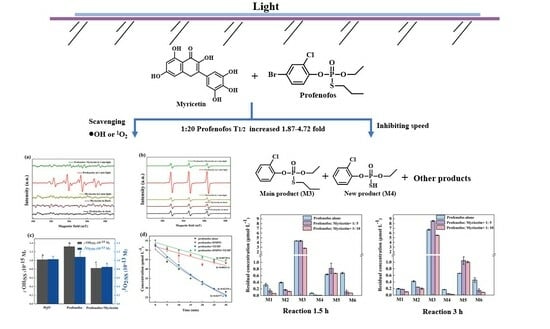
Myricetin Inhibits Photodegradation of Profenofos in Water: Pathways and Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Photolysis Experiments
2.3. Sample Analysis of Profenofos Photodegradation Kinetics
2.4. Identification and Quantification of Photoproducts
2.5. Detection of Free Radical Species and Steady-State Concentrations
2.6. Statistical Analysis and Comparison
3. Results and Discussion
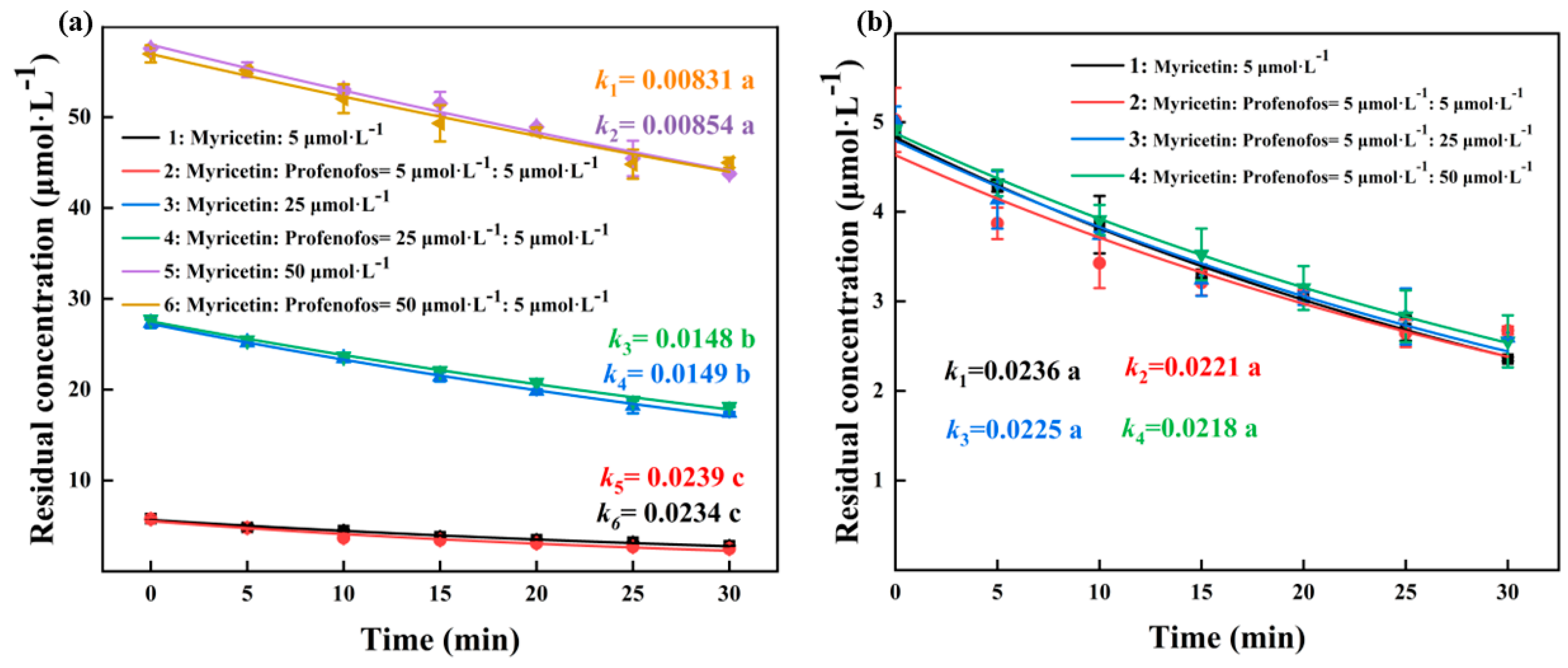
3.1. Myricetin Inhibited Profenofos Photolysis in Water under Artificial Light and Sunlight Irradiation
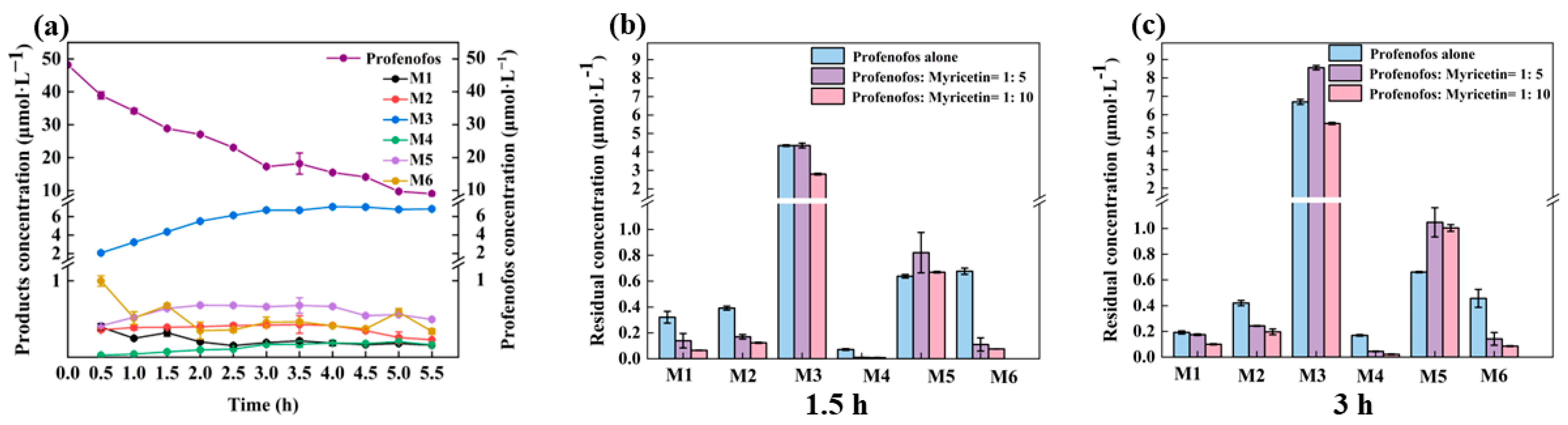
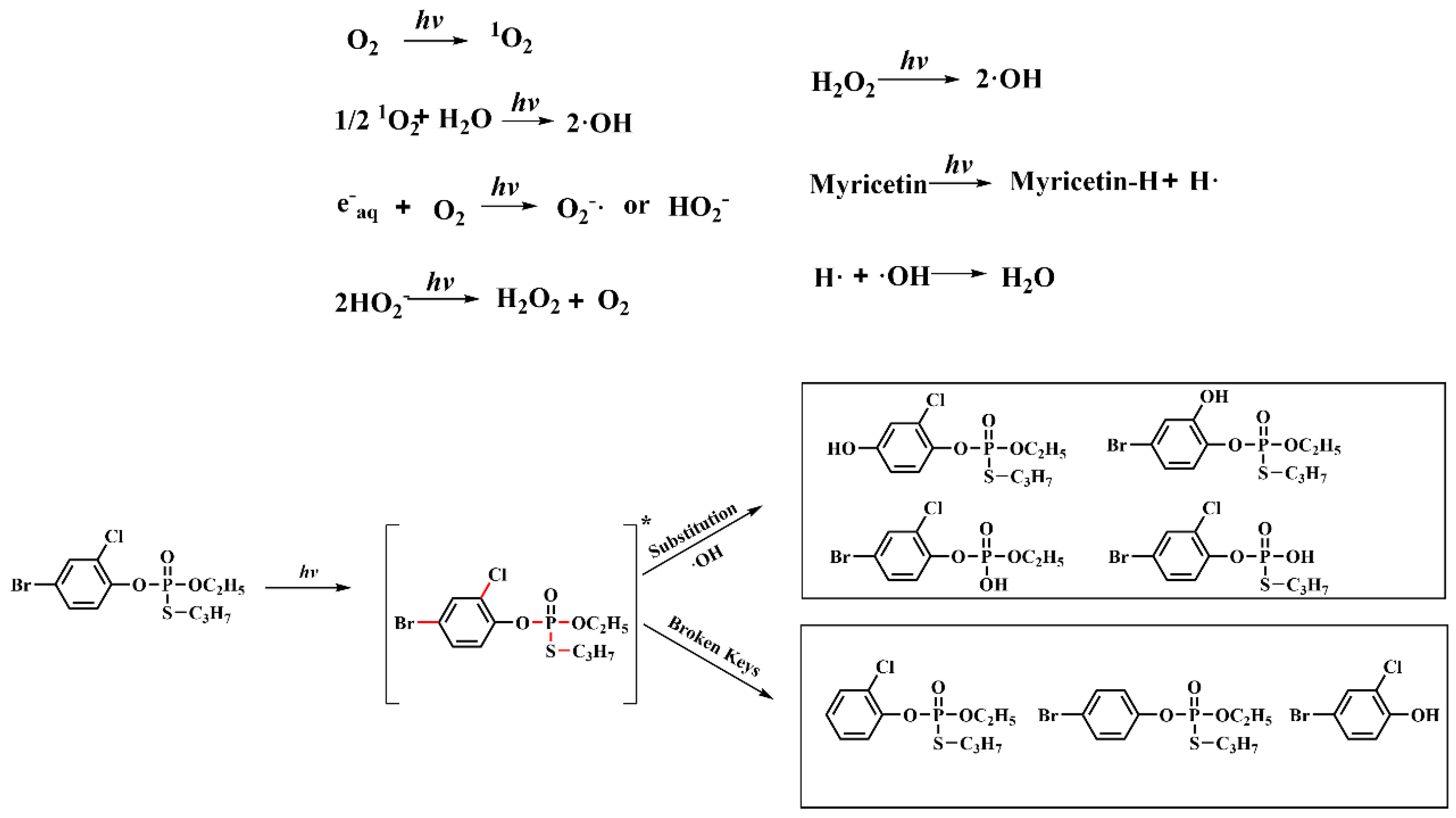
3.2. Photodegradation Pathway of Profenofos with and without Myricetin
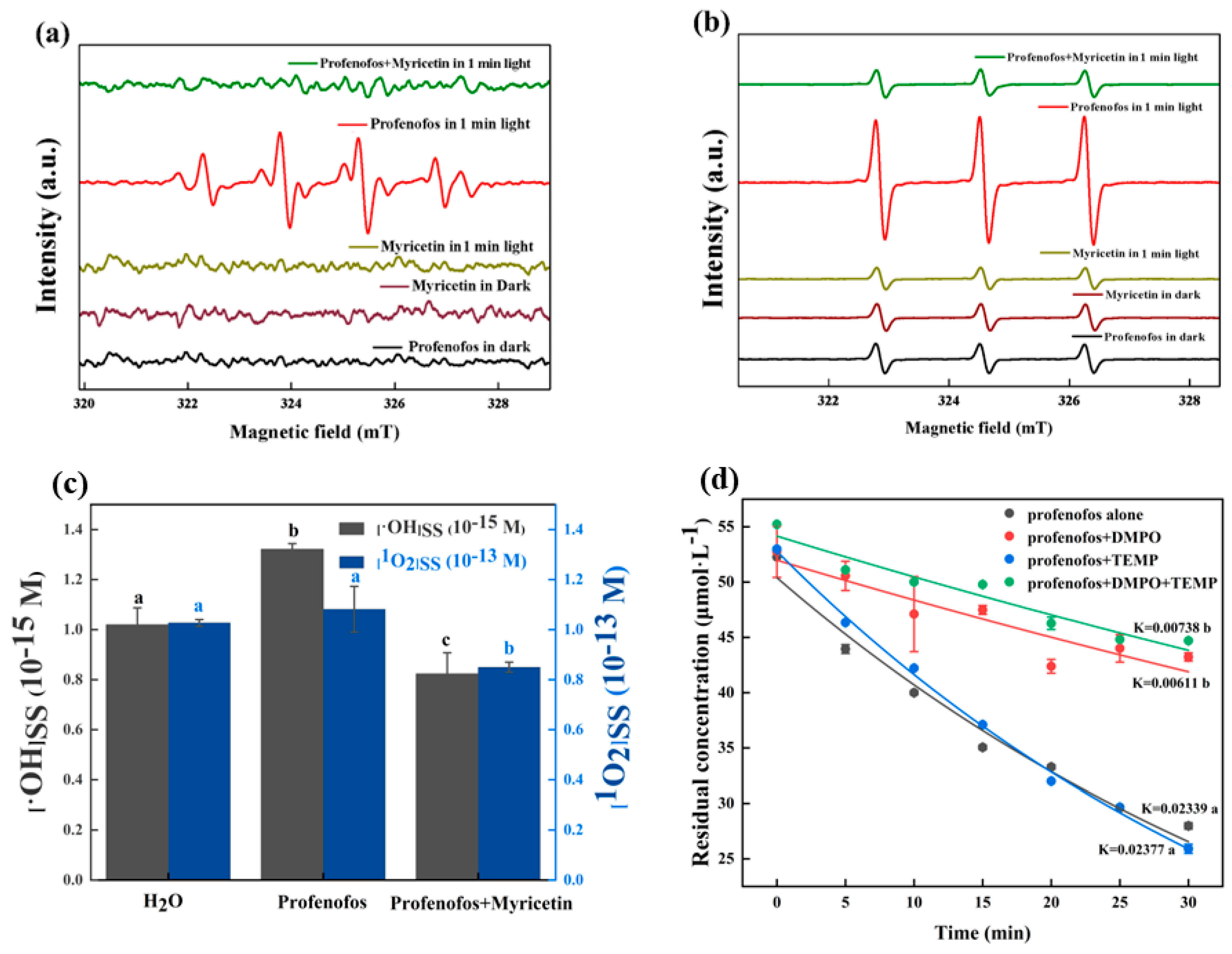
3.3. Scavenging Reactive Species of Myricetin on Profenofos Photodegradation
3.3.1. Generation of •OH and 1O2 during Profenofos Photolysis
3.3.2. Inhibition Mechanism of Myricetin on Profenofos Photodegradation
3.4. The Impact of Myricetin on the Photodegradation of Profenofos in Natural Water under Solar Irradiation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Dawson, A.H.; Eddleston, M.; Senarathna, L.; Mohamed, F.; Gawarammana, I.; Bowe, S.J.; Manuweera, G.; Buckley, N.A. Acute Human Lethal Toxicity of Agricultural Pesticides: A Prospective Cohort Study. PLoS Med. 2010, 7, 1000357. [Google Scholar] [CrossRef]
- Mostafalou, S.; Abdollahi, M. Pesticides: An update of human exposure and toxicity. Arch. Toxicol. 2017, 91, 549–599. [Google Scholar] [CrossRef]
- Kushwaha, M.; Verma, S.; Chatterjee, S. Profenofos, an Acetylcholinesterase-Inhibiting Organophosphorus Pesticide: A Short Review of Its Usage, Toxicity, and Biodegradation. J. Environ. Qual. 2016, 45, 1478–1489. [Google Scholar] [CrossRef]
- Clair, C.R.S.; Norris, E.J.; Masloski, K.E.; Coatsa, J.R.; Gassmann, A.J. Evaluation of pyrethroids and organophosphates in insecticide mixtures for management of western corn rootworm larvae. Pest Manag. Sci. 2020, 76, 3871–3878. [Google Scholar] [CrossRef]
- Loha, K.; Lamoree, M.; Boer, J. Pesticide residue levels in vegetables and surface waters at the Central Rift Valley (CRV) of Ethiopia. Environ. Monit. Assess. 2020, 192, 546. [Google Scholar] [CrossRef]
- Nyantakyi, J.A.; Wiafe, S.; Akoto, O. Seasonal Changes in Pesticide Residues in Water and Sediments from River Tano, Ghana. J. Environ. Public Health 2022, 2022, 8997449. [Google Scholar] [CrossRef]
- Chu, S.; Baker, M.R.; Leong, G.; Letcher, R.J.; Li, Q.X. Covalent binding of the organophosphate insecticide profenofos to tyrosine on α- and β-tubulin proteins. Chemosphere 2018, 199, 154–159. [Google Scholar] [CrossRef]
- Rajesh, L.; David, M. Hepatotoxic Potentials of Profenofos on Wistar Albino Rats: A Histopathological Study. Int. J. Pharm. Biol. Arch. 2014, 5, 70–75. Available online: http://www.ijpba.info/index.php/ijpba/article/download/1224/875/1996 (accessed on 26 September 2022).
- Pamanji, R.; Yashwanth, B.; Bethu, M.; Leelavathi, S.; Ravinder, K.; Rao, J.V. Toxicity effects of profenofos on embryonic and larval development of Zebrafish (Danio rerio). Environ. Toxicol. Pharmacol. 2015, 39, 887–897. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Liu, S.; Zhu, G. Lethal Effect and In Vivo Genotoxicity of Profenofos to Chinese Native Amphibian (Rana spinosa) Tadpoles. Arch. Environ. Contam. Toxicol. 2010, 59, 478–483. [Google Scholar] [CrossRef]
- Paidi, M.K.; Satapute, P.; Haider, M.S.; Udikeri, S.S.; Ramachandra, Y.L.; Vo, D.-V.N.; Govarthanan, M.; Jogaiah, S. Mitigation of organophosphorus insecticides from environment: Residual detoxification by bioweapon catalytic scavengers. Environ. Res. 2021, 200, 111368. [Google Scholar] [CrossRef] [PubMed]
- Zamy, C.; Mazellier, P.; Legube, B. Phototransformation of selected organophosphorus pesticides in dilute aqueous solutions. Water Res. 2004, 38, 2305–2314. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Kodikara, D.; Albi, L.S.; Hatano, Y.; Chen, G.; Yoshimura, C.; Wang, J. Photodegradation of organic micropollutants in aquatic environment: Importance, factors and processes. Water Res. 2023, 231, 118236. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.G.; Guo, Y.J.; Wang, Y.H.; Fan, S.J.; Wang, K.Q.; Han, W.H. A Systematic Review of Photolysis and Hydrolysis Degradation Modes, Degradation Mechanisms, and Identification Methods of Pesticides. J. Chem. 2022, 2022, 9552466. [Google Scholar] [CrossRef]
- Sarker, A.; Islam, T.; Kim, J.E. A pilot lab trial for enhanced oxidative transformation of procymidone fungicide and its aniline metabolite using heterogeneous MnO2 catalysts. Environ. Sci. Pollut. Res. 2023, 30, 3783–3794. [Google Scholar] [CrossRef]
- Lv, P.; Tao, Y.M.; Zhang, N.; Yang, Y.W.; Wu, X.W.; Li, Q.X.; Hua, R.M. Cyanidin-3-O-glucoside mediated photodegradation of profenofos in water. Chemosphere 2022, 308, 136170. [Google Scholar] [CrossRef] [PubMed]
- Samara, F.; Feghaly, F.; Kanan, S. Photocatalytic degradation of profenofos using silver-platinum doped zeolite. Catal. Today 2023, 424, 112602. [Google Scholar] [CrossRef]
- Ratpukdi, T.; Siripattanakul-Ratpukdi, S.; Thongprowh, P.; Soontharo, S.; Jenjaiwit, S.; Prasertwongchai, A.; Anotai, J.; Nongsung, P.; Khan, E. Photodegradation of profenofos in aqueous solution by vacuum ultraviolet. J. Photochem. Photobiol. A Chem. 2022, 433, 114179. [Google Scholar] [CrossRef]
- Cardoso, F.; Leal, I.; Nery, T. Characterization of Bioactive Compounds in Fruit and Vegetable Bagasse. J. Bioeng. 2021, 4, 58–62. [Google Scholar] [CrossRef]
- Kuppusamya, S.; Thavamani, P.; Megharaj, M.; Nirola, R.; Lee, Y.B.; Naidu, R. Assessment of antioxidant activity, minerals, phenols and flavonoid contents of common plant/tree waste extracts. Ind. Crops Prod. 2016, 83, 630–634. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, V.; Khan, M.; Balda, S.; Gupta, N.; Capalash, N.; Sharma, P. Flavonoid-rich agro-industrial residues for enhanced bacterial laccase production by submerged and solid-state fermentation. 3 Biotech 2017, 7, 200. [Google Scholar] [CrossRef]
- Corell, L.; Armenta, S.; Turrillas, F.; Guardia, M. Flavonoid determination in onion, chili and leek by hard cap espresso extraction and liquid chromatography with diode array detection. Microchem. J. 2018, 140, 74–79. [Google Scholar] [CrossRef]
- Jiang, H.; Engelhardt, U.; Thräne, C.; Maiwald, B.; Stark, J. Determination of flavonol glycosides in green tea, oolong tea and black tea by UHPLC compared to HPLC. Food Chem. 2015, 183, 30–35. [Google Scholar] [CrossRef]
- Zhu, T.; Yuri, L.; Kyungho, R. Preparation of a hybrid organic-inorganic monolith for extraction and purification of quercetin and myricetin from Chamaecyparis obtusa. Chem. Res. Chin. Univ. 2014, 30, 216–221. [Google Scholar] [CrossRef]
- He, J.; Giusti, M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A Dietary Molecule with Diverse Biological Activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.A.; Almeida, S.K.C.; Soares, I.N.; Barboza, C.A.; Freitas, R.G.; Brown, A.; de Souza, G.L.C. A computational investigation on the antioxidant potential of myricetin 3,4′-di-O-α-L-rhamnopyranoside. J. Mol. Model. 2018, 24, 133. [Google Scholar] [CrossRef]
- Lv, P.; Zhang, J.; Shi, T.; Dai, L.; Li, X.; Wu, X.; Li, X.; Tang, J.; Wang, Y.; Li, Q.X.; et al. Procyanidolic oligomers enhance photodegradation of chlorothalonil in water via reductive dechlorination. Appl. Catal. B 2017, 217, 137–143. [Google Scholar] [CrossRef]
- Lv, P.; Min, S.Y.; Wang, Y.; Zheng, X.Y.; Wu, X.W.; Li, Q.X.; Hua, R.M. Flavonoid-sensitized photolysis of chlorothalonil in water. Pest Manag. Sci. 2020, 76, 2972–2977. [Google Scholar] [CrossRef]
- Lv, P.; Wang, Y.; Zheng, X.Y.; Wu, X.W.; Li, Q.X.; Hua, R.M. Selective, stepwise photodegradation of chlorothalonil, dichlobenil and dichloro- and trichloro-isophthalonitriles enhanced by cyanidin in water. Sci. Total Environ. 2022, 805, 150157. [Google Scholar] [CrossRef]
- Pranczk, J.; Jacewicz, D.; Wyrzykowski, D.; Chmurzynski, L. Analytical Methods for Determination of Reactive Oxygen Species. Curr. Pharm. Anal. 2014, 10, 293–304. [Google Scholar] [CrossRef]
- Liu, K.; Gao, Y.; Liu, J.; Wen, Y.; Zhao, Y.; Zhang, K.; Yu, G. Photoreactivity of Metal–Organic Frameworks in Aqueous Solutions: Metal Dependence of Reactive Oxygen Species Production. Environ. Sci. Technol. 2016, 50, 3634–3640. [Google Scholar] [CrossRef]
- Zhao, Y.; Jafvert, C. Environmental photochemistry of single layered graphene oxide in water. Environ. Sci. Nano 2015, 2, 136–142. [Google Scholar] [CrossRef]
- Barzegar, A. The role of intramolecular H-bonds predominant effects in myricetin higher antioxidant activity. Comput. Theor. Chem. 2017, 1115, 239–247. [Google Scholar] [CrossRef]
- Abass, K.; Reponen, P.; Jalonen, J.; Pelkonen, O. In vitro metabolism and interaction of profenofos by human, mouse and rat liver preparations. Pestic. Biochem. Physiol. 2007, 87, 238–247. [Google Scholar] [CrossRef]
- Angthararuk, D.; Harir, M.; Schmitt-Kopplin, P.; Sutthivaiyakit, S.; Kettrup, A.; Sutthivaiyakit, P. Degradation products of profenofos as identified by high-field FTICR mass spectrometry: Isotopic fine structure approach. J. Environ. Sci. Health B 2016, 52, 10–22. [Google Scholar] [CrossRef]
- Fang, L.C.; Shi, Q.Y.; Xu, L.Y.; Shi, T.Z.; Wu, X.W.; Li, Q.X.; Hua, R.M. Enantioselective Uptake Determines Degradation Selectivity of Chiral Profenofos in Cupriavidus nantongensis X1T. J. Agric. Food Chem. 2020, 68, 6493–6501. [Google Scholar] [CrossRef]
- Liu, X.; Zhan, Y.; Zhang, Z.; Pan, L.; Hu, L.; Liu, K.; Zhou, X.; Bai, L. Photocatalytic Degradation of Profenofos and Triazophos Residues in the Chinese Cabbage, Brassica chinensis, Using Ce-Doped TiO2. Catalysts 2019, 9, 294. [Google Scholar] [CrossRef]
- Jabeen, H.; Lqbal, S.; Anwar, S.; Parales, R. Optimization of profenofos degradation by a novel bacterial consortium PBAC using response surface me ethodology. Int. Biodeterior. Biodegrad. 2015, 100, 89–97. [Google Scholar] [CrossRef]
- Li, X.; Ouyang, X.; Liang, M.; Chen, D. Comparative Analysis of Radical Adduct Formation (RAF) Products and Antioxidant Pathways between Myricetin-3-O-Galactoside and Myricetin Aglycone. Molecules 2019, 24, 2769. [Google Scholar] [CrossRef]
- Mostafa, S.; Rosario-Ortiz, F. Photochemical formation of singlet oxygen from wastewater derived organic matter. In Proceedings of the 245th National Spring Meeting of the American-Chemical-Society (ACS), New Orleans, LA, USA, 7–11 April 2013; Volume 245, pp. 187–192. Available online: https://www.researchgate.net/publication/293632940 (accessed on 18 June 2022).
- Zhang, D.; Yan, S.; Song, W. Photochemically Induced Formation of Reactive Oxygen Species (ROS) from Effluent Organic Matter. Environ. Sci. Technol. 2014, 48, 12645–12653. [Google Scholar] [CrossRef]
- Mostafa, S.; Rosario-Ortiz, F.L. Singlet Oxygen Formation from Wastewater Organic Matter. Environ. Sci. Technol. 2013, 47, 8179–8186. [Google Scholar] [CrossRef]
- Haag, W.; Hoigne, J. Singlet oxygen in surface waters. 3. Photochemical formation and steady-state concentrations in various types of waters. Environ. Sci. Technol. 1986, 20, 341–348. [Google Scholar] [CrossRef]
- Huang, F.; Gao, F.; Li, C.; Campos, L.C. Photodegradation of free estrogens driven by UV light: Effects of operation mode and water matrix. Sci. Total Environ. 2022, 835, 155515. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.F.; Chen, J.W.; Xie, Q. Direct and dissolved oxygen involved photodegradation of MeO-PBDEs in water. J. Hazard. Mater. 2016, 307, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Huang, C.P.; Doong, R. Photocatalytic degradation of bisphenol A over a ZnFe2O4/TiO2 nanocomposite under visible light. Sci. Total Environ. 2018, 646, 745–746. [Google Scholar] [CrossRef] [PubMed]


| Light Source | Ratio (Profenofos: Myricetin) | Kinetic Parameters | T1/2 (h) | Inhibition Rate (%) | ||
|---|---|---|---|---|---|---|
| C0 (μmol·L−1) | K/h−1 | R2 | ||||
| HPML | 1:0 | 4.70 | 0.402 | 0.99 | 1.73 (±0.07) a | / |
| 1:1 | 4.89 | 0.373 | 0.99 | 1.86 (±0.06) a | 7.21 | |
| 1:5 | 5.00 | 0.289 | 0.99 | 2.40 (±0.1) b | 28.11 | |
| 1:10 | 4.94 | 0.164 | 0.99 | 4.22 (±0.35) c | 59.20 | |
| 1:20 | 4.62 | 0.111 | 0.97 | 6.24 (±0.44) d | 72.39 a | |
| UV lamp | 1:0 | 5.06 | 0.141 | 0.98 | 4.92 (±0.31) a | / |
| 1:1 | 4.97 | 0.123 | 0.98 | 5.63 (±0.31) b | 12.77 | |
| 1:5 | 4.87 | 0.100 | 0.99 | 6.91 (±0.21) c | 29.08 | |
| 1:10 | 5.00 | 0.0988 | 0.99 | 7.02 (±0.34) c | 29.93 | |
| 1:20 | 5.28 | 0.0756 | 0.99 | 9.18 (±0.33) d | 46.38 b | |
| Xenon lamp | 1:0 | 4.75 | 0.0990 | 0.99 | 7.00 (±0.099) a | / |
| 1:1 | 4.90 | 0.0840 | 0.99 | 8.25 (±0.084) a | 15. 15 | |
| 1:5 | 5.13 | 0.0470 | 0.98 | 14.75 (±0.047) b | 52.53 | |
| 1:10 | 5.18 | 0.0310 | 0.97 | 22.36 (±0.031) c | 68.69 | |
| 1:20 | 4.91 | 0.0210 | 0.99 | 33.01 (±0.231) d | 78.79 c | |
| Sunlight | 1:0 | 5.02 | 0.00770 | 0.99 | 90.02 (±4.5) a | / |
| 1:1 | 4.99 | 0.00282 | 0.96 | 245.80 (±12.38) b | 63.38 | |
| 1:5 | 4.70 | 0.00271 | 0.98 | 255.80 (±11.95) b | 64.81 | |
| 1:10 | 4.67 | 0.00159 | 0.99 | 435.94 (±21.80) c | 79.35 | |
| 1:20 | 4.65 | 0.00101 | 0.99 | 686.28 (±34.31) d | 86.88 d | |
| Water | Ratio (Profenofos: Myricetin) | Kinetic Parameters | T1/2 (h) | Inhibition Rate (%) | ||
|---|---|---|---|---|---|---|
| C0 (μmol·L−1) | K/h−1 | R2 | ||||
| Purified Water | 1:0 | 4.89 | 0.0211 | 0.99 | 32.8 (±0.90) a | / |
| 1:5 | 4.93 | 0.0195 | 0.96 | 35.6 (±1.07) b | 7.95 | |
| Pond Water | 1:0 | 4.79 | 0.442 | 0.99 | 1.57 (±0.10) a | / |
| 1:5 | 4.75 | 0.189 | 0.99 | 3.68 (±0.27) b | 57.24 | |
| Field Ditch Water | 1:0 | 4.95 | 0.095 | 0.95 | 7.38 (±0.88) a | / |
| 1:5 | 5.01 | 0.079 | 0.94 | 8.84 (±0.80) b | 16.84 | |
| Paddy Water | 1:0 | 4.89 | 0.074 | 0.94 | 9.45 (±1.11) a | / |
| 1:5 | 4.87 | 0.045 | 0.99 | 15.45 (±2.10) b | 39.19 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Yang, Y.; Wang, X.; Shi, T.; Lv, P.; Li, Q.X.; Hua, R. Myricetin Inhibits Photodegradation of Profenofos in Water: Pathways and Mechanisms. Agronomy 2024, 14, 399. https://doi.org/10.3390/agronomy14020399
Zhang N, Yang Y, Wang X, Shi T, Lv P, Li QX, Hua R. Myricetin Inhibits Photodegradation of Profenofos in Water: Pathways and Mechanisms. Agronomy. 2024; 14(2):399. https://doi.org/10.3390/agronomy14020399
Chicago/Turabian StyleZhang, Nan, Yawei Yang, Xin Wang, Taozhong Shi, Pei Lv, Qing X. Li, and Rimao Hua. 2024. "Myricetin Inhibits Photodegradation of Profenofos in Water: Pathways and Mechanisms" Agronomy 14, no. 2: 399. https://doi.org/10.3390/agronomy14020399
APA StyleZhang, N., Yang, Y., Wang, X., Shi, T., Lv, P., Li, Q. X., & Hua, R. (2024). Myricetin Inhibits Photodegradation of Profenofos in Water: Pathways and Mechanisms. Agronomy, 14(2), 399. https://doi.org/10.3390/agronomy14020399