Variation in Nutritional Value of Diverse Wheat Genotypes
Abstract
1. Introduction
2. Materials and Methods
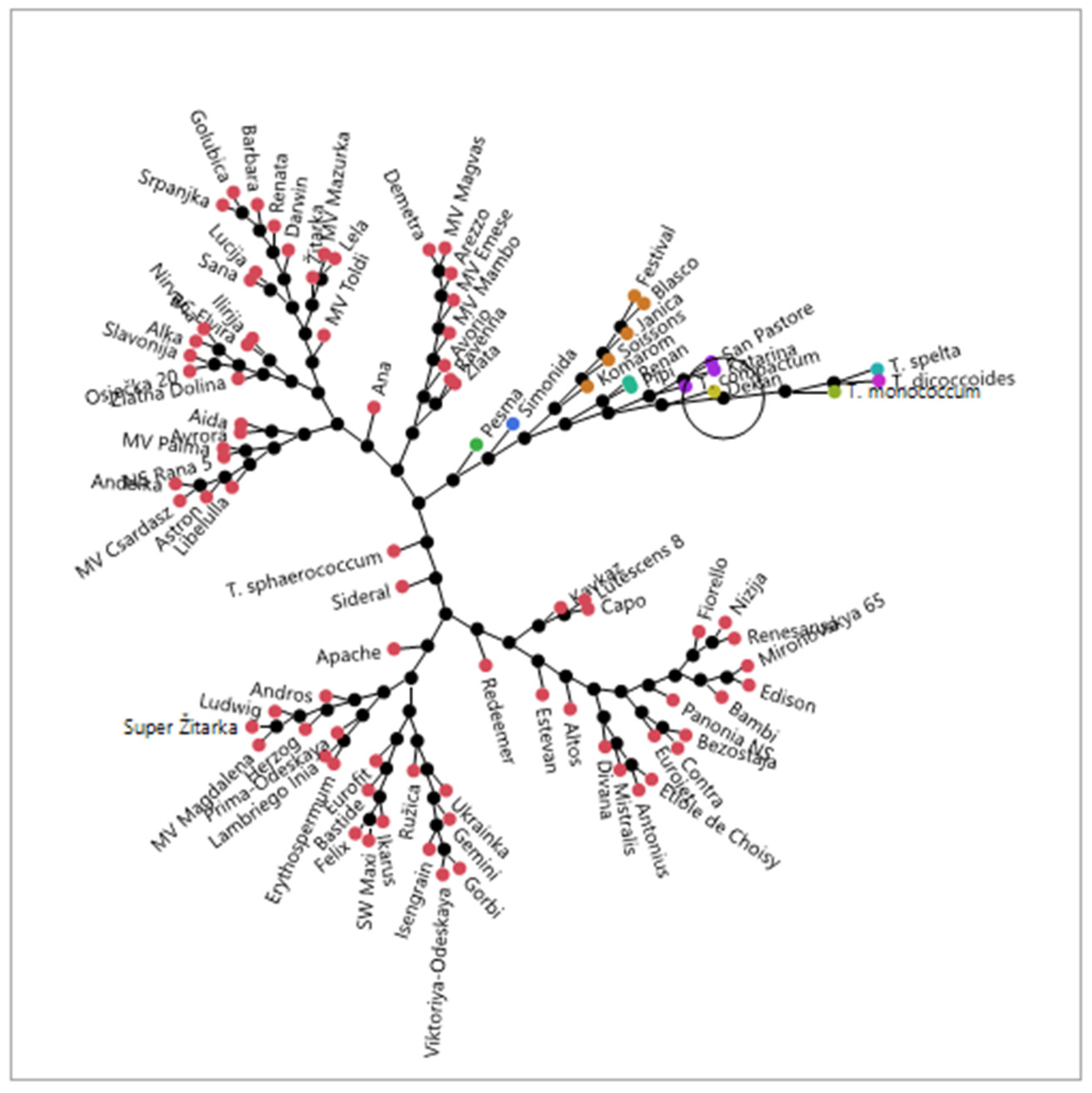
2.1. Germplasm
2.2. Determination of Mg, Fe, and Zn Concentration in Grain and Wheatgrass Juice
2.3. Determination of Protein Concentration in Grain and Wheatgrass Juice
2.4. Determination of Phytic Acid in Wheat Grain
2.5. Statistical Analysis
3. Results
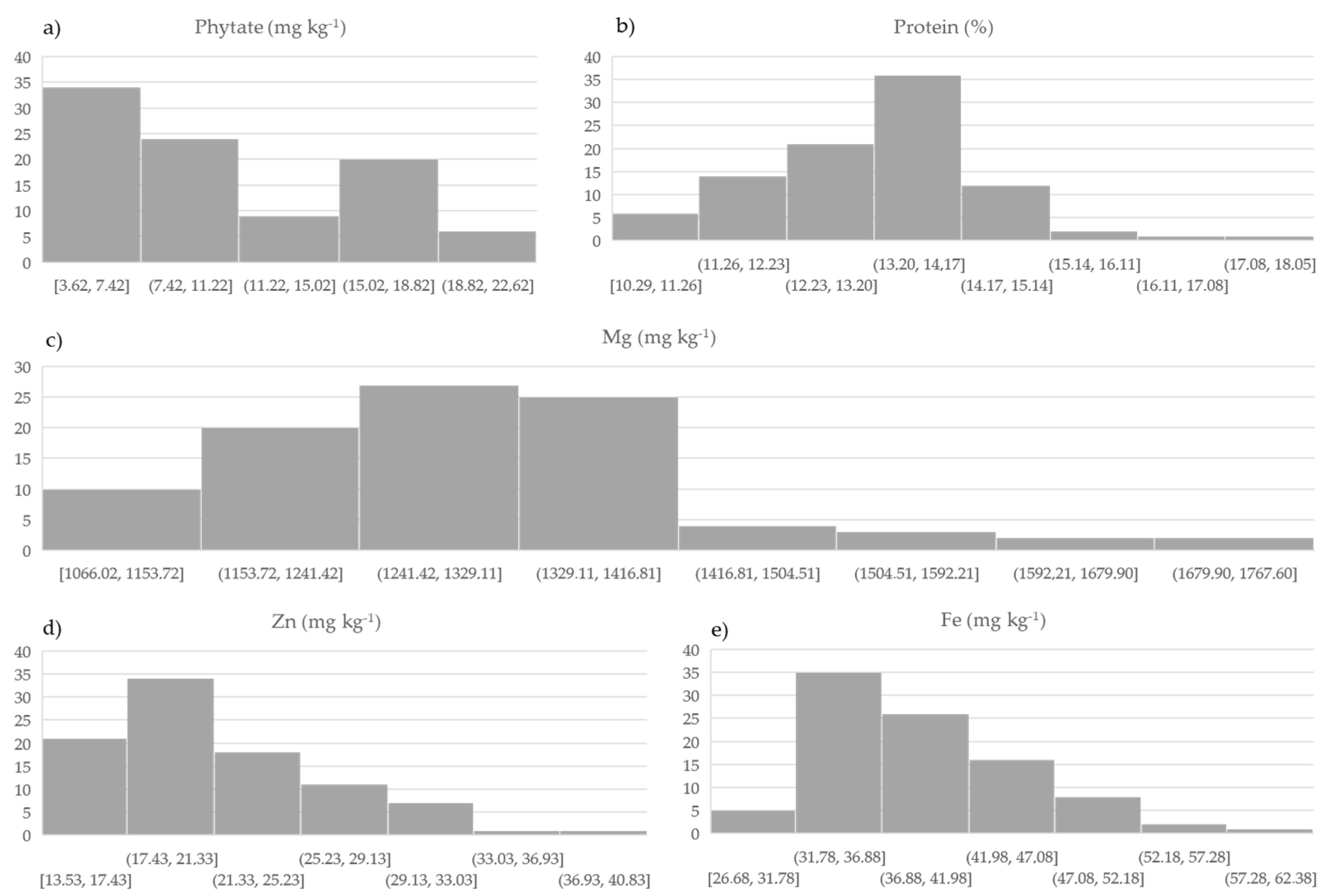
3.1. Variation in Protein, Phytate, Mg, Fe, and Zn in Grain
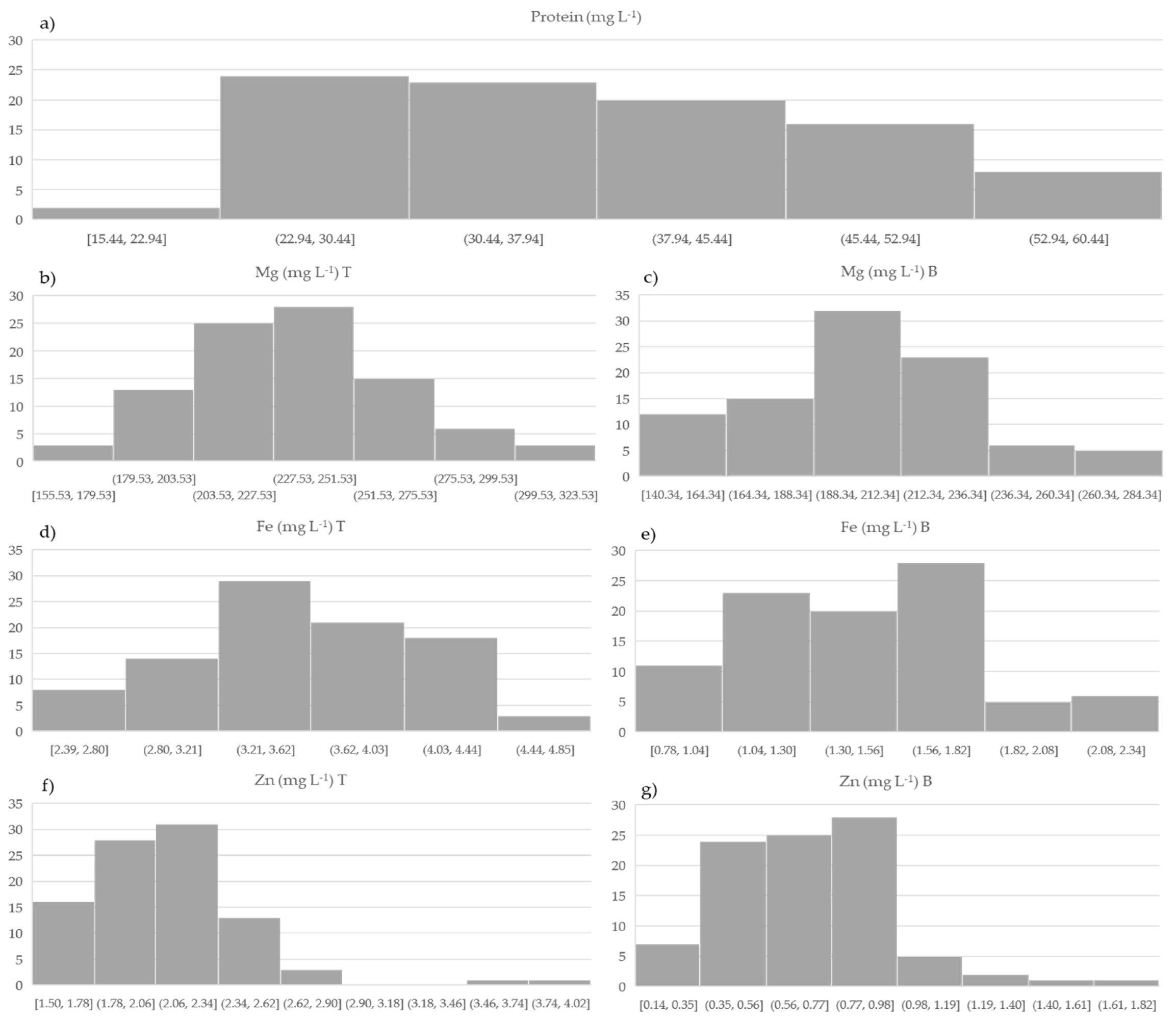
3.2. Variation in Total Protein and Total and In Vitro Bioavailable Concentration of Mg, Fe, and Zn in Wheatgrass Juice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. Food and Agriculture Organization of the United Nations FAOSTAT Database; FAOSTAT: Rome, Italy, 2021. [Google Scholar]
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Sec. 2015, 4, 178–202. [Google Scholar] [CrossRef] [PubMed]
- Padalia, S.; Drabu, S.; Raheja, I.; Gupta, A.; Dhamija, M. Multitude potential of wheatgrass juice (Green Blood): An overview. Chron. Young Sci. 2010, 1, 23–28. [Google Scholar]
- Sharma, S.; Shrivastav, V.K.; Shrivastav, A.; Shrivastav, B.R. Therapeutic potential of wheat grass (Triticum aestivum L.) for the treatmentof chronic diseases. South Asian J. Exp. Biol. 2013, 3, 308–313. [Google Scholar] [CrossRef]
- Mujoriya, R.; Bodla, R.B. A study on wheat grass and its nutritional value. Food Sci. Qual. Manag. 2011, 2, 1–8. [Google Scholar]
- Mathur, S.; Mathur, R.; Kohli, G.K. Therapeutic use of wheat grass juice for the treatment of anemia in young women of Ajmer city (Rajasthan, India). Int. J. Nutr. Sci. 2017, 2, 1014–1018. [Google Scholar]
- Lakshmeesha, D.R. Study to Assess the Efficacy of Wheatgrass Juice Therapy Intervention on Haemoglobin Level in Adolescent Anaemic Females. Indo Glob. J. Pharm. Sci. 2022, 12, 30–35. [Google Scholar] [CrossRef]
- Ben-Arye, E.; Goldin, E.; Wengrower, D.; Stamper, A.; Kohn, R.; Berry, E. Wheat grass juice in the treatment of active distal ulcerative colitis: A randomized double-blind placebo-controlled trial. Scand. J. Gastroenterol. 2002, 37, 444–449. [Google Scholar] [CrossRef]
- Bar-Sela, G.; Tsalic, M.; Fried, G.; Goldberg, H. Wheat grass juice may improve hematological toxicity related to chemotherapy in breast cancer patients: A pilot study. Nutr. Cancer 2007, 58, 43–48. [Google Scholar] [CrossRef]
- Avisar, A.; Cohen, M.; Brenner, B.; Bronshtein, T.; Machluf, M.; Bar-Sela, G.; Aharon, A. Extracellular Vesicles Reflect the Efficacy of Wheatgrass Juice Supplement in Colon Cancer Patients During Adjuvant Chemotherapy. Front. Oncol. 2020, 10, 1659. [Google Scholar] [CrossRef]
- Fatiukha, A.; Filler, N.; Lupo, I.; Lidzbarsky, G.; Klymiuk, V.; Korol, A.B.; Krugman, T. Grain protein content and thousand kernel weight QTLs identified in a durum× wild emmer wheat mapping population tested in five environments. Theor. Appl. Genet. 2019, 133, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.H.; Sun, D.; Nevo, E. Domestication evolution, genetics and genomics in wheat. Mol. Breed. 2011, 28, 281–301. [Google Scholar] [CrossRef]
- Longin, C.F.H.; Würschum, T. Back to the future–tapping into ancient grains for food diversity. Trends Plant Sci. 2016, 21, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Garvin, D.F.; Welch, R.M.; Finley, J.W. Historical shifts in the seed mineral micronutrient concentration of US hard red winter wheat germplasm. J. Sci. Food Agric. 2006, 86, 2213–2220. [Google Scholar] [CrossRef]
- Zhao, F.J.; Su, Y.H.; Dunham, S.J.; Rakszegi, M.; Bedo, Z.; McGrath, S.P.; Shewry, P.R. Variation in mineral micronutrient concentrations in grain of wheat lines of diverse origin. J. Cereal Sci. 2009, 49, 290–295. [Google Scholar] [CrossRef]
- Pandey, A.; Khan, M.K.; Hakki, E.E.; Thomas, G.; Hamurcu, M.; Gezgin, S.; Gizlenci, O.; Akkaya, M.S. Assessment of genetic variability for grain nutrients from diverse regions: Potential for wheat improvement. SpringerPlus 2016, 5, 1912. [Google Scholar] [CrossRef]
- Marcos-Barbero, E.L.; Pérez, P.; Martínez-Carrasco, R.; Arellano, J.B.; Morcuende, R. Genotypic variability on grain yield and grain nutritional quality characteristics of wheat grown under elevated CO2 and high temperature. Plants 2021, 10, 1043. [Google Scholar] [CrossRef]
- Thakur, N.; Raigond, P.; Singh, Y.; Mishra, T.; Singh, B.; Lal, M.K.; Dutt, S. Recent updates on bioaccessibility of phytonutrients. Trends Food Sci. Technol. 2020, 97, 366–380. [Google Scholar] [CrossRef]
- Grubišić, S.; Orkić, V.; Guberac, S.; Lisjak, M.; Petrović, S.; Rebekić, A. Optimalan način sjetve pšenice (Triticum aestivum L.) za uzgoj pšenične trave. Poljoprivreda 2019, 25, 31–37. [Google Scholar] [CrossRef]
- Grubišić, S.; Kristić, M.; Lisjak, M.; Mišković Špoljarić, K.; Petrović, S.; Vila, S.; Rebekić, A. Effect of wheatgrass juice on nutritional quality of apple, carrot, beet, orange and lemon juice. Foods 2022, 11, 445. [Google Scholar] [CrossRef] [PubMed]
- Kingston, H.M.; Jassie, L.B. Microwave energy for acid decomposition at elevated temperatures and pressures using biological and botanical samples. Anal. Chem. 1986, 58, 2534–2541. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Haugh, W.; Lantzsch, H.J. Sensitive method for the rapid determination of phytate in cereals and cereal products. J. Sci. Food Agric. 1983, 34, 1423–1426. [Google Scholar] [CrossRef]
- Fischer, T.R.A. History of wheat breeding: A personal view. In Wheat Improvement: Food Security in a Changing Climate; Reynolds, M.P., Braun, H.J., Eds.; Springer: Cham, Switzerland, 2022; pp. 17–30. [Google Scholar]
- Dubcovsky, J.; Lijavetzky, D.; Appendino, L.; Tranquilli, G. Comparative RFLP mapping of Triticum monococcum genes controlling vernalization requirement. Theor. Appl. Genet. 1998, 97, 968–975. [Google Scholar] [CrossRef]
- Sharma, R.; Crossa, J.; Ataei, N.; Lodin, R.; Joshi, A.K.; Vargas, M.; Braun, H.J.; Singh, R.P.; Bentley, A.R. Plant breeding increases spring wheat yield potential in Afghanistan. Crop Sci. 2021, 62, 167–177. [Google Scholar] [CrossRef]
- Govindan, V.; Atanda, S.; Singh, R.P.; Huerta-Espino, J.; Crespo-Herrera, L.A.; Juliana, P.; Mondal, S.; Joshi, A.K.; Bentley, A.R. Breeding increases grain yield, zinc, and iron, supporting enhanced wheat biofortification. Crop Sci. 2022, 62, 1912–1925. [Google Scholar] [CrossRef]
- Alomari, D.Z.; Eggert, K.; Von Wirén, N.; Polley, A.; Plieske, J.; Ganal, M.W.; Liu, F.; Pillen, K.; Röder, M.S. Whole-genome association mapping and genomic prediction for iron concentration in wheat grains. Int. J. Mol. Sci. 2018, 20, 76. [Google Scholar] [CrossRef] [PubMed]
- Amiri, R.; Bahraminejad, S.; Cheghamirza, K.; Arzani, A. Genetic analysis of iron and zinc concentrations in bread wheat grains. J. Cereal Sci. 2020, 95, 103077. [Google Scholar] [CrossRef]
- Zhang, H.; Mittal, N.; Leamy, L.J.; Barazani, O.; Song, B.H. Back into the wild—Apply untapped genetic diversity of wild relatives for crop improvement. Evol. Appl. 2017, 10, 5–24. [Google Scholar] [CrossRef]
- Çakmak, İ.; Torun, A.Y.F.E.R.; Millet, E.; Feldman, M.; Fahima, T.; Korol, A.; Nevo, H.J.; Özkan, H. Triticum dicoccoides: An important genetic resource for increasing zinc and iron concentration in modern cultivated wheat. Soil Sci. Plant Nutr. 2004, 50, 1047–1054. [Google Scholar] [CrossRef]
- Monasterio, I.; Graham, R.D. Breeding for trace minerals in wheat. Food Nutr. Bull. 2000, 21, 392–396. [Google Scholar] [CrossRef]
- Uauy, C.; Distelfeld, A.; Fahima, T.; Blechl, A.; Dubcovsky, J. A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science 2006, 314, 1298–1301. [Google Scholar] [CrossRef] [PubMed]
- Erba, D.; Hidalgo, A.; Bresciani, J.; Brandolini, A. Environmental and genotypic influences on trace element and mineral concentrations in whole meal flour of einkorn (Triticum monococcum L. subsp. monococcum). J. Cereal Sci. 2011, 54, 250–254. [Google Scholar] [CrossRef]
- Velu, G.; Ortiz-Monasterio, I.; Singh, R.P.; Payne, T. Variation for Grain Micronutrients Concentration in Wheat Core-collection Accessions of Diverse Origin. Asian J. Crop Sci. 2011, 3, 43–48. [Google Scholar] [CrossRef]
- Kumar, A.; Kapoor, P.; Chunduri, V.; Sharma, S.; Garg, M. Potential of Aegilops sp. for improvement of grain processing and nutritional quality in wheat (Triticum aestivum). Front. Plant Sci. 2019, 10, 308–326. [Google Scholar] [CrossRef]
- Cakmak, I.; Ozkan, H.; Braun, H.J.; Welch, R.M.; Romheld, V. Zinc and iron concentrations in seeds of wild, primitive, and modern wheats. Food Nutr. Bull. 2000, 21, 401–403. [Google Scholar] [CrossRef]
- Peleg, Z.; Saranga, Y.; Yazici, A.; Fahima, T.; Ozturk, L.; Cakmak, I. Grain zinc, iron and protein concentrations and zinc-efficiency in wild emmer wheat under contrasting irrigation regimes. Plant Soil 2008, 306, 57–67. [Google Scholar] [CrossRef]
- Velu, G.; Ortiz-Monasterio, I.; Cakmak, I.; Hao, Y.; Singh, R.Á. Biofortification strategies to increase grain zinc and iron concentrations in wheat. J. Cereal Sci. 2014, 59, 365–372. [Google Scholar] [CrossRef]
- Bálint, A.F.; Kovács, G.; Erdei, L.; Sutka, J. Comparison of the Cu, Zn, Fe, Ca and Mg contents of the grains of wild, ancient and cultivated wheat species. Cereal Res. Commun. 2001, 29, 375–382. [Google Scholar] [CrossRef]
- Kartseva, T.; Alqudah, A.M.; Aleksandrov, V.; Alomari, D.Z.; Doneva, D.; Arif, M.A.R.; Borner, A.; Misheva, S. Nutritional Genomic Approach for Improving Grain Protein Content in Wheat. Foods 2023, 12, 1399. [Google Scholar] [CrossRef]
- Ma, J.; Ye, M.; Liu, Q.; Yuan, M.; Zhang, D.; Li, C.; Zeng, Q.; Wu, J.; Han, D.; Jiang, L. Genome-wide association study for grain zinc concentration in bread wheat (Triticum aestivum L.). Front. Plant Sci. 2023, 14, 1169858. [Google Scholar] [CrossRef] [PubMed]
- Brouns, F. Phytic acid and whole grains for health controversy. Nutrients 2021, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Ma, G.S. Bioavailability of phytic acid and minerals. Foreign Med. Sci. 2005, 32, 141–144. [Google Scholar]
- Shewry, P.R.; Hey, S. Do “ancient” wheat species differ from modern bread wheat in their contents of bioactive components? J. Cereal Sci. 2015, 65, 236–243. [Google Scholar] [CrossRef]
- De Brier, N.; Gomand, S.V.; Donner, E.; Paterson, D.; Smolders, E.; Delcour, J.A.; Lombi, E. Element distribution and iron speciation in mature wheat grains (Triticum aestivum L.) using synchrotron X-ray fluorescence microscopy mapping and X-ray absorption near-edge structure (XANES) Edwards imaging. Plant Cell Environ. 2016, 39, 1835–1847. [Google Scholar] [CrossRef]
- Latunde-Dada, G.O.; Li, X.; Parodi, A.; C., H.; Ellis, P.R.; Sharp, P.A. Micromilling enhances iron bioaccessibility from wholegrain wheat. J. Agric. Food Chem. 2014, 62, 11222–11227. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrović, S.; Vila, S.; Grubišić Šestanj, S.; Rebekić, A. Variation in Nutritional Value of Diverse Wheat Genotypes. Agronomy 2024, 14, 311. https://doi.org/10.3390/agronomy14020311
Petrović S, Vila S, Grubišić Šestanj S, Rebekić A. Variation in Nutritional Value of Diverse Wheat Genotypes. Agronomy. 2024; 14(2):311. https://doi.org/10.3390/agronomy14020311
Chicago/Turabian StylePetrović, Sonja, Sonja Vila, Sanja Grubišić Šestanj, and Andrijana Rebekić. 2024. "Variation in Nutritional Value of Diverse Wheat Genotypes" Agronomy 14, no. 2: 311. https://doi.org/10.3390/agronomy14020311
APA StylePetrović, S., Vila, S., Grubišić Šestanj, S., & Rebekić, A. (2024). Variation in Nutritional Value of Diverse Wheat Genotypes. Agronomy, 14(2), 311. https://doi.org/10.3390/agronomy14020311




