Utilizing Remote Sensing to Quantify the Performance of Soybean Insecticide Seed Treatments
Abstract
:1. Introduction
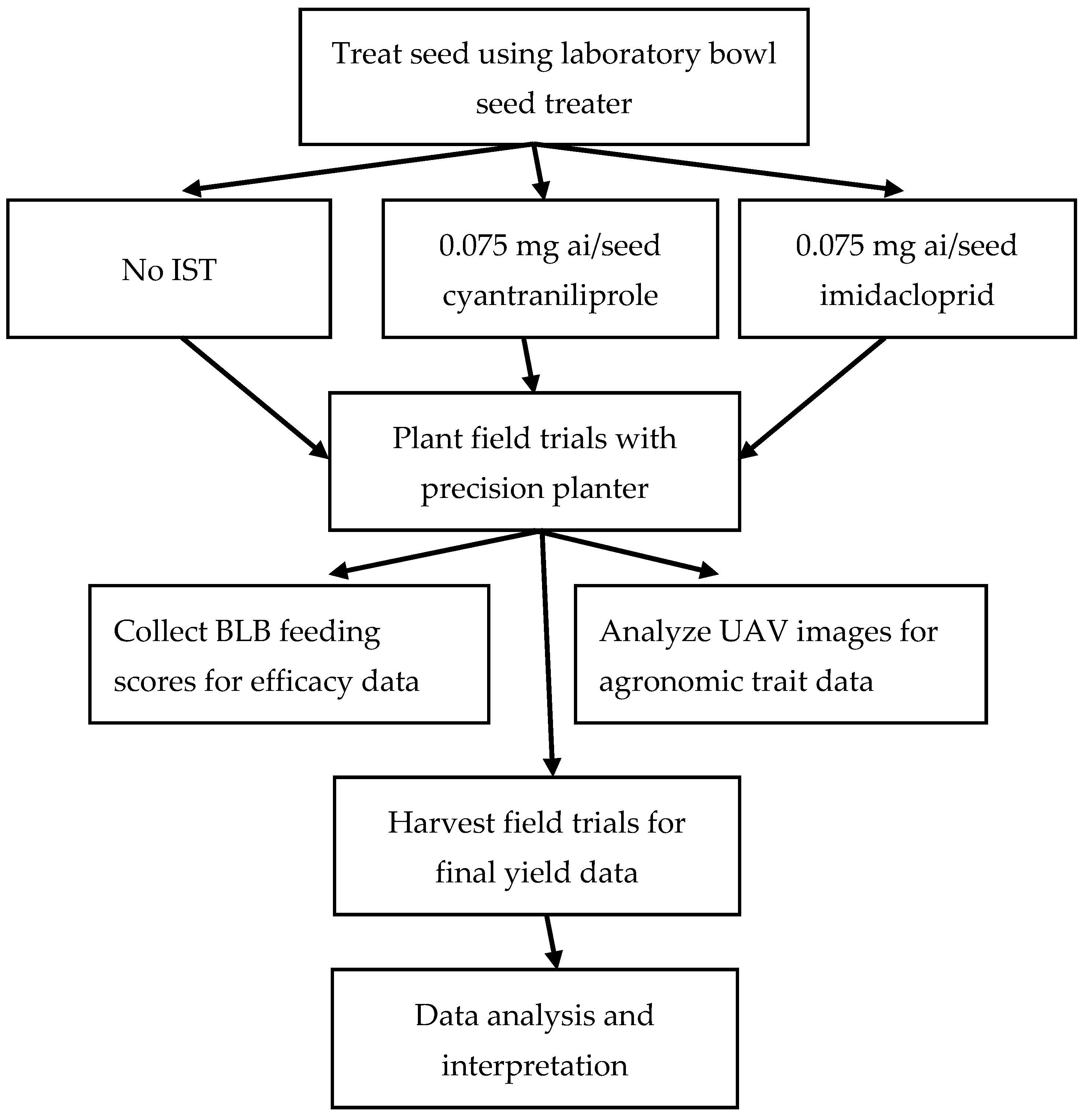
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Mailer, R.J. Oilseeds: Overview. In Encyclopedia of Food Grains: The World of Food Grains; Wrigley, C., Corke, H., Seetharaman, K., Faubion, J., Eds.; Academic Press: Oxford, UK, 2016; Volume 1, pp. 221–222. [Google Scholar]
- McVetty, P.B.; Lukow, O.M.; Hall, L.M.; Rajcan, I.; Rahman, H. Grain production and consumption: Oilseeds in North America. In Encyclopedia of Food Grains, 2nd ed.; Wrigley, C., Corke, H., Seetharaman, K., Faubion, J., Eds.; Academic Press: Oxford, UK, 2016; pp. 401–408. [Google Scholar]
- Beszterda, M.; Nogala-Kalucka, M. Current research developments on the processing and improvement of the nutritional quality of rapeseed (Brassica napus L.). Eur. J. Lipid Sci. Technol. 2019, 121, 1800045. [Google Scholar] [CrossRef]
- Hudson, K.A.; Hudson, M.E. Genetic variation for seed oil biosynthesis in soybean. Plant Mol. Bio. Rep. 2021, 39, 700–709. [Google Scholar] [CrossRef]
- USDA-ERS. Oil Crops Yearbook. 2023. Available online: https://www.ers.usda.gov/data-products/oil-crops-yearbook/oil-crops-yearbook (accessed on 28 November 2023).
- Statistics Canada. Principal Field Crop Areas, June 2023. Available online: https://www150.statcan.gc.ca/n1/daily-quotidien/230628/dq230628a-eng.htm (accessed on 28 November 2023).
- Kogan, M.; Waldbauer, G.P.; Boiteau, G.; Eastman, C.E. Sampling bean leaf beetles on soybean. In Sampling Methods in Soybean Entomology; Kogan, M., Herzog, D.C., Eds.; Springer: New York, NY, USA, 1980; pp. 202–215. [Google Scholar]
- Pedigo, L.P.; Zeiss, M.R. Effect of soybean planting date on bean leaf beetle (Coleoptera: Chrysomelidae) abundance and pod injury. J. Econ. Entomol. 1996, 89, 183–188. [Google Scholar] [CrossRef]
- French, B.W.; Hammack, L. Sexual dimorphism of basitarsi in pest species of Diabrotica and Cerotoma spp. (Coleoptera: Chrysomelidae). Ann. Entomol. Soc. Am. 2007, 100, 59–63. [Google Scholar]
- Bradshaw, J.D.; Rice, M.E.; Hill, J.H. Evaluation of management strategies for bean leaf beetles (Coleoptera: Chrysomelidae) and bean pod mottle virus (Comoviridae) in soybean. J. Econ. Entomol. 2008, 101, 1211–1227. [Google Scholar] [CrossRef]
- Varenhorst, A. Scout Soybeans for Bean Leaf Beetle Feeding. SDSU Extension. 2020. Available online: https://extension.sdstate.edu/scout-soybeans-bean-leaf-beetle-feeding (accessed on 13 January 2024).
- Bradshaw, J.; Rice, M.; Dean, A.; Hodgson, E. Bean Leaf Beetle. ISU Extension and Outreach Integrated Crop Management. 2022. Available online: https://crops.extension.iastate.edu/bean-leaf-beetle (accessed on 13 January 2024).
- Musser, F.; Hodgson, E.; Mueller, D. Bean Leaf Beetle in Soybean. USDA Crop Protect. Network. 2023. Available online: https://cropprotectionnetwork.org/encyclopedia/bean-leaf-beetle-in-soybean (accessed on 13 January 2024).
- Hadi, B.A.; Bradshaw, J.D.; Rice, M.E.; Hill, J.H. Bean leaf beetle (Coleoptera: Chysomelidae) and bean pod mottle virus in soybean: Biology, ecology, and management. J. Integr. Pest Mgmt. 2012, 3, B1–B7. [Google Scholar] [CrossRef]
- Smelser, R.B.; Pedigo, L.P. Bean leaf beetle (Coleoptera: Chrysomelidae) herbivory on leaf, stem, and pod components of soybean. J. Econ. Entomol. 1992, 85, 2408–2412. [Google Scholar] [CrossRef]
- Smelser, R.B.; Pedigo, L.P. Soybean seed yield and quality reduction by bean leaf beetle (Coleoptera, Chrysomelidae) pod injury. J. Econ. Entomol. 1992, 85, 2399–2403. [Google Scholar] [CrossRef]
- Giesler, L.J.; Ghabrial, S.A.; Hunt, T.E.; Hill, J.H. Bean pod mottle virus: A threat to US soybean production. Plant Dis. 2002, 86, 1280–1289. [Google Scholar] [CrossRef]
- Krell, R.K.; Pedigo, L.P.; Hill, J.H.; Rice, M.E. Bean leaf beetle (Coleoptera: Chrysomelidae) management for reduction of bean pod mottle virus. J. Econ. Entomol. 2004, 97, 192–202. [Google Scholar] [CrossRef]
- Hill, J.H.; Koval, N.C.; Gaska, J.M.; Grau, C.R. Identification of field tolerance to bean pod mottle and soybean mosaic viruses in soybean. Crop Sci. 2007, 47, 212–218. [Google Scholar] [CrossRef]
- Sikora, E.J.; Murphy, J.F.; Conner, K.N. Monitoring bean pod mottle virus and soybean mosaic virus incidence at different soybean growth stages in Alabama. Plant Health Prog. 2017, 18, 166. [Google Scholar] [CrossRef]
- Vojvodic, M.; Bazok, R. Future of insecticide seed treatment. Sustainability 2021, 13, 8792. [Google Scholar] [CrossRef]
- Jeschke, P.; Nauen, R. Neonicotinoids—From zero to hero in insecticide chemistry. Pest Manag. Sci. 2008, 64, 1084–1098. [Google Scholar] [CrossRef]
- Nauen, R.; Jeschke, P.; Copping, L. In focus: Neonicotinoid insecticides. Pest Manag. Sci. 2008, 64, 1081. [Google Scholar] [CrossRef] [PubMed]
- Knodel, J.J.; Lubenow, L.A.; Olson, D.L. Integrated Pest Management of Flea Beetles in Canada. NDSU Extension Serv. 2017. Available online: https://www.ag.ndsu.edu/publications/crops/integrated-pest-management-of-flea-beetles-in-canola (accessed on 24 November 2023).
- Hladik, M.L.; Main, A.R.; Goulson, D. Environmental risks and challenges associated with neonicotinoid insecticides. Environ. Sci. Technol. 2018, 52, 3329–3335. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 2011, 59, 2897–2908. [Google Scholar] [CrossRef]
- Zhang, L.; Greenberg, S.M.; Zhang, Y.; Liu, T. Effectiveness of thiamethoxam and imidacloprid seed treatments against Bemisia tabaci (Hemiptera: Aleyrodidae) on cotton. Pest Manag. Sci. 2011, 67, 226–232. [Google Scholar] [CrossRef]
- Lahm, G.P.; Cordova, D.; Barry, J.D. New and selective ryanodine receptor activators for insect control. Bioorg. Med. Chem. 2009, 17, 4127–4133. [Google Scholar] [CrossRef] [PubMed]
- Thrash, B.; Adamczyk, J.J.; Lorenz, G.; Scott, A.W.; Armstrong, J.S.; Pfannenstiel, R.; Taillon, N. Laboratory evaluation of lepidopteran-active soybean seed treatments on survivorship of fall armyworm (Lepidopter: Noctuidae) larvae. Fla. Entomol. 2013, 96, 724–730. [Google Scholar] [CrossRef]
- Tiwari, S.; Stelinski, L.L. Effects of cyantraniliprole, a novel anthranilic diamide insecticide, against Asian citrus psyllid under laboratory and field conditions. Pest Manag. Sci. 2013, 69, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, Z.; Cui, K.; Zhao, Y.; Han, J.; Liu, F.; Mu, W. Effects of sublethal concentrations of cyantraniliprole on the development, fecundity, and nutritional physiology of the black cutworm Agrotis ipsilon (Lepidoptera: Noctuidae). PLoS ONE 2016, 11, e0156555. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Ding, J.; Zhao, Y.; Luo, J.; Mu, W.; Zhang, Z. Cyantraniliprole at sublethal dosages negatively affects the development, reproduction, and nutrient utilization of Ostrinia furnacalis (Lepidoptera: Crambidae). J. Econ. Entomol. 2017, 110, 230–238. [Google Scholar] [PubMed]
- Pes, M.P.; Melo, A.A.; Stacke, R.S.; Zanella, R.; Perini, C.R.; Silva, F.M.A.; Carus Guedes, J.V. Translocation of chlorantraniliprole and cyantraniliprole applied to corn as seed treatment and foliar spraying to control Spodoptera frugiperda (Lepidoptera: Noctuidae). PLoS ONE 2020, 15, e0229151. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.E.; Villegas, J.M.; Way, M.O.; Pearson, R.A.; Stout, M.J. Cyantraniliprole: A new insecticidal seed treatment for U.S. rice. Crop Prot. 2021, 140, 105410. [Google Scholar] [CrossRef]
- Barry, J.D.; Protillo, H.E.; Annan, I.B.; Cameron, R.A.; Clagg, D.G.; Dietrich, R.F.; Watson, L.J.; Leighty, R.M.; Ryan, D.L.; McMillan, J.A.; et al. Movement of cyantraniliprole in plants after foliar applications and its impact on the control of sucking and chewing insects. Pest Manag. Sci. 2014, 71, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Li, P.; Yao, X.; Sun, S.; Li, X.; Zhang, F.; Jiang, X. Cyantraniliprole seed treatment effectively controls wireworms (Ipleonomus canaliculatus Faldermann) and white grubs (Anomala corpulenta Motschulsky) in maize fields. Heliyon 2023, 9, e17302. [Google Scholar] [CrossRef]
- Trybom, J.; Jeschke, M.; Butzen, S. Seed treatment effects on stand establishment and yield in soybean. Pioneer Agron. Sci. Field Facts 2010, 9, 2–4. [Google Scholar]
- Gaspar, A.P.; Marburger, D.A.; Mourzinis, S.; Conley, S.P. Soybean seed yield response to multiple seed treatment components across diverse environments. Agron. J. 2014, 106, 1955–1962. [Google Scholar] [CrossRef]
- Cook, D.R.; Crow, W.; Gore, J.; Threet, M. Impact of selected insecticide seed treatments on soybean stand establishment and yield, 2020. Arthropod Manag. Tests 2021, 46, tsab086. [Google Scholar] [CrossRef]
- Cox, W.J.; Shields, E.; Cherney, J.H. Planting dates and seed treatment effects on soybean in the Northeastern United States. Agron. J. 2008, 100, 1662–1665. [Google Scholar] [CrossRef]
- Cox, W.J.; Cherney, J.H. Location, variation, and seedling rate interactions with soybean seed-applied insecticide/fungicides. Agron. J. 2011, 103, 1366–1371. [Google Scholar] [CrossRef]
- Labrie, G.; Gagnon, A.V.; Vanasse, A.; Latraverse, A.; Tremblay, G. Impacts of neonicotinoid seed treatments on soil-dwelling pest populations and agronomic parameters in corn and soybean in Quebec (Canada). PLoS ONE 2020, 15, e0229136. [Google Scholar] [CrossRef] [PubMed]
- Puri, V.; Nayyar, A.; Raja, L. Agriculture drones: A modern breakthrough in precision agriculture. J. Stat. Manag. Syst. 2017, 20, 4507–4518. [Google Scholar] [CrossRef]
- Daponte, P.; De Vito, L.; Glielmo, L.; Iannelli, L.; Liuzza, D.; Picariello, F.; Silano, G. A review on the use of drones for precision agriculture. IOP Conf. Ser. Earth Environ. Sci. 2019, 275, 012022. [Google Scholar] [CrossRef]
- Milics, G. Application of UAVs in Precision Agriculture. In International Climate Protection; Palocz-Andresen, M., Szalay, D., Gosztom, A., Sipos, L., Taligas, T., Eds.; Springer: Cham, Switzerland, 2019; pp. 93–97. [Google Scholar]
- Narayanan, B.; Floyd, B.; Tu, K.; Ries, L.; Hausmann, N. Improving soybean breeding using UAS measurements of physiological maturity. Autonomous Air and Ground Sensing Systems for Agricultural Optimization and Phenotyping IV. In Proceedings of the SPIE Defense + Commercial Sensing, Baltimore, MD, USA, 3–7 April 2019; Volume 110080U. [Google Scholar] [CrossRef]
- Olson, D.; Anderson, J. Review on unmanned aerial vehicles, remote sensors, imagery processing, and their applications in agriculture. Agron. J. 2021, 113, 971–992. [Google Scholar] [CrossRef]
- Hegstad, J.M.; Gaspar, A.P.; Feng, L.; Lackermann, K.; Hudson, A.; Howieson, M. Agronomic and efficacy evaluations of oxathiapiprolin as a soybean seed treatment. Agron. J. 2021, 113, 4850–4864. [Google Scholar] [CrossRef]
- Stroup, W.W.; Milliken, G.A.; Claassen, E.A.; Wolfinger, R.D. SAS® for Mixed Models: Introduction and Basic Applications; SAS Institute Inc.: Cary, NC, USA, 2019. [Google Scholar]
- Koch, R.L.; Burkness, E.C.; Hutchinson, W.D.; Rabeay, T.L. Efficacy of systemic insecticide seed treatments for protection of early-growth stage snap beans from bean leaf beetle (Coleoptera: Chrysomelidae) foliar feeding. Crop Prot. 2005, 24, 734–742. [Google Scholar] [CrossRef]
- Bradshaw, J.; Rice, M.; Hill, J. Seed Treatments in Soybean: Managing Bean Leaf Beetles; Integrated Crop Management; Iowa State University: Ames, IA, USA, 2007; Available online: https://crops.extension.iastate.edu/encyclopedia/seed-treatments-soybean-managing-bean-leaf-beetles (accessed on 13 January 2024).
- Conceicao, G.M.; Barbieri, A.P.P.; Lucio, A.D.; Martin, T.N.; Mertz, L.M.; Mattioni, N.M.; Lorentz, L.H. Seedlings performance and yield of soybean submitted to different chemical treatment in seeds. Biosci. J. 2014, 30, 1711–1720. [Google Scholar]
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef] [PubMed]
- Virk, G.; Snider, J.L.; Pilon, C. Physiological contributors to early season whole-crop vigor in cotton. Crop Sci. 2019, 59, 2774–2783. [Google Scholar] [CrossRef]
- Nazari, R.; Parsa, S.; Afshari, R.T.; Mahmoodi, S.; Seyyedi, S.M. Salacylic acid priming before and after accelerated aging process increases seedling vigor in aged soybean seed. J. Crop Improv. 2020, 34, 218–237. [Google Scholar] [CrossRef]
- Smith, J.L.; Baute, T.S.; Schaafsma, A.W. Quantifying early season pest injury and yield protection of insecticide seed treatments in corn and soybean production in Ontario, Canada. J. Econ. Entom. 2020, 113, 2197–2212. [Google Scholar] [CrossRef]
- Esker, P.D.; Conley, S.P. Probability of yield response and breaking even for soybean seed treatments. Crop Sci. 2012, 52, 351–359. [Google Scholar] [CrossRef]
- Seagraves, M.P.; Lundgren, J.G. Effects of neonicotinoid seed treatments on soybean aphid and its natural enemies. J. Pest Sci. 2012, 85, 125–132. [Google Scholar] [CrossRef]
- Gaspar, A.P.; Mitchell, P.D.; Conley, S.P. Economic risk and profitability of soybean fungicide and insecticide seed treatments at reduced seeding rates. Crop Sci. 2015, 55, 924–933. [Google Scholar] [CrossRef]
- Alford, A.M.; Krupke, C.H. A meta-analysis and economic evaluation of neonicotinoid seed treatments and other prophylactic insecticides in Indiana maize from 2000–2015 with IPM recommendations. J. Econ. Entomol. 2018, 111, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Averitt, B.J.; Welbaum, G.E.; Li, X.Y.; Prenger, E.; Qin, J.; Zhang, B. Evaluating genotypes and seed treatments to increase field emergence of low phytic acid soybeans. Agriculture 2020, 10, 516. [Google Scholar] [CrossRef]
- North, J.H.; Gore, J.; Cachot, A.L.; Stewart, S.D.; Lorenz, G.M.; Musser, F.R.; Cook, D.R.; Kerns, D.L.; Dodds, D.M. Value of neonicotinoid insecticide seed treatments in mid-south soybean (Glycine max) production systems. J. Econ. Entom. 2016, 109, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, A.P.; Mueller, D.S.; Wise, K.A.; Chilvers, M.I.; Tenuta, A.U.; Conley, S.P. Response of broad-spectrum and target-specific seed treatment and seeding rate on soybean seed yield, profitability, and economic risk. Crop Sci. 2016, 56, 2251–2262. [Google Scholar] [CrossRef]

| Year | City, State | Variety | Planting Date | Avg. BLB Feeding Score/Group 1 | Harvest Date 2 |
|---|---|---|---|---|---|
| 2018 | Mansfield, Illinois | P36T36X | 30 April 2018 | 6 Low | 11 October 2018 |
| 2018 | Pesotum, Illinois | P36T36X | 28 April 2018 | 6 Low | 12 October 2018 |
| 2018 | Seymour, Illinois | P36T36X | 26 April 2018 | 7 Low | 28 October 2018 |
| 2018 | Colfax, Indiana | P36T36X | 1 May 2018 | 6 Low | 24 October 2018 |
| 2018 | Windfall1, Indiana | P36T36X | 15 May 2018 | 5 High | 22 October 2018 |
| 2018 | Windfall2, Indiana | P36T36X | 4 May 2018 | 5 High | 25 October 2018 |
| 2018 | Atlantic, Iowa | P28T71X | 10 May 2018 | 6 Low | 22 October 2018 |
| 2018 | Johnston, Iowa | P28T71X | 24 April 2018 | 5 High | 17 October 2018 |
| 2018 | Montezuma, Iowa | P28T71X | 25 April 2018 | 5 High | Not Harvested |
| 2018 | Prairie City, Iowa | P31A22X | 30 April 2018 | 6 Low | 25 October 2018 |
| 2018 | Winterset, Iowa | P28T71X | 25 April 2018 | 6 Low | 21 October 2018 |
| 2019 | Morrisonville, Illinois | P38A98X | 3 June 2019 | 6 Low | 23 October 2019 |
| 2019 | Seymour, Illinois | P38A98X | 18 May 2019 | 6 Low | 15 October 2019 |
| 2019 | Colfax, Indiana | P38A98X | 2 June 2019 | 6 Low | 11 October 2019 |
| 2019 | Windfall, Indiana | P38A98X | 16 May 2019 | 6 Low | 28 October 2019 |
| 2019 | Atlantic, Iowa | P31A22X | 4 May 2019 | 6 Low | 15 October 2019 |
| 2019 | Dallas Center, Iowa | P31A22X | 3 June 2019 | 7 Low | 23 October 2019 |
| 2019 | Johnston1, Iowa | P31A22X | 15 April 2019 | 7 Low | 18 October 2019 |
| 2019 | Johnston2, Iowa | P31A22X | 15 April 2019 | 6 Low | 20 October 2019 |
| 2019 | Johnston3, Iowa | P31A22X | 15 April 2019 | 6 Low | 22 October 2019 |
| 2019 | Reasoner, Iowa | P31A22X | 3 June 2019 | 6 Low | 18 October 2019 |
| 2019 | Washington, Iowa | P31A22X | 16 April 2019 | 5 High | 16 October 2019 |
| 2019 | Winterset, Iowa | P31A22X | 3 June 2019 | 5 High | 27 October 2019 |
| 2019 | Silverthorn, Missouri | P48A60X | 10 May 2019 | 5 High | 3 October 2019 |
| 2019 | Obion, Tennessee | P48A60X | 14 May 2019 | 6 Low | 5 October 2019 |
| 2020 | Champaign, Illinois | P36A83X | 10 April 2020 | 7 Low | 12 October 2020 |
| 2020 | Windfall1, Indiana | P36A83X | 9 May 2020 | 6 Low | Not Harvested |
| 2020 | Windfall2, Indiana | P36A83X | 25 April 2020 | 7 Low | 10 October 2020 |
| 2020 | DeSoto, Iowa | P31A22X | 2 May 2020 | 7 Low | 30 October 2020 |
| 2020 | Johnston1, Iowa | P31A22X | 20 April 2020 | 4 High | 30 October 2020 |
| 2020 | Johnston2, Iowa | P31A22X | 20 April 2020 | 5 High | 30 October 2020 |
| 2020 | Montezuma, Iowa | P31A22X | 12 May 2020 | 7 Low | 7 October 2020 |
| 2021 | Seymour, Illinois | P36A83X | 16 April 2021 | 2 High | 24 September 2021 |
| 2021 | Groomsville, Indiana | P36A83X | 27 April 2021 | 6 Low | 4 November 2021 |
| 2021 | Atlantic, Iowa | P33A53X | 24 April 2021 | 6 Low | 27 September 2021 |
| 2021 | Johnston1, Iowa | P33A53X | 6 May 2021 | 4 High | 5 October 2021 |
| 2021 | Johnston2, Iowa | P33A53X | 22 April 2021 | 5 High | 5 October 2021 |
| 2021 | Johnston3, Iowa | P33A53X | 7 April 2021 | 5 High | 5 October 2021 |
| 2021 | Johnston4, Iowa | P33A53X | 7 April 2021 | 4 High | 5 October 2021 |
| 2021 | Reasoner1, Iowa | P33A53X | 6 May 2021 | 4 High | 14 October 2021 |
| 2021 | Reasoner2, Iowa | P33A53X | 6 May 2021 | 5 High | 8 October 2021 |
| 2021 | Winterset1, Iowa | P33A53X | 5 May 2021 | 4 High | 5 October 2021 |
| 2021 | Winterset2, Iowa | P33A53X | 5 May 2021 | 4 High | 4 October 2021 |
| 2022 | Colfax, Indiana | P29A25X | 28 April 2022 | 1 High | 2 October 2022 |
| 2022 | Windfall, Indiana | P29A25X | 22 April 2022 | 1 High | 14 October 2022 |
| 2022 | Desoto, Iowa | P29A25X | 19 May 2022 | 3 High | 13 October 2022 |
| 2022 | Johnston1, Iowa | P29A25X | 31 May 2022 | 4 High | 17 October 2022 |
| 2022 | Johnston2, Iowa | P29A25X | 27 April 2022 | 1 High | 17 October 2022 |
| 2022 | Obion, Tennessee | P27A64X | 30 April 2022 | 5 High | 26 October 2022 |
| 2023 | Homer, Illinois | P35T15E | 13 April 2023 | 3 High | 3 October 2023 |
| 2023 | Perry, Indiana | P35T15E | 19 April 2023 | 3 High | 23 October 2023 |
| 2023 | Windfall, Indiana | P35T15E | 13 April 2023 | 6 Low | 30 September 2023 |
| 2023 | Baxter1, Iowa | P29A18E | 27 April 2023 | 6 Low | 10 October 2023 |
| 2023 | Baxter2, Iowa | P28A21X | 27 April 2022 | 5 High | 10 October 2023 |
| 2023 | Johnston1, Iowa | P28A65E | 27 April 2023 | 5 High | 22 October 2023 |
| 2023 | Johnston2, Iowa | P28A65E | 27 April 2023 | 6 Low | 22 October 2023 |
| 2023 | Johnston3, Iowa | P28A21X | 27 April 2023 | 5 High | 22 October 2023 |
| 2023 | Johnston4, Iowa | P28A21X | 13 April 2023 | 4 High | 30 September 2023 |
| 2023 | Johnston5, Iowa | P28A21X | 27 April 2023 | 4 High | 30 September 2023 |
| 2023 | Redfield, South Dakota | P09A62X | 17 May 2023 | 7 Low | 4 October 2023 |
| Trait | BLB Pressure 1 | Effect | Num DF | Den DF | F Value | Prob F |
|---|---|---|---|---|---|---|
| BLB feeding score | Low | Seed Treatment | 2 | 49.7594 | 283.563 | 0.000 |
| High | Seed Treatment | 2 | 59.9497 | 268.625 | 0.000 | |
| UAV emergence gaps 2 | Low | Seed Treatment | 2 | 38.1304 | 1.34745 | 0.272 |
| High | Seed Treatment | 2 | 62.0686 | 10.512 | 0.000 | |
| UAV vigor 3 | Low | Seed Treatment | 2 | 37.5057 | 1.62668 | 0.210 |
| High | Seed Treatment | 2 | 70.5389 | 17.4571 | 0.000 | |
| UAV greenness 4 | Low | Seed Treatment | 2 | 30.4635 | 6.06129 | 0.006 |
| High | Seed Treatment | 2 | 63.1174 | 25.0777 | 0.000 | |
| Yield (kg/ha) | Low | Seed Treatment | 2 | 348.69 | 16.6767 | 0.000 |
| High | Seed Treatment | 2 | 386.051 | 16.7091 | 0.000 |
| BLB Feeding Score 1 | UAV Emergence Gaps 2 | UAV Vigor 3 | UAV Greenness 4 | Yield (kg/ha) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Seed Treatment | Low 5 | High 5 | Low 5 | High 5 | Low 5 | High 5 | Low 5 | High 5 | Low 5 | High 5 |
| FST No IST | 6.3 b | 4.2 c | 61.5 a | 78.5 a | 11.8 a | 12.0 c | 60.8 b | 59.7 b | 4536.1 c | 4369.0 c |
| Cyantraniliprole + FST | 8.4 a | 8.0 a | 52.5 a | 46.7 c | 12.2 a | 13.6 a | 61.1 a | 60.5 a | 4790.6 a | 4582.7 a |
| Imidacloprid + FST | 8.3 a | 7.4 b | 60.2 a | 63.9 b | 12.1 a | 12.8 b | 61.2 a | 60.2 a | 4699.5 b | 4490.4 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hegstad, J.M.; Mo, H.; Gaspar, A.P.; Rule, D. Utilizing Remote Sensing to Quantify the Performance of Soybean Insecticide Seed Treatments. Agronomy 2024, 14, 340. https://doi.org/10.3390/agronomy14020340
Hegstad JM, Mo H, Gaspar AP, Rule D. Utilizing Remote Sensing to Quantify the Performance of Soybean Insecticide Seed Treatments. Agronomy. 2024; 14(2):340. https://doi.org/10.3390/agronomy14020340
Chicago/Turabian StyleHegstad, Jeffrey M., Hua Mo, Adam P. Gaspar, and Dwain Rule. 2024. "Utilizing Remote Sensing to Quantify the Performance of Soybean Insecticide Seed Treatments" Agronomy 14, no. 2: 340. https://doi.org/10.3390/agronomy14020340





