The Identification of Significant Single Nucleotide Polymorphisms for Shoot Sulfur Accumulation and Sulfur Concentration Using a Genome-Wide Association Analysis in Wild Soybean Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Planting
2.2. Traits Measurement
2.3. Data Analysis
2.4. Genome-Wide Association Study
2.5. Candidate Gene Prediction and Genetic Diversity Analysis
3. Results
3.1. Shoot Sulfur Concentration and Sulfur Accumulation in Wild Soybean Seedling
3.2. SA Was Significantly Associated with Multiple Traits
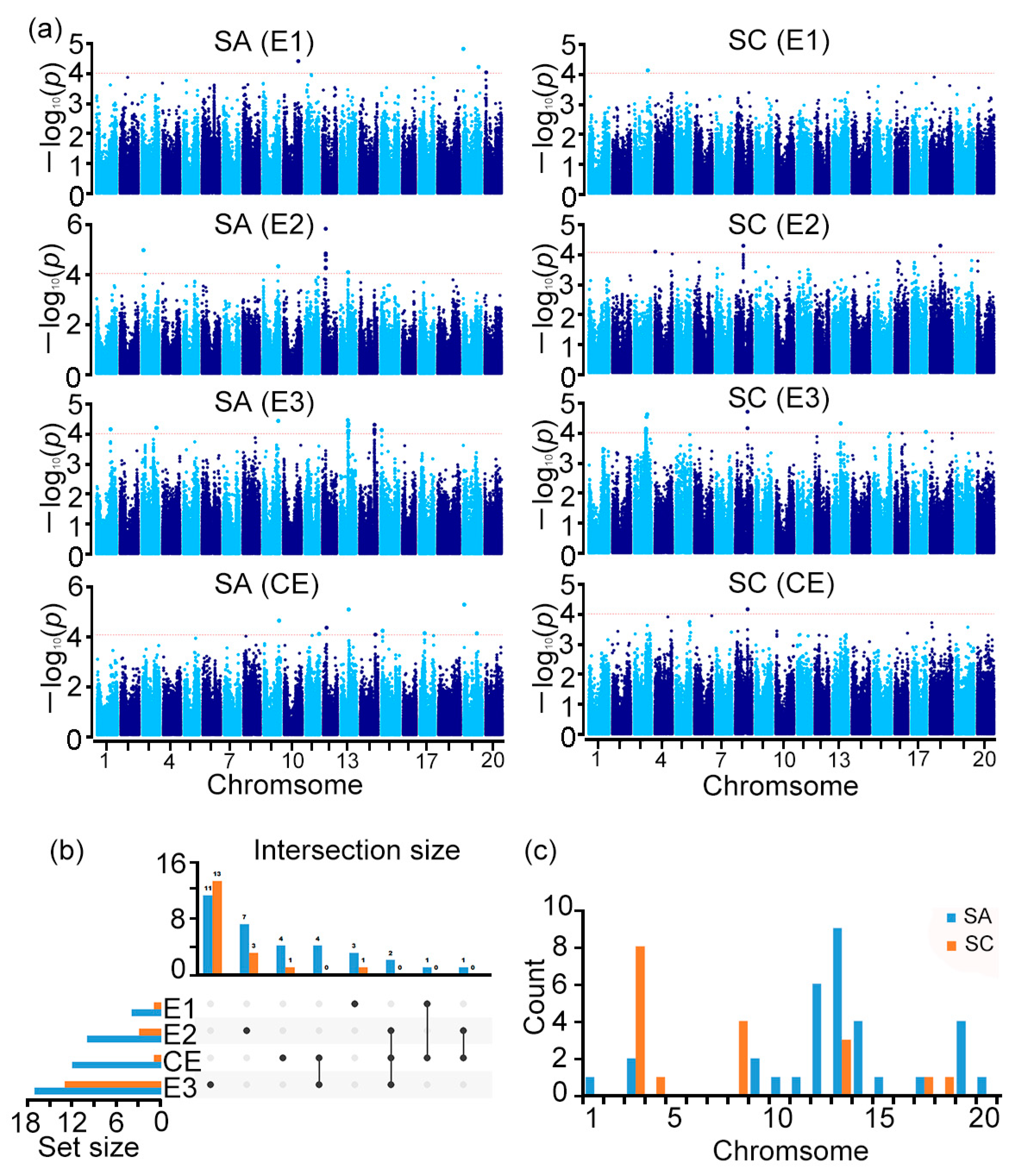
3.3. Six Stable Loci Were Identified by GWAS
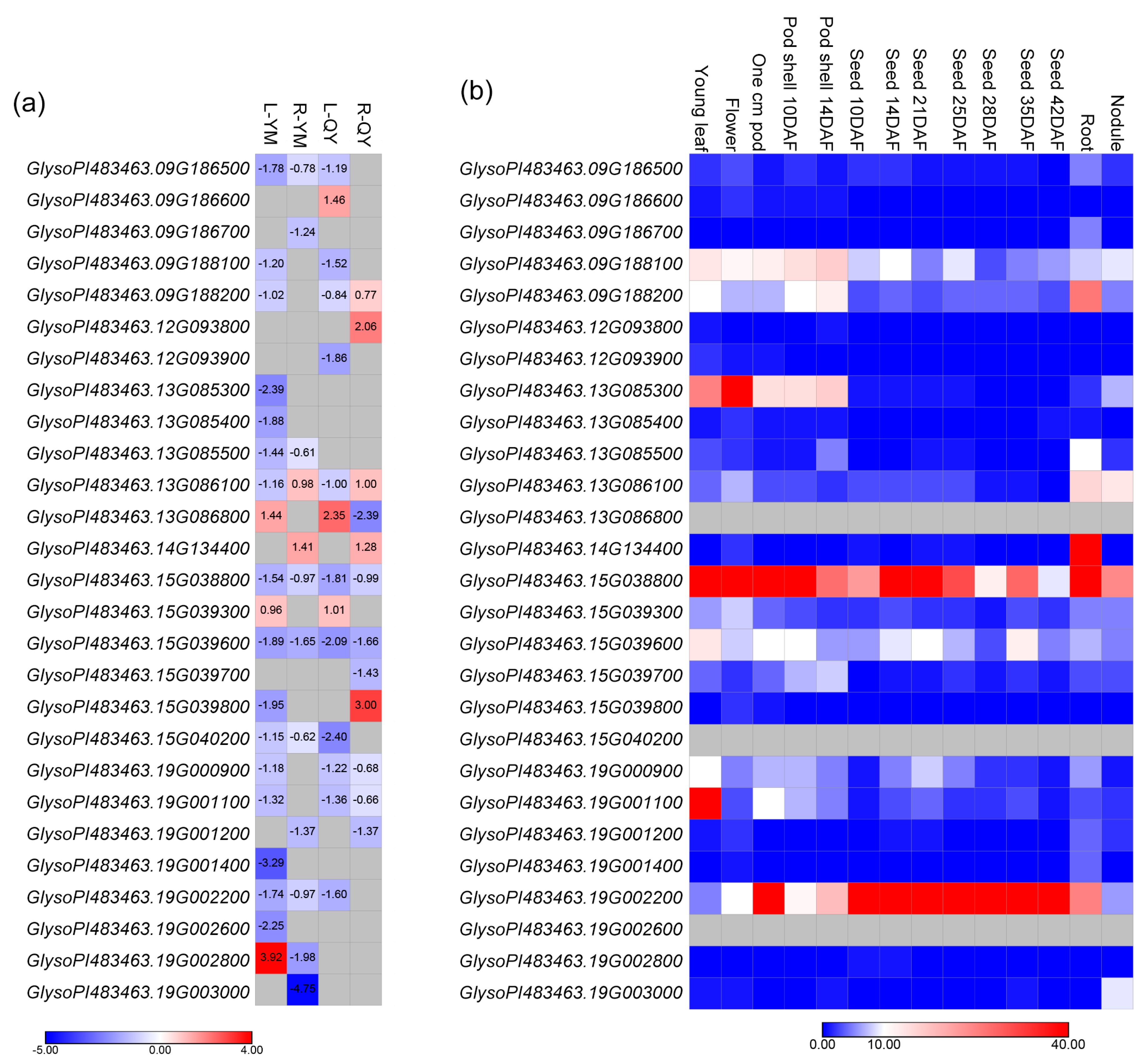
3.4. Candidate Genes
4. Discussion
4.1. Four New Loci Correlated with Shoot Sulfur Accumulation in Wild Soybean
4.2. Candidate Genes Associated with Shoot Sulfur Accumulation
4.3. Ribosomal Genes May Play a Key Role in Sulfur Accumulation in Wild Soybean
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y. Characteristics of soybean requirement to sulphur and effects of sulphur on the physiology of soybean. Heilongjiang Agric. Sci. 1998, 5, 12–15. [Google Scholar]
- Li, J.; Zhang, Y.; Wang, J. Study on the effect of sulfur on the growth and physiology of soybean. Chin. J. Soil. Sci. 2004, 35, 612–616. [Google Scholar]
- Liu, L. Effect of Sulfur on Yields and Quality of Soybean (Glycine max (L.) Merrill). Ph.D. Thesis, Northeast Agricultural University, Harbin, China, 2005. [Google Scholar]
- Wu, M.; Xiao, C. Sulphur nutrition of soybean. Soybean Sci. 1998, 17, 299–304. [Google Scholar]
- Wang, H.; Wu, Z.; Zhang, Y.; Yu, D. Transcriptional expression profiling of soybean genes under sulfur-starved conditions by RNA-seq. Acta Agron. Sin. 2023, 49, 105–118. [Google Scholar]
- Kopriva, S.; Malagoli, M.; Takahashi, H. Sulfur nutrition: Impacts on plant development, metabolism, and stress responses. J. Exp. Bot. 2019, 70, 4069–4073. [Google Scholar] [CrossRef]
- Liu, C.Q.; Cao, S.Q.; Chen, G.A.; Wu, X.J. Sulphur in the agriculture of China. Acta Pedol. Sin. 1990, 27, 398–404. [Google Scholar]
- Qian, X.H.; Yang, P.; Zhou, X.J.; Hu, R.G.; Sun, H.L.; Zhang, Z.K.; Sun, X.J. Current situation and spatial-temporal distribution of soil available sulfur in Anhui province. J. Plant Nutr. Fert. 2018, 24, 1357–1364. [Google Scholar]
- Li, Q.; Gao, Y.; Yang, A. Sulfur homeostasis in plants. Int. J. Mol. Sci. 2020, 21, 8926. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhou, X.; Zuo, L.; Wang, H.; Yu, D. Identification and functional characterization of the sulfate transporter gene GmSULTR1;2b in soybean. BMC Genom. 2016, 17, 373. [Google Scholar] [CrossRef] [PubMed]
- Phartiyal, P.; Kim, W.S.; Cahoon, R.E.; Jez, J.M.; Krishnan, H.B. The role of 5′-adenylylsulfate reductase in the sulfur assimilation pathway of soybean: Molecular cloning, kinetic characterization, and gene expression. Phytochemistry 2008, 69, 356–364. [Google Scholar] [CrossRef]
- Chronis, D.; Krishnan, H.B. Sulfur assimilation in soybean (Glycine max [L.] Merr): Molecular cloning and characterization of a cytosolic isoform of serine acetyltransferase. Planta 2003, 218, 417–426. [Google Scholar] [CrossRef]
- Chronis, D.; Krishnan, H.B. Sulfur assimilation in soybean: Molecular cloning and characterization of O-acetylserine (thiol) lyase (Cysteine synthase). Crop Sci. 2003, 43, 1819–1827. [Google Scholar] [CrossRef]
- Krishnana, H.B.; Jez, J.M. Review: The promise and limits for enhancing sulfur-containing amino acid content of soybean seed. Plant Sci. 2018, 272, 14–21. [Google Scholar] [CrossRef]
- Ramamurthy, R.K.; Jedlicka, J.; Graef, G.L.; Waters, B.M. Identification of new QTLs for seed mineral, cysteine, and methionine concentrations in soybean [Glycine max (L.) Merr.]. Mol. Breed. 2014, 34, 431–445. [Google Scholar] [CrossRef]
- Malle, S.; Morrison, M.; Belzile, F. Identification of loci controlling mineral element concentration in soybean seeds. BMC Plant Biol. 2020, 20, 419. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, G.; Nelson, R.; Granada, S.; Krishnan, H.B.; Gillman, J.D.; Baxter, I. Genomewide association study of ionomic traits on diverse soybean populations from germplasm collections. Plant Direct. 2018, 2, e00033. [Google Scholar] [CrossRef] [PubMed]
- Dhanapal, A.P.; Ray, J.D.; Smith, J.R.; Purcell, L.C.; Fritschi, F.B. Identification of novel genomic loci associated with soybean shoot tissue macro- and micronutrient concentrations. Plant Genome 2018, 11, 170066. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Zhou, G.; Ma, J.; Jiang, W.; Jin, L.G.; Zhang, Z.; Guo, Y.; Zhang, J.; Sui, Y.; Zheng, L.; et al. De novo assembly of soybean wild relatives for pan-genome analysis of diversity and agronomic traits. Nat. Biotechnol. 2014, 32, 1045–1052. [Google Scholar] [CrossRef]
- Liu, Y.; Du, H.; Li, P.; Shen, Y.; Peng, H.; Liu, S.; Zhou, G.A.; Zhang, H.; Liu, Z.; Shi, M.; et al. Pan-genome of wild and cultivated soybeans. Cell 2020, 182, 162–176. [Google Scholar] [CrossRef]
- Wang, J.; Chu, S.S.; Zhang, H.R.; Zhu, Y.; Cheng, H.; Yu, D. Development and application of a novel genome-wide SNP array reveals domestication history in soybean. Sci. Rep. 2016, 6, 20728. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J.; Yang, Y.; Du, W.; Zhang, D.; Yu, D.; Cheng, H. A genome-wide expression profile analysis reveals active genes and pathways coping with phosphate starvation in soybean. BMC Genom. 2016, 17, 192. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.J.; Chang, R.Z. Descriptors and Data Standard for Soybean (Glycine spp.); China Agriculture Press Co., Ltd.: Beijing, China, 2006; pp. 54–63. [Google Scholar]
- Ziegler, G.; Terauchi, A.; Becker, A.; Armstrong, P.; Hudson, K.; Baxter, I. Ionomic screening of field-grown soybean identifies mutants with altered seed elemental composition. Plant Genome 2013, 6, 1–9. [Google Scholar] [CrossRef]
- Chea, L.; Meijide, A.; Meinen, C.; Pawelzik, E.; Naumann, M. Cultivar-dependent responses in plant growth, leaf physiology, phosphorus use efficiency, and tuber quality of potatoes under limited phosphorus availability conditions. Front. Plant Sci. 2021, 12, 723862. [Google Scholar] [CrossRef] [PubMed]
- Lipka, A.E.; Tian, F.; Wang, Q.; Peiffer, J.; Li, M.; Bradbury, P.J.; Gore, M.A.; Buckler, E.S.; Zhang, Z. GAPIT: Genome association and prediction integrated tool. Bioinformatics 2012, 28, 2397–2399. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, H.; Tang, Z.; Xu, J.; Yin, D.; Zhang, Z.; Yuan, X.; Zhu, M.; Zhao, S.; Li, X.; et al. rMVP: A memory-efficient, visualization-enhanced, and parallel-accelerated tool for genome-wide association study. Genom. Proteom. Bioinform. 2021, 19, 619–628. [Google Scholar] [CrossRef]
- Conway, J.R.; Lex, A.; Gehlenborg, N. UpSetR: An R package for the visualization of intersecting sets and their properties. Bioinformatics 2017, 33, 2938–2940. [Google Scholar] [CrossRef]
- Severin, A.J.; Woody, J.L.; Bolon, Y.T.; Joseph, B.; Diers, B.W.; Farmer, A.D.; Muehlbauer, G.J.; Nelson, R.T.; Grant, D.; Specht, J.E.; et al. RNA-Seq Atlas of Glycine max: A guide to the soybean transcriptome. BMC Plant Biol. 2010, 10, 160. [Google Scholar] [CrossRef]
- Zhang, D.; Li, H.; Wang, J.; Zhang, H.; Hu, Z.; Chu, S.; Lv, H.; Yu, D. High-density genetic mapping identifies new major loci for tolerance to low-phosphorus stress in soybean. Front. Plant Sci. 2016, 7, 372. [Google Scholar] [CrossRef]
- Lin, S.; Cianzio, S.; Shoemaker, R. Mapping genetic loci for iron deficiency chlorosis in soybean. Mol. Breed. 1997, 3, 219–229. [Google Scholar] [CrossRef]
- Lin, S.F.; Grant, D.; Cianzio, S.R.; Shoemaker, R.C. Molecular characterization of iron deficiency chlorosis in soybean. J. Plant Nutr. 2000, 23, 1929–1939. [Google Scholar] [CrossRef]
- Liang, Q.; Cheng, X.; Mei, M.; Yan, X.; Liao, H. QTL analysis of root traits as related to phosphorus efficiency in soybean. Ann. Bot. 2010, 106, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Charlson, D.V.; Bailey, T.B.; Cianzio, S.R.; Shoemaker, R.C. Molecular marker Satt481 is associated with iron-deficiency chlorosis resistance in a soybean breeding population. Crop Sci. 2005, 45, 2394–2399. [Google Scholar] [CrossRef]
- Yu, L.H.; Miao, Z.Q.; Qi, G.F.; Wu, J.; Cai, X.T.; Mao, J.L.; Xiang, C.B. MADS-Box transcription factor AGL21 regulates lateral root development and responds to multiple external and physiological signals. Mol. Plant 2014, 7, 1653–1669. [Google Scholar] [CrossRef]
- Huang, S.; Liang, Z.; Chen, S.; Sun, H.; Fan, X.; Wang, C.; Xu, G.; Zhang, Y.A. Transcription factor, OsMADS57, regulates long-distance nitrate transport and root elongation. Plant Physiol. 2019, 180, 882–895. [Google Scholar] [CrossRef]
- Xing, Y.; Wang, N.; Zhang, T.; Zhang, Q.; Du, D.; Chen, X.; Lu, X.; Zhang, Y.; Zhu, M.; Liu, M.; et al. SHORT-ROOT 1 is critical to cell division and tracheary element development in rice roots. Plant J. 2021, 105, 1179–1191. [Google Scholar] [CrossRef]
- Tu, B.; Hu, L.; Chen, W.; Li, T.; Hu, B.; Zheng, L.; Lv, Z.; You, S.; Wang, Y.; Ma, B.; et al. Disruption of OsEXO70A1 causes irregular vascular bundles and perturbs mineral nutrient assimilation in rice. Sci. Rep. 2015, 5, 18609. [Google Scholar] [CrossRef]
- Astolfi, S.; Zuchi, S.; Cesco, S.; Di Toppi, L.S.; Pirazzi, D.; Badiani, M.; Varanini, Z.; Pinton, R. Iron deficiency induces sulfate uptake and modulates redistribution of reduced sulfur pool in barley plants. Funct. Plant Biol. 2006, 33, 1055–1061. [Google Scholar] [CrossRef]
- Ciaffi, M.; Paolacci, A.R.; Celletti, S.; Catarcione, G.; Kopriva, S.; Astolfi, S. Transcriptional and physiological changes in the S assimilation pathway due to single or combined S and Fe deprivation in durum wheat (Triticum durum L.) seedlings. J. Exp. Bot. 2013, 64, 1663–1675. [Google Scholar] [CrossRef]
- Paolacci, A.R.; Celletti, S.; Catarcione, G.; Hawkesford, M.J.; Astolfi, S.; Ciaffi, M. Iron deprivation results in a rapid but not sustained increase of the expression of genes involved in iron metabolism and sulfate uptake in tomato (Solanum lycopersicum L.) seedlings. J. Integr. Plant Biol. 2014, 56, 88–100. [Google Scholar] [CrossRef]
- Burow, M.; Halkier, B.A. How does a plant orchestrate defense in time and space? Using glucosinolates in Arabidopsis as case study. Curr. Opin. Plant Biol. 2017, 38, 142–147. [Google Scholar] [CrossRef]
- Chan, K.X.; Phua, S.Y.; Van Breusegem, F. Secondary sulfur metabolism in cellular signalling and oxidative stress responses. J. Exp. Bot. 2019, 70, 4237–4250. [Google Scholar] [CrossRef]
- Del Carmen Martínez-Ballesta, M.; Moreno, D.A.; Carvajal, M. The physiological importance of glucosinolates on plant response to abiotic stress in Brassica. Int. J. Mol. Sci. 2013, 14, 11607–11625. [Google Scholar] [CrossRef]
- Ristova, D.; Kopriva, S. Sulfur signaling and starvation response in Arabidopsis. iScience 2022, 25, 104242. [Google Scholar] [CrossRef]
- Ahmad, N.; Malagoli, M.; Wirtz, M.; Hell, R. Drought stress in maize causes differential acclimation responses of glutathione and sulfur metabolism in leaves and roots. BMC Plant Biol. 2016, 16, 247. [Google Scholar] [CrossRef]
- Barakat, A.; Szick-Miranda, K.; Chang, I.F.; Guyot, R.; Blanc, G.; Cooke, R.; Delseny, M.; Bailey-Serres, J. The organization of cytoplasmic ribosomal protein genes in the Arabidopsis genome. Plant Physiol. 2001, 127, 398–415. [Google Scholar] [CrossRef]
- Luo, A.; Zhan, H.; Zhang, X.; Du, H.; Zhang, Y.; Peng, X. Cytoplasmic ribosomal protein L14B is essential for fertilization in Arabidopsis. Plant Sci. 2020, 292, 110394. [Google Scholar] [CrossRef]
- Sha, A.H.; Chen, Y.H.; Shan, Z.H.; Zhang, X.J.; Wu, X.J.; Qiu, D.Z.; Zhou, X.A. Identification of photoperiod-regulated gene in soybean and functional analysis in Nicotiana benthamiana. J. Genet. 2014, 93, 43–51. [Google Scholar]
- Zhang, J.; Yuan, H.; Yang, Y.; Fish, T.; Lyi, S.M.; Thannhauser, T.W.; Zhang, L.; Li, L. Plastid ribosomal protein S5 is involved in photosynthesis, plant development, and cold stress tolerance in Arabidopsis. J. Exp. Bot. 2016, 67, 2731–2744. [Google Scholar] [CrossRef]
- Dong, X.; Duan, S.; Wang, H.B.; Jin, H.L. Plastid ribosomal protein LPE2 is involved in photosynthesis and the response to C/N balance in Arabidopsis thaliana. J. Integr. Plant Biol. 2020, 62, 1418–1432. [Google Scholar] [CrossRef] [PubMed]
- Tarnowski, L.; Rodriguez, M.C.; Brzywczy, J.; Cysewski, D.; Wawrzynska, A.; Sirko, A. Overexpression of the selective autophagy cargo receptor NBR1 modifies plant response to sulfur deficit. Cells 2020, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Z.; Yuan, W.; Kan, G.; Huang, F.; Yu, D.; Wang, H. Research on the regulation effect of ribosomal gene GmRPL12 on low sulfur tolerance in soybean. Soybean Sci. 2020, 39, 518–526. [Google Scholar]
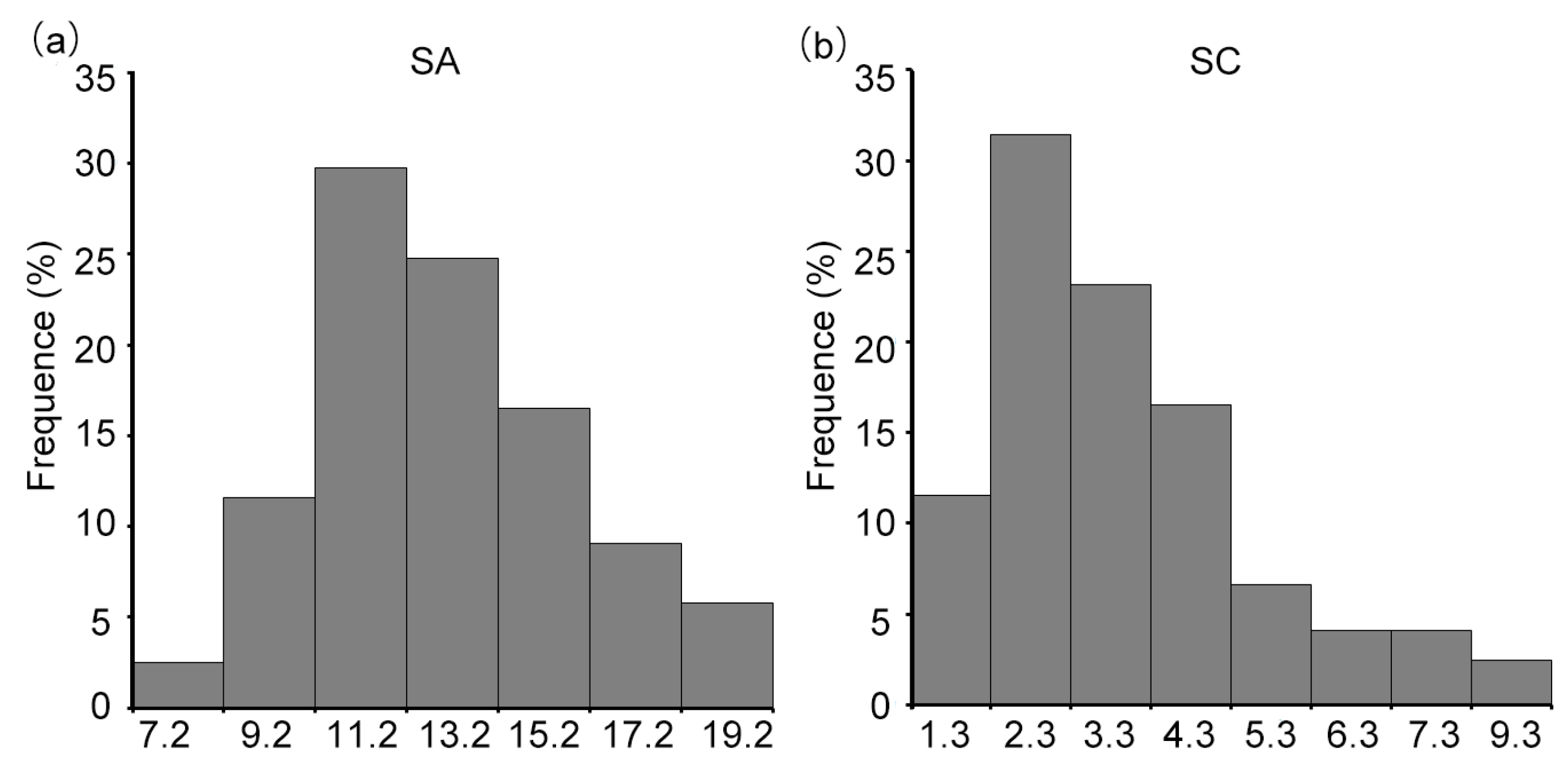

| Trait | Env. | Max. | Min. | Mean | SD | CV (%) | G | G × E | H2 (%) |
|---|---|---|---|---|---|---|---|---|---|
| SA | E1 | 9.83 mg | 0.94 mg | 3.80 mg | 2.00 | 52.65 | *** | *** | 68.01 |
| E2 | 9.21 mg | 0.42 mg | 2.15 mg | 1.29 | 60.04 | ||||
| E3 | 14.75 mg | 0.54 mg | 4.32 mg | 2.88 | 66.79 | ||||
| SC | E1 | 25.23 mg/g | 5.26 mg/g | 13.25 mg/g | 3.70 | 27.95 | *** | *** | 38.23 |
| E2 | 27.84 mg/g | 4.41 mg/g | 10.82 mg/g | 3.29 | 30.41 | ||||
| E3 | 40.73 mg/g | 6.03 mg/g | 15.20 mg/g | 7.56 | 49.74 |
| Traits | SA | SC |
|---|---|---|
| PH | 0.19 * 0.04 | −0.063 0.49 |
| RL | 0.32 ** 0.0004 | −0.09 0.28 |
| FWS | 0.88 ** <0.0001 | 0.10 0.27 |
| FWR | 0.79 ** <0.0001 | 0.16 0.08 |
| DWS | 0.86 ** <0.0001 | 0.02 0.79 |
| DWR | 0.79 ** <0.0001 | −0.01 0.95 |
| SC | 0.46 ** <0.0001 |
| QTL | Representative SNP | Chr. | Position (bp) a | Number of Significant SNPs | Env. | Genes Containing the SNP | Neighbor/Overlapping QTL |
|---|---|---|---|---|---|---|---|
| qSA9 | AX-94068640 | 9 | 43,282,109 | 2 | E2/E3/CE | GlysoPI483463.09G187200 | Shoot S 1-g8 [18]; Shoot K 1-g19 [18]; Acid phosphatase activity, variable P [30] |
| qSA12 | AX-93799340 | 12 | 9,828,900 | 3 | E2/CE | Fe effic 8-3, 4-3, 11-3 [31,32] | |
| qSA13 | AX-94108622 | 13 | 22,470,359 | 2 | E3/CE | GlysoPI483463.13G085900 | Shoot S 1-g22 [18], shoot P [33]; shoot P 1-3 [18]; Root weight, dry, variable P [30] |
| qSA14 | AX-94127410 | 14 | 42,849,138 | 1 | E3/CE | Root weight, dry, variable P 1-16 [30]; Fe effic 3-1, 10-3 [31,32] | |
| qSA15 | AX-93664677 | 15 | 3,334,704 | 1 | E3/CE | GlysoPI483463.15G039600 | P use efficiency, variable P 1-4, 1-6, 1-10 [30] |
| qSA19 | AX-94183129 | 19 | 144,267 | 1 | E1/CE | GlysoPI483463.19G132700 | Fe effic 13-2 [34]; |
| No. | Gene ID in G. soja v1.1 | GO Biological Process Descriptions |
|---|---|---|
| 1 | GlysoPI483463.09G186500 | Trehalose biosynthetic process; crop yield; Improving photosynthesis |
| 2 | GlysoPI483463.09G186600 | Sucrose metabolic process; response to water deprivation; response to bacterium; response to salt stress; response to abscisic acid; abscisic acid-activated signaling pathway; cellular response to gibberellin stimulus |
| 3 | GlysoPI483463.09G186700 | Multicellular organism development; flower development; vegetative to reproductive phase transition of meristem; regulation of growth; positive regulation of transcription by RNA polymerase II; root development |
| 4 | GlysoPI483463.09G188100 | Spliceosomal snrnp assembly; chloride transport; cell volume homeostasis |
| 5 | GlysoPI483463.09G188200 | Chloroplast organization; chromoplast organization; carotene biosynthetic process; protein stabilization; positive regulation of carotenoid biosynthetic process; resistance to TYLCV; response to biotic and abiotic stresses |
| 6 | GlysoPI483463.12G093800 | Response to cold; cell differentiation; positive regulation of transcription, DNA-templated; response to freezing |
| 7 | GlysoPI483463.12G093900 | Regulation of cyclin-dependent protein serine/threonine kinase activity; mitotic cell cycle phase transition; cell division |
| 8 | GlysoPI483463.13G085300 | Biological process; MAPK phosphatase |
| 9 | GlysoPI483463.13G085400 | Biological process |
| 10 | GlysoPI483463.13G085500 | Purine nucleoside catabolic process; response to drought |
| 11 | GlysoPI483463.13G086100 | Triglyceride biosynthetic process; plant development |
| 12 | GlysoPI483463.13G086800 | Transmembrane transport; Fe homeostasis; Cd-responsive gene; respond to drought, cadmium, and salt stresses |
| 13 | GlysoPI483463.14G134400 | Unknown |
| 14 | GlysoPI483463.15G038800 | Ribosomal large subunit assembly; translation; response to oxidative stress; response to cold; response to high light intensity; tolerance to drought and salt stresses |
| 15 | GlysoPI483463.15G039300 | Seed germination; root development |
| 16 | GlysoPI483463.15G039600 | Maturation of LSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA); translation |
| 17 | GlysoPI483463.15G039700 | Exocytosis; protein transport; cell division to polar growth; response to biotic/abiotic stresses; seed development; defense response (the soybean cyst nematode); root development |
| 18 | GlysoPI483463.15G039800 | Fatty acid biosynthetic process; cuticular wax formation; late stage of leaf and flower development |
| 19 | GlysoPI483463.15G040200 | Unknown |
| 20 | GlysoPI483463.19G000900 | Unknown |
| 21 | GlysoPI483463.19G001100 | Translation; ribosome assembly |
| 22 | GlysoPI483463.19G001200 | Formation of translation preinitiation complex; formation of cytoplasmic translation initiation complex; cold temperatures and freezing tolerance; regulating cell division, cell growth, and cell death; anti-virus |
| 23 | GlysoPI483463.19G001400 | Cellular response to hypoxia |
| 24 | GlysoPI483463.19G002200 | Lipid transport; response to disease and salt stress; tolerance to abiotic and biotic stress |
| 25 | GlysoPI483463.19G002600 | Carbohydrate metabolic process; phosphorylation |
| 26 | GlysoPI483463.19G002800 | Regulation of transcription, DNA-templated; regulate nitrogen deficiency-induced leaf senescence; response to Pi deficiency; affects grain nutrient concentrations; promote lateral root number |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhang, Y.; Chen, Y.; Ren, K.; Chen, J.; Kan, G.; Yu, D. The Identification of Significant Single Nucleotide Polymorphisms for Shoot Sulfur Accumulation and Sulfur Concentration Using a Genome-Wide Association Analysis in Wild Soybean Seedlings. Agronomy 2024, 14, 292. https://doi.org/10.3390/agronomy14020292
Wang H, Zhang Y, Chen Y, Ren K, Chen J, Kan G, Yu D. The Identification of Significant Single Nucleotide Polymorphisms for Shoot Sulfur Accumulation and Sulfur Concentration Using a Genome-Wide Association Analysis in Wild Soybean Seedlings. Agronomy. 2024; 14(2):292. https://doi.org/10.3390/agronomy14020292
Chicago/Turabian StyleWang, Hui, Yu’e Zhang, Yudan Chen, Kaili Ren, Jialuo Chen, Guizhen Kan, and Deyue Yu. 2024. "The Identification of Significant Single Nucleotide Polymorphisms for Shoot Sulfur Accumulation and Sulfur Concentration Using a Genome-Wide Association Analysis in Wild Soybean Seedlings" Agronomy 14, no. 2: 292. https://doi.org/10.3390/agronomy14020292
APA StyleWang, H., Zhang, Y., Chen, Y., Ren, K., Chen, J., Kan, G., & Yu, D. (2024). The Identification of Significant Single Nucleotide Polymorphisms for Shoot Sulfur Accumulation and Sulfur Concentration Using a Genome-Wide Association Analysis in Wild Soybean Seedlings. Agronomy, 14(2), 292. https://doi.org/10.3390/agronomy14020292






