Exogenous 24-Epibrassinolide Improves Resistance to Leaf Spot Disease Through Antioxidant Regulation and Phenylpropanoid Metabolism in Oats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth and Experimental Conditions
2.2. Measurement of Disease Index
2.3. Determination of Chlorophyll Content
2.4. Determination of Antioxidant Enzyme Activity and ROS Production
2.5. Determination of Enzyme Activity and Metabolite Content Related to Phenylpropane Metabolism
2.6. RNA Sequencing and DEG Identification
2.7. Gene Expression Confirmation
2.8. Statistical Analysis
3. Results
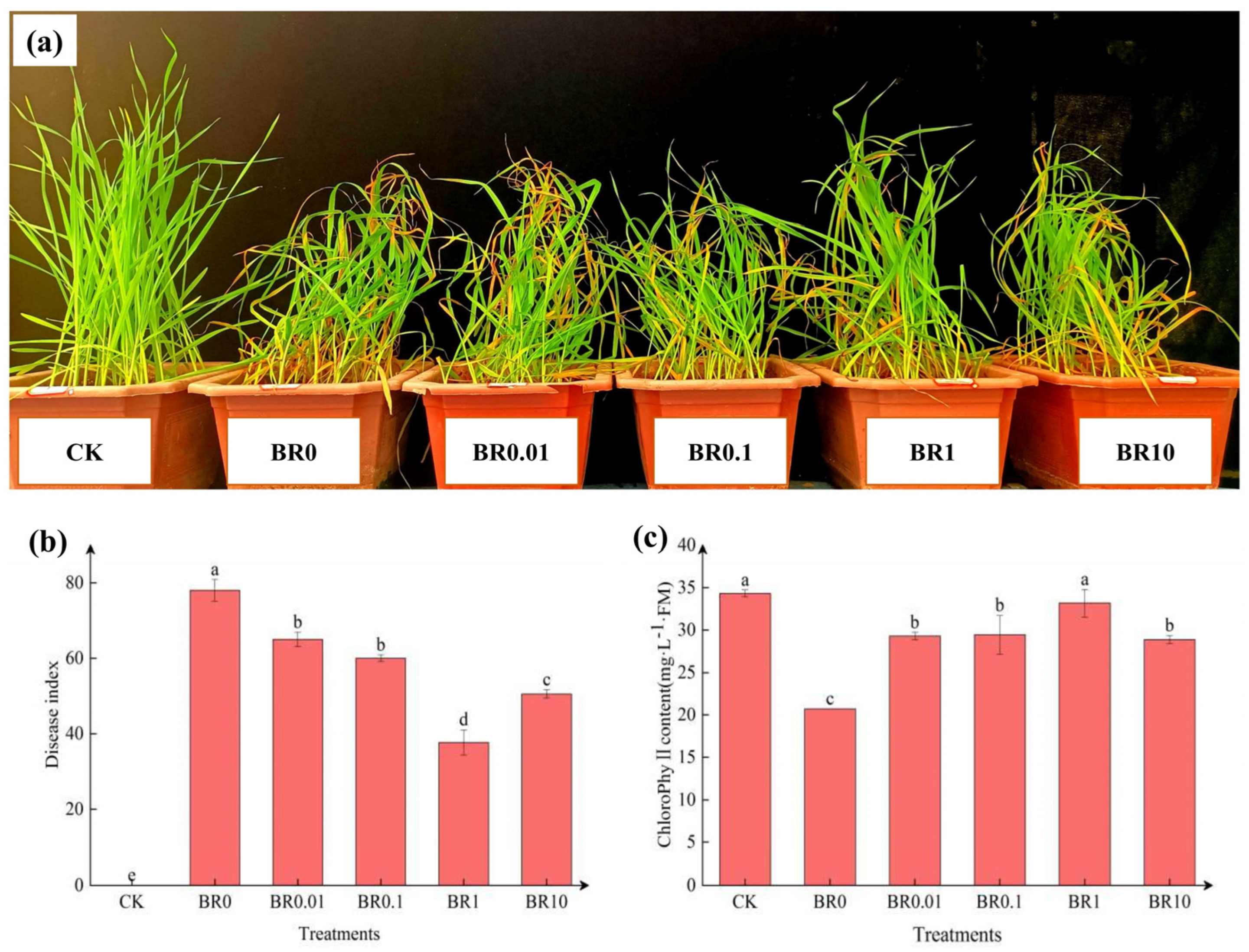
3.1. Effect of Exogenous EBR on Disease Index and Chlorophyll Content
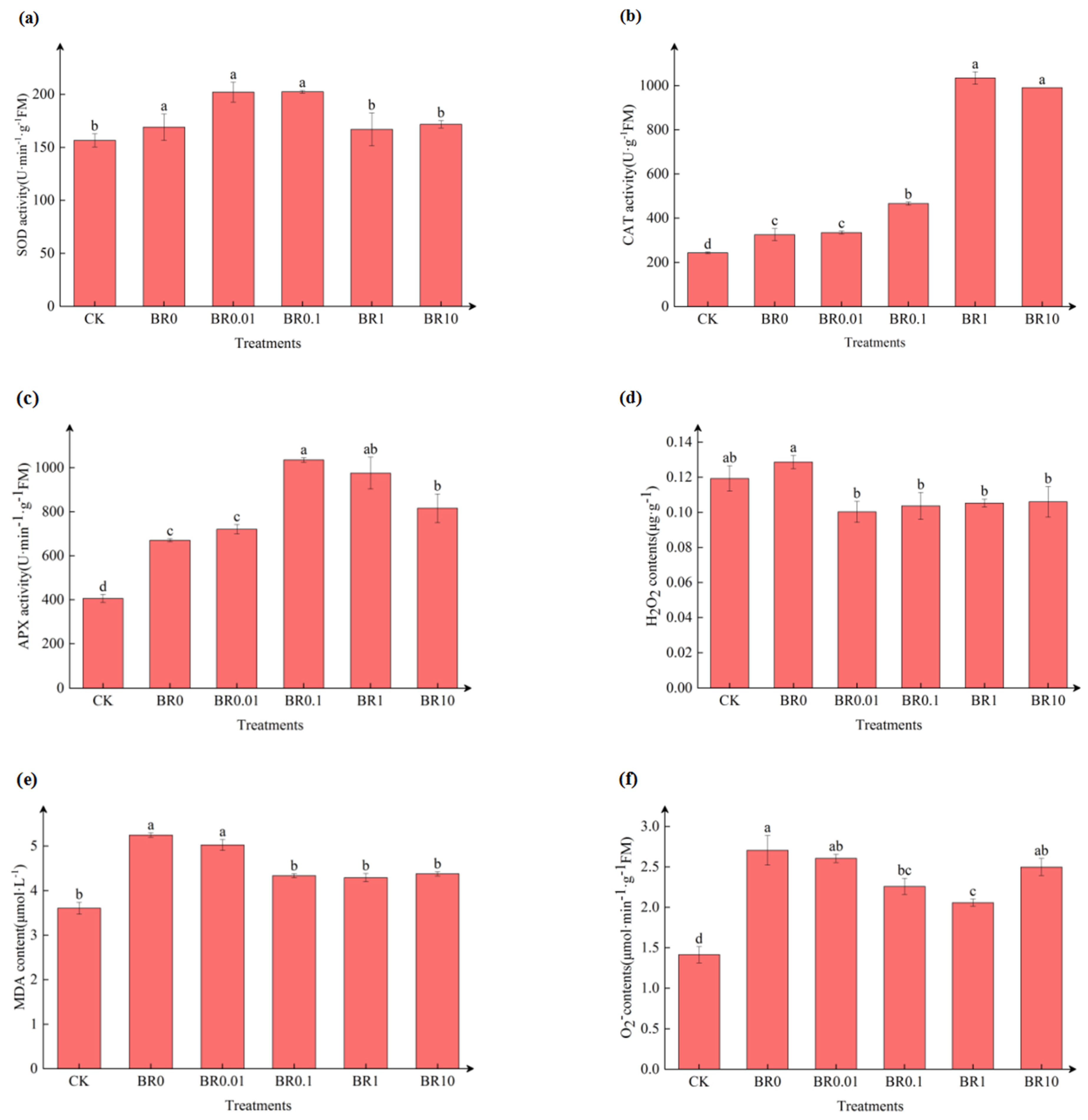
3.2. Effect of Exogenous EBR on Antioxidant Enzyme Activity and ROS Production
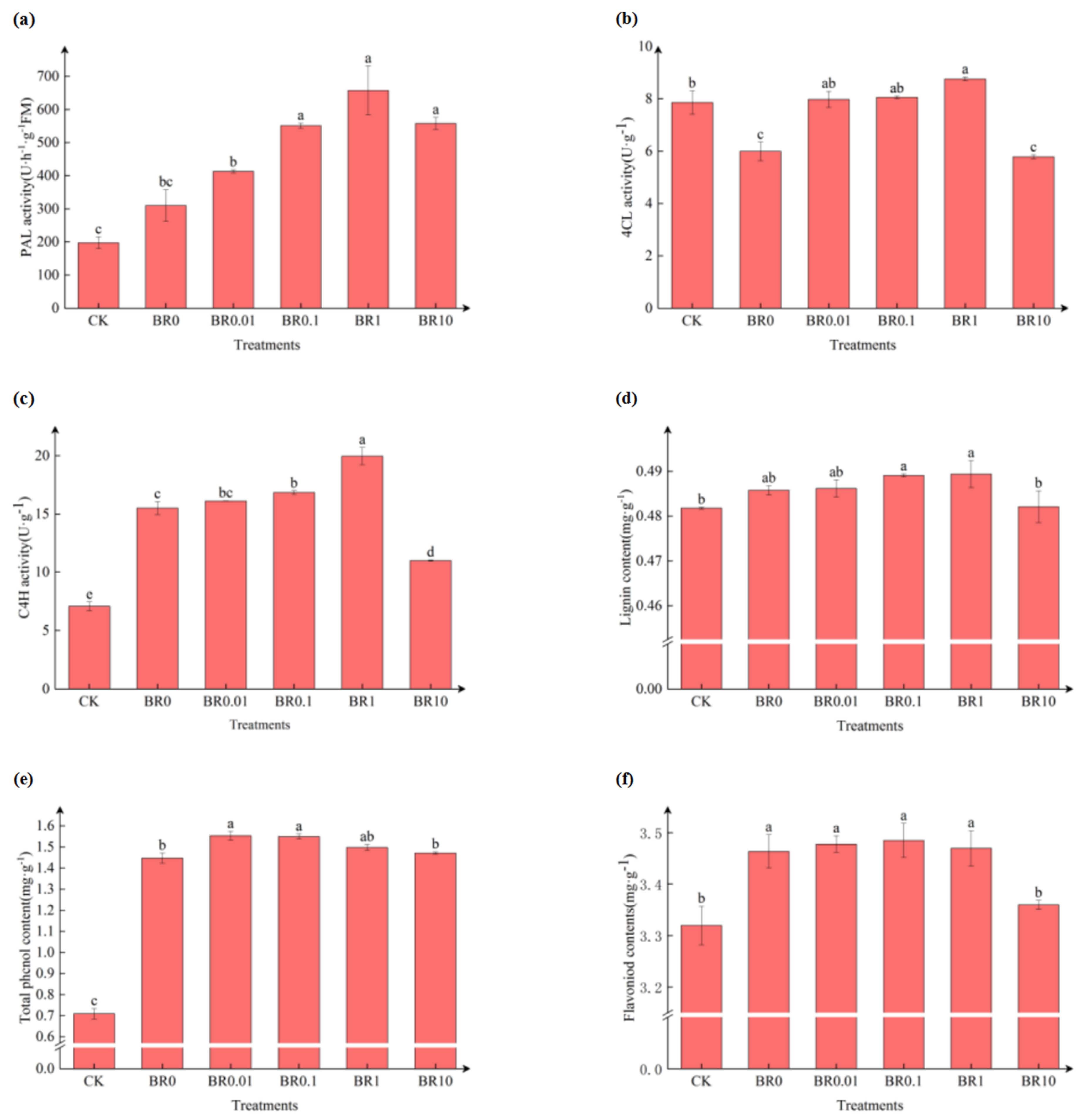
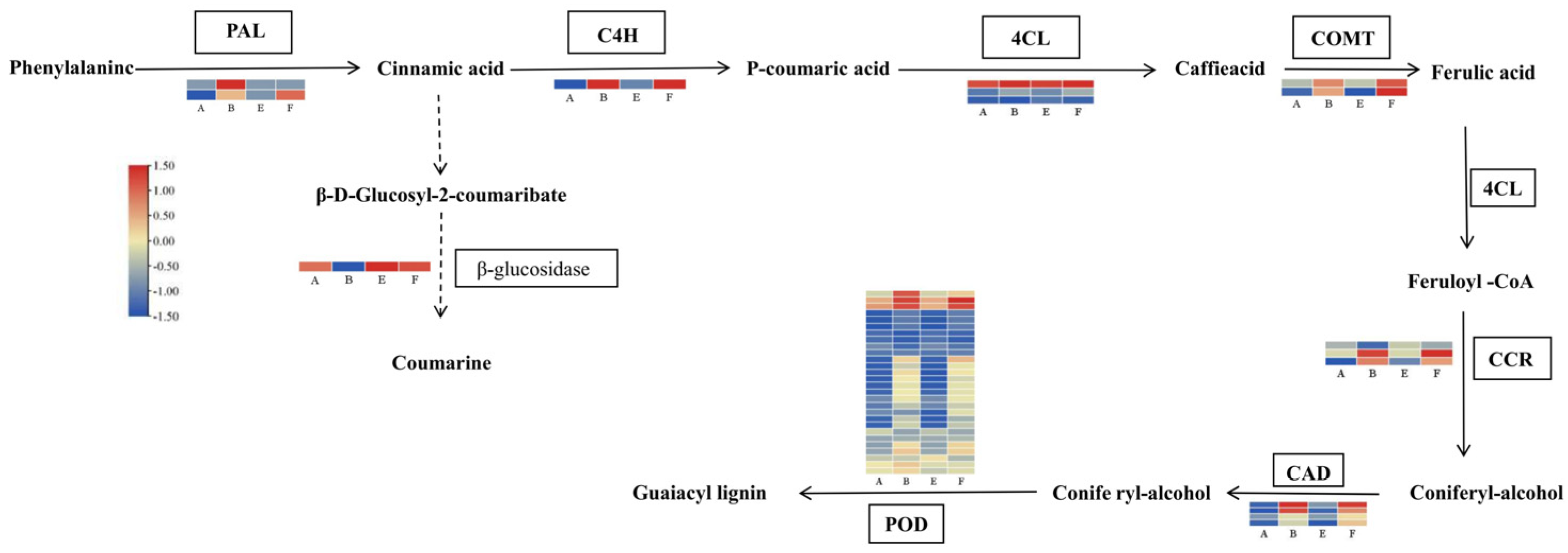
3.3. Effect of Exogenous EBR on Enzyme Activity and Metabolite Contents for Phenylpropanoid Metabolism
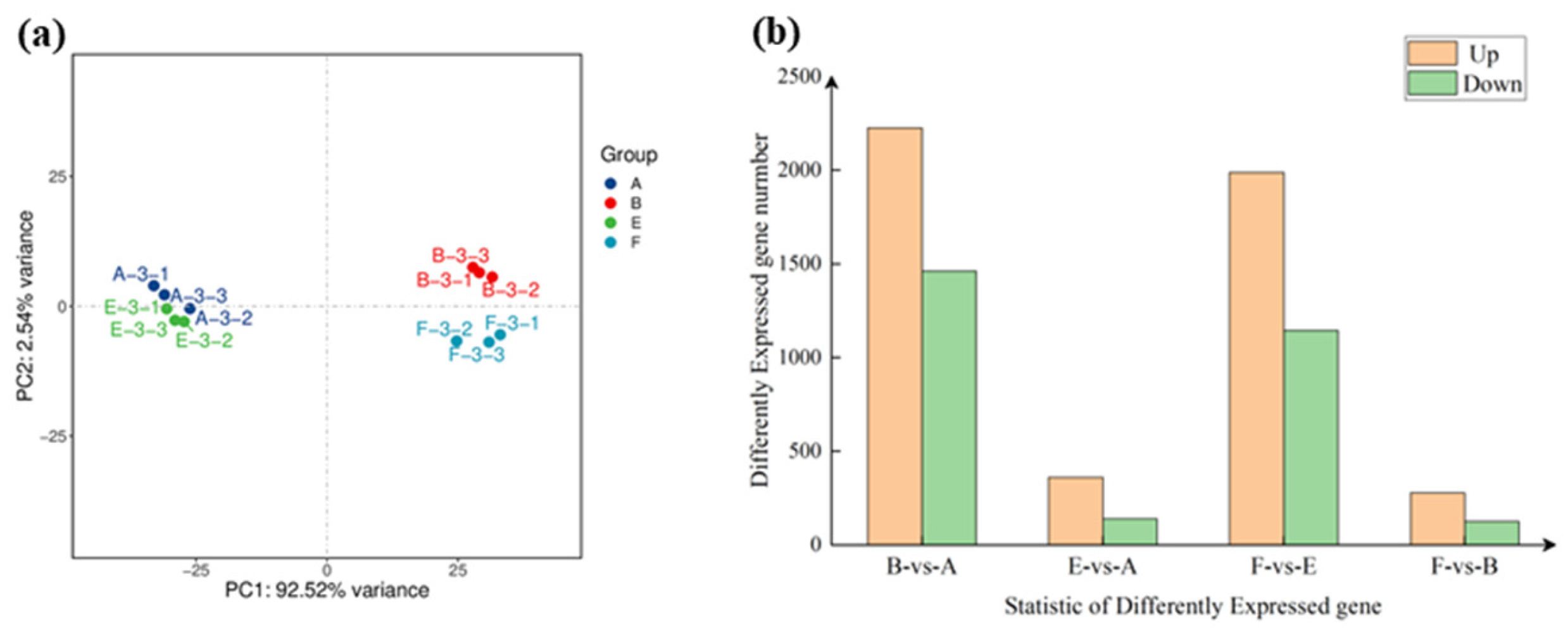
3.4. DEGs Responding to Leaf Spot Disease and Exogenous EBR
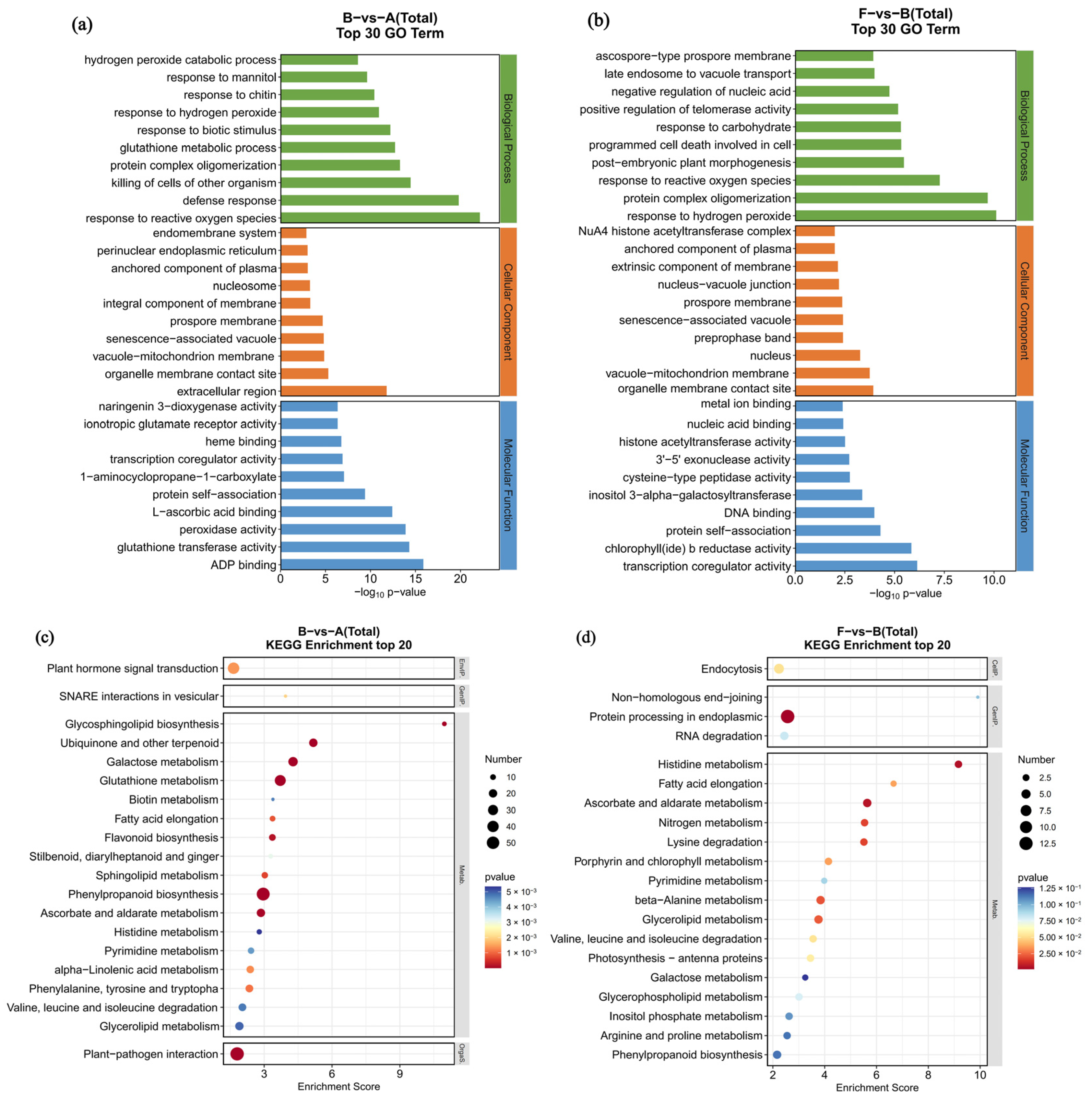
3.5. GO and KEGG Enrichment of DEGs
3.6. MapMan Analysis of DEGs
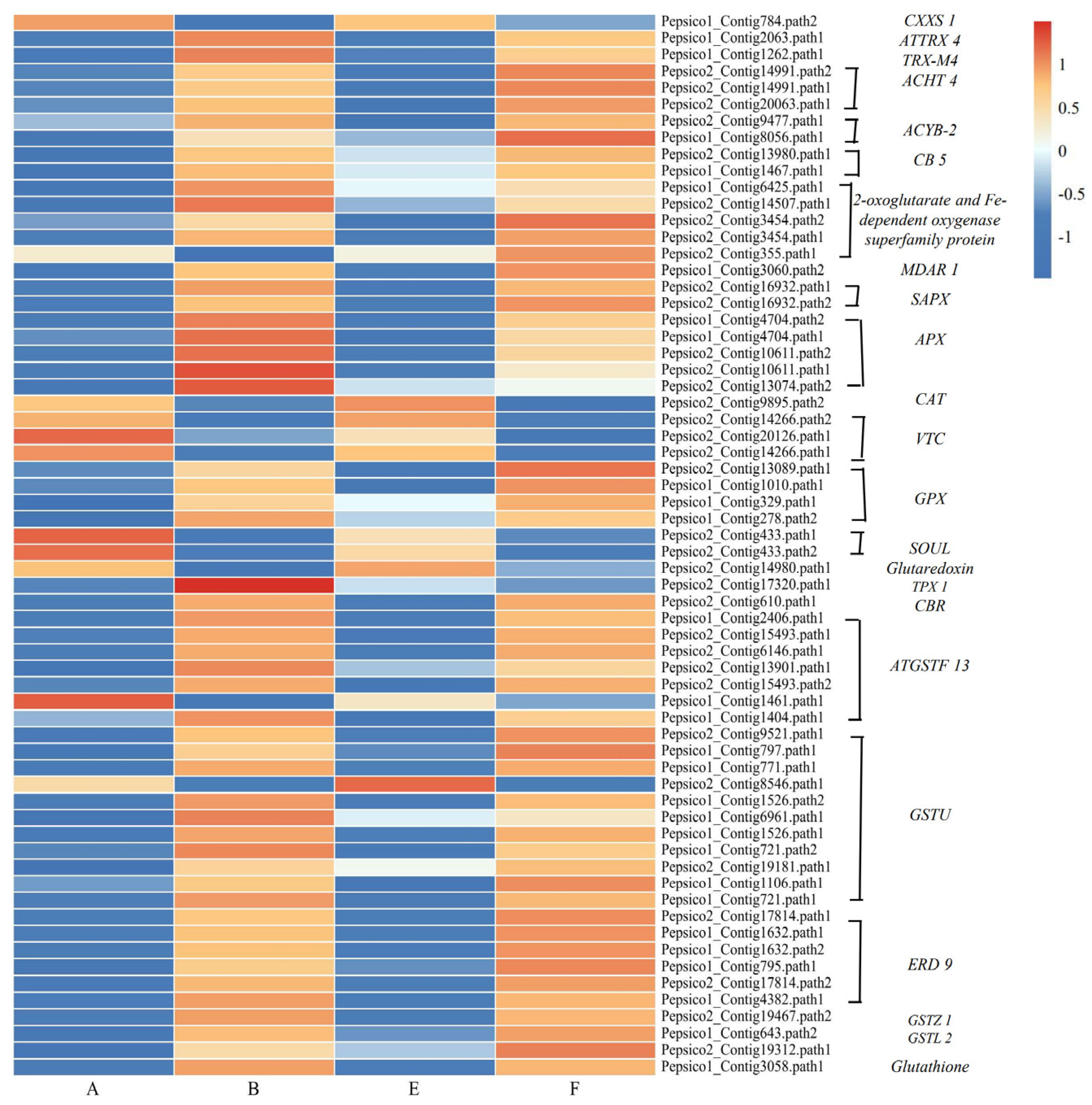
3.7. DEGs Involved in Redox Reactions
3.8. DEGs Involved in Signal Transduction
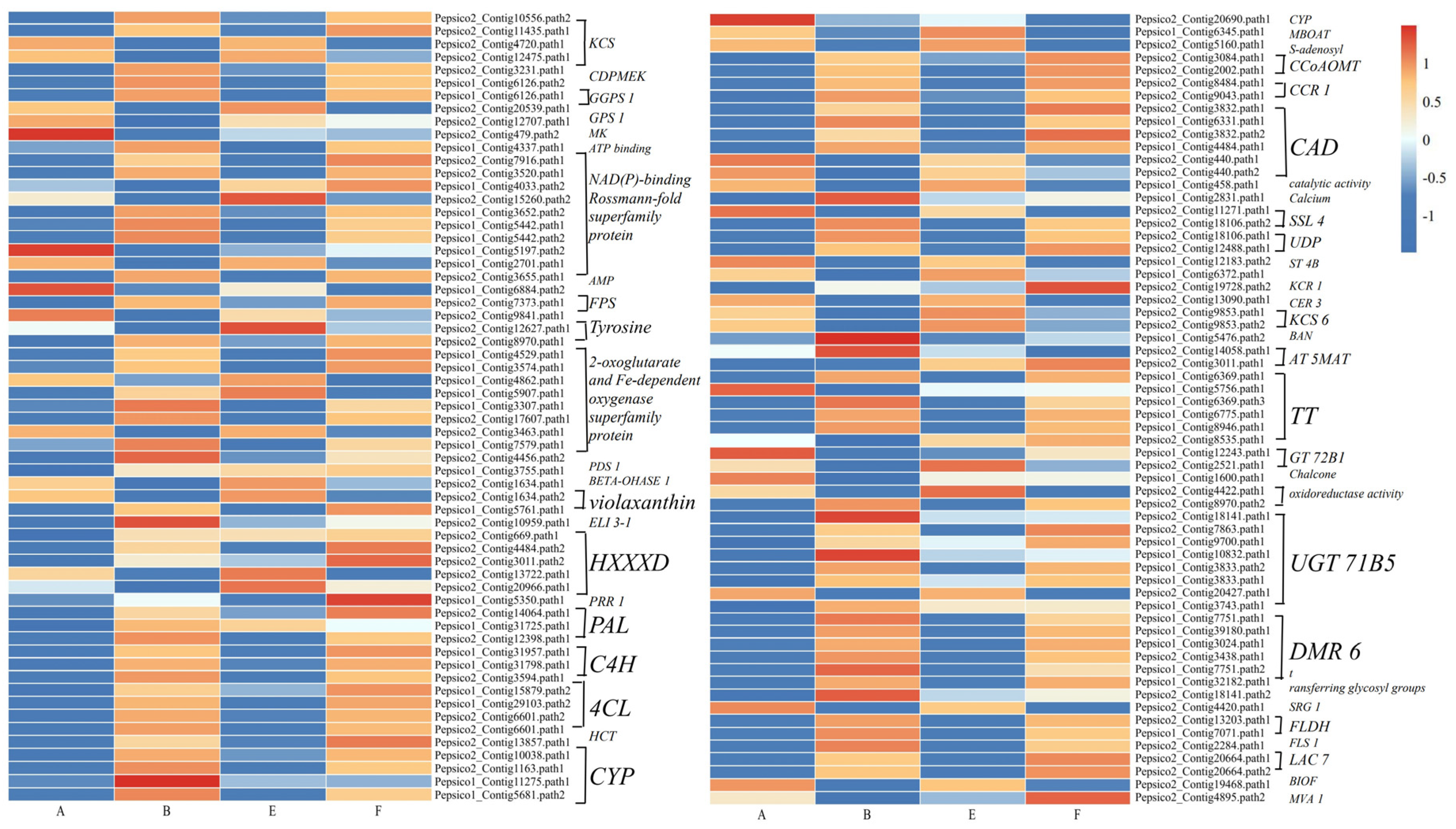
3.9. DEGs Involved in Secondary Metabolism
4. Discussion
4.1. BR Signaling Involved in Improving Plant Defense Responses Against Plant Disease
4.2. Exogenous EBR Alleviates Oxidative Damage by Enhancing Antioxidant Defense System
4.3. Exogenous EBR Improves Defense Responses by Mediating Phenylpropanoid and Lignin Pathways
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Primer | Upstream Primer | Downstream Primer |
|---|---|---|
| Actin | GAGCGGGAAATTGTAAGGGAC | ATGGATGGCTGGAAGAGGAC |
| pepsico2_contig1860.path1 | TCGCACTTCTACTTCGTCGG | TGCAGGTATGTCATGGCGAG |
| pepsico1_contig1010.path1 | CTGGCACCAACGAGGAGATT | CGCCGAAAAGACCACCTTTG |
| pepsico1_contig31601.path1 | ATACTTCGCGCTACTGTGGG | TGCAACCTGCGAGTCATCTT |
| pepsico1_contig2831.path1 | GTGAGAGCTGTGAGCGAAGT | TTACCCAGGGCAGCTAGGAT |
| Pepsico1_Contig11380.path2 | ACCACCGATGCCGTATGTTT | GCCACCTCAGTCTCCAACTC |
| pepsico1_contig3446.path1 | GCCATATCTCTGACCCTCGC | TACCAAGCTGAGGAGCAAGC |
| pepsico1_contig4053.path1 | ACGACATATGGCTCTGCCAC | GATCGTGATCACCTCCGACG |
| Pepsico1_Contig4086.path1 | CTTTCAGCAGTAATCGCCGC | TCCCGTTCCATTGTCTGAGC |
| Pepsico1_Contig4133.path1 | CCCCTTCCAGAGTGGTTCAC | GGATCTTCAAGTGGCTGCCT |
| Pepsico1_Contig4140.path2 | CGCACCTGGCGATTTGTATG | TGAAGTGGCAGTAGCTGACG |
| Pepsico1_Contig4179.path2 | AAGGCCGACGTCTTCAGTTT | TCTTGTACAGCTTCCAGGCG |
| Pepsico1_Contig4239.path2 | GGACGCCATCCCATAAGTGT | TCCCTCATGCTCTTGCACTG |
| Pepsico1_Contig426.path1 | TGGGCTTTATGCTCGTCTGG | ACCTGCATCGCCTCGATATG |
| Pepsico1_Contig4293.path3 | GGTGGAGCATGCCTATGAGT | TTCTGGTCAAGTGCCGTTGT |
| Pepsico1_Contig4326.path1 | CAGCAGTTGTGCAGGTAAGC | TCTGTGGACAGACAGTTCGC |
| Pepsico1_Contig4396.path2 | CAACGTCACCACCCTCCTAC | GTCTTGGGTGGGGAATAGCC |
| Pepsico2_Contig20595.path1 | AGAACTTCAGCCAGCTCGTC | ATGCCTCAGCAGGATGTTCC |
| Pepsico2_Contig2061.path1 | CACATCTACGAGAACGGGGG | ATAGAGCGACCCTTCCCGAT |
| Pepsico2_Contig20671.path1 | AGGCCAGACCCCTATTCCAT | GAATTCCGACGACATGCAGC |
| Pepsico2_Contig8925.path1 | GACGGCTCCACTAGAACCAC | GGGCAACAAACGTCAGCATT |
Appendix B

References
- Ibrahim, M.; Ahmad, A.; Sohail, A.; Asad, M. Nutritional and functional characterization of different oat (Avena sativa L.) cultivars. Int. J. Food Prop. 2020, 23, 1373–1385. [Google Scholar] [CrossRef]
- Marshall, A.; Cowan, S.; Edwards, S.; Griffiths, S.; White, E. Crops that feed the world 9. Oats a cereal crop for human and livestock feed with industrial applications. Food Secur. 2013, 5, 13–33. [Google Scholar] [CrossRef]
- Butt, M.; Tahir-Nadeem, M.; Khan, M.; Shabir, R.; Butt, M. Oat: Unique among the cereals. Eur. J. Nutr. 2008, 47, 68–79. [Google Scholar] [CrossRef]
- Shakeel, Q.; Raheel, D.; Bajwa, R.; Rashid, I.; Raza, H.Y.; Saleem, S.R. Etiology and Management of Economically Significant Diseases of Avena sativa; CRC Press: Boca Raton, FL, USA, 2021; pp. 131–163. ISBN 9781003055365. [Google Scholar]
- Dietz, J.; Schierenbeck, M.; Simón, M. Impact of foliar diseases and its interaction with nitrogen fertilization and fungicides mixtures on green leaf area dynamics and yield in oat genotypes with different resistance. Crop Prot. 2019, 121, 80–88. [Google Scholar] [CrossRef]
- Islam, T.; Danishuddin; Tamanna, N.; Matin, M.N.; Barai, H.R.; Haque, M.A. Resistance mechanisms of plant pathogenic fungi to fungicide, environmental impacts of fungicides, and sustainable solutions. Plants 2024, 13, 2737. [Google Scholar] [CrossRef]
- Demiwal, P.; Nabi, S.; Mir, J.; Verma, M.; Yadav, S.; Roy, P.; Sircar, D. Methyl jasmonate improves resistance in scab-susceptible Red Delicious apple by altering ROS homeostasis and enhancing phenylpropanoid biosynthesis. Plant Physiol. Bioch. 2024, 207, 108371. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Coll, Y.; Coll, F.; Amorós, A.; Pujol, M. Brassinosteroids roles and applications: An up-date. Biologia 2015, 70, 726–732. [Google Scholar] [CrossRef]
- Yu, M.; Zhao, Z.; He, J. Brassinosteroid signaling in plant–microbe interactions. Int. J. Mol. Sci. 2018, 19, 4091. [Google Scholar] [CrossRef]
- Nakashita, H.; Yasuda, M.; Nitta, T.; Asami, T.; Fujioka, S.; Arai, T.; Sekimata, K.; Takatsuto, S.; Yamaguchi, I.; Yoshida, S. Brassinosteroid functions in a broad range of disease resistance in tobacco and rice. Plant J. 2003, 33, 887–898. [Google Scholar] [CrossRef]
- Shi, H.; Shen, Q.; Qi, Y.; Yan, H.; Nie, H.; Chen, Y.; Zhao, T.; Katagiri, F.; Tang, D. BR-SIGNALING KINASE1 physically associates with FLAGELLIN SENSING2 and regulates plant innate immunity in Arabidopsis. Plant Cell 2013, 25, 1143–1157. [Google Scholar] [CrossRef]
- Shi, H.; Li, Q.; Luo, M.; Yan, H.; Xie, B.; Li, X.; Zhong, G.; Chen, D.; Tang, D. BRASSINOSTEROID-SIGNALING KINASE1 modulates MAP KINASE15 phosphorylation to confer powdery mildew resistance in Arabidopsis. Plant Cell 2022, 34, 1768–1783. [Google Scholar] [CrossRef]
- Bharat, B.; Shivakumar, S.; Guido, S. BRASSINOSTEROID-SIGNALING KINASE5 associates with immune receptors and is required for immune responses. Plant Physiol. 2019, 180, 1166–1184. [Google Scholar]
- Huang, S.; Nie, S.; Wang, S.; Liu, J.; Zhang, Y.; Wang, X. SlBIR3 negatively regulates PAMP responses and cell death in tomato. Int. J. Mol. Sci. 2017, 18, 1966. [Google Scholar] [CrossRef]
- Liu, T.; Xu, H.; Amanullah, S.; Du, T.; Hu, X.; Che, Y.; Zhang, L.; Jiang, Z.; Zhu, L.; Wang, D. Deciphering the enhancing impact of exogenous brassinolide on physiological indices of melon plants under downy mildew-induced stress. Plants 2024, 13, 779. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Ahammed, G.J.; Wang, X.; Li, X. 2,4-Epibrassinolide enhances resistance against Colletotrichum fructicola by promoting lignin biosynthesis in Camellia sinensis L. J. Plant Growth Regul. 2023, 42, 1558–1566. [Google Scholar] [CrossRef]
- Kang, Y.; Jiang, Z.; Meng, C.; Ning, X.; Pan, G.; Yang, X.; Zhong, M. A multifaceted crosstalk between brassinosteroid and gibberellin regulates the resistance of cucumber to phytophthora melonis. Plant J. 2024, 119, 1353–1368. [Google Scholar] [CrossRef]
- Zhang, L.; Ahammed, G.; Li, X.; Wei, J.; Li, Y.; Yan, P.; Zhang, L.; Wen, Y. Exogenous brassinosteroid enhances plant defense against Colletotrichum gloeosporioides by activating phenylpropanoid pathway in Camellia sinensis L. J. Plant Growth Regul. 2018, 37, 1235–1243. [Google Scholar] [CrossRef]
- Song, Y.; Hu, C.; Xue, Y.; Gu, J.; He, J.; Ren, Y. 2,4-epibrassinolide enhances mango resistance to Colletotrichum gloeosporioides via activating multiple defense response. Sci. Hortic. 2022, 303, 111249. [Google Scholar] [CrossRef]
- Senanayake, I.C.; Rathnayaka, A.R.; Marasinghe, D.S.; Calabon, M.S.; Gentekaki, E.; Lee, H.B. Morphological approaches in studying fungi: Collection, examination, isolation, sporulation and preservation. Mycosphere 2020, 11, 2678–2754. [Google Scholar] [CrossRef]
- Niu, K.; Ma, X.; Liang, G.; Ma, H.; Jia, Z.; Liu, W.; Yu, Q. 5-Aminolevulinic acid modulates antioxidant defense systems and mitigates drought-induced damage in Kentucky bluegrass seedlings. Protoplasma 2017, 254, 2083–2094. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Roberts, A.; Trapnell, C.; Donaghey, J.; Rinn, J.; Pachter, L. Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol. 2011, 12, R22. [Google Scholar] [CrossRef]
- Simon, A.; Theodor, P.; Wolfgang, H. HTSeq—A python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar]
- Love, M.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Pan, Y.; Niu, K.; Miao, P.; Zhao, G.; Zhang, Y.; Ju, Z.; Chai, J.; Cui, X.; Zhang, R. Genome-wide analysis of the SWEET gene family and its response to powdery mildew and leaf spot infection in the common oat (Avena sativa L.). BMC Genom. 2024, 25, 995. [Google Scholar] [CrossRef]
- Madden, L.; Wheelis, M. The threat of plant pathogens as weapons against U.S. crops. Annu. Rev. Phytopathol. 2003, 41, 155–176. [Google Scholar] [CrossRef]
- Lozano-Durán, R.; Zipfel, C. Trade-off between growth and immunity: Role of brassinosteroids. Trends Plant Sci. 2015, 20, 12–19. [Google Scholar] [CrossRef]
- Nafisi, M.; Fimognari, L.; Sakuragi, Y. Interplays between the cell wall and phytohormones in interaction between plants and necrotrophic pathogens. Phytochemistry 2015, 112, 63–71. [Google Scholar] [CrossRef]
- Sharma, N.; Kour, S.; Kumar, D.; Kaur, R.; Khajuria, A.; Ohri, P. Role of brassinosteroids (BRs) in modulating antioxidative defense mechanism in plants growing under abiotic and biotic stress conditions. Antioxid. Def. Plants 2022, 1, 325–367. [Google Scholar]
- Checker, V.; Kushwaha, H.; Kumari, P.; Yadav, S. Role of Phytohormones in Plant Defense: Signaling and Cross Talk; Springer: Singapore, 2018. [Google Scholar]
- Janeczko, A.; Saja, D.; Dziurka, M.; Gábor, G.; Balázs, B. Brassinosteroid deficiency caused by the mutation of the HvDWARF gene influences the reactions of barley to powdery mildew. Physiol. Mol. Plant Pathol. 2019, 108, 101438. [Google Scholar] [CrossRef]
- Li, J.; Chory, J. A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 1997, 90, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Goddard, R.; Peraldi, A.; Ridout, C.; Nicholson, P. Enhanced disease resistance caused by BRI1 mutation is conserved between Brachypodium distachyon and barley (Hordeum vulgare). Mol. Plant Microbe Interact. 2014, 27, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Long, L.; Zhu, L.; Xu, L.; Gao, W.; Sun, L.; Liu, L.; Zhang, X. Proteomic and virus-induced gene silencing (VIGS) analyses reveal that gossypol, brassinosteroids, and jasmonic acid contribute to the resistance of cotton to Verticillium dahliae. Mol. Cell. Proteom. 2013, 12, 3690–3703. [Google Scholar] [CrossRef]
- Song, Y.; Zhai, Y.; Li, L.; Yang, Z.; Ge, X.; Yang, Z.; Zhang, C.; Li, F.; Ren, M. BIN2 negatively regulates plant defence against Verticillium dahliae in Arabidopsis and cotton. Plant Biotechnol. J. 2021, 19, 2097–2112. [Google Scholar] [CrossRef]
- Huot, B.; Yao, J.; Montgomery, B. Growth-defense tradeoffs in plants: A balancing act to optimize fitness. Mol. Plant. 2014, 7, 1267–1287. [Google Scholar] [CrossRef]
- Nolan, T.; Vukainovi, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional regulators of plant growth, development, and stress responses. Plant Cell 2019, 32, 295–318. [Google Scholar] [CrossRef]
- Thornton, L.; Peng, H.; Neff, M. Rice CYP734A cytochrome P450s inactivate brassinosteroids in Arabidopsis. Planta 2011, 234, 1151–1162. [Google Scholar] [CrossRef]
- Ohnishi, T.; Nomura, T.; Watanabe, B.; Ohta, D.; Yokota, T.; Miyagawa, H.; Sakata, K.; Mizutani, M. Tomato cytochrome P450 CYP734A7 functions in brassinosteroid catabolism. Phytochemistry 2006, 67, 1895–1906. [Google Scholar] [CrossRef]
- Wei, L.; Zhong, Y.; Wu, X.; Wei, S.; Liu, Y. Roles of nitric oxide and brassinosteroid in improving fruit quality during postharvest: Potential regulators? J. Agr. Food Chem. 2024, 72, 23671–23688. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Liu, Y.; Ji, L.; Xiao, Z.; He, J.; Qiu, Z.; Liu, D.; Sun, Z.; Xu, T. Brassinosteroids mediate susceptibility to brown planthopper by integrating with the salicylic acid and jasmonic acid pathways in rice. J. Exp. Bot. 2018, 69, 4433–4442. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.; Shang, Y.; Nam, K. Brassinosteroids modulate ABA-induced stomatal closure in Arabidopsis. J. Exp. Bot. 2016, 67, 6297–6308. [Google Scholar] [CrossRef] [PubMed]
- Bharath, P.; Gahir, S.; Raghavendra, A. Abscisic acid-induced stomatal closure: An important component of plant defense against abiotic and biotic stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef] [PubMed]
- Postiglione, A.; Muday, G. The role of ROS homeostasis in ABA-Induced guard cell signaling. Front. Plant Sci. 2020, 11, 968. [Google Scholar] [CrossRef]
- Mauch-Mani, B.; Mauch, F. The role of abscisic acid in plant–pathogen interactions. Curr. Opin. Plant Biol. 2005, 8, 409–414. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, S. MAPK cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 2013, 51, 245–266. [Google Scholar] [CrossRef]
- Yan, H.; Zhao, Y.; Shi, H.; Li, J.; Wang, Y.; Tang, D. BRASSINOSTEROID-SIGNALING KINASE1 phosphorylates MAPKKK5 to regulate immunity in Arabidopsis. Plant Physiol. 2018, 176, 2991–3002. [Google Scholar] [CrossRef]
- Torres, M.; Jones, J.; Dangl, J. Reactive oxygen species signaling in response to pathogens. Plant Physiol. 2006, 141, 373–378. [Google Scholar] [CrossRef]
- Ullah, F.; Yi, M. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar]
- Apel, K.; Hirt, H. REACTIVE OXYGEN SPECIES: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Smirnoff, N. Reactive Oxygen Species in Plant Development and Pathogen Defence; Blackwell Publishing Ltd.: Oxford, UK, 2007. [Google Scholar]
- Gill, S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.; Zulfiqar, F.; Raza, A.; Mohsin, S.; Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Mhamdi, A.; Foyer, C. Oxidative stress and antioxidative systems: Recipes for successful data collection and interpretation. Plant Cell Environ. 2016, 39, 1140–1160. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhang, S.; Sun, L.; Liang, S.; Zheng, X.; Ren, H.; Qi, X. Effects of enhanced resistance and transcriptome analysis of twig blight disease by exogenous brassinolide in Myrica rubra. Antioxidants 2024, 13, 61. [Google Scholar] [CrossRef]
- Zhang, D.; Deng, X.; Fu, F.; Lin, H. Induction of plant virus defense response by brassinosteroids and brassinosteroid signaling in Arabidopsis thaliana. Planta 2015, 241, 875–885. [Google Scholar] [CrossRef]
- Ding, J.; Shi, K.; Zhou, Y.; Yu, J. Effects of root and foliar applications of 2,4-epibrassinolide on Fusarium wilt and antioxidant metabolism in cucumber roots. Hortscience 2009, 44, 1340–1345. [Google Scholar] [CrossRef]
- Upadhyay, R.; Saini, R.; Shukla, P.; Tiwari, K. Role of secondary metabolites in plant defense mechanisms: A molecular and biotechnological insights. Phytochem. Rev. 2024, 1–31. [Google Scholar] [CrossRef]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.; Bahadar, K. Role of secondary metabolites in plant defense against pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Dixon, R.; Achnine, L.; Kota, P.; Liu, C. The phenylpropanoid pathway and plant defence—A genomics perspective. Mol. Plant Pathol. 2002, 3, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Treutter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Neutelings, G. Lignin variability in plant cell walls: Contribution of new models. Plant Sci. 2011, 181, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Bonawitz, N.; Chapple, C. The genetics of lignin biosynthesis: Connecting genotype to phenotype. Annu. Rev. Genet. 2010, 44, 337–363. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, N.; Selvaraj, G.; Wei, Y.; King, J. Role of lignification in plant defense. Plant Signal. Behav. 2009, 4, 158–159. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Niu, K.; Zhao, G.; Zhang, Y.; Chai, J.; Ju, Z. Exogenous 24-Epibrassinolide Improves Resistance to Leaf Spot Disease Through Antioxidant Regulation and Phenylpropanoid Metabolism in Oats. Agronomy 2024, 14, 3035. https://doi.org/10.3390/agronomy14123035
Wang Z, Niu K, Zhao G, Zhang Y, Chai J, Ju Z. Exogenous 24-Epibrassinolide Improves Resistance to Leaf Spot Disease Through Antioxidant Regulation and Phenylpropanoid Metabolism in Oats. Agronomy. 2024; 14(12):3035. https://doi.org/10.3390/agronomy14123035
Chicago/Turabian StyleWang, Zheng, Kuiju Niu, Guiqin Zhao, Yuehua Zhang, Jikuan Chai, and Zeliang Ju. 2024. "Exogenous 24-Epibrassinolide Improves Resistance to Leaf Spot Disease Through Antioxidant Regulation and Phenylpropanoid Metabolism in Oats" Agronomy 14, no. 12: 3035. https://doi.org/10.3390/agronomy14123035
APA StyleWang, Z., Niu, K., Zhao, G., Zhang, Y., Chai, J., & Ju, Z. (2024). Exogenous 24-Epibrassinolide Improves Resistance to Leaf Spot Disease Through Antioxidant Regulation and Phenylpropanoid Metabolism in Oats. Agronomy, 14(12), 3035. https://doi.org/10.3390/agronomy14123035






