Construction and Identification of Cold Tolerance in Different Broccoli Cultivars at the Seedling Stage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plants, Instruments, and Reagents
2.2. Cold-Resistant Test and Phenotypic Investigation
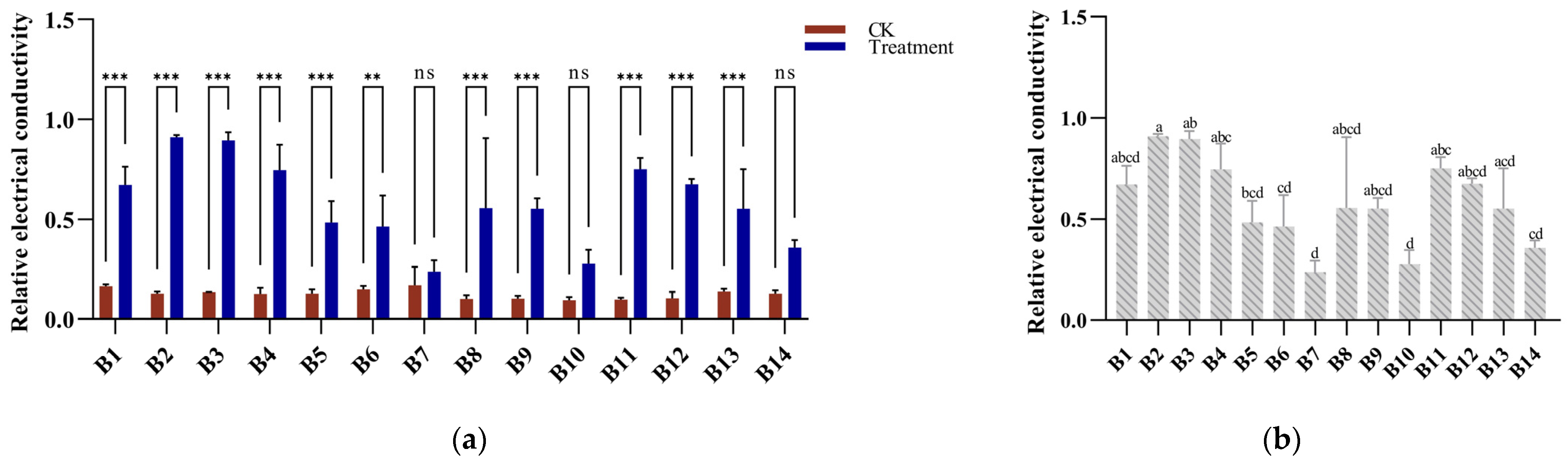
2.3. Determination of Electrical Conductivity
2.4. Determination of MDA
2.5. Determination of the Soluble Sugar Level
2.6. Determination of H2O2 Content
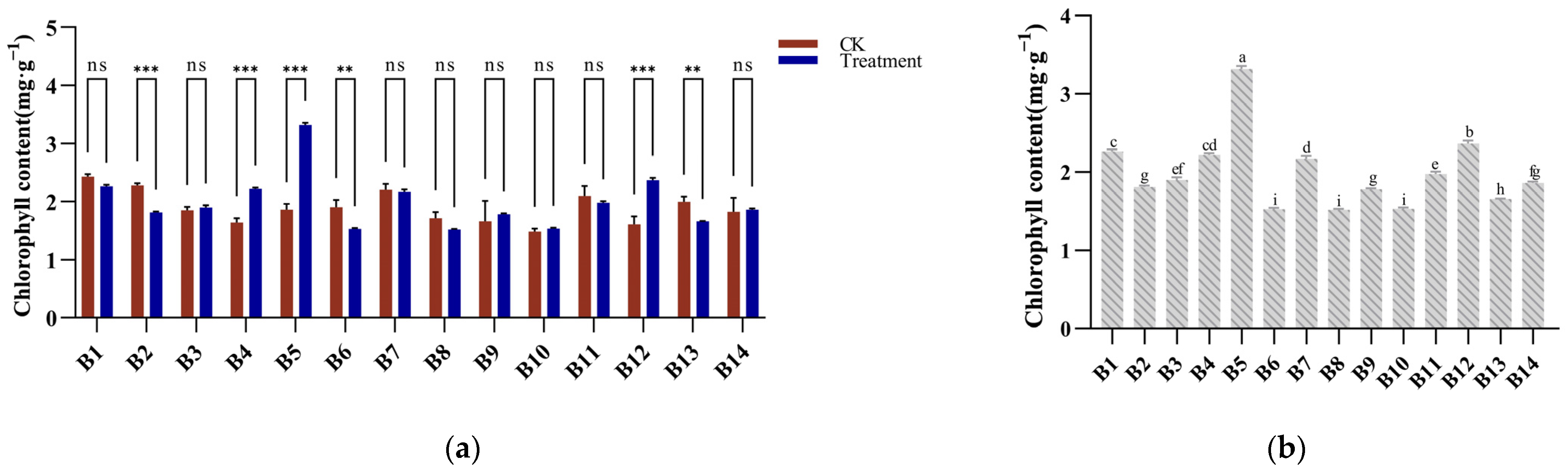
2.7. Determination of Chlorophyll Content
2.8. Statistical Analysis
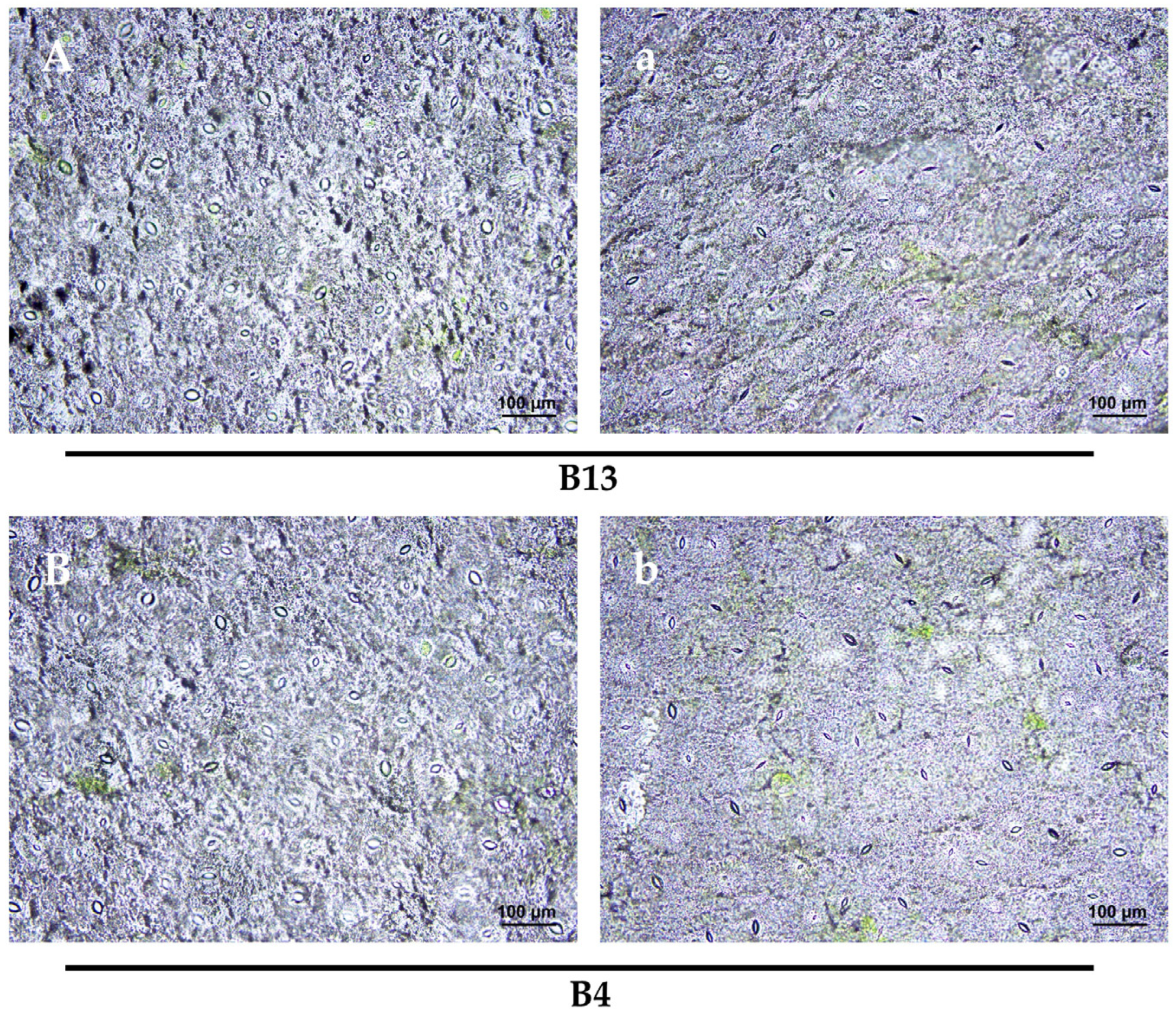
2.9. Stomatal Observation
3. Results
3.1. The Phenotypic Profiles of Broccoli Response to Cold Stress
3.2. Electrical Conductivity Response to Cold Stress
3.3. MDA Response to Cold Stress
3.4. Soluble Sugar Response to Cold Stress
3.5. Hydrogen Peroxide Response to Cold Stress
3.6. Chlorophyll Response to Cold Stress
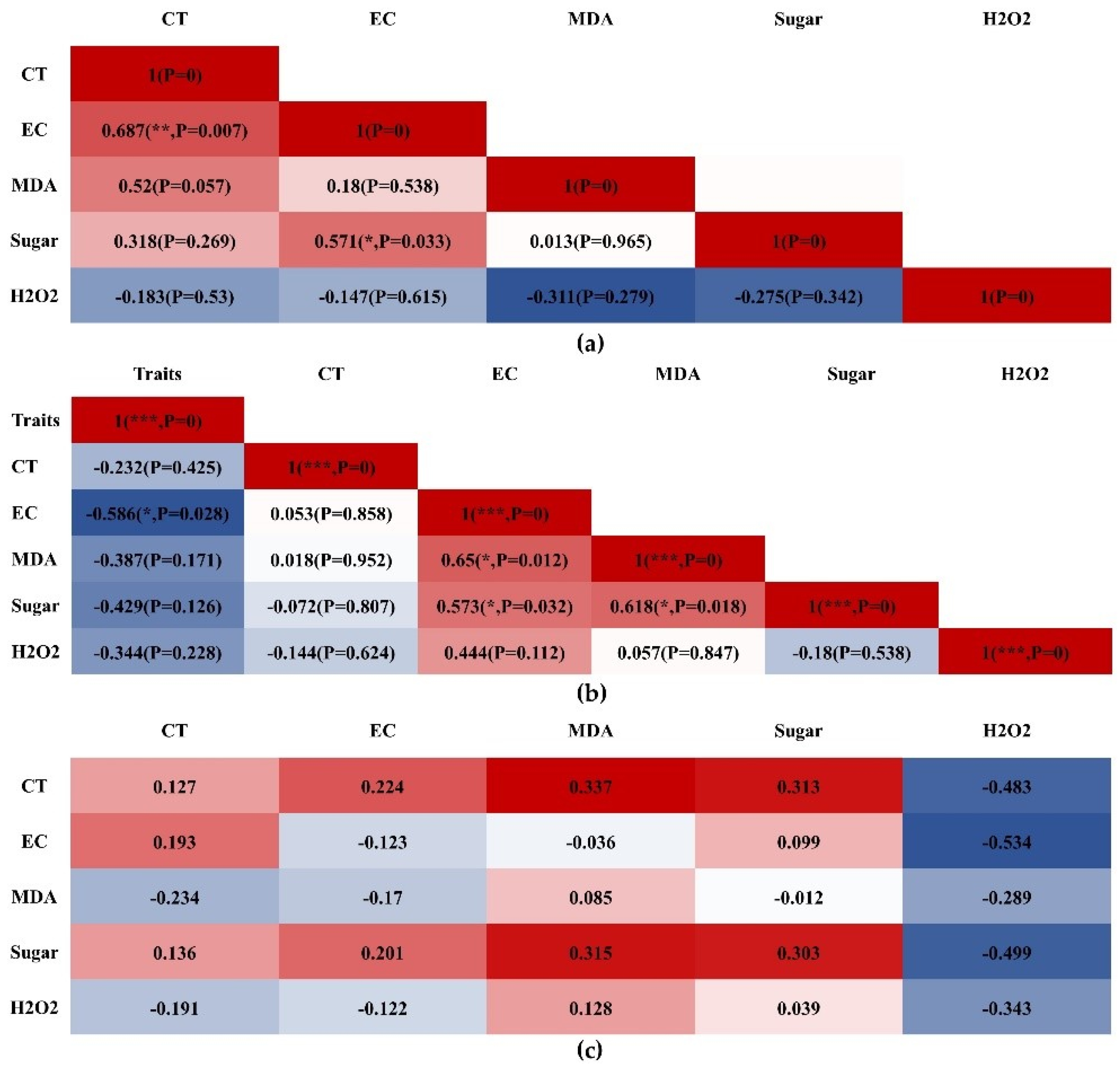
3.7. Correlation Analysis of CT, EC, MDA, Sugar, and H2O2
3.8. Stomatal Response to Cold Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- James, D.; Devaraj, S.; Bellur, P.; Lakkanna, S.; Vicini, J.; Boddupalli, S. Novel concepts of broccoli sulforaphanes and disease: Induction of phase II antioxidant and detoxification enzymes by enhanced-glucoraphanin broccoli. Nutr. Rev. 2012, 70, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Conzatti, A.; Froes, F.C.; Schweigert, P.I.; Souza, C.G. Clinical and molecular evidence of the consumption of broccoli, glucoraphanin and sulforaphane in humans. Nutr. Hosp. 2014, 31, 559–569. [Google Scholar] [PubMed]
- Darand, M.; Alizadeh, S.; Mansourian, M. The effect of Brassica vegetables on blood glucose levels and lipid profiles in adults. A systematic review and meta-analysis. Phytother. Res. 2022, 36, 1914–1929. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Feng, L.; Zhang, S.; Li, L.; Guan, R.; Long, W.; Xian, Z.; Zhang, J.; Shen, W. Ammonia borane positively regulates cold tolerance in Brassica napus via hydrogen sulfide signaling. BMC Plant Biol. 2022, 22, 585. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, Y.; Yu, Z.; Gao, Z.; Ding, Q.; Shah, S.; Lin, W.; Li, Y.; Hou, X. BcMYB111 Responds to BcCBF2 and Induces Flavonol Biosynthesis to Enhance Tolerance under Cold Stress in Non-Heading Chinese Cabbage. Int. J. Mol. Sci. 2023, 24, 8670. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Wang, F.; Ma, L.; Qi, Z.; Liu, S.; Chen, C.; Wu, J.; Wang, P.; Yang, C.; Wu, Y.; et al. Physiological and Biochemical Mechanisms and Cytology of Cold Tolerance in Brassica napus. Front. Plant Sci. 2020, 11, 1241. [Google Scholar] [CrossRef]
- Farooq, M.A.; Li, L.; Ali, B.; Gill, R.A.; Wang, J.; Ali, S.; Gill, M.B.; Zhou, W. Oxidative injury and antioxidant enzymes regulation in arsenic-exposed seedlings of four Brassica napus L. cultivars. Environ. Sci. Pollut. Res. 2015, 22, 10699–10712. [Google Scholar] [CrossRef]
- Goswami, A.K.; Maurya, N.K.; Goswami, S.; Bardhan, K.; Singh, S.K.; Prakash, J.; Pradhan, S.; Kumar, A.; Chinnusamy, V.; Kumar, P.; et al. Physio-biochemical and molecular stress regulators and their crosstalk for low-temperature stress responses in fruit crops: A review. Front. Plant Sci. 2022, 13, 1022167. [Google Scholar] [CrossRef]
- Pu, Y.; Liu, L.; Wu, J.; Zhao, Y.; Bai, J.; Ma, L.; Yue, J.; Jin, J.; Niu, Z.; Fang, Y.; et al. Transcriptome Profile Analysis of Winter Rapeseed (Brassica napus L.) in Response to Freezing Stress, Reveal Potentially Connected Events to Freezing Stress. Int. J. Mol. Sci. 2019, 20, 2771. [Google Scholar] [CrossRef]
- Zhang, C.; Yao, X.; Zhang, Y.; Zhao, S.; Liu, J.; Wu, G.; Yan, X.; Luo, J. Transcriptomic Profiling Highlights the ABA Response Role of BnSIP1-1 in Brassica napus. Int. J. Mol. Sci. 2023, 24, 11180. [Google Scholar] [CrossRef]
- Wei, Q.; Xie, K.; Wang, H.; Shao, X.; Wei, Y.; Chen, Y.; Jiang, S.; Cao, M.; Chen, J.; Xu, F. Calcium Involved in the Enrichment of gamma-Aminobutyric Acid (GABA) in Broccoli Sprouts under Fructose Treatment. Plants 2023, 12, 224. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Hui, Q.; Gu, Z. Effects of ABA and CaCl2 on GABA accumulation in fava bean germinating under hypoxia-NaCl stress. Biosci. Biotechnol. Biochem. 2016, 80, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, J.; Shen, Y.; Sheng, X.; Shaw, R.K.; Branca, F.; Gu, H. A 43 Bp-Deletion in the F3′H Gene Reducing Anthocyanins Is Responsible for Keeping Buds Green at Low Temperatures in Broccoli. Int. J. Mol. Sci. 2023, 24, 11391. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Yang, W.; Zhang, J.; Zhao, L.; Quan, Y.; He, Z.; Xu, Y.; Zhang, F.; Yin, M.; Wang, Y.; et al. Overexpression of ClRAP2.4 in Chrysanthemum enhances tolerance to cold stress. Funct. Plant Biol. 2023, 50, 470–481. [Google Scholar] [CrossRef]
- Hong, E.; Xia, X.; Ji, W.; Li, T.; Xu, X.; Chen, J.; Chen, X.; Zhu, X. Effects of High Temperature Stress on the Physiological and Biochemical Characteristics of Paeonia ostii. Int. J. Mol. Sci. 2023, 24, 11180. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, H.; Wang, L.; Zhao, Q.; Wang, D.; Zhang, T. Cold acclimation alleviates cold stress-induced PSII inhibition and oxidative damage in tobacco leaves. Plant Signal. Behav. 2022, 17, 2013638. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, K.; Tang, Q.; Zeng, L.; Wu, Z. Light Intensity Regulates Low-Temperature Adaptability of Tea Plant through ROS Stress and Developmental Programs. Int. J. Mol. Sci. 2023, 24, 9852. [Google Scholar] [CrossRef]
- Chazaux, M.; Schiphorst, C.; Lazzari, G.; Caffarri, S. Precise estimation of chlorophyll a, b and carotenoid content by deconvolution of the absorption spectrum and new simultaneous equations for Chl determination. Plant J. 2022, 109, 1630–1648. [Google Scholar] [CrossRef]
- Agurla, S.; Gahir, S.; Munemasa, S.; Murata, Y.; Raghavendra, A.S. Mechanism of Stomatal Closure in Plants Exposed to Drought and Cold Stress. Adv. Exp. Med. Biol. 2018, 1081, 215–232. [Google Scholar]
- Deng, Y.; Kashtoh, H.; Wang, Q.; Zhen, G.; Li, Q.; Tang, L.; Gao, H.; Zhang, C.; Qin, L.; Su, M.; et al. Structure and activity of SLAC1 channels for stomatal signaling in leaves. Proc. Natl. Acad. Sci. USA 2021, 118, e2015151118. [Google Scholar] [CrossRef]
- Movahedi, M.; Zoulias, N.; Casson, S.A.; Sun, P.; Liang, Y.; Hetherington, A.M.; Gray, J.E.; Chater, C.C.C. Stomatal responses to carbon dioxide and light require abscisic acid catabolism in Arabidopsis. Interface Focus 2021, 11, 20200036. [Google Scholar] [CrossRef] [PubMed]
- Biel, A.; Moser, M.; Meier, I. A Role for Plant KASH Proteins in Regulating Stomatal Dynamics1 [OPEN]. Plant Physiol. 2020, 182, 1100–1113. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Rahman, M.A.; Skalicky, M.; Alabdallah, N.M.; Waseem, M.; Jahan, M.S.; Ahammed, G.J.; El-Mogy, M.M.; El-Yazied, A.A.; Ibrahim, M.F.M.; et al. Ozone Induced Stomatal Regulations, MAPK and Phytohormone Signaling in Plants. Int. J. Mol. Sci. 2021, 22, 6304. [Google Scholar] [CrossRef] [PubMed]
- Schulze, S.; Dubeaux, G.; Ceciliato, P.H.O.; Munemasa, S.; Nuhkat, M.; Yarmolinsky, D.; Aguilar, J.; Diaz, R.; Azoulay-Shemer, T.; Steinhorst, L.; et al. A role for calcium-dependent protein kinases in differential CO2- and ABA-controlled stomatal closing and low CO2-induced stomatal opening in Arabidopsis. New Phytol. 2021, 229, 2765–2779. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Han, J.; Guo, B.; Zhao, W.; Zhou, S.; Zhou, C.; Zhang, L.; Li, X.; Han, D. MbICE1 Confers Drought and Cold Tolerance through Up-Regulating Antioxidant Capacity and Stress-Resistant Genes in Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 16072. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A.; Rahman, A. Cold stress response in Arabidopsis thaliana is mediated by GNOM ARF-GEF. Plant J. 2019, 97, 500–516. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, R.; Huang, K.; Huang, S.; Wang, H.; Wei, Z.; Li, Z.; Bian, M.; Jiang, W.; Wu, T.; et al. The OsWRKY63-OsWRKY76-OsDREB1B module regulates chilling tolerance in rice. Plant J. 2022, 112, 383–398. [Google Scholar] [CrossRef]
- Lou, Q.; Guo, H.; Li, J.; Han, S.; Khan, N.U.; Gu, Y.; Zhao, W.; Zhang, Z.; Zhang, H.; Li, Z.; et al. Cold-adaptive evolution at the reproductive stage in Geng/japonica subspecies reveals the role of OsMAPK3 and OsLEA9. Plant J. 2022, 111, 1032–1051. [Google Scholar] [CrossRef]
- Mu, J.; Fu, Y.; Liu, B.; Zhang, Y.; Wang, A.; Li, Y.; Zhu, J. SiFBA5, a cold-responsive factor from Saussurea involucrata promotes cold resilience and biomass increase in transgenic tomato plants under cold stress. BMC Plant Biol. 2021, 21, 75. [Google Scholar] [CrossRef]
- Nai, G.; Liang, G.; Ma, W.; Lu, S.; Li, Y.; Gou, H.; Guo, L.; Chen, B.; Mao, J. Overexpression VaPYL9 improves cold tolerance in tomato by regulating key genes in hormone signaling and antioxidant enzyme. BMC Plant Biol. 2022, 22, 344. [Google Scholar] [CrossRef]
- Xu, D.; Zuo, J.; Fang, Y.; Yan, Z.; Shi, J.; Gao, L.; Wang, Q.; Jiang, A. Effect of folic acid on the postharvest physiology of broccoli during storage. Food Chem. 2021, 339, 127981. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, M.S.; Flores, F.B. Employing phytosulfokine α (PSKα) for delaying broccoli florets yellowing during cold storage. Food Chem. 2021, 355, 129626. [Google Scholar] [CrossRef] [PubMed]
- Rastegar, S.; Shojaie, A.; Koy, R.A.M. Foliar application of salicylic acid and calcium chloride delays the loss of chlorophyll and preserves the quality of broccoli during storage. J. Food Biochem. 2022, 46, e14154. [Google Scholar] [CrossRef] [PubMed]
- Šola, I.; Davosir, D.; Kokic, E.; Zekirovski, J. Effect of Hot-and Cold-Water Treatment on Broccoli Bioactive Compounds, Oxidative Stress Parameters and Biological Effects of Their Extracts. Plants 2023, 12, 1135. [Google Scholar] [CrossRef] [PubMed]
- Rácz, I.; Páldi, E.; Szalai, G.; Janda, T.; Pál, M.; Lásztity, D. S-methylmethionine reduces cell membrane damage in higher plants exposed to low-temperature stress. J. Plant Physiol. 2008, 165, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Ma, K.; Zhao, Y.; Mei, C.; Mamat, A.; Wang, J.; Qin, L.; He, T. The cold-stress responsive gene DREB1A involved in low-temperature tolerance in Xinjiang wild walnut. PeerJ 2022, 10, e14021. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Hou, R.; Yang, N.; Li, L.; Deng, J.; Qin, G.; Ding, D. Physiological and TMT-labeled proteomic analyses reveal important roles of sugar and secondary metabolism in Citrus junos under cold stress. J. Proteom. 2021, 237, 104145. [Google Scholar] [CrossRef]
- Meng, A.; Wen, D.; Zhang, C. Dynamic Changes in Seed Germination under Low-Temperature Stress in Maize. Int. J. Mol. Sci. 2022, 23, 5495. [Google Scholar] [CrossRef]
- Gu, Y.; He, L.; Zhao, C.; Wang, F.; Yan, B.; Gao, Y.; Li, Z.; Yang, K.; Xu, J. Biochemical and Transcriptional Regulation of Membrane Lipid Metabolism in Maize Leaves under Low Temperature. Front. Plant Sci. 2017, 8, 2053. [Google Scholar] [CrossRef]
- Qin, H.; Cui, X.; Shu, X.; Zhang, J. The transcription factor VaNAC72–regulated expression of the VaCP17 gene from Chinese wild Vitis amurensis enhances cold tolerance in transgenic grape (V. vinifera). Plant Physiol. Biochem. 2023, 200, 107768. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, X.; Zhang, Z.; Wang, A.; Zhu, J. Cold-regulated gene LeCOR413PM2 confers cold stress tolerance in tomato plants. Gene 2021, 764, 145097. [Google Scholar] [CrossRef] [PubMed]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of sugars under abiotic stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Li, Y.; Wang, P.; Jiao, S.; Wang, H.; Mao, J.; Chen, B. VaAPL1 Promotes Starch Synthesis to Constantly Contribute to Soluble Sugar Accumulation, Improving Low Temperature Tolerance in Arabidopsis and Tomato. Front. Plant Sci. 2022, 13, 920424. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, S.J.; Min, W.K.; Cha, S.; Song, J.T.; Seo, H.S. COP1 mutation causes low leaf temperature under various abiotic stresses in Arabidopsis thaliana. Plant Direct 2022, 6, e473. [Google Scholar] [CrossRef]




| Number | Cultivar | Generation | Source | Maturity |
|---|---|---|---|---|
| B1 | Naihanyouxiu | F1 | Sakada, Japan | Medium early |
| B2 | Yan Xiu | F1 | Sakada, Japan | Medium to late |
| B3 | Qianghan | F1 | Saint Denis, USA | Medium to late |
| B4 | Jade No. 5 | F1 | Syngenta | Medium |
| B5 | Lvxiong 90 | F1 | Shida, Japan | Late |
| B6 | King 11 | F1 | Zhaofeng, Wenzhou | Extremely late |
| B7 | Zheqing 80 | F1 | Zhejiang Meizhiao Seed Industry | Extremely late |
| B8 | Zhongqing 11 | F1 | Chinese Academy of Agricultural Sciences | Extremely precocious |
| B9 | Zhongqing 15 | F1 | Chinese Academy of Agricultural Sciences | Medium to late |
| B10 | Zhongqing 16 | F1 | Chinese Academy of Agricultural Sciences | Precocity |
| B11 | Zhongqing 318 | F1 | Chinese Academy of Agricultural Sciences | Extremely late |
| B12 | Zhongqing 319 | F1 | Chinese Academy of Agricultural Sciences | Late |
| B13 | Meiqing | F1 | Zhejiang Meizhiao Seed Industry | Medium to late |
| B14 | Meiao 7172 | F1 | Zhejiang Meizhiao Seed Industry | Medium to late |
| Cold Resistance Rating (0–5) | Performance | Cold Resistance |
|---|---|---|
| 0 | There is little or only slight damage to the leaves of broccoli seedlings. Most leaves have no obvious yellowing, wilting, or necrosis. | Highly cold tolerant |
| 1 | ||
| 2 | The leaves of broccoli seedlings are damaged to a certain extent, and some leaves are slightly yellowed, wilted, or edge browned. | Moderate cold tolerance |
| 3 | ||
| 4 | The leaves of broccoli seedlings were severely damaged, and most of the leaves showed obvious yellowing, wilting, browning, or necrosis. | Low cold tolerance |
| 5 |
| Number | Cultivar | Cold Resistance Rating (0–5) |
|---|---|---|
| B1 | Naihanyouxiu | 2 |
| B2 | Yan Xiu | 1 |
| B3 | Qianghan | 1 |
| B4 | Jade No. 5 | 0 |
| B5 | Lvxiong 90 | 3 |
| B6 | King 11 | 4 |
| B7 | Zheqing 80 | 3 |
| B8 | Zhongqing 11 | 2 |
| B9 | Zhongqing 15 | 4 |
| B10 | Zhongqing 16 | 3 |
| B11 | Zhongqing 318 | 4 |
| B12 | Zhongqing 319 | 3 |
| B13 | Meiqing | 5 |
| B14 | Meiao 7172 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, D.; Han, F.; Zhao, Y.; Liu, Y.; Liu, Y.; Huang, J.; Li, Z. Construction and Identification of Cold Tolerance in Different Broccoli Cultivars at the Seedling Stage. Agronomy 2024, 14, 237. https://doi.org/10.3390/agronomy14020237
Wen D, Han F, Zhao Y, Liu Y, Liu Y, Huang J, Li Z. Construction and Identification of Cold Tolerance in Different Broccoli Cultivars at the Seedling Stage. Agronomy. 2024; 14(2):237. https://doi.org/10.3390/agronomy14020237
Chicago/Turabian StyleWen, Dongna, Fengqing Han, Yongyu Zhao, Yuxiang Liu, Yumei Liu, Jianxin Huang, and Zhansheng Li. 2024. "Construction and Identification of Cold Tolerance in Different Broccoli Cultivars at the Seedling Stage" Agronomy 14, no. 2: 237. https://doi.org/10.3390/agronomy14020237
APA StyleWen, D., Han, F., Zhao, Y., Liu, Y., Liu, Y., Huang, J., & Li, Z. (2024). Construction and Identification of Cold Tolerance in Different Broccoli Cultivars at the Seedling Stage. Agronomy, 14(2), 237. https://doi.org/10.3390/agronomy14020237









