Phytotoxic Effects and Potential Allelochemicals from Water Extracts of Paulownia tomentosa Flower Litter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Water Extract of P. tomentosa Flower Litter
2.3. Seed Germination
2.4. Seedling Growth
2.5. Cell Viability
2.6. Reactive Oxygen Species (ROS)
2.7. Malondialdehyde (MDA)
2.8. Free Proline
2.9. LC-HRMS
2.10. Statistical Analysis
3. Results
3.1. Effects of EPF on Seed Germination
3.2. Effects of EPF on Seedlings’ Growth
3.3. Cell Viability in Root Tips of Lettuce Seedlings after EPF Treatment
3.4. ROS Production and Lipid Peroxidation in Lettuce Seedlings after Treatment with EPF
3.5. Effect of EPF on Free Proline Accumulation in Lettuce Seedling Roots
3.6. Analysis of Active Compounds in EPF
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984. [Google Scholar]
- Qin, F.; Liu, S.; Yu, S. Effects of Allelopathy and Competition for Water and Nutrients on Survival and Growth of Tree Species in Eucalyptus urophylla Plantations. For. Ecol. Manag. 2018, 424, 387–395. [Google Scholar] [CrossRef]
- Bonanomi, G.; Zotti, M.; Idbella, M.; Mazzoleni, S.; Abd-ElGawad, A.M. Microbiota Modulation of Allelopathy Depends on Litter Chemistry: Mitigation or Exacerbation? Sci. Total Environ. 2021, 776, 145942. [Google Scholar] [CrossRef] [PubMed]
- Kimura, F.; Sato, M.; Kato-Noguchi, H. Allelopathy of Pine Litter: Delivery of Allelopathic Substances into Forest Floor. J. Plant Biol. 2015, 58, 61–67. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, H.; Wei, M.; Wu, B.; Wang, C. Litter Decomposition Process Dramatically Declines the Allelopathy of Soli-dago canadensis L. on the Seed Germination and Seedling Growth of Lactuca sativa L. Int. J. Phytoremediat. 2020, 22, 1295–1303. [Google Scholar] [CrossRef]
- De Jesus Jatoba, L.; Varela, R.M.; Molinillo, J.M.G.; Ud Din, Z.; Juliano Gualtieri, S.C.; Rodrigues-Filho, E.; Macías, F.A. Alle-lopathy of Bracken Fern (Pteridium arachnoideum): New Evidence from Green Fronds, Litter, and Soil. PLoS ONE 2016, 11, e0161670. [Google Scholar] [CrossRef] [PubMed]
- Hiruki, C. Paulownia Witches’-Broom Disease Important in East Asia. Acta Hortic. 1999, 496, 63–68. [Google Scholar] [CrossRef]
- Ipekci, Z.; Gozukirmizi, N. Direct Somatic Embryogenesis and Synthetic Seed Production from Paulownia elongata. Plant Cell Rep. 2003, 22, 16–24. [Google Scholar] [CrossRef]
- Jakubowski, M. Cultivation Potential and Uses of Paulownia Wood: A Review. Forests 2022, 13, 668. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, G.; Fan, J.; Zhou, Y.; Gao, Z. Relationship between the Witches Broom and the Growth of Paulownia. J. Northeast For. Univ. 2007, 22, 109–110. [Google Scholar]
- He, T.; Vaidya, B.; Perry, Z.; Parajuli, P.; Joshee, N. Paulownia as a Medicinal Tree: Traditional Uses and Current Advances. Eur. J. Med. Plants 2016, 14, 1–15. [Google Scholar] [CrossRef]
- Dżugan, M.; Miłek, M.; Grabek-Lejko, D.; Hęclik, J.; Jacek, B.; Litwińczuk, W. Antioxidant Activity, Polyphenolic Profiles and Antibacterial Properties of Leaf Extract of Various Paulownia spp. Clones. Agronomy 2021, 11, 2001. [Google Scholar] [CrossRef]
- Nowak, B.; Moniuszko-Szajwaj, B.; Skorupka, M.; Puchalska, J.; Kozłowska, M.; Bocianowski, J.; Kołodziejski, P.A.; Szumach-er-Strabel, M.; Patra, A.K.; Stochmal, A.; et al. Effect of Paulownia Leaves Extract Levels on In Vitro Ruminal Fermentation, Microbial Population, Methane Production, and Fatty Acid Biohydrogenation. Molecules 2022, 27, 4288. [Google Scholar] [CrossRef]
- Kazi, M.; Simabayasi, T. A glucoside from Paulownia. Japan 1931, 93, 735. [Google Scholar]
- Guo, N.; Zhai, X.; Fan, G. Chemical Composition, Health Benefits and Future Prospects of Paulownia Flowers: A Review. Food Chem. 2023, 412, 135496. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xiao, C.; Li, J.; Wu, S.; Zhang, W.; Kong, L.; Tang, W.Z.; Jia, X. Two New Dicyclic C-geranylflavanones Isolated from Paulownia fortunei and Their Cytotoxicity. Phytochem. Lett. 2023, 54, 97–100. [Google Scholar] [CrossRef]
- Molčanová, L.; Treml, J.; Brezáni, V.; Maršík, P.; Kurhan, S.; Trávníček, Z.; Uhrin, P.; Šmejkal, K. C-geranylated Flavonoids from Paulownia tomentosa Steud. Fruit as Potential Anti-inflammatory Agents. J. Ethnopharmacol. 2022, 296, 115509. [Google Scholar] [CrossRef]
- Molčanová, L.; Kauerová, T.; Dall’Acqua, S.; Maršík, P.; Kollár, P.; Šmejkal, K. Antiproliferative and Cytotoxic Activities of C-geranylated Flavonoids from Paulownia tomentosa Steud. Fruit. Bioorg. Chem. 2021, 111, 104797. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Wang, Y.; Gao, T.; Wang, X.; Zhao, Y. Identification of C-geranylated flavonoids from Paulownia catalpifolia Gong Tong fruits by HPLC-DAD-ESI-MS/MS and their anti-aging effects on 2BS cells induced by H2O2. Chin. J. Nat. Med. 2017, 15, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Hanáková, Z.; Hošek, J.; Babula, P.; Dall’Acqua, S.; Václavík, J.; Šmejkal, K. C-geranylated Flavanones from Paulownia tomentosa Fruits as Potential Anti-inflammatory Compounds Acting via Inhibition of TNF-α Production. J. Nat. Prod. 2015, 78, 850–863. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Moon, H.I.; Ohk, J.; Lee, S.; Li, C.; Kim, S.K.; Lee, M.S. Inhibitory Effect and Mechanism on Antiproliferation of Iso-atriplicolide Tiglate (PCAC) from Paulownia coreana. Molecules 2012, 17, 5945–5951. [Google Scholar] [CrossRef]
- Tian, N.; Liu, Z.; Shi, T. Allelopathic Effects of 12 Trees Leaf Litter Decomposition on Maize in Interplanting of Trees (Fruits) and Crops. J. Agro-Environ. Sci. 2013, 32, 1000–1008. [Google Scholar]
- Zhao, Y.; Chen, Z.; Wang, K.; Wang, Q.; Fan, W. Allelopathy of Paulownia and poplar leaves aqueous extracts on crop seed germination. Trans. ASAE 2010, 26, 400–405. [Google Scholar]
- Yuan, Z.; Luo, L.; Zang, A.; Meng, Z. Isolation and Bioassay of Herbicidal Active Ingredient from Pauownia tomentosa. Chin. J. Pestic. Sci. 2009, 11, 239–243. [Google Scholar]
- Pan, J.; Zhu, M.; Chen, H. Aluminum-Induced Cell Death in Root-Tip Cells of Barley. Environ. Exp. Bot. 2001, 46, 71–79. [Google Scholar] [CrossRef]
- Jacyn Baker, C.; Mock, N.M. An Improved Method for Monitoring Cell Death in Cell Suspension and Leaf Disc Assays Using Evans Blue. Plant Cell Tissue Organ Cult. 1994, 39, 7–12. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kobayashi, Y.; Devi, S.R.; Rikiishi, S.; Matsumoto, H. Aluminum Toxicity is Associated with Mitochondrial Dysfunction and the Production of Reactive Oxygen Species in Plant Cells. Plant Physiol. 2002, 128, 63–72. [Google Scholar] [CrossRef]
- Frew, J.E.; Jones, P.; Scholes, G. Spectrophotometric Determination of Hydrogen Peroxide and Organic Hydropheroxides at Low Concentrations in Aqueous Solution. Anal. Chim. Acta 1983, 155, 139–150. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the Thiobarbituric Acid-Reactive-Substances Assay for Estimating Lipid Peroxidation in Plant Tissues Containing Anthocyanin and other Interfering Compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Duke, S.O. Allelopathy: Current Status of Research and Future of the Discipline: A Commentary. Allelopath. J. 2010, 25, 17–30. [Google Scholar]
- Hierro, J.L.; Callaway, R.M. The Ecological Importance of Allelopathy. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 25–45. [Google Scholar] [CrossRef]
- Niakan, M.; Saberi, K. Effects of Eucalyptus Allelopathy on Growth Characters and Antioxidant Enzymes Activity in Phalaris Weed. Asian J. Plant Sci. 2009, 8, 440–446. [Google Scholar] [CrossRef]
- Yao, S.; Li, F.; Peng, L.; Feng, X.; Dong, J.; Feng, Z.; Zhang, J.; Zhao, Q.; Feng, S.; Xu, Y.; et al. A study of the allelopathic effect of extracts from different parts of Iva xanthiifolia on five Brassicaceae species. Acta Pratacult. Sin. 2018, 27, 56–66. [Google Scholar] [CrossRef]
- Hager, H.A. Differential Effects of Typha Litter and Plants on Invasive Lythrum salicaria Seedling Survival and Growth. Biol. Invasions 2004, 6, 433–444. [Google Scholar] [CrossRef]
- Wan, F.; Liu, W.; Guo, J.; Qiang, S.; Li, B.; Wang, J.; Yang, G.; Niu, H.; Gui, F.; Huang, W.; et al. Invasive Mechanism and Con-trol Strategy of Ageratina adenophora (Sprengel). Sci. China Life Sci. 2010, 53, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Turk, M.A.; Tawaha, A.M. Allelopathic Effect of Black Mustard (Brassica nigra L.) on Germination and Growth of Wild Oat (Avena fatua L.). Crop Prot. 2003, 22, 673–677. [Google Scholar] [CrossRef]
- Chon, S.U.; Choi, S.K.; Jung, S.; Jang, H.G.; Pyo, B.S.; Kim, S.M. Effects of Alfalfa Leaf Extracts and Phenolic Allelochemicals on Early Seedling Growth and Root Morphology of Alfalfa and Barnyard Grass. Crop Prot. 2002, 21, 1077–1082. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as Key Players in Plant Stress Signaling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and Artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, D.; Ding, L.; Cui, H.; Jin, H.; Yang, X.; Yang, J.; Qin, B. Mechanism of Artemisinin Phytotoxicity Action: Induc-tion of Reactive Oxygen Species and Cell Death in Lettuce Seedlings. Plant Physiol. Biochem. 2015, 88, 53–59. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, D.; Cui, H.; Zhang, D.; Sun, Y.; Jin, H.; Li, X.; Yang, X.; Guo, H.; He, X.; et al. Phytotoxicity Mechanisms of Two Coumarin Allelochemicals from Stellera chamaejasme in Lettuce Seedlings. Acta Physiol. Plant. 2016, 38, 248. [Google Scholar] [CrossRef]
- Yan, Z.; Tan, J.; Guo, K.; Yao, L. Phytotoxic Mechanism of Allelochemical Liquiritin on Root Growth of Lettuce Seedlings. Plant Signal. Behav. 2020, 15, 1795581. [Google Scholar] [CrossRef]
- Yan, Z.; Li, P.; Xiao, Y.; Cao, L.; Yao, L. Phytotoxic Effects of Allelochemical Acacetin on Seed Germination and Seedling Growth of Selected Vegetables and Its Potential Physiological Mechanism. Agronomy 2022, 12, 1038. [Google Scholar] [CrossRef]
- Li, Z.H.; Wang, Q.; Ruan, X.; Pan, C.D.; Jiang, D.A. Phenolics and Plant Allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Wang, J.; Cai, D. Effects of Citrate and Malate on the Seed Germination and Membrane Permeability of Pak Choi. Chin. Agric. Sci. Bull. 2007, 23, 312–316. [Google Scholar]
- Pinho, I.A.; Lopes, D.V.; Martins, R.C.; Quina, M.J. Phytotoxicity assessment of olive mill solid wastes and the influence of phenolic compounds. Chemosphere 2017, 185, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Yan, Z.Q.; Jin, H.; Li, X.Z.; He, X.F.; Yang, X.Y.; Qin, B. Allelopathic effect and mechanism of Cinnamic acid and Caffeic acid on the growth of lettuce. Acta Bot. Boreal.-Occident. Sin. 2016, 36, 93–99. [Google Scholar] [CrossRef]
- Chen, E.; Zhang, D.H.; Wang, D.D.; Jin, H.; Li, X.Z.; He, X.F.; Yan, Z.Q.; Shen, H.M. Allelopathic effect and mechanism of action of three phenolic acids on lettuce seedlings. Chin. J. Pestic. Sci. 2016, 18, 317–322. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.M.; Hashinaga, F. Allelopathic Potential of Two Sesquiterpene Lactones from Magnolia grandiflora L. Biochem. Syst. Ecol. 2007, 35, 737–742. [Google Scholar] [CrossRef]
- Pan, L.; Li, X.; Yan, Z.; Guo, H.; Qin, B. Phytotoxicity of Umbelliferone and its Analogs: Structure–Activity Relationships and Action Mechanisms. Plant Physiol. Biochem. 2015, 97, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Cipollini, D.; Stevenson, R.; Enright, S.; Eyles, A.; Bonello, P. Phenolic Metabolites in Leaves of the Invasive Shrub, Lonicera maackii, and Their Potential Phytotoxic and Anti-herbivore Effects. J. Chem. Ecol. 2008, 34, 144–152. [Google Scholar] [CrossRef]
- Sasamoto, H.; Ashihara, H. Effect of Nicotinic acid, Nicotinamide and Trigonelline on the Proliferation of Lettuce Cells Derived from Protoplasts. Phytochem. Lett. 2014, 7, 38–41. [Google Scholar] [CrossRef]
- Cheng, Y.; Sun, W.; Lu, J. Allelopathic Effects Luteolin on Seed Germination and Seedling Growth of Wheat. Hortic. Seed 2016, 7, 75–77. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Buta, J.G.; Spaulding, D.W. Allelochemicals in tall fescue-abscisic and phenolic acids. J. Chem. Ecol. 1989, 15, 1629–1636. [Google Scholar] [CrossRef]
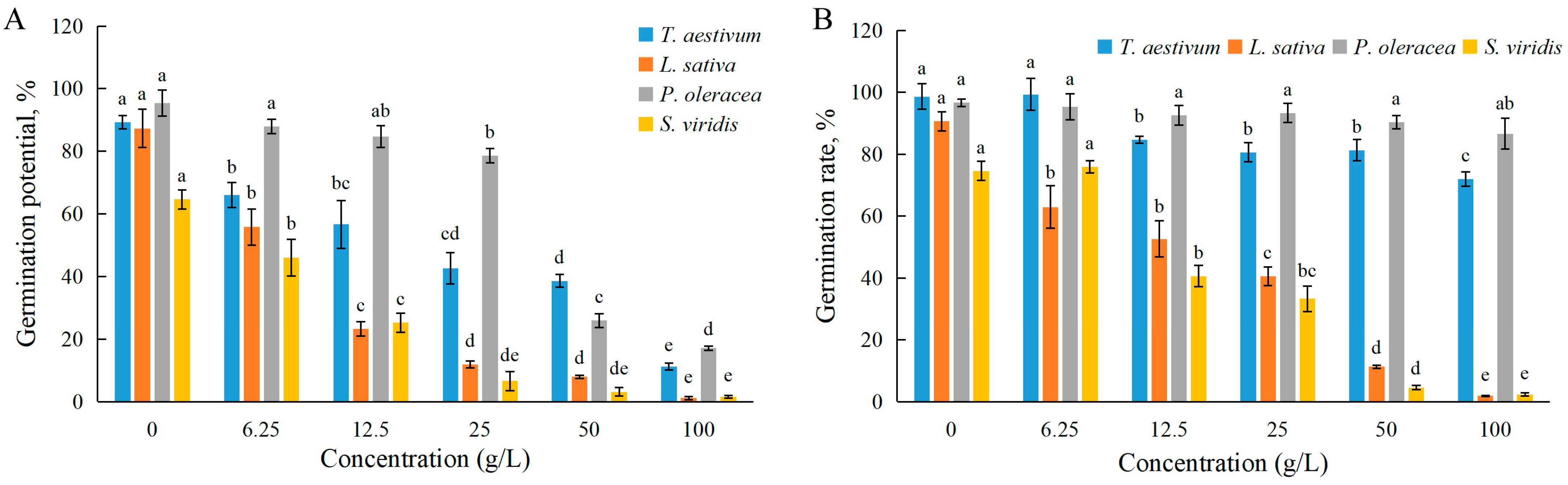
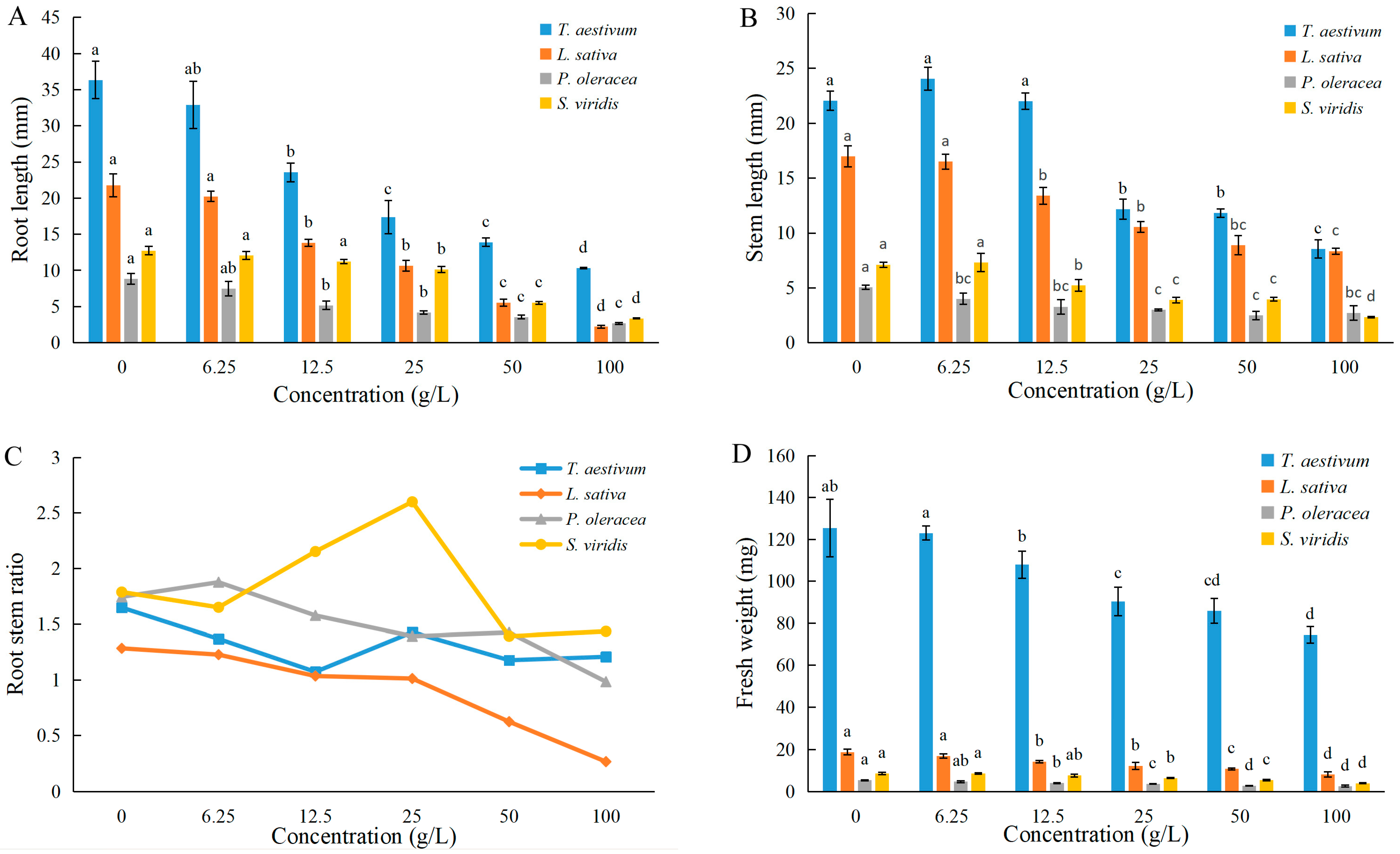
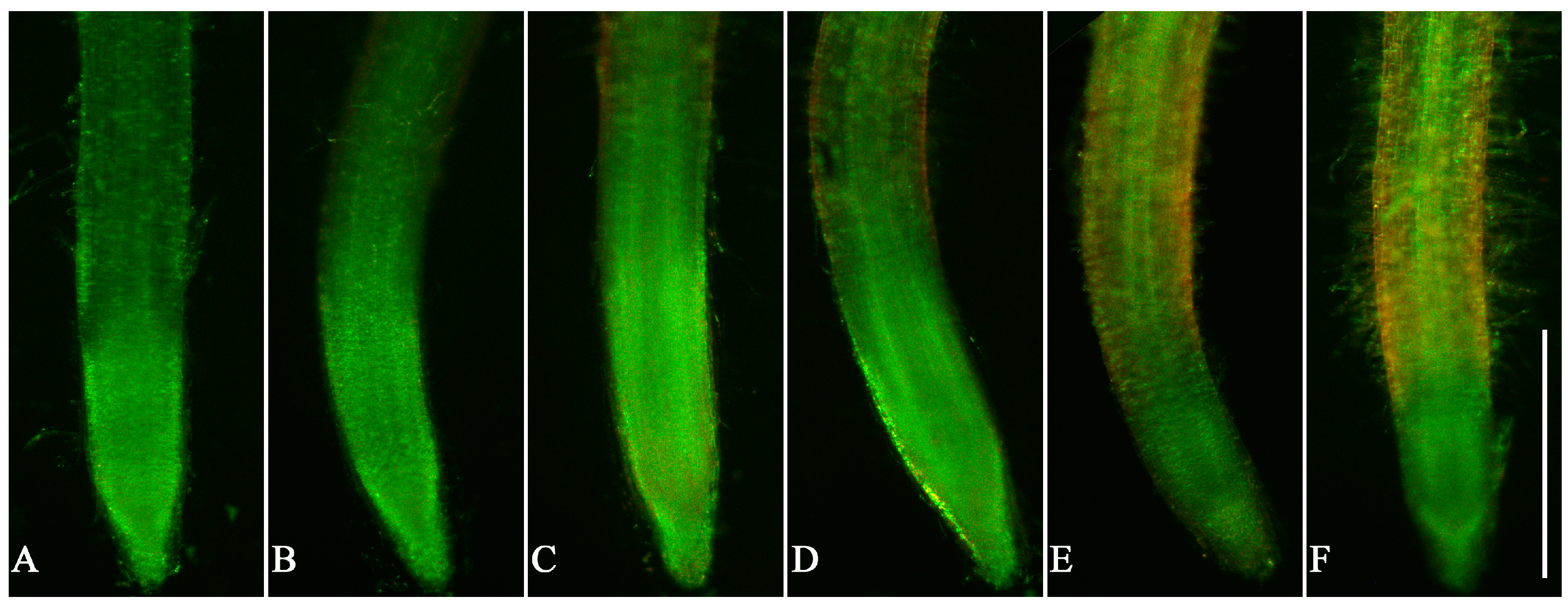
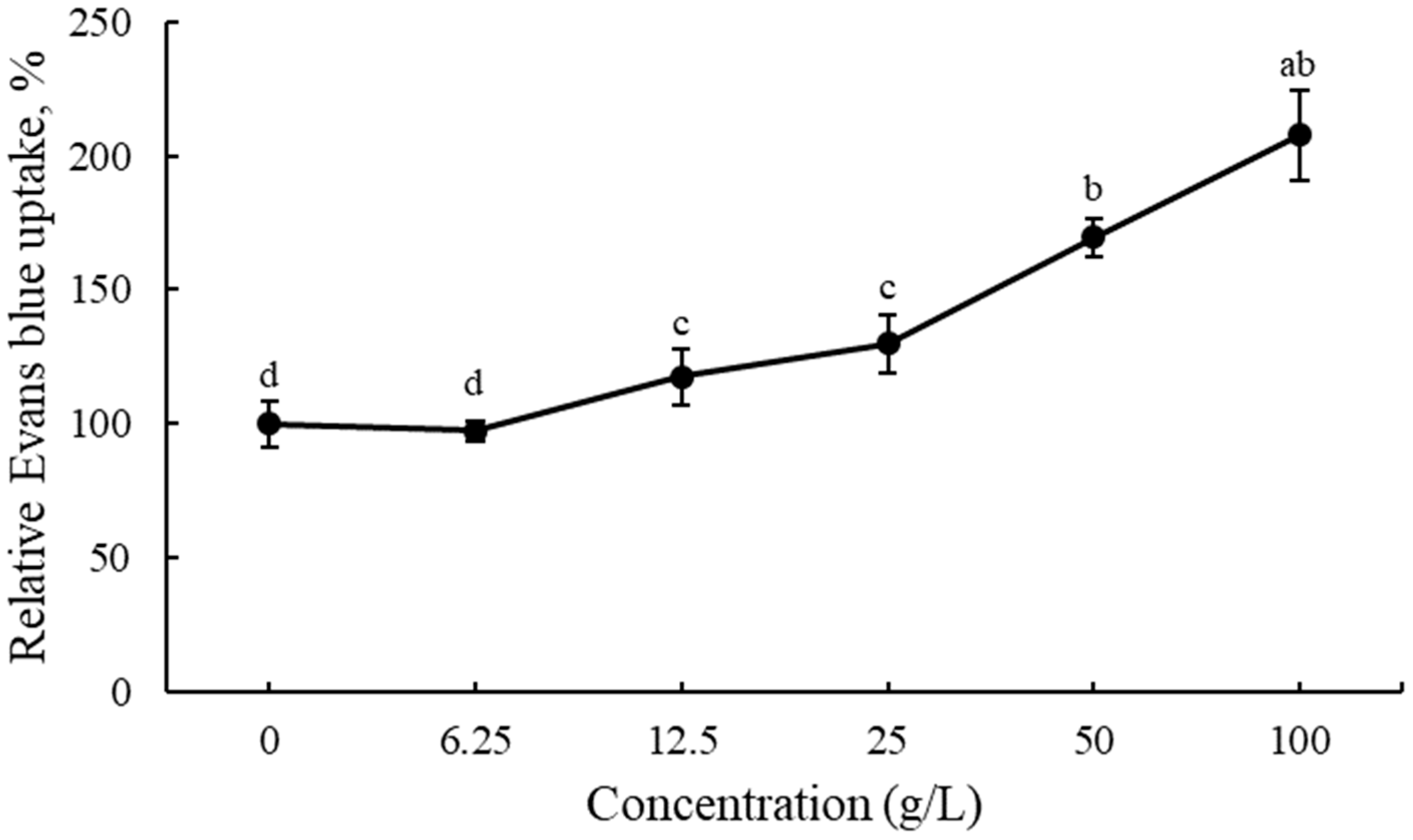
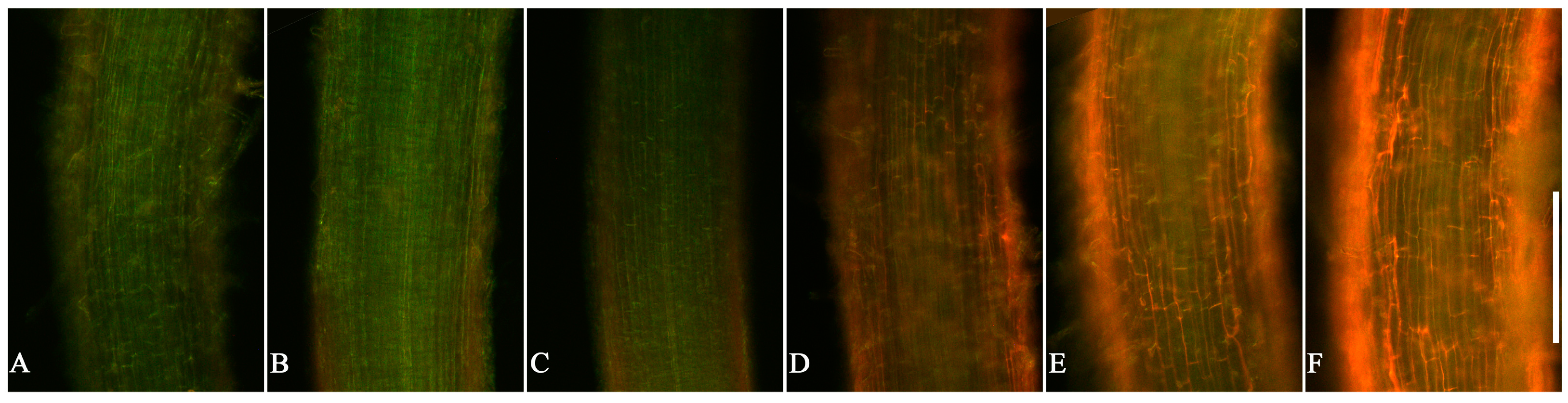
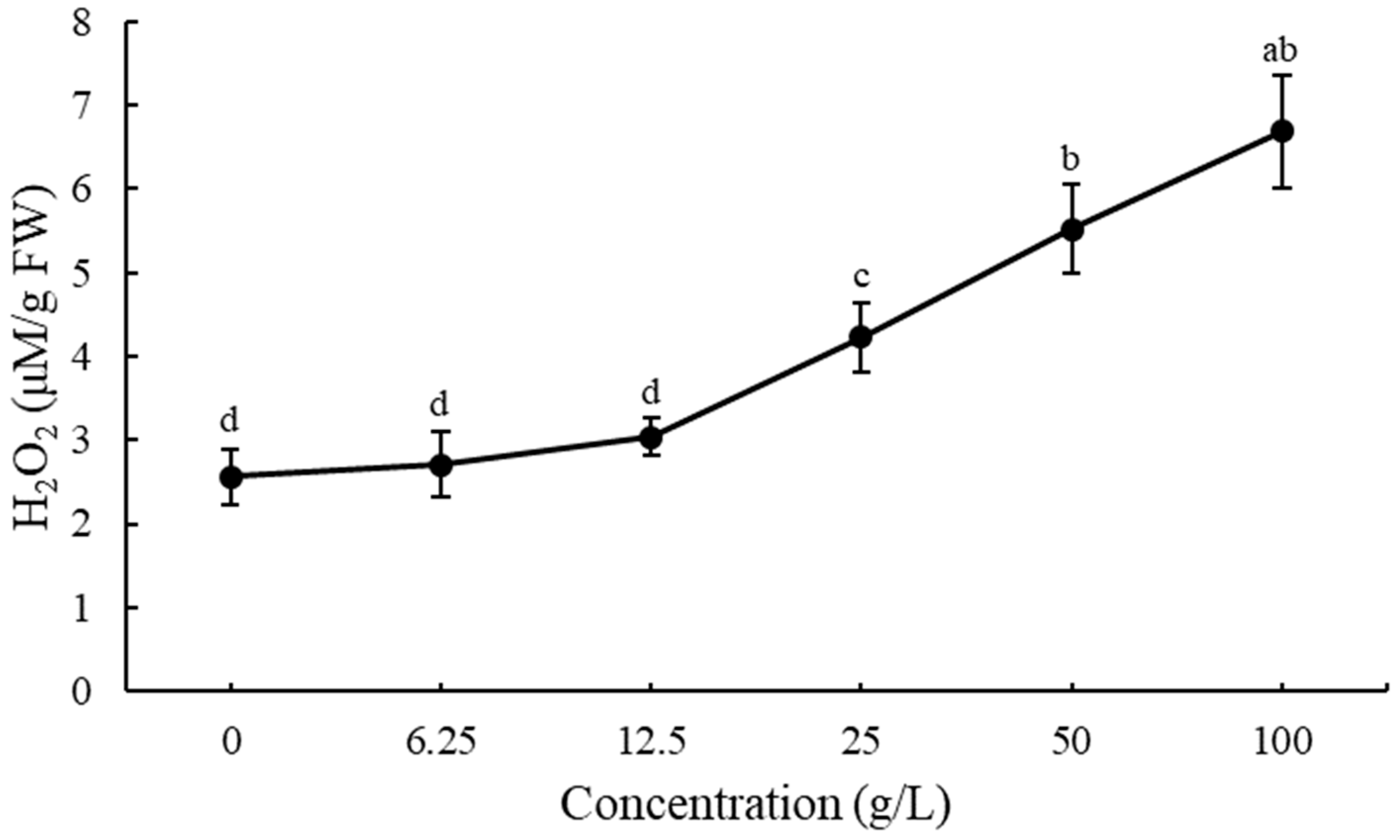

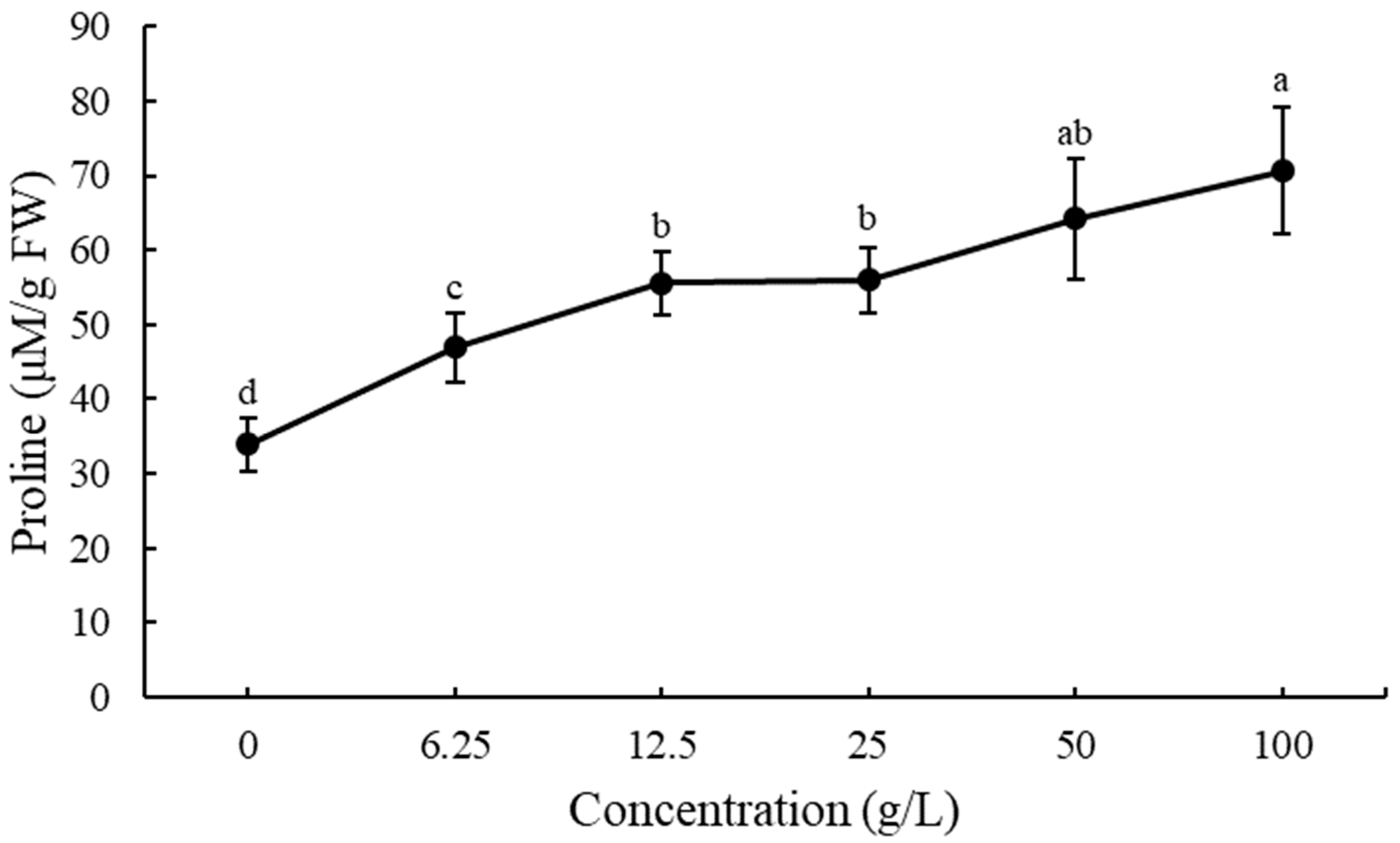
| Germination Potential | Germination Rate | Root Length | Stem Length | Fresh Weight | |
|---|---|---|---|---|---|
| Wheat | 22.7 | >100 | 25.6 | 53.1 | >100 |
| Lettuce | 8.9 | 17.5 | 23.4 | 89.0 | 91.2 |
| Purslane | 34.6 | >100 | 21.5 | >100 | 95.7 |
| Green bristlegrass | 9.2 | 18.2 | 47.8 | 77.4 | 93.9 |
| No. | Proposed Compounds | Molecular Formula | MW | Mass Error (ppm) | Main Fragment MS2 | RT (min) | Peak Area (%) |
|---|---|---|---|---|---|---|---|
| 1 | 2-Pyrrolidinecarboxylic acid | C5H9NO2 | 115.06315 | −1.55 | 70.0652, 68.0496, 98.0599 | 2.21 | 2.57 |
| 2 | Nicotinic acid | C6H5NO2 | 123.0319 | −1.04 | 80.0494, 96.0344, 124.0393 | 2.23 | 5.31 |
| 3 | Quinic acid | C7H12O6 | 192.06316 | −1.21 | 173.0088, 111.0086, 191.0195, 85.0293 | 2.23 | 0.56 |
| 4 | Maleic acid | C4H4O4 | 116.01076 | −1.7 | 115.0035, 113.0244, 71.0137, 87.0086 | 2.26 | 4.01 |
| 5 | 7-Hydroxycoumarin | C9H6O3 | 162.03147 | −1.41 | 149.0450, 145.0282, 107.0490, 135.0440, 95.0490, 79.0542, 53.0390 | 2.32 | 6.45 |
| 6 | Citric acid | C6H8O7 | 192.0268 | −1.03 | 173.0090, 154.9982, 129.0193, 111.0086, 87.0086, 85.0293, 67.0188, 57.0344 | 2.38 | 14.84 |
| 7 | Fumaric acid | C4H4O4 | 116.01077 | −1.66 | 71.0138 | 4.72 | 1.07 |
| 8 | Harpagide | C15H24O10 | 364.13657 | −1.04 | 183.0660, 89.0243, 201.0771, 165.0555, 157.0504 | 21.15 | 6.82 |
| 9 | Paeonol | C9H10O3 | 166.06284 | −0.94 | 149.0596, 91.0543 | 21.15 | 1.65 |
| 10 | Caffeic acid | C9H8O4 | 180.04207 | −1.07 | 135.0451, 133.0296, 117.0344, 107.0501, 89.0396 | 23.55 | 5.16 |
| 11 | Protocatechuic acid | C7H6O4 | 154.02643 | −1.18 | 109.0293, 91.0188, 80.9650, 65.0396, 81.0344 | 23.72 | 1.88 |
| 12 | Rosarin | C20H28O10 | 428.16767 | −1.34 | 110.0242 | 25.24 | 2.30 |
| 13 | Cinnamic acid | C9H8O2 | 148.0522 | −1.54 | 121.0647, 93.0698, 81.0696, 65.0389, 107.0853 | 25.56 | 2.08 |
| 14 | 4-Methoxysalicylic acid | C8H8O4 | 168.04203 | −1.38 | 167.0347, 108.0215 | 26.29 | 0.50 |
| 15 | Azelaic acid | C9H16O4 | 188.10461 | −1.32 | 125.0970, 123.0815, 97.0658, 83.0502, 57.0345 | 26.84 | 1.11 |
| 16 | Parthenolide | C15H20O3 | 248.14088 | −1.47 | 249.1481, 231.1378, 213.1273, 203.1431, 185.1325, 145.1011, 119.0855, 105.0698 | 26.92 | 2.46 |
| 17 | Salicylic acid | C7H6O3 | 138.03152 | −1.24 | 108.0214, 94.0377, 93.0344, 65.0395 | 27.37 | 2.70 |
| 18 | Methylparaben | C8H8O3 | 152.04717 | −1.17 | 153.0546, 125.0597 | 27.54 | 0.71 |
| 19 | Abscisic acid | C15H20O4 | 264.13569 | −1.78 | 219.1388, 204.1153, 51.0763, 97.0291 | 28.77 | 15.64 |
| 20 | Luteolin | C15H10O6 | 286.04745 | −1 | 241.0502, 217.0501, 134.0327, 152.0068, 267.0289, 257.0456, 213.0561, 151.0035, 197.0608, 283.0315, 175.0398, 133.0293 | 29.21 | 3.99 |
| 21 | Apigenin | C15H10O5 | 270.05238 | −1.62 | 269.0456 | 31.14 | 9.87 |
| 22 | Diosmetin | C16H12O6 | 300.06299 | −1.32 | 299.0558, 284.0323 | 31.58 | 0.87 |
| 23 | Isorhamnetin | C16H12O7 | 316.058 | −0.96 | 300.0271, 271.0242, 163.0025, 151.0035 | 31.88 | 1.04 |
| 24 | Clareolide | C16H26O2 | 250.19287 | −1.63 | 121.1011 | 33.94 | 0.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Tan, J.; Yu, Y.; Dong, J.; Cao, L.; Yao, L.; Zhang, Y.; Yan, Z. Phytotoxic Effects and Potential Allelochemicals from Water Extracts of Paulownia tomentosa Flower Litter. Agronomy 2024, 14, 367. https://doi.org/10.3390/agronomy14020367
Xiao Y, Tan J, Yu Y, Dong J, Cao L, Yao L, Zhang Y, Yan Z. Phytotoxic Effects and Potential Allelochemicals from Water Extracts of Paulownia tomentosa Flower Litter. Agronomy. 2024; 14(2):367. https://doi.org/10.3390/agronomy14020367
Chicago/Turabian StyleXiao, Yali, Jing Tan, Yi Yu, Jiajia Dong, Lingling Cao, Lunguang Yao, Yingjun Zhang, and Zhiqiang Yan. 2024. "Phytotoxic Effects and Potential Allelochemicals from Water Extracts of Paulownia tomentosa Flower Litter" Agronomy 14, no. 2: 367. https://doi.org/10.3390/agronomy14020367





