Genetic Gains of Grain Yield among the Maize Cultivars Released over a Century from the National Breeding Program of Zimbabwe
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Testing Locations
2.3. Experimental Design
2.4. Trial Management
2.5. Data Collection
2.6. Statistical Analysis
3. Results
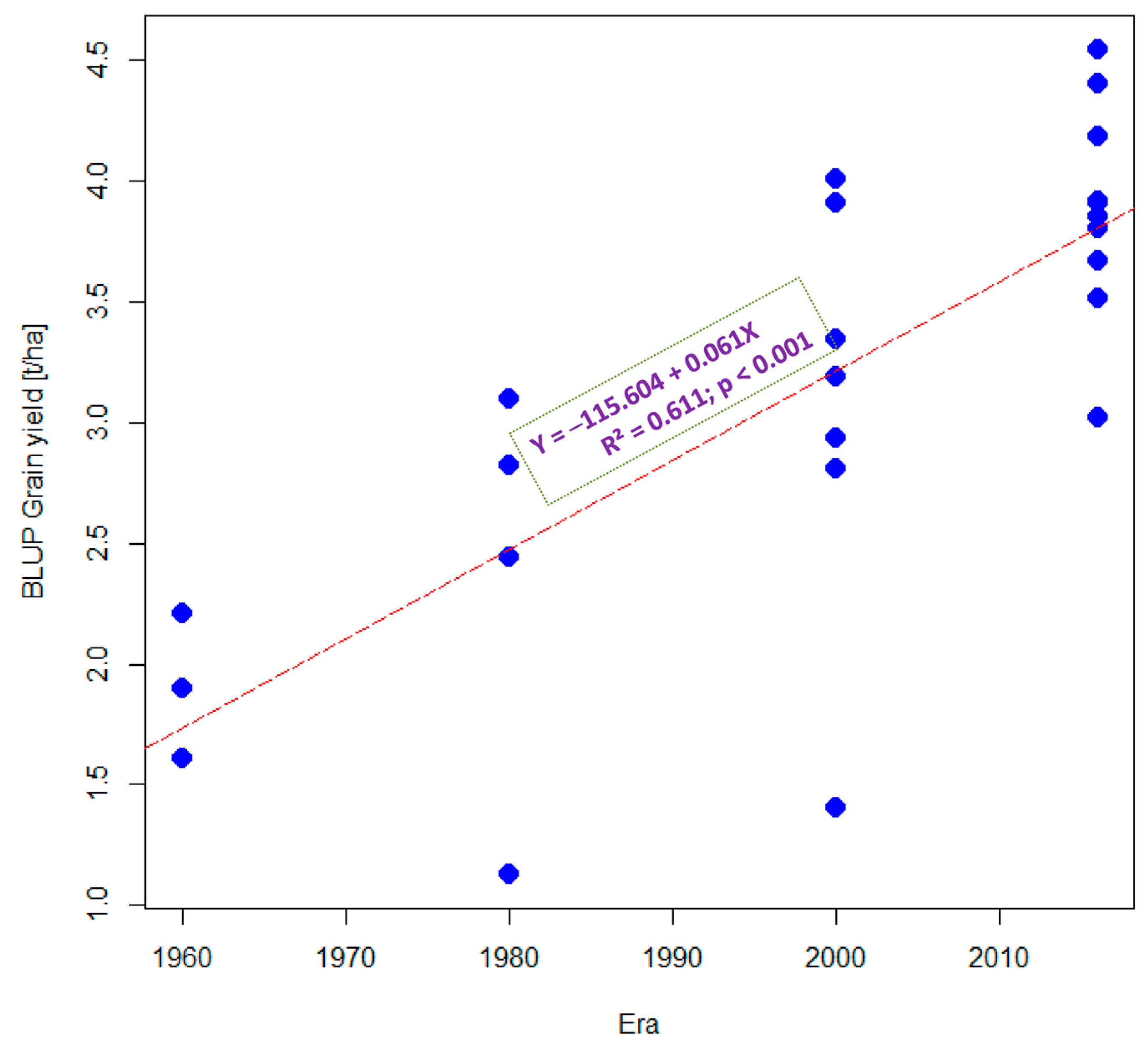
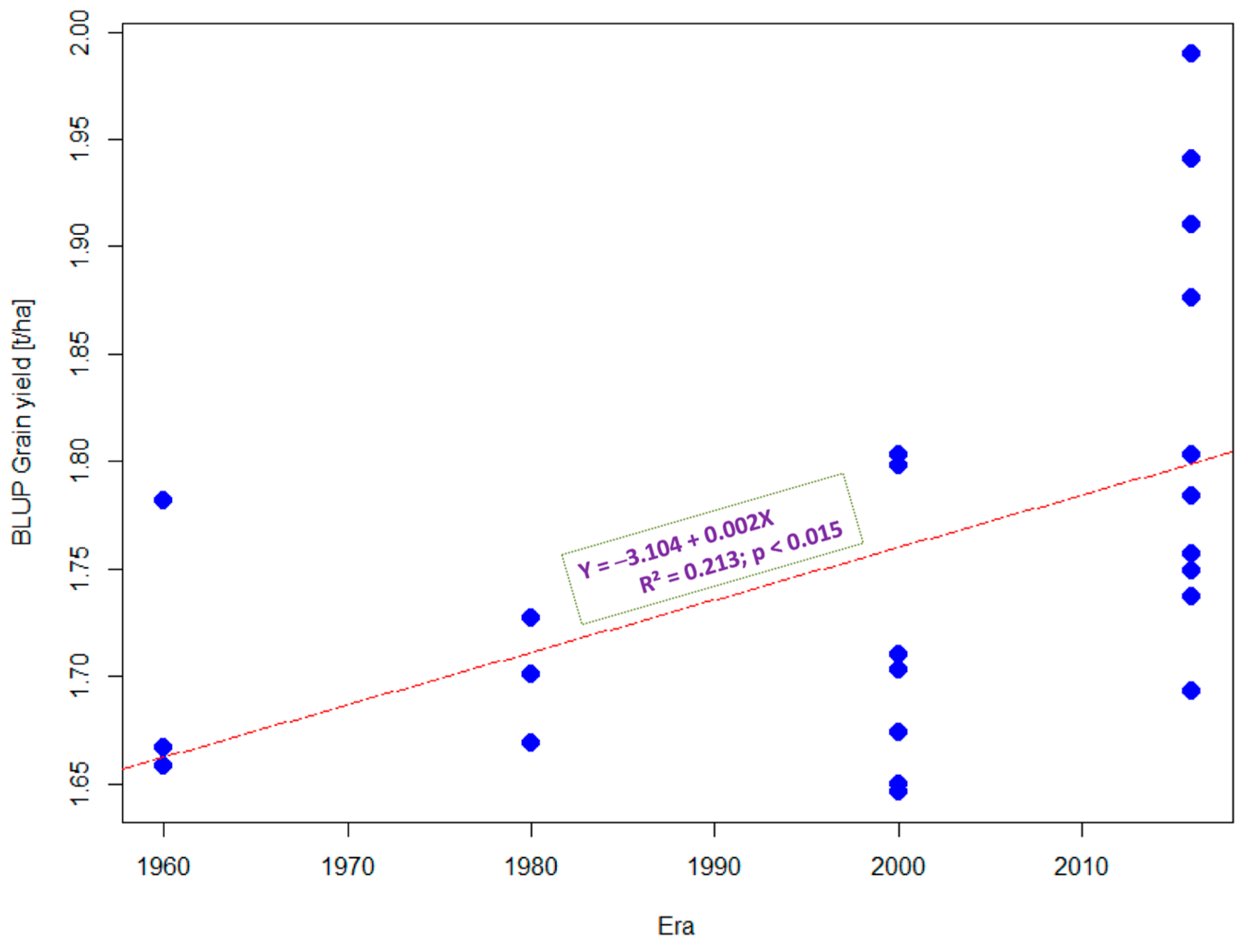
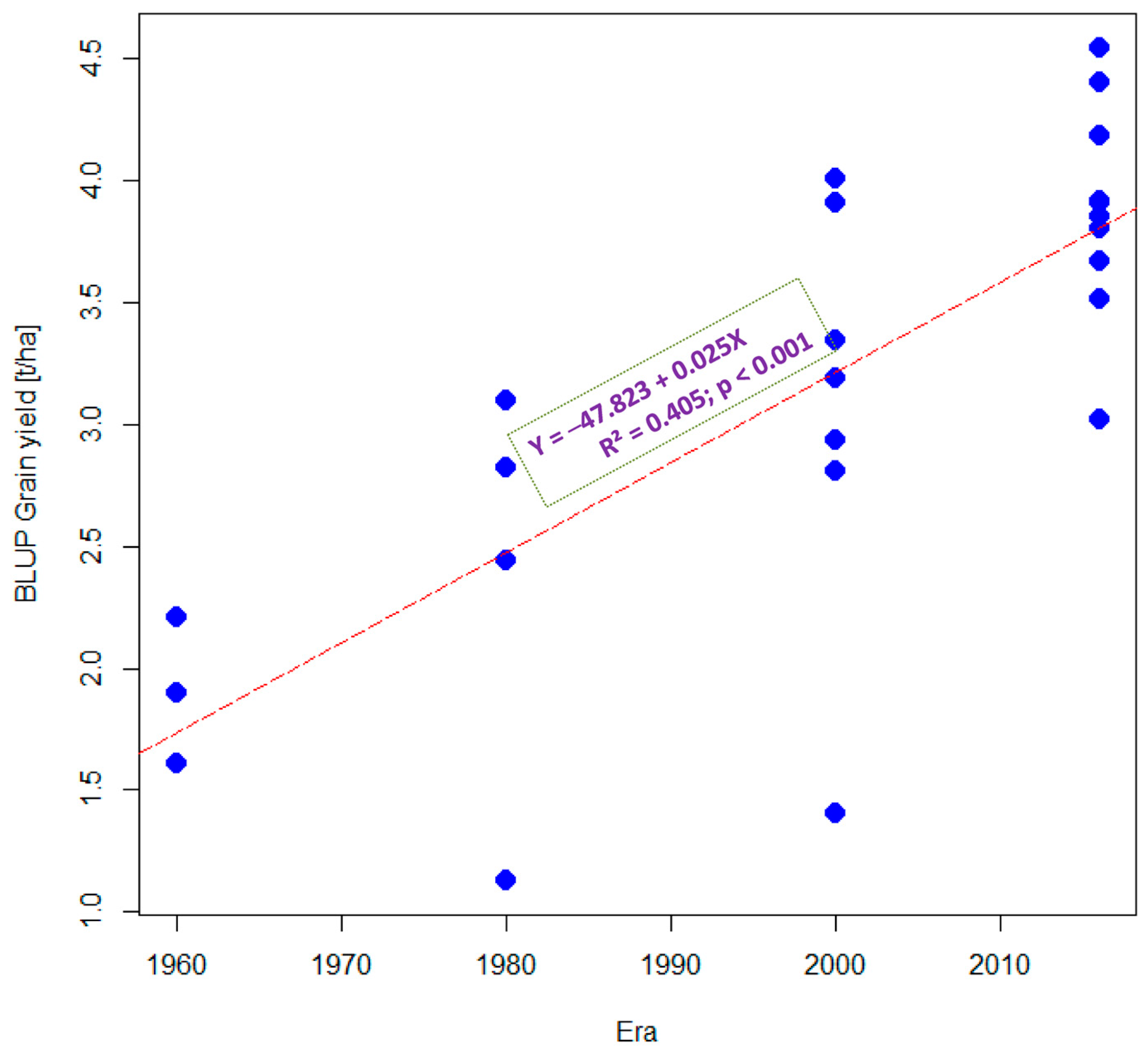
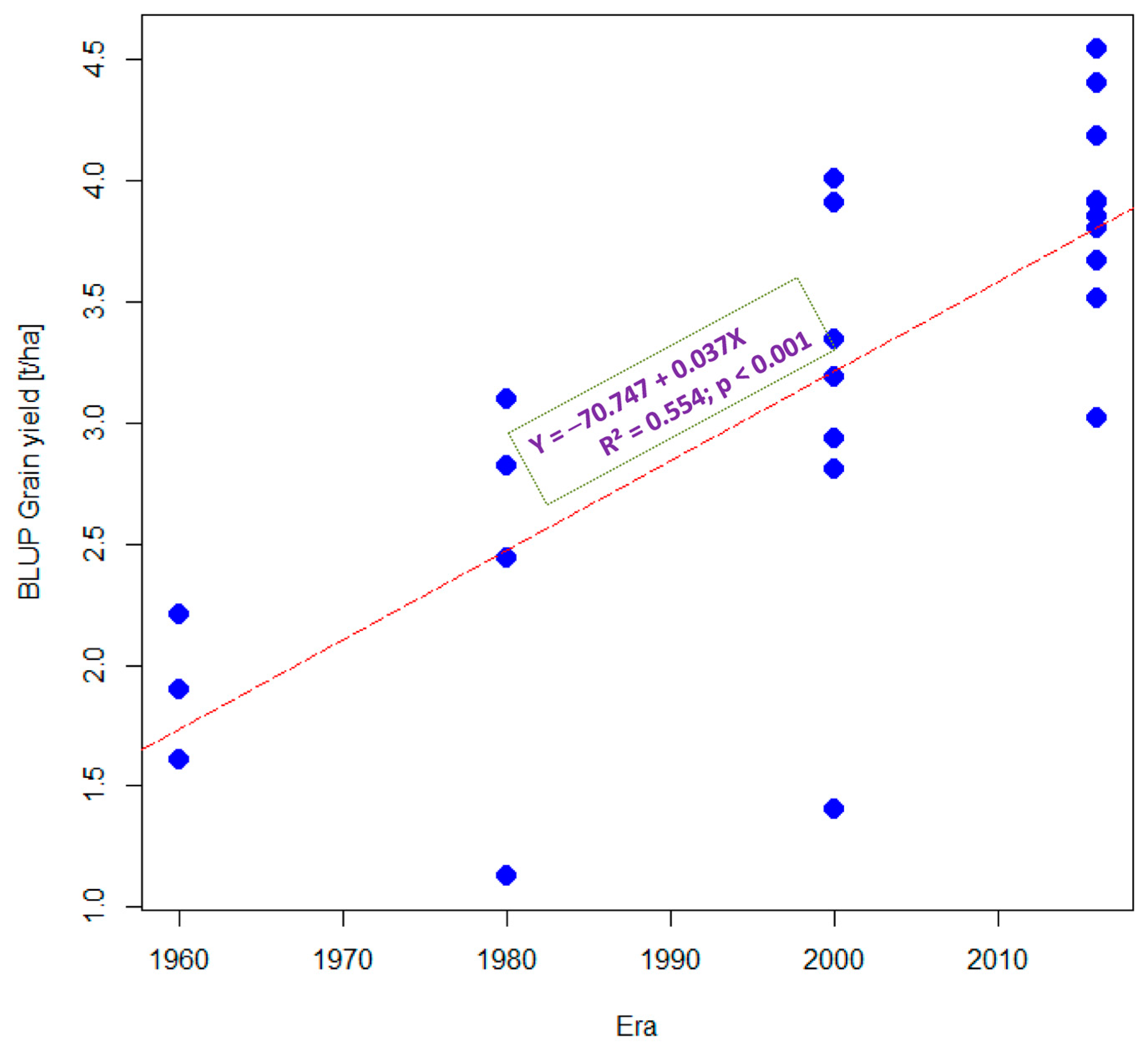
3.1. Grain Yield Performance under Optimal and Stress Conditions
3.2. Estimated Genetic Gains under Optimal and Stress Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rukuni, M.; Tawonezvi, P.; Munyuki-hungwe, M.; Dube, E.; Kilian, W.; Mwadzingeni, L.; Sosibo, N.Z.; Barnard, A.; Tsilo, T.J.; Rice, E.; et al. Agricultura revolution. Crop Sci. 2016, 51, 185–192. [Google Scholar] [CrossRef]
- Masuka, B.; Atlin, G.N.; Olsen, M.; Magorokosho, C.; Labuschagne, M.; Crossa, J.; Bänziger, M.; Pixley, K.V.; Vivek, B.S.; Von Biljon, A.; et al. Gains in maize genetic improvement in eastern and southern Africa: I. CIMMYT hybrid breeding pipeline. Crop Sci. 2017, 57, 168–179. [Google Scholar] [CrossRef]
- Cairns, J.E.; Crossa, J.; Zaidi, P.H.; Grudloyma, P.; Sanchez, C.; Luis Araus, J.; Thaitad, S.; Makumbi, D.; Magorokosho, C.; Bänziger, M.; et al. Identification of drought, heat, and combined drought and heat tolerant donors in maize. Crop Sci. 2013, 53, 1335–1346. [Google Scholar] [CrossRef]
- Huang, S.; Gao, Y.; Li, Y.; Xu, L.; Tao, H.; Wang, P. Influence of plant architecture on maize physiology and yield in the Heilonggang River valley. Crop J. 2017, 5, 52–62. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Fakorede, M.A.; Oyekunle, M. Agronomic traits associated with genetic gains in maize yield during three breeding eras in West Africa. Maydica 2014, 59, 49–57. [Google Scholar]
- Badu-Apraku, B.; Oyekunle, M.; Menkir, A.; Obeng-Antwi, K.; Yallou, C.G.; Usman, I.S.; Alidu, H. Comparative Performance of Early-maturing Maize Cultivars Developed in Three Eras under Drought Stress and Well-watered Environments in West Africa. Crop Sci. 2013, 53, 1298. [Google Scholar] [CrossRef]
- Hall, A.J.; Richards, R.A. Prognosis for genetic improvement of yield potential and water-limited yield of major grain crops. Field Crops Res. 2013, 143, 18–33. [Google Scholar] [CrossRef]
- Duvick, D.N. Genetic progress in yield of United States maize (Zea mays L.). Maydica 2005, 50, 193–202. [Google Scholar]
- Fan, X.M.; Kang, M.S.; Chen, H.; Zhang, Y.; Tan, J.; Xu, C. Yield stability of maize hybrids evaluated in multi-environment trials in Yunnan, China. Agron. J. 2007, 99, 220–228. [Google Scholar] [CrossRef]
- Gupta, H.S.; Hossain, F.; Muthusamy, V. Biofortification of maize: An Indian perspective. Indian J. Genet. Plant Breed. 2015, 75, 1–22. [Google Scholar] [CrossRef]
- Tripp, R. Athropology and on-farm research. Hum. Organ. 1985, 44, 114–124. [Google Scholar] [CrossRef]
- Payne, R.W.; Murray, D.A.; Harding, S.A. An Introduction to the Genstat Command Language, 18th ed.; VSN International: Hemel Hempstead, UK, 2015. [Google Scholar]
- Wickham, H. Teaching safe-stats, not statistical abstinence. Am. Stat. 2015, 69, 266–282. [Google Scholar]
- Prasanna, B.M.; Burgueño, J.; Beyene, Y.; Makumbi, D.; Asea, G.; Woyengo, V.; Tarekegne, A.; Magorokosho, C.; Wegary, D.; Ndhlela, T.; et al. Genetic trends in CIMMYT’ s tropical maize breeding pipelines. Sci. Rep. 2022, 12, 20110. [Google Scholar] [CrossRef] [PubMed]
- Eicher, C.K.; Lansing, E. Zimbabwe ’s Maize-Based Green Revolution: Preconditions for Replication. World Dev. 1995, 23, 805–818. [Google Scholar] [CrossRef]
- Kol, C. Genomic Designing of Climate-Smart Cereal Crops; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Gasura, E.; Setimela, P.S.; Souta, C.M. Evaluation of the performance of sorghumgenotypes using gge biplot. Can. J. Plant Sci. 2015, 95, 1205–1214. [Google Scholar] [CrossRef]
- Ci, X.; Li, M.; Liang, X.; Xie, Z.; Zhang, D.; Li, X.; Lu, Z.; Ru, G.; Bai, L.; Xie, C.; et al. Genetic Contribution to Advanced Yield for Maize Hybrids Released from 1970 to 2000 in China. Crop Sci. 2011, 51, 13. [Google Scholar] [CrossRef]

| Genotype | Source | Year of Release | Period |
|---|---|---|---|
| Hickory King (open-pollinated variety) | Crop Breeding Institute, ZNBP | 1900 | Before 1960 |
| Salisbury White (open-pollinated variety) | Crop Breeding Institute, ZNBP | 1900 | 1st period |
| Southern Cross (open-pollinated variety) | Crop Breeding Institute, ZNBP | 1900 | |
| R200 (hybrid) | Crop Breeding Institute, ZNBP | 1971 | 1960–1980 |
| R201 (hybrid) | Crop Breeding Institute, ZNBP | 1971 | 2nd period |
| R215 (hybrid) | Crop Breeding Institute, ZNBP | 1971 | |
| SR52 (hybrid) | Crop Breeding Institute, ZNBP | 1960 | |
| ZS107 (hybrid) | Crop Breeding Institute, ZNBP | 1985 | 1981–2000 |
| ZS108 (hybrid) | Crop Breeding Institute, ZNBP | 1998 | 3rd period |
| ZS255 (hybrid) | Crop Breeding Institute, ZNBP | 1998 | |
| ZS206 (hybrid) | Crop Breeding Institute, ZNBP | 1988 | |
| ZS232 (hybrid) | Crop Breeding Institute, ZNBP | 1985 | |
| ZS240 (hybrid) | Crop Breeding Institute, ZNBP | 1992 | |
| SC513 * (hybrid) | Seed Co | 1999 | |
| ZS263 (hybrid) | Crop Breeding Institute, ZNBP | 2011 | 2001–2016 |
| ZS265 (hybrid) | Crop Breeding Institute, ZNBP | 2011 | 4th period |
| ZS269 (hybrid) | Crop Breeding Institute, ZNBP | 2014 | |
| ZS271 (hybrid) | Crop Breeding Institute, ZNBP | 2014 | |
| ZS273 (hybrid) | Crop Breeding Institute, ZNBP | 2014 | |
| ZS275 (hybrid) | Crop Breeding Institute, ZNBP | 2014 | |
| ZS242 (hybrid) | Crop Breeding Institute, ZNBP | 2015 | |
| SC529 * (hybrid) | Seed Co | 2012 | |
| SC533 * (hybrid) | Seed Co | 2007 | |
| SC543 * (hybrid) | Seed Co | 2009 |
| Site Name | Latitude | Longitude | Soil Type | Rainfall (mm) | Altitude (masl) | Natural Region | Management | Temperature (min~max, °C) |
|---|---|---|---|---|---|---|---|---|
| Chiredzi Research Station | 21°58′ S | 31°17′ E | Vertisols | <450 m | 425 m | IV | Managed heat stress | 12 min~35 max |
| Chiredzi Research Station | 21°58′ S | 31°17′ E | Vertisols | <450 m | 425 m | IV | Managed drought stress | 12 min~35 max |
| Chiredzi Research Station | 21°58′ S | 31°17′ E | Vertisols | <450 m | 425 m | IV | Random drought stress | 12 min~35 max |
| Kadoma Research Station | 18°19′ S | 29°53′ S | Ferralsols | 550–800 | 1149 m | IIB | Optimal | 20 min~28 max |
| Kadoma Research Station | 18°19′ S | 29°53′ S | Ferralsols | 550–800 | 1149 m | IIB | Random drought | 20 min~28 max |
| Art Farm | 17°26′ S | 31°05′ E | Ferralsols | 750–1000 | 1480 m | IIA | Optimal | 18 min~25 max |
| Ratray Arnold Research Station | 17°14′ S | 31°14′ E | Ferralsols | 918 | 1300 m | IIB | Optimal | 12.8 min~28.6 max |
| CIMMYT Harare | 17°49′ S | 31°01′ E | Ferralsols | 750–1000 | 1480 m | IIA | Optimal | 10 min~25 max |
| Bindura | 17°30′ S | 31°32′ S | Ferralsols | 400–600 | 1070 m | IIB | Random drought | 17 min~28 max |
| Muzarabani Estate | 16°33′ S | 31°17′ E | Ferralsols | <450 | 400 m | V | Managed drought stress | 15 min~40 max |
| Traits | Method of Measurement |
|---|---|
| Grain yield (GY) | Shelled grain weight per plot adjusted to 12.5% grain moisture and converted to tons per hectare. |
| Anthesis date (AD) | Number of days from planting to the time when 50% of the tassels of plants shedding pollen. |
| Silking date (SD) | Number of days from planting to the time when 50% of plants have emerged silks. |
| Anthesis silking interval (ASI) h | Calculated as the difference between days to 50% silking and 50% anthesis. |
| Cobs per plant (EPP) | Obtained by dividing the total number of cobs per plot by the number of plants harvested. |
| Number of plants (NP) | The total number of plants harvested per plot. |
| Root lodging (RL) | Percentage of plants that show stems that are inclining by more than 45°. |
| Plant height (PH) | Average height of five randomly selected plants in centimetres from the ground level to the node bearing of the flag leaf using graduated measuring stick. |
| Cob height (EH) | Average height of the upper most cobs of the same plants used for plant height measurement from ground level to the node bearing the cob, measured in cm using graduated measuring stick. |
| Leaf senescence (Sen) | The proportion of dead leaves to that of the whole plant expressed as a percentage using a scale of 1–10. A score of 10 is when 100% or all the leaves have senesced while 1 is when 10% of leaves senesce. |
| Moisture (MOI) | Water content percentage of grain as measured at harvest. |
| Tassel blasting (TB) | Tassel blast is the drying of the complete tassel (or most of it) without pollen extrusion. It will be recorded at 1–2 weeks after tassel emergence. Tassel blast is recorded as the percentage of plants in a plot with tassel blast symptoms. |
| Leaf firing (Lf) | Recorded as the percentage of plants in a plot with leaf symptoms, i.e., leaves burned starting from the top of the plant and progressing downward due to heat stress. This will be recorded 1–2 weeks after anthesis. |
| Across-Site Analysis | Managed Drought Stress | Random Drought Stress | Optimal Management | Managed Heat Stress | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source of variation | DF | MS | DF | MS | DF | MS | DF | MS | DF | MS |
| Site | 9 | 222.8 *** | 1 | 30.2 *** | 2 | 57.4 *** | 2 | 63.7 *** | 1 | 6.3 |
| Replication (Site) | 10 | 3.828 | 2 | 0.2375 | 3 | 5.513 | 3 | 1.23 | 2 | 8.788 *** |
| Block (Replication × Site) | 100 | 3.368 *** | 20 | 1.9615 | 30 | 3.026 | 30 | 5.205 | 20 | 2.5315 |
| Cultivar | 23 | 17.483 *** | 23 | 2.396 | 23 | 4.98 *** | 23 | 16.6 *** | 23 | 2.5349 |
| Cultivar × Site | 207 | 2.422 *** | 23 | 1.610 | 46 | 1.313 | 46 | 3.479 | 23 | 1.090 |
| Heritability | 0.8999 | 0.1326 | 0.7487 | 0.8197 | 0.4669 | |||||
| Genotypic variance | 1.0548 | 0.0795 | 0.797 | 2.8056 | 0.2447 | |||||
| Error variance | 1.1759 | 0.6114 | 1.0434 | 2.1279 | 0.7824 | |||||
| G x E variance | 0.7807 | 0.8368 | 0.281 | 0.7875 | 0.2982 | |||||
| CV | 34.637 | 45.279 | 56.087 | 23.662 | 40.453 | |||||
| LSD | 2.1254 | 1.5325 | 2.002 | 2.8591 | 1.7336 | |||||
| Grand mean | 3.1308 | 1.7268 | 1.8212 | 6.1647 | 2.1865 | |||||
| Minimum | 31 | 0.3 | −0.54 | 0.69 | 0.4 | |||||
| Maximum | 163 | 2.57 | 7.22 | 10.58 | 6.45 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazibuko, P.; Mutengwa, C.; Magorokosho, C.; Kutywayo, D.; Kamutando, C.N. Genetic Gains of Grain Yield among the Maize Cultivars Released over a Century from the National Breeding Program of Zimbabwe. Agronomy 2024, 14, 246. https://doi.org/10.3390/agronomy14020246
Mazibuko P, Mutengwa C, Magorokosho C, Kutywayo D, Kamutando CN. Genetic Gains of Grain Yield among the Maize Cultivars Released over a Century from the National Breeding Program of Zimbabwe. Agronomy. 2024; 14(2):246. https://doi.org/10.3390/agronomy14020246
Chicago/Turabian StyleMazibuko, Purity, Charles Mutengwa, Cosmos Magorokosho, Dumisani Kutywayo, and Casper Nyaradzai Kamutando. 2024. "Genetic Gains of Grain Yield among the Maize Cultivars Released over a Century from the National Breeding Program of Zimbabwe" Agronomy 14, no. 2: 246. https://doi.org/10.3390/agronomy14020246





