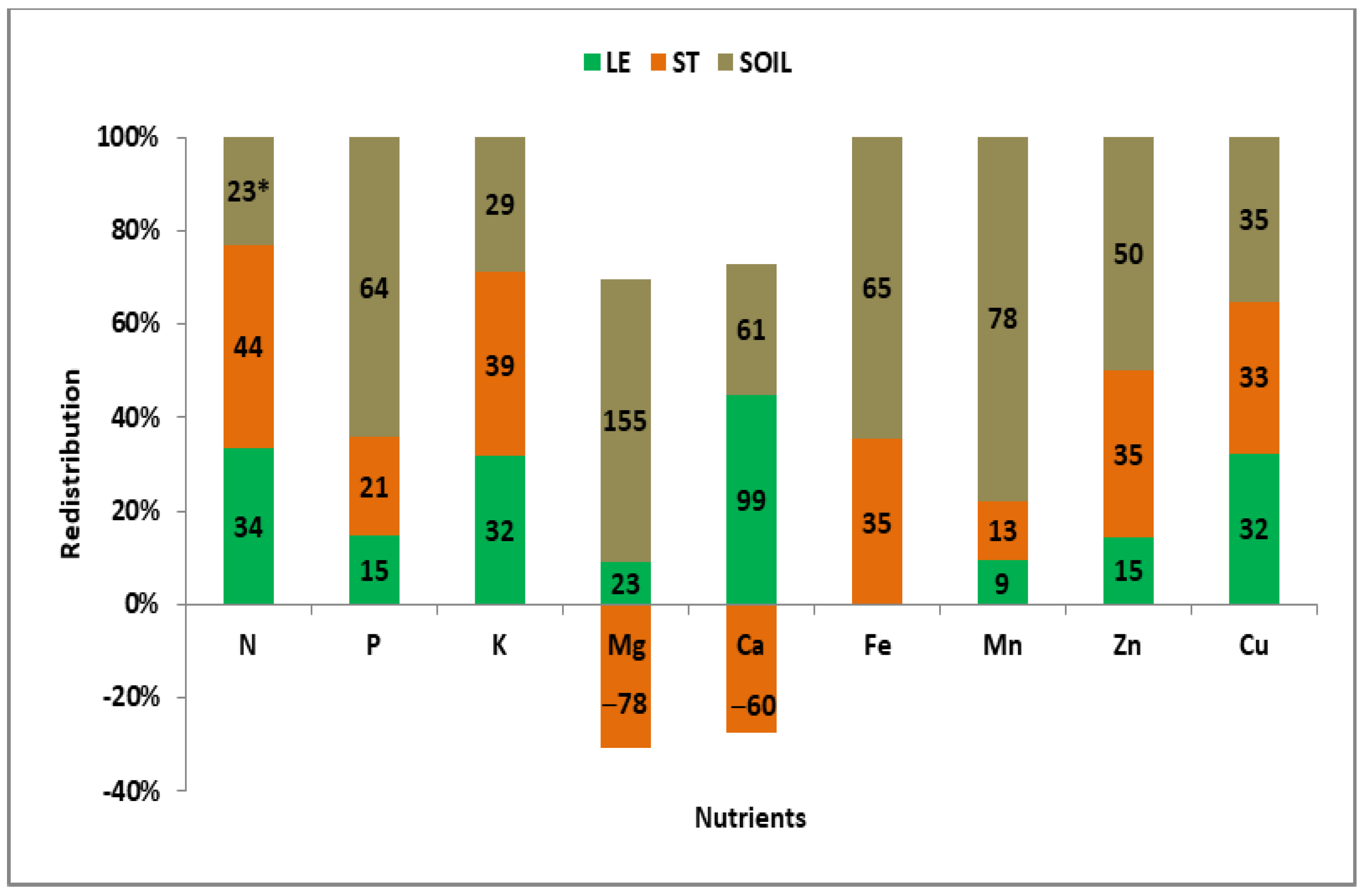
3.5. Redistribution of Nutrients Taken up by Winter Wheat in the CCW
4. Discussion
4.1. Ear Nutrients—Yield Prediction
- Effective N—2014. This season was characterized by a low amount of N in the ears and high yield. The productivity of N accumulated in ears was 141 kg grain kg−1 ear N.
- Optimal N—2015. This season was characterized by a moderate amount of N in the ears and a very high yield. The productivity of N accumulated in ears was 120 kg of grain kg−1 N.
- Ineffective N—2016. This season was characterized by a very high amount of N in the ears and moderate yield. The productivity of N accumulated in ears was only 60 kg grain kg−1 ear N.
- Moderate: Cu, N, K, and P; range: 160–180 kg N ha−1;
- High: Mg, Ca, and Zn; range: 190–210 kg N ha−1;
- Very high: Fe and Mn; range: 210–230 kg N ha−1.
4.2. Nutrient Economy of Winter Wheat Canopy During CCW
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Wheat Trait | Three-Year System, 3YS, n = 21 | R2 | Two-Year System, 2YS, n = 14 | R2 |
|---|---|---|---|---|
| Ear biomass, EAB | EAB = 0.83 + 0.3N + 0.21Mg − 1.46Ca | 0.98 | EAB = 0.06 − 0.01N + 0.24P + 0.04K + 1.11Ca − 0.009Fe | 0.997 |
| Grain yield, GY | GY = 4.82 − 0.87P + 8.92Ca + 0.08Zn | 0.89 | GY = 5.78 + 0.17N − 1.28P + 0.05Mn | 0.85 |
| Grain protein content, CP | CPC = 1.1 + 0.06N | 0.73 | CPC = 8.76 + 0.14N − 1.31P + 0.05Mn | 0.83 |
| Grain protein yield, CPY | GPY = 0.11 − 0.11P + 1.49Ca + 0.01Zn | 0.80 | GPY = 0.48 + 0.04N − 0.3P + 0.01Mn | 0.87 |
| Canopy ear density, CED | CED = 426 + 2.28N − 29.2Mg + 1.8Zn | 0.72 | CED = 311 + 29Cu | 0.69 |
| Grain per ear, GE | GE = 25.7 − 2.29P + 22.5Ca + 0.13Mn | 0.79 | Nonsignificant | - |
| Canopy grain density, CGD | GD = 10.1 − 1.88Mg + 8.2Ca + 0.13Zn | 0.88 | CGD = 10.6 + 0.13N | 0.68 |
| Thousand grain weight | TGW = 45.9 + 4.3Ca | 0.49 | TGW = 51.6 + 0.07N − 2.1P + 0.28K | 0.78 |
| Wheat Trait | Leaves | R2 | Stems | R2 |
|---|---|---|---|---|
| Ear biomass, EAB | EAB = 3.51 − 0.015Fe + 0.094Zn | 0.70 | EAB = 1.7 + 0.23P − 0.31Mg | 0.91 |
| Grain yield, GY | GY = 6.53 + 0.83P − 0.82Mg | 0.78 | GY = 9.28 − 0.17Cu | 0.26 |
| Grain protein content, GPC | GPC = 8.65 + 0.095N | 0.31 | GPC = 8.84 + 0.06N + 0.0Zn − 0.34Cu | 0.59 |
| Grain protein yield, GPY | GPY = 0.41 + 0.019N − 0.067Mg | 0.60 | Nonsignificant | - |
| Canopy ear density, CED | CED = 422 + 53P − 67Mg − 1.9Fe + 9.6Zn | 0.71 | Nonsignificant | - |
| Grain per ear, GE | GE = 26.4 − 0.96Mg + 0.07Fe | 0.84 | GE = 34.3 − 0.35Cu | 0.27 |
| Canopy grain density, CGD | CGD = 12.4 + 1.95P − 1.66Mg | 0.84 | Nonsignificant | - |
| Thousand grain weight | TGW = 50.1 − 0.53K | 0.32 | TGW = 48 − 0.28Mg − 0.007Fe | 0.58 |
| Wheat Trait | Three-Year System, 3YS, n = 21 | R2 |
|---|---|---|
| Ear biomass, EAB | EAB = 1.06 − 0.11P + 0.3Mg + 0.1Zn − 0.07Cu | 0.94 |
| Grain yield, GY | GY = 8.2 + 0.22Ca | 0.45 |
| Grain protein content, GPC | GPC = 0.4 + 0.24Mg | 0.53 |
| Grain protein yield, GPY | GPY = 1.0 + 0.028Ca | 0.31 |
| Canopy ear density, CED | CD = 623 + 6.0Ca | 0.23 |
| Grain per ear, GE | GE = 26.6 − 0.41P + 0.49Ca + 0.2Mn | 0.73 |
| Canopy grain density, CGD | CGR = 18 + 0.63Ca − 0.024Fe | 0.59 |
| Thousand grain weight | TGW = 48.3 + 0.01Fe | 0.59 |
Appendix B


References
- FAOSTAT. Available online: https://faostat.fao.org/site/567/default.aspx#ancor (accessed on 25 August 2024).
- Sharma, I.; Tyagi, B.S.; Singh, G.; Venkatesh, K.; Gupta, O.P. Enhancing wheat production—A global perspective. Indian J. Agric. Sci. 2015, 85, 3–13. [Google Scholar] [CrossRef]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef]
- Dadrasi, A.; Chaichi, M.; Nehbandani, A.; Soltani, E.; Nemati, A.; Salmani, F.; Heydari, M.; Yousefi, A.R. Global insight into understanding wheat yield and production in agro-ecological zoning. Sci. Rep. 2023, 13, 15898. [Google Scholar] [CrossRef] [PubMed]
- Grzebisz, W.; Łukowiak, R. Nitrogen gap amelioration is a core for sustainable intensification of agriculture—A concept. Agronomy 2021, 11, 419. [Google Scholar] [CrossRef]
- Senapati, N.; Semenov, M.A. Large genetic yield potential and genetic yield gap estimated for wheat in Europe. Glob. Food Secur. 2020, 24, 100340. [Google Scholar] [CrossRef] [PubMed]
- Slafer, G.A.; Garcia, G.A.; Serrago, R.A.; Miralles, D.J. Physiological drivers of responses of grains per m2 to environmental and genetic factors in wheat. Field Crops Res. 2022, 285, 108593. [Google Scholar] [CrossRef]
- Kirby, E.J.M. Analysis of leaf, stem and ear growth in wheat from terminal spikelet stage to anthesis. Field Crops. Res. 1988, 18, 127–140. [Google Scholar] [CrossRef]
- González, F.G.; Miralles, D.J.; Slafer, G.A. Wheat floret survival as related to pre-anthesis spike growth. J. Exp. Bot. 2011, 62, 4889–4901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Wu, Y.; Zheng, X.; Guo, X.; Sun, W.; Sun, Z.; Wang, Z.; Zhang, Y. A dynamic regulation of nitrogen on floret primordia development in wheat. Crop J. 2024, 12, 271–280. [Google Scholar] [CrossRef]
- Ferrante, A.; Savin, R.; Slafer, G.A. Floret development and spike fertility in wheat: Differences between cultivars of contrasting yield potential and their sensitivity to photoperiod and soil N. Funct. Plant Biol. 2005, 32, 189–197. [Google Scholar] [CrossRef]
- Prieto, P.; Ochagavia, H.; Savin, R.; Griffiths, S.; Slafer, G.A. Dynamics of floret initiation/death determining spike fertility in wheat as affected by PpG genes under field conditions. J. Exp. Bot. 2018, 69, 2633–2645. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Tahir Ata-ul-Karim, S.; Lemaire, G.; Duan, A.; Liu, Z.; Guo, Y.; Qin, A.; Ning, D.; Liu, Z. Exploring the nitrogen source-sink ratio to quantify ear nitrogen accumulation in maize and wheat using critical nitrogen dilution curve. Field Crops Res. 2021, 274, 108332. [Google Scholar] [CrossRef]
- Grzebisz, W.; Szczepaniak, W.; Potarzycki, J.; Biber, M. Prediction of grain yield and gluten content in winter bread wheat based on nutrient content in plant parts during the Critical Cereal Window. Agronomy 2023, 13, 2649. [Google Scholar] [CrossRef]
- Duan, J.; Wu, Y.; Zhou, Y.; Ren, X.; Shao, Y.; Feng, W.; Zhu, Y.; Wang, Y.; Guo, T. Grain number response to pre-anthesis dry matter and nitrogen in improving wheat yield in the Huang-Huai Plain. Sci. Rep. 2018, 8, 7126. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, W.; Nowicki, B.; Bełka, D.; Kazimierowicz, A.; Kulwicki, M.; Grzebisz, W. Effect of foliar application of micronutrients and fungicides on the nitrogen use efficiency in winter wheat. Agronomy 2022, 12, 257. [Google Scholar] [CrossRef]
- Grzebisz, W. Technology of Crop Plants Fertilization—Yielding Physiology. Part II. Cereals and Maize; PWRiL: Poznań, Poland, 2012; 279p. (In Polish) [Google Scholar]
- Iwańska, M.; Paderewski, J.; Stępień, M.; Rodrigues, P.C. Adaptation of winter wheat cultivars to different environments: A case study in Poland. Agronomy 2020, 10, 632. [Google Scholar] [CrossRef]
- Hlisnikovsky, L.; Menšik, L.; Kunzová, E. The development of winter wheat yield and quality under different fertilizer regimes and soil-climatic conditions in the Czech Republic. Agronomy 2020, 10, 1160. [Google Scholar] [CrossRef]
- Koppensteiner, L.K.; Kaul, H.-P.; Piepho, H.-P.; Barta, N.; Euteneuer, P.; Bernas, J.; Klimek-Kopyra, A.; Gronauer, A.; Neugschwandtner, R.W. Yield and yield components of facultative wheat are affected by sowing time, nitrogen fertilization and environment. Europ. J. Agron. 2022, 140, 126591. [Google Scholar] [CrossRef]
- Khalid, A.; Hameed, A.; Farrukh Tahir, M. Wheat quality: A review on chemical composition, nutritional attributes, grain anatomy, types, classification, and function of seed storage proteins in bread making quality. Front. Nutr. 2023, 10, 1053196. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, D.; Schnurbusch, T. Plant and floret growth at distinct developmental stages during the stem elongation phase in wheat. Front. Plant Sci. 2018, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- Gaju, O.; Allard, V.; Martre, P.; Le Gouis, J.; Moreau, D.; Bogard, M.; Hubbart, S.; Foulkes, M.J. Nitrogen partitioning and remobilization in relation to leaf senescence, grain yield and grain nitrogen concentration in wheat cultivars. Field Crops Res. 2014, 155, 213–223. [Google Scholar] [CrossRef]
- Kong, L.G.; Xie, Y.; Hu, L.; Feng, B.; Li, S.D. Remobilization of vegetative nitrogen to developing grain in wheat (Triticum aestivum L.). Field Crops Res. 2016, 196, 134–144. [Google Scholar] [CrossRef]
- Masoni, A.; Ercoli, L.; Mariotti, M.; Arduini, I. Post-anthesis accumulation and remobilization of dry matter, nitrogen and phosphorus in durum wheat as affected by soil type. Europ. J. Agron. 2007, 26, 179–186. [Google Scholar] [CrossRef]
- Meng, Q.; Yue, S.; Chen, X.; Cui, Z.; Ye, Y.; Ma, W.; Tong, Y.; Zhang, F. Understanding dry matter and nitrogen accumulation with time-course for high-yielding wheat production in China. PLoS ONE 2013, 8, e68783. [Google Scholar] [CrossRef] [PubMed]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Com. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Kęsik, K. Application of the Mehlich 3 method in the fertilizer advisory system. Stud. I Rap. IUNG-PIB 2016, 48, 95–104. (In Polish) [Google Scholar]
- Trávník, K.; Zbíral, J.; Němec, P. Agrochemical Soil Testing—Mehlich III; Central Institute for Supervising and Testing in Agriculture: Brno, Czech Republic, 1999. (In Czech)
- Zbíral, J. Determination of plant-available micronutrients by the Mehlich 3 soil extractant—A proposal of critical values. Plant Soil Environ. 2016, 62, 527–531. [Google Scholar] [CrossRef]
- PN-EN ISO 20483:2014-02; Cereal Grains and Pulses—Determination of Nitrogen Content and Conversion to Crude Protein—Kjeldahl Method. Polish Committee for Standardization: Warsaw, Poland, 2014; 24p.
- Grzebisz, W.; Biber, M. Nutrient mass in winter wheat in the Cereal Critical Window under different nitrogen levels—Effect on grain yield and grain protein concentration. Agronomy 2024, 14, 2435. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Hu, N.; Li, W.; Qin, W.; Gao, Y.; Liu, Y.; Sun, Z.; Yu, K.; Wang, Z.; et al. Spike growth affects spike fertility through the number of florets with green anthers before floret abortion. Field Crops Res. 2021, 260, 108007. [Google Scholar] [CrossRef]
- Szczepaniak, W.; Potarzycki, J. Impact of increasing nitrogen rates on the course of the nitrogen critical concentration curve during the vegetative growth of winter wheat. J. Elem. 2014, 19, 549–566. [Google Scholar]
- Wu, W.; Wang, Y.; Wang, L.; Xu, H.; Zörb, C.; Geiflus, C.-M.; Xue, C.; Sun, Z.; Ma, W. Booting stage is the key for timing split nitrogen application in improving yield and quality of wheat—A global meta-analysis. Field Crops Res. 2022, 287, 108665. [Google Scholar] [CrossRef]
- Hamnér, K.; Weih, M.; Eriksson, J.; Kirchmann, H. Influence of nitrogen supply on macro- and micronutrient accumulation during growth of winter wheat. Field Crops Res. 2017, 213, 118–129. [Google Scholar] [CrossRef]
- Adams, C.B.; Graebner, R.; Marschall, J.; Neely, C.; Long, D.S.; Reardon, C.L.; Rogers, C.W. Yield has minimal effect on whole-grain yield mineral density of modern soft wheat, compared to production environment. Field Crops Res. 2024, 312, 109403. [Google Scholar] [CrossRef]
- Bindraban, P.S.; Sayre, K.D.; Solismoya, E. Identifying factors that determine kernel number in wheat. Field Crops Res. 1998, 58, 223–234. [Google Scholar] [CrossRef]
- Demotes-Mainard, S.; Jeuffroy, M.-H. Effects of nitrogen and radiation on dry matter and nitrogen accumulation in the spike of winter wheat. Field Crops Res. 2004, 87, 221–233. [Google Scholar] [CrossRef]
- Grzebisz, W.; Barłóg, P.; Kryszak, J.; Łukowiak, R. Pre-anthesis nutritional status of spelt wheat as a tool for predictong the attinable yield. Agronomy 2019, 9, 558. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Guan, H.-W.; Liu, L.-R.; He, L.; Duan, J.-H.; Wang, C.-Y.; Guo, T.-C.; Feng, C. Effect of phosphorus application on spike and fertile floret development and yield of winter wheat under different water treatments. Acta. Agron. Sinica 2023, 49, 2753–2765. [Google Scholar]
- Grzebisz, W.; Szczepaniak, W.; Barłog, P.; Przygocka-Cyna, K.; Potarzycki, J. Phosphorus sources for winter oilseed rape (Brassica napus L.) during reproductive growth-magnesium sulfate management impact on P use efficiency. Arch. Agron. Soil Sci. 2018, 64, 1646–1662. [Google Scholar] [CrossRef]
- Lemaire, G.; Jeuffroy, M.-H.; Fstal, F. Diagnosis tool for plant an crop N status in vegetative stage: Theory and practices for crop N management. Eur. J. Agron. 2008, 28, 614–624. [Google Scholar] [CrossRef]
- Barraclough, P.B. The growth and activity of winter wheat roots in the field: Nutrient uptakes of high-yielding crops. J. Agric. Sci. Camb. 1986, 106, 45–52. [Google Scholar] [CrossRef]
- Grzebisz, W.; Diatta, J.; Barłóg, P.; Biber, M.; Potarzycki, J.; Łukowiak, R.; Przygocka-Cyna, K.; Szczepaniak, W. Soil fertility clock-crop rotation as a paradigm in nitrogen fertilizer productivity control. Plants 2022, 11, 2841. [Google Scholar] [CrossRef] [PubMed]
- Potarzycki, J.; Grzebisz, W.; Szczepaniak, W. Magnesium fertilization increases nitrogen use efficiency in winter wheat (Triticum aestivum L.). Plants 2022, 11, 2600. [Google Scholar] [CrossRef] [PubMed]
- Ram, S.; Malik, V.K.; Gupta, V.; Narwal, S.; Sirohi, M.; Ankush; Pandey, V.; Gupta, O.P.; Mista, A.K.; Singh, G. Impact of foliar application of iron and zinc fertilizers on grain iron, zinc and protein contents in bread wheat (Triticum aestivum L.). Front. Nutr. 2024, 11, 1378937. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.G.; Slader, G.A.; Miralles, D.J. Grain and floret number in response mto the photoperiod during stem elongation in fully and slightly nvernalized wheats. Field Crops Res. 2003, 81, 17–27. [Google Scholar] [CrossRef]
- Xie, Q.; Mayes, S.; Sparkes, D.L. Preanthesis biomass accumulation and plant organs defines yield components in wheat. Eur. J. Agron. 2016, 81, 15–26. [Google Scholar] [CrossRef]
- Dreccer, M.F.; Wockner, K.B.; Palta, J.A.; McIntyre, C.L.; Borgognone, M.G.; Bourgault, M.; Reynolds, M.; Miralles, D.J. More fertile florests and grains per spike can be achieved at higher temperature in wheat lines with high spike biomass and sugar content at booting. Funct. Plant Biol. 2014, 41, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Körner, C. Paradigm shift in plant growth control. Curr. Opin. Plant Biol. 2015, 25, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Slafer, G.A.; Elia, M.; Savin, R.; García, G.A.; Terrile, I.I.; Ferrante, A.; Miralles, D.J.; González, F.G. Fruiting efficiency: An alternative trait to further rise in wheat yield. Food Energy Secur. 2015, 4, 92–109. [Google Scholar] [CrossRef]
- Fradgley, N.S.; Bentley, A.R.; Swarbreck, S.M. Defining the physiological determinants of low nitrogen requirement in wheat. Bioch. Soc. Trans. 2021, 49, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Grzebisz, W.; Łukowiak, R.; Kotnis, K. Evaluation of nitrogen fertilization systems based on the in-season variability of the nitrogenous growth factors and soil fertility factors—A case of winter oilseed rape (Brassica napus L.). Agronomy 2020, 10, 1701. [Google Scholar] [CrossRef]
- White, P.J. Calcium. In Handbook of Plant Nutrition, 2nd ed.; Barker, A.V., Pilbeam, D.J., Eds.; CRC Press, Taylor and Francis Group: Bocan Raton, FL, USA, 2015; pp. 165–198. [Google Scholar]
- Liu, Y.; Xu, G. Nitrogen-iron interaction as an emerging factor influencing crop productivity and nutrient use efficiency. Mol. Plant 2023, 16, 1727–1729. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-Y.; Liu, Y.-M.; Zhang, W.; Chen, X.-P.; Zou, C.-Q. Zinc uptake, translocation, and remobilization in winter wheat as affected by soil application of Zn fertilizer. Front. Plant Sci. 2019, 10, 426. [Google Scholar] [CrossRef] [PubMed]

| Soil, cm | pH | Corg % | P | K | Mg | Ca | Cu | Mn | Zn | Fe | Nmin |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mg kg−1 | kg ha−1 | ||||||||||
| 2013/2014 | |||||||||||
| 0–30 | 6.9 | 1.3 | 234 VH 5 | 231 H | 105 M | 988 L | 0.4 L | 27.2 L | 3.6 M | 536 H | 86.4 |
| 30–60 | 6.7 | 1.1 | 234 VH | 237 H | 103 VM | 876 L | 0.4 L | 25.7 L | 3.5 M | 541 H | |
| 2014/2015 | |||||||||||
| 0–30 | 7.1 | 2.2 | 185 VH | 185 M | 165 MVH | 2045 M | 3.5 M | 85.5 M | 6.3 H | 268 M | 129.0 |
| 30–60 | 7.2 | 2.1 | 161 VH | 157 M | 155 VH | 2063 M | 3.5 M | 93.8 M | 5.6 H | 269 M | |
| 2015/2016 | |||||||||||
| 0–30 | 6.6 | 1.6 | 202 VH | 281 VH | 165 VH | 1480 L | 2.8 M | 61.9 M | 6.1 H | 347 M | 110.0 |
| 30–60 | 6.6 | 1.4 | 139 VH | 222 VH | 163 VH | 1504 L | 2.5 M | 62.0 M | 3.7 M | 231 M | |
| Factor | Level of Factor | N | P | K | Mg | Ca | Fe | Mn | Zn | Cu |
|---|---|---|---|---|---|---|---|---|---|---|
| kg ha−1 | g ha−1 | |||||||||
| Year | 2014 | 69.6c | 11.6b | 53.2a | 5.71b | 0.54c | 131.8b | 155.3b | 132.1b | 12.7b |
| (Y) | 2015 | 93.3b | 10.0b | 43.4b | 3.40c | 0.94b | 114.5c | 61.3c | 87.8c | 12.8b |
| 2016 | 120.8a | 21.5a | 54.0a | 12.40a | 1.08a | 230.0a | 175.9a | 148.2a | 22.4a | |
| Fc, p | 46.4 *** | 143 *** | 12.8 *** | 363 *** | 92.6 *** | 90.9 *** | 138 *** | 41.6 *** | 84.2 *** | |
| Nitrogen | 0 | 63.9c | 10.4c | 33.5c | 5.46c | 0.58c | 111.1d | 85.5d | 77.4c | 11.5d |
| Rates (N) | 40 | 76.5c | 12.4bc | 40.2c | 6.35b | 0.72b | 130.5cd | 109.6c | 95.1bc | 14.5c |
| kg N ha−1 | 80 | 93.5b | 13.4b | 48.0b | 6.54b | 0.80b | 143.8c | 124.9bc | 115.1b | 15.7bc |
| 120 | 107.1ab | 16.3a | 57.7a | 7.93a | 0.96a | 173.2b | 141.9ab | 142.0a | 17.1ab | |
| 160 | 110.7a | 16.2a | 60.3a | 8.10a | 0.96a | 180.7ab | 150.1a | 147.5a | 19.3a | |
| 200 | 105.3ab | 16.1a | 57.6a | 7.99a | 0.98a | 194.3a | 145.6ab | 137.8a | 17.6ab | |
| 240 | 105.1ab | 15.6a | 54.1ab | 7.81a | 0.95a | 177.7b | 158.3a | 144.1a | 16.1bc | |
| Fc, p | 9.6 *** | 8.7 *** | 16.2 *** | 7.7 *** | 12.2 *** | 9.4 *** | 10.6 *** | 13.8 *** | 7.4 *** | |
| Source variation for the studied interaction | ||||||||||
| Y × N | ns | ns | ns | ns | ns | ns | ns | ns | ns | |
| Factor | Level of Factor | N | P | K | Mg | Ca | Fe | Mn | Zn | Cu |
|---|---|---|---|---|---|---|---|---|---|---|
| kg ha−1 | g ha−1 | |||||||||
| Year | 2014 | 34.6 | 4.7a | 31.9a | 0.00b | −1.78b | 81.7a | −13.3b | 15.4a | 4.4b |
| (Y) | 2015 | 41.6 | 2.2b | −6.0c | −3.33c | −11.34c | 44.7b | −27.9b | 2.8b | 1.2c |
| 2016 | 40.5 | 3.2b | 17.1b | 2.84a | 1.07a | 1.7c | 16.5a | 21.5a | 7.2a | |
| Fc. p | 0.8 ns | 11.4 *** | 31.2 *** | 145.6 *** | 228 *** | 33.5 *** | 7.4 *** | 23.0 *** | 18.6 *** | |
| Nitrogen | 0 | 24.2 | 1.7 | 12.2 | −0.05 | −1.91a | 8.1b | −1.6 | 5.4 | 2.0d |
| Rates (N) | 40 | 32.5 | 3.2 | 14.1 | 0.48 | −2.47ab | 23.0ab | 2.1 | 11.4 | 3.5c |
| kg N ha−1 | 80 | 38.8 | 3.2 | 14.2 | −0.13 | −3.48a–c | 48.1ab | −6.7 | 13.0 | 4.8ab |
| 120 | 36.8 | 3.4 | 10.9 | −0.36 | −5.51c | 47.5ab | −16.5 | 16.6 | 5.8a | |
| 160 | 48.7 | 4.0 | 17.6 | −0.24 | −5.28bc | 58.0a | −19.9 | 18.5 | 5.0ab | |
| 200 | 45.4 | 4.0 | 11.3 | −0.47 | −4.96bc | 60.6a | 1.9 | 14.9 | 4.4bc | |
| 240 | 45.9 | 3.9 | 20.1 | −0.37 | −4.50a–c | 53.8a | −16.8 | 12.7 | 4.5bc | |
| Fc. p | 1.9 ns | 2.0 ns | 0.2 ns | 0.7 ns | 4.7 *** | 3.5 ** | 0.6 ns | 1.9 ns | 2.9 ** | |
| Source variation for the studied interaction | ||||||||||
| Y × N | ns | ns | ns | ** | *** | ns | ns | * | ns | |
| Factor | Level of Factor | N | P | K | Mg | Ca | Fe | Mn | Zn | Cu |
|---|---|---|---|---|---|---|---|---|---|---|
| kg ha−1 | g ha−1 | |||||||||
| Year | 2014 | 35.1b | 4.58a | 21.0a | −1.88a | 0.82a | 79.1a | −125.1b | −16.8c | −4.7b |
| (Y) | 2015 | 6.5c | 0.90b | −29.7b | −4.35b | −2.77c | −118.6b | 38.4a | 13.7b | −5.3b |
| 2016 | 52.6a | 4.58a | 21.2a | −9.69c | −0.65b | 81.5a | 22.7a | 52.5a | 7.3a | |
| Fc, p | 51.0 *** | 30.1 *** | 37.7 *** | 217 *** | 295 *** | 79.4 *** | 112 *** | 97.4 *** | 117 *** | |
| Nitrogen | 0 | 22.5ab | 0.24c | −4.0c | −4.81 | −1.02ab | −6.1 | −23.7ab | −3.6c | −3.4b |
| Rates (N) | 40 | 18.7b | 4.61a | 16.4ab | −4.06 | −0.72a | 15.8 | −13.7ab | 8.2bc | −1.6b |
| kg N ha−1 | 80 | 39.5a | 4.47ab | 20.5a | −5.39 | −0.83a–c | 38.2 | −16.4ab | 34.6a | 4.2a |
| 120 | 36.3ab | 2.03bc | −9.7bc | −6.82 | −1.20c | 43.8 | −60.8b | 9.3bc | −0.7b | |
| 160 | 33.5ab | 3.34ab | −8.5bc | −6.37 | −1.03bc | −34.4 | −40.5b | 19.4a–c | −2.7b | |
| 200 | 33.4ab | 4.21ab | −1.8bc | −5.24 | −0.74a–c | 27.0 | 15.0a | 24.7ab | −2.4b | |
| 240 | 36.0ab | 4.58a | 16.4ab | −4.44 | −0.54a | 13.8 | −9.2ab | 22.6ab | 0.2ab | |
| Fc, p | 2.4 * | 7.7 *** | 3.2 ** | 5.8 *** | 2.0 ns | 1.9 ns | 3.5 ** | 5.5 *** | 8.1 *** | |
| Source variation for the studied interaction | ||||||||||
| Y × N | *** | *** | *** | *** | *** | *** | ** | *** | *** | |
| Factor | Level of Factor | N | P | K | Mg | Ca | Fe | Mn | Zn | Cu |
|---|---|---|---|---|---|---|---|---|---|---|
| kg ha−1 | g ha−1 | |||||||||
| Year | 2014 | −0.2b | 2.2c | −0.1b | 7.6c | 1.5b | −28.9c | 292.4a | 133.0a | 13.0ab |
| (Y) | 2015 | 45.2a | 6.9b | 79.1a | 11.1b | 15.0a | 188.4a | 50.7c | 71.4b | 16.9a |
| 2016 | 27.7ab | 13.7a | 15.6b | 19.2a | 0.7b | 146.8a | 136.8b | 74.2b | 7.9b | |
| Fc. p | 5.9 * | 32.9 *** | 17.7 *** | 67.2 *** | 215 *** | 24.0 *** | 30.5 *** | 17.8 *** | 9.2 *** | |
| Nitrogen | 0 | 17.1 | 8.2 | 23.4 | 10.3b | 3.5c | 109.3 | 107.8 | 74.4c | 12.6 |
| Rates (N) | 40 | 25.2 | 4.6 | 9.7 | 9.9b | 3.9bc | 91.7 | 121.2 | 75.4c | 12.6 |
| kg N ha−1 | 80 | 15.1 | 5.8 | 13.3 | 12.0ab | 5.1a–c | 57.5 | 148.1 | 67.6cd | 6.7 |
| 120 | 34.0 | 10.9 | 56.5 | 15.1a | 7.7a | 81.9 | 219.2 | 116.1a | 12.0 | |
| 160 | 28.4 | 8.9 | 51.2 | 14.7ab | 7.3ab | 157.1 | 210.5 | 109.5ab | 17.0 | |
| 200 | 26.5 | 7.9 | 48.1 | 13.7ab | 6.7a–c | 106.8 | 128.6 | 98.2b | 15.7 | |
| 240 | 23.2 | 7.2 | 17.5 | 12.6ab | 6.0a–c | 110.2 | 184.4 | 108.7ab | 11.5 | |
| Fc. p | 0.2 ns | 1.8 ns | 1.7 ns | 3.3 * | 3.8 ** | 1.5 ns | 1.8 ns | 2.5 * | 1.4 ns | |
| Source variation for the studied interaction | ||||||||||
| Y × N | ns | ns | ns | ** | ns | *** | ns | ns | * | *** |
| Growing | Nutrient Sources for the Growing Ear—Summary | Nutrient Invested in Vegetative Organs | ||||
|---|---|---|---|---|---|---|
| Season | Type of Source | Source | Sources | |||
| Year | Acronym | of Nutrients | Nutrients | Leaves (LE) | Stems (ST) | |
| 2014 | Multi | A | LE + ST + SO | P | ||
| Double—VE | B | LE + ST | N, Fe, K | Ca, Mn | Mg, Mn, Zn, Cu | |
| Double—SOLE | C | SO + LE | Zn, Cu | |||
| Single—ST | D | ST | Ca | |||
| Single—SO | E | SO | Mg, Mn | |||
| 2015 | Multi | A | LE + ST + SO | N, P, Zn | ||
| Double—SOLE | C | SO + LE | Fe, Cu | K, Mg, Ca, Mn | K, Mg, Ca, Fe, Cu | |
| Double—SOST | D | SO + ST | Mn | |||
| Single | E | SO | K, Mg, Ca | |||
| 2016 | Multi | A | LE + ST + SO | N, P, K, Mn, Zn, Cu | ||
| Double—SOLE | C | SO + LE | Mg | — | Mg, Ca | |
| Double—SOST Single—LE | D F | SO + ST LE | Fe | |||
| Mean | Multi | A | LE + ST + SO | N, P, K, Fe, Zn | ||
| 2014— | Double—SOLE | C | SO + LE | Cu | Mg (trace), Ca, Mn | Mg, Mn, Cu |
| 2016 | Single | E | SO | Mg, Ca, Mn | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzebisz, W.; Szczepaniak, W.; Przygocka-Cyna, K.; Biber, M.; Spiżewski, T. The Sources of Nutrients for the Growing Ear of Winter Wheat in the Critical Cereal Window. Agronomy 2024, 14, 3018. https://doi.org/10.3390/agronomy14123018
Grzebisz W, Szczepaniak W, Przygocka-Cyna K, Biber M, Spiżewski T. The Sources of Nutrients for the Growing Ear of Winter Wheat in the Critical Cereal Window. Agronomy. 2024; 14(12):3018. https://doi.org/10.3390/agronomy14123018
Chicago/Turabian StyleGrzebisz, Witold, Witold Szczepaniak, Katarzyna Przygocka-Cyna, Maria Biber, and Tomasz Spiżewski. 2024. "The Sources of Nutrients for the Growing Ear of Winter Wheat in the Critical Cereal Window" Agronomy 14, no. 12: 3018. https://doi.org/10.3390/agronomy14123018
APA StyleGrzebisz, W., Szczepaniak, W., Przygocka-Cyna, K., Biber, M., & Spiżewski, T. (2024). The Sources of Nutrients for the Growing Ear of Winter Wheat in the Critical Cereal Window. Agronomy, 14(12), 3018. https://doi.org/10.3390/agronomy14123018






