The Substitution of Sphagnum for Peat as a Culture Substrate Reduces N2O Emissions from Vegetable Production Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Design
2.2. Collection of Substrate and Gas Samples
2.3. Analysis of Substrate Samples
2.4. Gas Sample Analysis and Microbial Pathways of N2O Emissions
2.5. qPCR Assay and High-Throughput Sequencing
2.6. Contribution Analysis of Microbial Pathways for N2O Emissions
2.7. Statistical Analysis
3. Results
3.1. Physicochemical Properties, Lettuce Yields, and Nitrogen Use Efficiency of All the Treatments
3.2. Mineral Nitrogen and N2O Emissions of All the Treatments
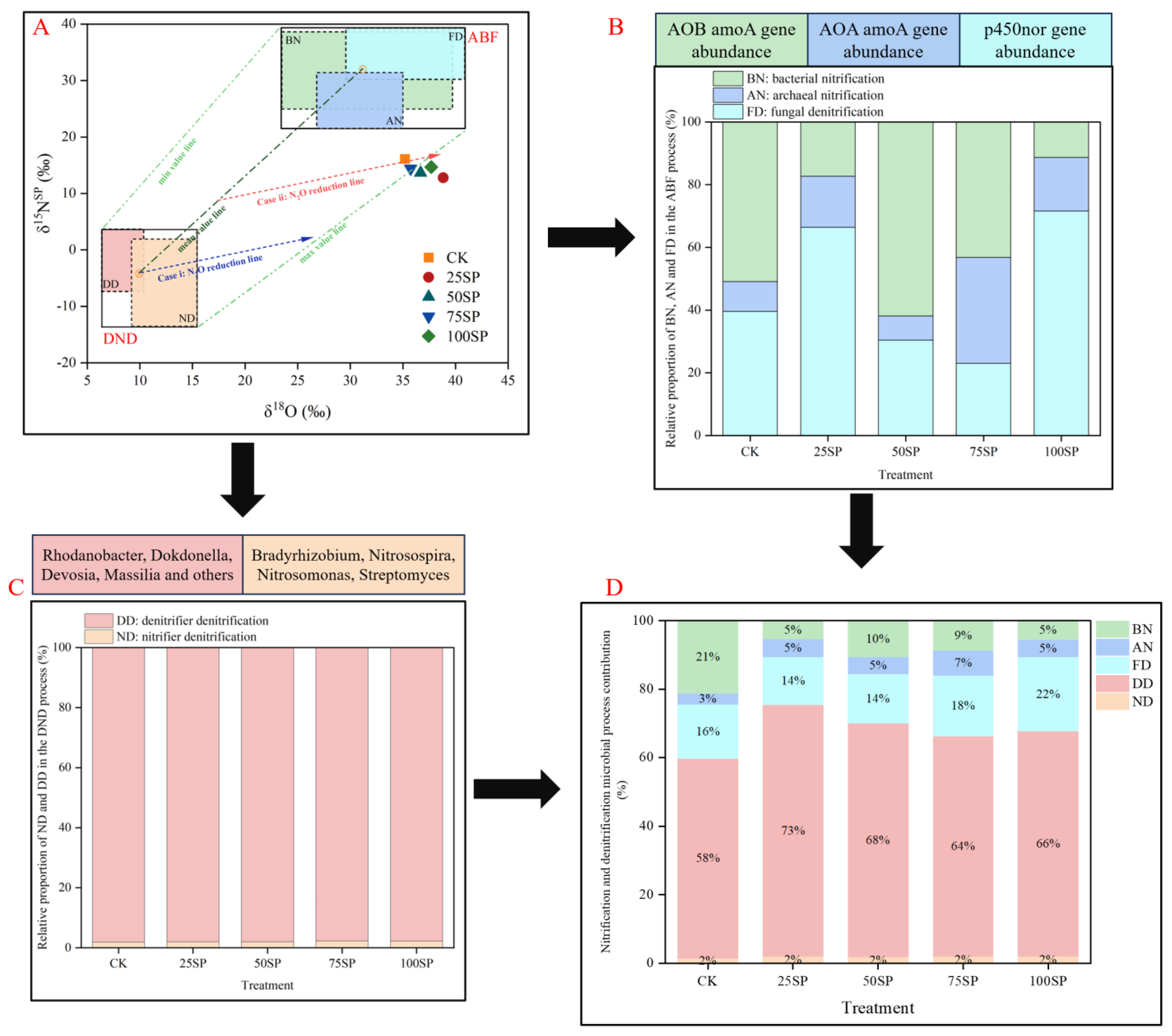
3.3. Isotope Analysis of All the Treatments
3.4. qPCR Assay of All the Treatments
3.5. Microbial Community Composition of Genera
3.6. Correlation Analysis between Microbial Communities and Physicochemical Properties
4. Discussion
4.1. Effects of Different Sphagnum Substitution Rates on N2O Emissions
4.2. Microbial Mechanisms of N2O Production and Consumption
4.3. The Correlation between Microbial Action and Environmental Factors due to Sphagnum Substitution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gruda, N.S. Advances in Horticultural Soilless Culture; Burleigh Dodds Science Publishing: London, UK, 2021. [Google Scholar] [CrossRef]
- Taskila, S.; Sarkela, R.; Tanskanen, J. Valuable applications for peat moss. Biomass Convers. Biorefinery 2016, 6, 115–126. [Google Scholar] [CrossRef]
- Baranyai, B.; Krebs, M.; Oehmke, C.; Joosten, H. Total biomass and annual yield of Drosera on cultivated Sphagnum in north-west Germany. Mires Peat 2022, 28, 19. [Google Scholar] [CrossRef]
- Wichmann, S.; Krebs, M.; Kumar, S.; Gaudig, G. Paludiculture on former bog grassland: Profitability of Sphagnum farming in North West Germany. Mires Peat 2020, 26, 18. [Google Scholar] [CrossRef]
- Muller, R.; Glatzel, S. Sphagnum farming substrate is a competitive alternative to traditional horticultural substrates for achieving desired hydro-physical properties. Mires Peat 2021, 27, 1–12. [Google Scholar] [CrossRef]
- Lin, W.; Li, Q.; Zhou, W.; Yang, R.; Zhang, D.; Wang, H.; Li, Y.; Qi, Z.; Li, Y. Insights into production and consumption processes of nitrous oxide emitted from soilless culture systems by dual isotopocule plot and functional genes. Sci. Total Environ. 2023, 856, 159046. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, N.C.; Tenesaca, C.G.; VanLoocke, A.; Hall, S.J. Nitrous oxide emissions from agricultural soils challenge climate sustainability in the US Corn Belt. Proc. Natl. Acad. Sci. USA 2021, 118, e2112108118. [Google Scholar] [CrossRef] [PubMed]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Aksenov, A.S.; Shirokova, L.S.; Kisil, O.Y.; Kolesova, S.N.; Lim, A.G.; Kuzmina, D.; Pouillé, S.; Alexis, M.A.; Castrec-Rouelle, M.; Loiko, S.V.; et al. Bacterial Number and Genetic Diversity in a Permafrost Peatland (Western Siberia): Testing a Link with Organic Matter Quality and Elementary Composition of a Peat Soil Profile. Diversity 2021, 13, 328. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, Z.; Zhang, Z. Effects of heavy metal pollution and soil physicochemical properties on the Sphagnum farmland soil microbial community structure in Southern Guizhou, China. Int. J. Phytoremediation 2023, 25, 1762–1773. [Google Scholar] [CrossRef]
- Liang, X.; Zhou, W.; Yang, R.; Zhang, D.; Wang, H.; Li, Q.; Qi, Z.; Li, Y.; Lin, W. Microbial mechanism of biochar addition to reduce N2O emissions from soilless substrate systems. J. Environ. Manag. 2023, 348, 119326. [Google Scholar] [CrossRef]
- Johannes, H.; Hans-Martin, K.; Stefanie, S.; Reiner, R.; Markus, F.; Thomas, S.; Andreas, K.; Sebastian, B. Linking N2O emissions from biochar-amended soil to the structure and function of the N-cycling microbial community. ISME J. 2014, 8, 660–674. [Google Scholar]
- Wang, J.; Zou, J. No-till increases soil denitrification via its positive effects on the activity and abundance of the denitrifying community. Soil Biol. Biochem. 2020, 142, 107706. [Google Scholar] [CrossRef]
- Aldossari, N.; Ishii, S. Fungal denitrification revisited—Recent advancements and future opportunities. Soil Biol. Biochem. 2021, 157, 108250. [Google Scholar] [CrossRef]
- Lin, W.; Ding, J.; Xu, C.; Zheng, Q.; Zhuang, S.; Mao, L.; Li, Q.; Liu, X.; Li, Y. Evaluation of N2O sources after fertilizers application in vegetable soil by dual isotopocule plots approach. Environ. Res. 2020, 188, 109818. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Ding, J.; Li, Y.; Zhang, W.; Ahmad, R.; Xu, C.; Mao, L.; Qiang, X.; Zheng, Q.; Li, Q. Partitioning of sources of N2O from soil treated with different types of fertilizers by the acetylene inhibition method and stable isotope analysis. Eur. J. Soil Sci. 2019, 70, 1037–1048. [Google Scholar] [CrossRef]
- Masta, M.; Espenberg, M.; Gadegaonkar, S.S.; Pärn, J.; Sepp, H.; Kirsimäe, K.; Sgouridis, F.; Müller, C.; Mander, Ü. Integrated isotope and microbiome analysis indicates dominance of denitrification in N2O production after rewetting of drained fen peat. Biogeochemistry 2022, 161, 119–136. [Google Scholar] [CrossRef]
- Lewicka-Szczebak, D.; Dyckmans, J.; Kaiser, J.; Marca, A.; Augustin, J.; Well, R. Oxygen isotope fractionation during N2O production by soil denitrification. Biogeosciences 2016, 13, 1129–1144. [Google Scholar] [CrossRef]
- Lewicka-Szczebak, D.; Augustin, J.; Giesemann, A.; Well, R. Quantifying N2O reduction to N2 based on N2O isotopocules–validation with independent methods (helium incubation and 15N gas flux method). Biogeosciences 2017, 14, 711–732. [Google Scholar] [CrossRef]
- Guo, S.; Wu, J.; Han, Z.; Li, Z.; Xu, P.; Liu, S.; Wang, J.; Zou, J. The legacy effect of biochar application on soil nitrous oxide emissions. GCB Bioenergy 2022, 15, 478–493. [Google Scholar] [CrossRef]
- Malghani, S.; Yoo, G.-y.; Giesemann, A.; Well, R.; Kang, H. Combined application of organic manure with urea does not alter the dominant biochemical pathway producing N2O from urea treated soil. Biol. Fertil. Soils 2019, 56, 331–343. [Google Scholar] [CrossRef]
- Masta, M.; Espenberg, M.; Kuusemets, L.; PÄRn, J.; Thayamkottu, S.; Sepp, H.; KirsimÄE, K.; Sgouridis, F.; Kasak, K.; Soosaar, K.; et al. 15N tracers and microbial analyses reveal in situ N2O sources in contrasting water regimes on drained peatland forest. Pedosphere 2023, in press. [CrossRef]
- Xuan, Y.; Mai, Y.; Xu, Y.; Zheng, J.; He, Z.; Shu, L.; Cao, Y. Enhanced microbial nitrification-denitrification processes in a subtropical metropolitan river network. Water Res. 2022, 222, 118857. [Google Scholar] [CrossRef]
- Mateus-Barros, E.; Meneghine, A.K.; Bagatini, I.L.; Fernandes, C.C.; Kishi, L.T.; Vieira, A.A.H.; Sarmento, H. Comparison of two DNA extraction methods widely used in aquatic microbial ecology. J. Microbiol. Methods 2019, 159, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Hernandez-Ramirez, G. Nitrous oxide emissions from manured soils as a function of various nitrification inhibitor rates and soil moisture contents. Sci. Total Environ. 2020, 738, 139669. [Google Scholar] [CrossRef]
- Guo, Y.; Geng, J.; Cheng, S.; Fang, H.; Li, X.; Yang, Y.; Li, Y.; Zhou, Y. Soil acidification and ammonia-oxidizing archaeal abundance dominate the contrasting responses of soil N2O emissions to NH4+ and NO3− enrichment in a subtropical plantation. Eur. J. Soil Biol. 2023, 116, 103491. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, T.; Wang, S.; Wang, Z. Soil pH and C/N ratio determines spatial variations in soil microbial communities and enzymatic activities of the agricultural ecosystems in Northeast China: Jilin Province case. Appl. Soil Ecol. 2020, 155, 103629. [Google Scholar] [CrossRef]
- Bagheri Novair, S.; Mirseyed Hosseini, H.; Etesami, H.; Razavipour, T. Rice straw and composted azolla alter carbon and nitrogen mineralization and microbial activity of a paddy soil under drying–rewetting cycles. Appl. Soil Ecol. 2020, 154, 103638. [Google Scholar] [CrossRef]
- Liu, X.; Rezaei Rashti, M.; Van Zwieten, L.; Esfandbod, M.; Rose, M.T.; Chen, C. Microbial carbon functional responses to compaction and moisture stresses in two contrasting Australian soils. Soil Tillage Res. 2023, 234, 105825. [Google Scholar] [CrossRef]
- Volpi, I.; Laville, P.; Bonari, E.; di Nasso, N.N.o.; Bosco, S. Improving the management of mineral fertilizers for nitrous oxide mitigation: The effect of nitrogen fertilizer type, urease and nitrification inhibitors in two different textured soils. Geoderma 2017, 307, 181–188. [Google Scholar] [CrossRef]
- Yamamoto, A.; Akiyama, H.; Nakajima, Y.; Hoshino, Y.T. Estimate of bacterial and fungal N2O production processes after crop residue input and fertilizer application to an agricultural field by 15N isotopomer analysis. Soil Biol. Biochem. 2017, 108, 9–16. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Q.; Yang, R.; Zhuang, S.; Lin, W.; Li, Y. Evaluating microbial role in reducing N2O emission by dual isotopocule mapping following substitution of inorganic fertilizer for organic fertilizer. J. Clean. Prod. 2021, 326, 129442. [Google Scholar] [CrossRef]
- Liao, J.; Hu, A.; Zhao, Z.; Liu, X.; Jiang, C.; Zhang, Z. Biochar with large specific surface area recruits N2O-reducing microbes and mitigate N2O emission. Soil Biol. Biochem. 2021, 156, 108212. [Google Scholar] [CrossRef]
- Wu, D.; Well, R.; Cardenas, L.M.; Fuss, R.; Lewicka-Szczebak, D.; Koster, J.R.; Bruggemann, N.; Bol, R. Quantifying N2O reduction to N2 during denitrification in soils via isotopic mapping approach: Model evaluation and uncertainty analysis. Environ. Res 2019, 179, 108806. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Wang, D.; Lui Lauren, M.; Nielsen, T.; Tian, R.; Kempher Megan, L.; Tao, X.; Pan, C.; Chakraborty, R.; Deutschbauer Adam, M.; et al. Genomic Features and Pervasive Negative Selection in Rhodanobacter Strains Isolated from Nitrate and Heavy Metal Contaminated Aquifer. Microbiol. Spectr. 2022, 10, e02591-21. [Google Scholar] [CrossRef] [PubMed]
- Dahal, R.H.; Kim, J. Rhodanobacter humi sp. nov., an acid-tolerant and alkalitolerant gammaproteobacterium isolated from forest soil. Int. J. Syst. Evol. Microbiol. 2017, 67, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, Y.H. Comparative analysis of bacteria associated with different mosses by 16S rRNA and 16S rDNA sequencing. J. Basic Microbiol. 2017, 57, 57–67. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, W.; Wang, X.; Wang, F.; Dong, W.; Hu, C.; Liu, B.; Sun, R. Nitrogen leaching greatly impacts bacterial community and denitrifiers abundance in subsoil under long-term fertilization. Agric. Ecosyst. Environ. 2020, 294, 106885. [Google Scholar] [CrossRef]
- Yang, Z.; She, R.; Hu, L.; Yu, Y.; Yao, H. Effects of biochar addition on nitrous oxide emission during soil freeze–thaw cycles. Front. Microbiol. 2022, 13, 1033210. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, Y.; Wang, B.; Zhang, H.; Ding, J. Effects of Stepwise Adjustment of C/N during the Start-Up of Submerged Membrane Bioreactors (SMBRs) on the Aerobic Denitrification of Wastewater. Water 2021, 13, 3251. [Google Scholar] [CrossRef]
- van den Heuvel, R.N.; van der Biezen, E.; Jetten, M.S.; Hefting, M.M.; Kartal, B. Denitrification at pH 4 by a soil-derived Rhodanobacter-dominated community. Environ. Microbiol. 2010, 12, 3264–3271. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, Z.T.; Shen, R.F.; Zhao, X.Q. Nitrate has a stronger rhizobacterial-based effect on rice growth and nitrogen use than ammonium in acidic paddy soil. Plant Soil 2023, 487, 605–621. [Google Scholar] [CrossRef]
- Qin, H.; Xing, X.; Tang, Y.; Zhu, B.; Wei, X.; Chen, X.; Liu, Y. Soil moisture and activity of nitrite- and nitrous oxide-reducing microbes enhanced nitrous oxide emissions in fallow paddy soils. Biol. Fertil. Soils 2019, 56, 53–67. [Google Scholar] [CrossRef]
- Harpenslager, S.F.; van den Elzen, E.; Kox, M.A.R.; Smolders, A.J.P.; Ettwig, K.F.; Lamers, L.P.M. Rewetting former agricultural peatlands: Topsoil removal as a prerequisite to avoid strong nutrient and greenhouse gas emissions. Ecol. Eng. 2015, 84, 159–168. [Google Scholar] [CrossRef]
- Kamarainen, A.; Jokinen, K.; Linden, L. Adding Sphagnum to peat growing medium improves plant performance under water restricting conditions. Mires Peat 2020, 26, 13. [Google Scholar] [CrossRef]


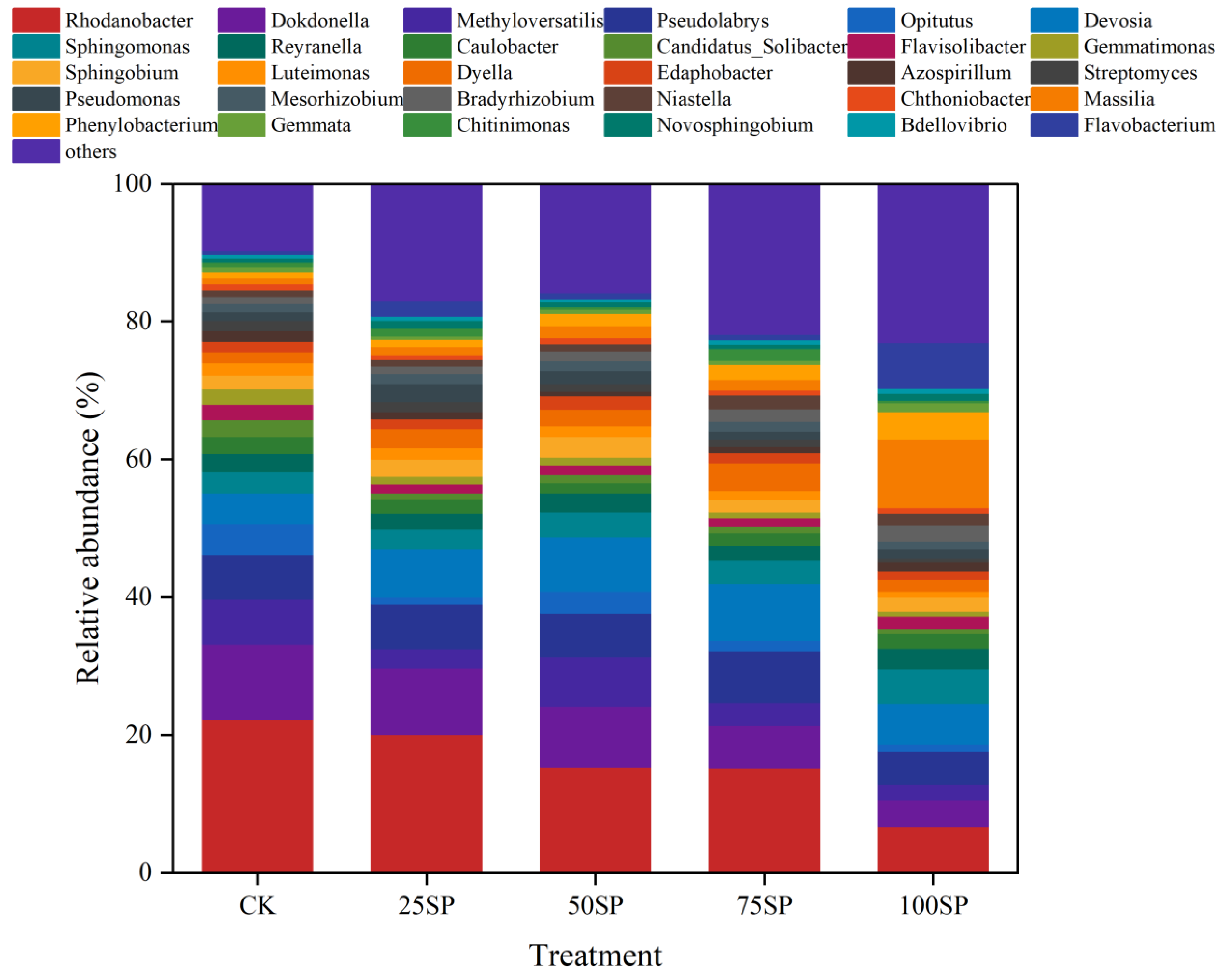

| Treatment | WFPS % | pH | EC μS cm−1 | C/N | NO3− mg kg−1 | NH4+ mg kg−1 | NO2− mg kg−1 | Bulk Density g cm−3 |
|---|---|---|---|---|---|---|---|---|
| CK | 62.5 ± 1.6 b | 6.4 ± 0.1 | 841.0 ± 353.6 b | 42.3 ± 2.3 a | 34.3 ± 10.5 c | 2.7 ± 0.5 c | 0.8 ± 0.3 | 0.17 ± 0.04 |
| 25SP | 62.1 ± 1.3 b | 6.5 ± 0.1 | 738.0 ± 137.4 b | 37.0 ± 2.6 b | 41.4 ± 4.8 c | 4.9 ± 0.7 c | 0.9 ± 0.2 | 0.15 ± 0.03 |
| 50SP | 70.6 ± 5.2 a | 6.5 ± 0.1 | 1016.7 ± 192.2 ab | 34.6 ± 1.5 bc | 55.4 ± 3.2 b | 12.6 ± 2.5 b | 1.3 ± 0.4 | 0.13 ± 0.03 |
| 75SP | 71.9 ± 6.0 a | 6.5 ± 0.1 | 1328.5 ± 377.8 a | 30.6 ± 4.3 cd | 61.2 ± 9.0 b | 23.2 ± 2.0 a | 1.3 ± 0.3 | 0.11 ± 0.02 |
| 100SP | 70.3 ± 3.5 a | 6.4 ± 0.2 | 1352.4 ± 104.6 a | 25.9 ± 2.8 d | 74.1 ± 3.6 a | 22.3 ± 2.6 a | 1.1 ± 0.2 | 0.08 ± 0.02 |
| NO3− | NH4+ | NO2− | pH | EC | C/N | WFPS | δ15Nα | δ15N | δ18O | |
|---|---|---|---|---|---|---|---|---|---|---|
| NO3− | 1 | 0.78 ** | 0.57 ** | −0.7 ** | 0.91 *** | −0.49 ** | 0.58 ** | −0.44 ** | −0.15 | −0.24 |
| NH4+ | 0.78 ** | 1 | 0.56 ** | −0.28 | 0.76 ** | −0.61 ** | 0.18 | 0.01 | −0.16 | 0.13 |
| NO2− | 0.57 ** | 0.56 ** | 1 | −0.34 * | 0.57 ** | −0.4 ** | 0.25 | −0.28 | −0.24 | −0.23 |
| N2O | 0.16 | 0.41 * | 0.21 | −0.08 | 0.35 ** | −0.28 | 0.06 | −0.11 | −0.14 | 0.05 |
| Treatment | δ15Nbulk ‰ | δ15Nα ‰ | δ18O ‰ | δ15NSP ‰ | ABF % | DND % | Scenario i % | Scenario ii % |
|---|---|---|---|---|---|---|---|---|
| CK | −5.5 | −13.6 | 35.2 | 16.1 | 40.0 | 60.0 | 67.2 | 84.6 |
| 25SP | −4.7 | −11.0 | 38.8 | 12.8 | 24.5 | 75.5 | 79.3 | 87.7 |
| 50SP | −3.2 | −10.7 | 36.7 | 13.7 | 30.0 | 70.0 | 74.2 | 85.7 |
| 75SP | −2.6 | −9.8 | 35.7 | 14.4 | 33.59 | 66.4 | 71.1 | 84.7 |
| 100SP | −0.1 | −7.4 | 37.7 | 14.7 | 32.25 | 67.8 | 75.1 | 87.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Wang, H.; Zhang, Y.; Yang, R.; Zhang, D.; Zhou, W.; Qi, Z.; Lin, W. The Substitution of Sphagnum for Peat as a Culture Substrate Reduces N2O Emissions from Vegetable Production Systems. Agronomy 2024, 14, 369. https://doi.org/10.3390/agronomy14020369
Liang X, Wang H, Zhang Y, Yang R, Zhang D, Zhou W, Qi Z, Lin W. The Substitution of Sphagnum for Peat as a Culture Substrate Reduces N2O Emissions from Vegetable Production Systems. Agronomy. 2024; 14(2):369. https://doi.org/10.3390/agronomy14020369
Chicago/Turabian StyleLiang, Xiaofeng, Hong Wang, Yudan Zhang, Rui Yang, Dongdong Zhang, Wanlai Zhou, Zhiyong Qi, and Wei Lin. 2024. "The Substitution of Sphagnum for Peat as a Culture Substrate Reduces N2O Emissions from Vegetable Production Systems" Agronomy 14, no. 2: 369. https://doi.org/10.3390/agronomy14020369
APA StyleLiang, X., Wang, H., Zhang, Y., Yang, R., Zhang, D., Zhou, W., Qi, Z., & Lin, W. (2024). The Substitution of Sphagnum for Peat as a Culture Substrate Reduces N2O Emissions from Vegetable Production Systems. Agronomy, 14(2), 369. https://doi.org/10.3390/agronomy14020369










