Changes in the Root Architecture of Oil Palm Seedlings in Response to Nitrogen Starvation
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Conditions and Experimental Design
2.2. Root Phenotyping
2.3. Statistical Analyses
3. Results and Discussion
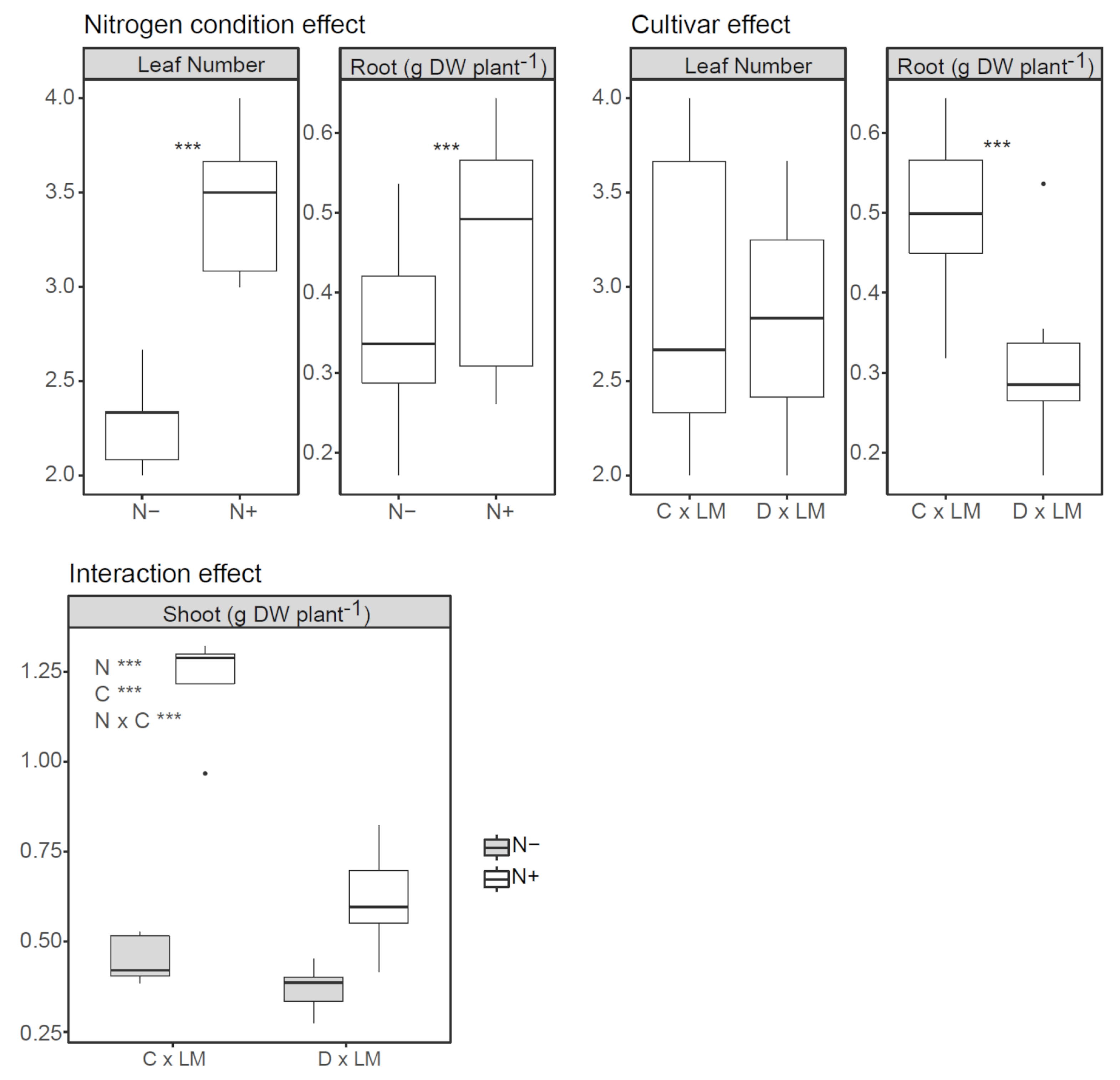
3.1. Effect of Nitrogen Starvation on Biomass Allocation
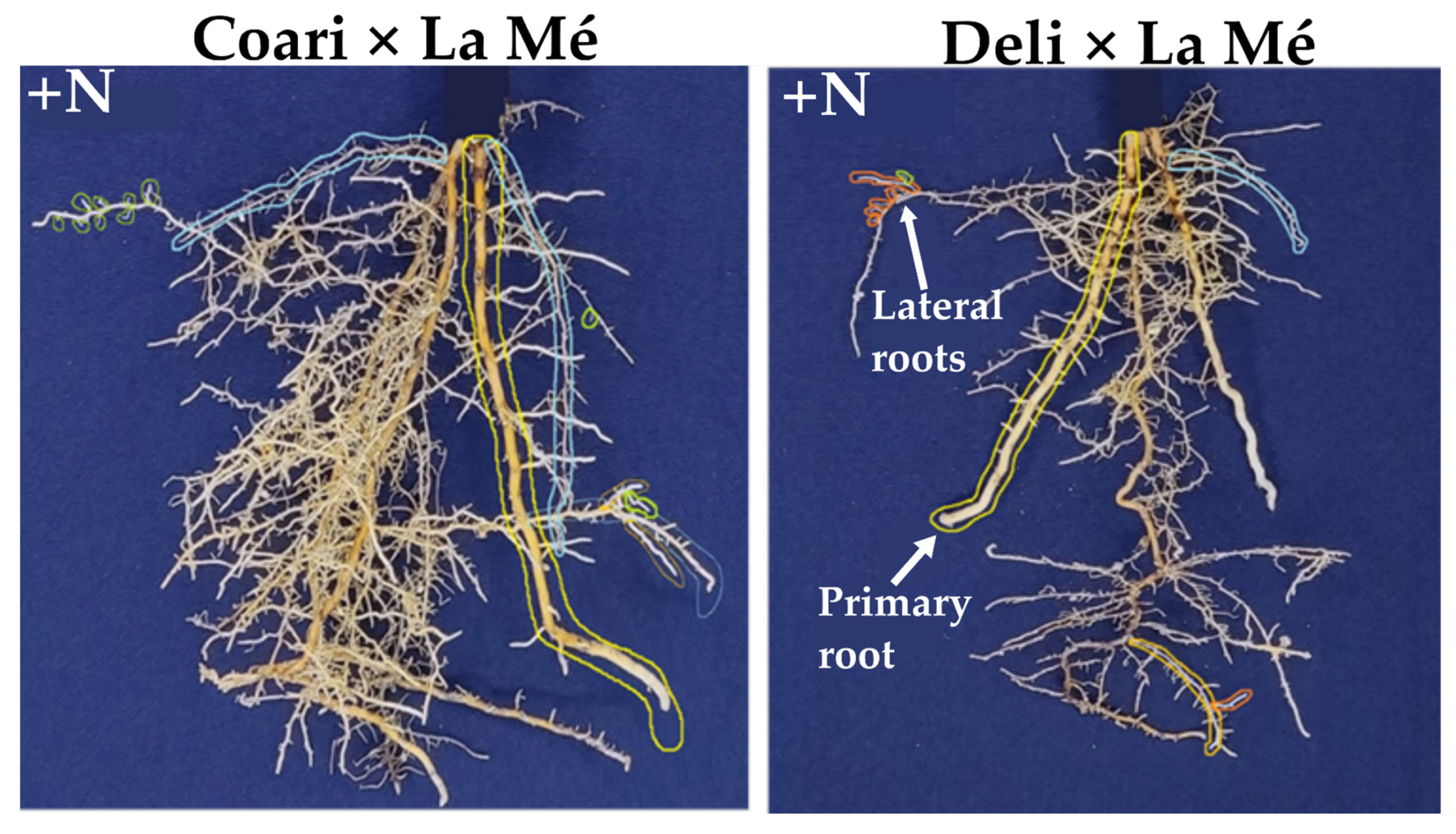
3.2. Root System Overview
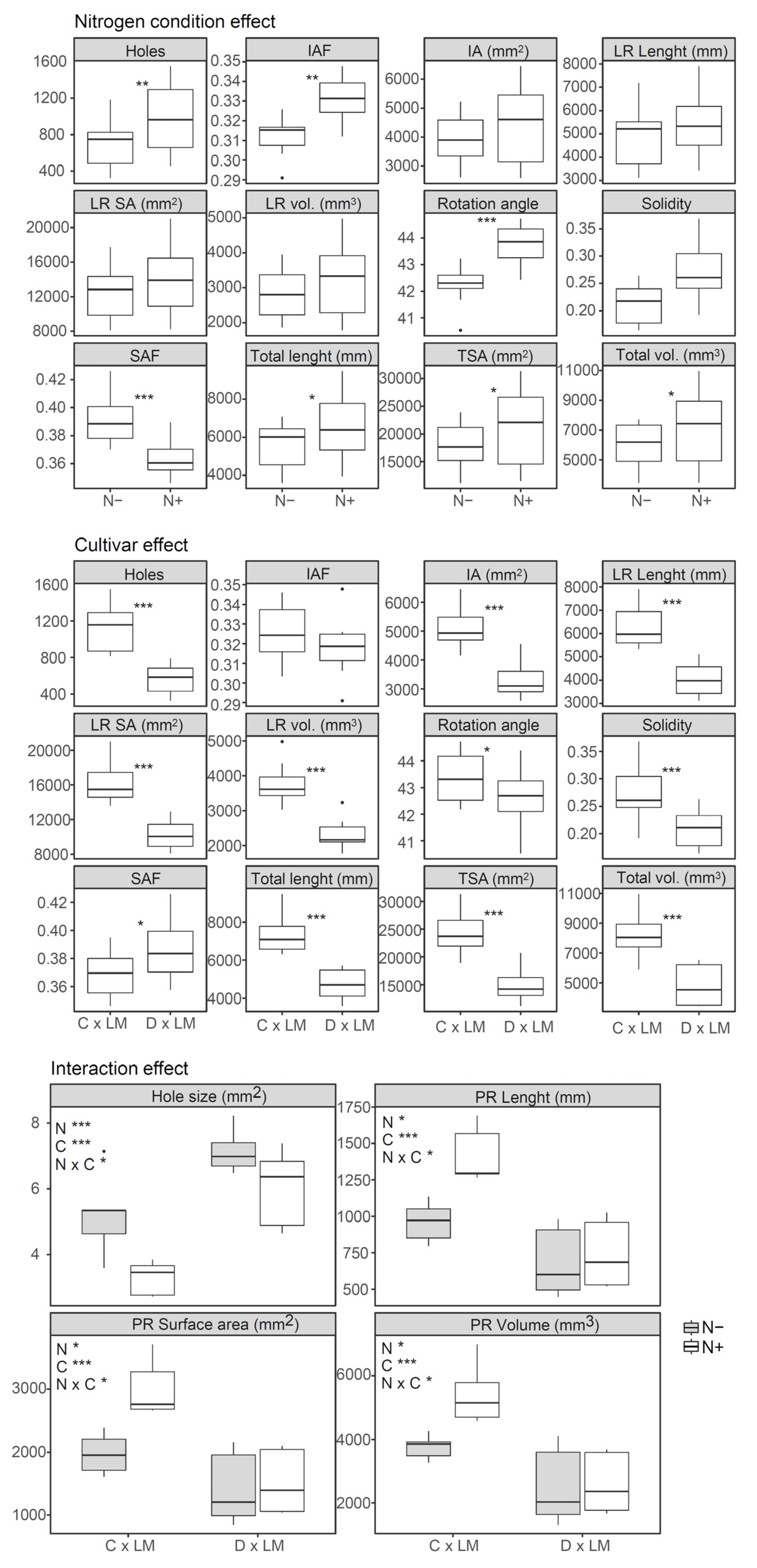
3.3. Root Plasticity in Response to Nitrogen Fertilization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corley, R.H.V.; Tinker, P.B. The Oil Palm, 5th ed.; Wiley Blackwell: Oxford, UK, 2016. [Google Scholar]
- Mannucci, P.M.; Jolliet, O.; Meijaard, E.; Slavin, J.; Rasetti, M.; Aleta, A.; Moreno, Y.; Agostoni, C. Sustainable nutrition and the case of vegetable oils to match present and future dietary needs. Front. Public Health 2023, 11, 1454. [Google Scholar] [CrossRef]
- Sibhatu, K.T. Oil palm boom: Its socioeconomic use and abuse. Front. Sustain. Food Syst. 2023, 7, 1083022. [Google Scholar] [CrossRef]
- Monzon, J.P.; Slingerland, M.A.; Rahutomo, S.; Agus, F.; Oberthür, T.; Andrade, J.F.; Couëdel, A.; Rattalino Edreira, J.I.; Hekman, W.; Van Den Beuken, R.; et al. Fostering a climate-smart intensification for oil palm. Nat. Sustain. 2021, 4, 595–601. [Google Scholar] [CrossRef]
- Zhou, Z.; Lu, J.-Z.; Widyastuti, R.; Scheu, S.; Potapov, A.; Krashevska, V. Plant roots are more strongly linked to microorganisms in leaf litter rather than in soil across tropical land-use systems. Soil Biol. Biochem. 2024, 190, 109320. [Google Scholar] [CrossRef]
- Acevedo, E.; Galindo-Castañeda, T.; Prada, F.; Navia, M.; Romero, H.M. Phosphate-solubilizing microorganisms associated with the rhizosphere of oil palm (Elaeis guineensis Jacq.) in Colombia. Appl. Soil Ecol. 2014, 80, 26–33. [Google Scholar] [CrossRef]
- Kong, S.-L.; Abdullah, S.N.A.; Ho, C.-L.; Musa, M.H.b.; Yeap, W.-C. Comparative transcriptome analysis reveals novel insights into transcriptional responses to phosphorus starvation in oil palm (Elaeis guineensis) root. BMC Genom. Data 2021, 22, 6. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, M.S.; Chong, K.P. Effects of Liming on Soil Properties and Its Roles in Increasing the Productivity and Profitability of the Oil Palm Industry in Malaysia. Agriculture 2022, 12, 322. [Google Scholar] [CrossRef]
- Mejia-Alvarado, F.S.; Botero-Rozo, D.; Araque, L.; Bayona, C.; Herrera-Corzo, M.; Montoya, C.; Ayala-Díaz, I.; Romero, H.M. Molecular network of the oil palm root response to aluminum stress. BMC Plant Biol. 2023, 23, 346. [Google Scholar] [CrossRef] [PubMed]
- de Araújo Brito, A.E.; Cardoso, K.P.S.; Costa, T.C.; da Silva Martins, J.T.; Machado, L.C.; dos Santos Nogueira, G.A.; Conceição, S.S.; de Oliveira, J.T.; Silva, P.A.; da Silva, R.T.L. The effect of aluminum treatment on metabolism in oil palm seedlings. Biosci. J. 2023, 39, 1981–3163. [Google Scholar] [CrossRef]
- Munar, A.; Sumarta, D.J.; Fajar, M. Growth of Palm Oil Seeds (Elaeis Guineensis Jacq.) on Solid Organic Fertilizer and Waste Tea Compost in Pre Nursery. In Proceedings International Conference Sustainable Agriculture and Natural Resources Management (ICoSAaNRM); UMSU: Medan, Indonesia, 2017. [Google Scholar]
- Fedepalma. Statistical Yearbook 2023. The Oil Palm Agroindustry in Colombia and the World 2018–2022; Fedepalma: Bogota, Colombia, 2023; p. 237. [Google Scholar]
- Mosquera-Montoya, M.; Ruiz-Alvarez, E.; Castros-Zamudio, L.E.; López-Alfonso, D.F.; Munevar-Martínez, D.E. Estimación del costo de producción para productores de palma de aceite de Colombia que han adoptado buenas prácticas agrícolas. Rev. Palmas 2019, 40, 3–15. [Google Scholar]
- Mohidin, H.; Hanafi, M.M.; Rafii, Y.M.; Abdullah, S.N.A.; Idris, A.S.; Man, S.; Idris, J.; Sahebi, M. Determination of optimum levels of nitrogen, phosphorus and potassium of oil palm seedlings in solution culture. Bragantia 2015, 74, 247–254. [Google Scholar] [CrossRef]
- Jourdan, C.; Michaux-Ferrière, N.; Perbal, G. Root system architecture and gravitropism in the oil palm. Ann. Bot. 2000, 85, 861–868. [Google Scholar] [CrossRef]
- Maqbool, S.; Hassan, M.A.; Xia, X.; York, L.M.; Rasheed, A.; He, Z. Root system architecture in cereals: Progress, challenges and perspective. Plant J. 2022, 110, 23–42. [Google Scholar] [CrossRef]
- Martin, K.A.; Brennan, F.P.; Schmidt, O.; Tracy, S. Comparison of two image analysis software for root trait analysis of single and mixed species grasslands. Plant Phenome J. 2022, 5, e20034. [Google Scholar] [CrossRef]
- De la Peña, M.; Ruiz-Romero, R.; Romero, H.M. Nitrogen Use Efficiency in Oil Palm Seedlings: Unraveling the Untapped Potential of Elevated External Ammonium Supply. Plants 2023, 12, 2819. [Google Scholar] [CrossRef] [PubMed]
- Pinto Gloria, G.C.; Silva Vasconcelos, S.; Gomes Junior, R.A.; Boari, A.d.J.; Murad Magalhães, M. Root morphological traits of oil palm genotypes with differential resistance to fatal yellowing. Semin. Cienc. Agrar. 2021, 42, 3073–3087. [Google Scholar] [CrossRef]
- Hormaza, P.; Fuquen, E.M.; Romero, H.M. Phenology of the oil palm interspecific hybrid Elaeis oleifera × Elaeis guineensis. Sci. Agric. 2012, 69, 275–280. [Google Scholar] [CrossRef]
- Forero, D.; Hormaza, P.; Romero, H. Phenological growth stages of African oil palm (Elaeis guineensis). Ann. Appl. Biol. 2012, 160, 56–65. [Google Scholar] [CrossRef]
- Ruíz-Romero, R.; De la Peña, M.; Romero, H.M. Metodología para evaluar en etapa de vivero la respuesta al déficit hídrico y nutrición de plántulas de Elaeis guineensis y de híbridos interespecíficos OxG. Ceniavances 2023, 196, 7. [Google Scholar]
- Seethepalli, A.; Dhakal, K.; Griffiths, M.; Guo, H.; Freschet, G.T.; York, L.M. RhizoVision Explorer: Open-source software for root image analysis and measurement standardization. AoB Plants 2021, 13, plab056. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.Y.; Razak, M.F.A.; Sim, C.C. Effects of Different Growing Media under Soilless Culture on the Growth and Nutrient Uptake of Oil Palm Seedlings in the Pre-Nursery Stage. Sci. Technol. Asia 2023, 28, 256–263. [Google Scholar]
- Jackson, L.E.; Burger, M.; Cavagnaro, T.R. Roots, nitrogen transformations, and ecosystem services. Annu. Rev. Plant Biol. 2008, 59, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Ruales, L.N.; Reyes-Cuesta, R. Crecimiento en vivero de las palmas aceiteras Elaeis oleífera x Elaeis guineensis y Elaeis guineensis x Elaeis guineensis en Tumaco Colombia. Ciencia y Tecnología Agropecuaria 2015, 16, 239–250. [Google Scholar] [CrossRef]
- Naulin, P.A.; Armijo, G.I.; Vega, A.S.; Tamayo, K.P.; Gras, D.E.; de la Cruz, J.; Gutiérrez, R.A. Nitrate induction of primary root growth requires cytokinin signaling in Arabidopsis thaliana. Plant Cell Physiol. 2020, 61, 342–352. [Google Scholar] [CrossRef]
- Gruber, B.D.; Giehl, R.F.; Friedel, S.; von Wirén, N. Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol. 2013, 163, 161–179. [Google Scholar] [CrossRef]
- Müller, L.M. NIT proteins regulate rice root plasticity in response to nitrate and ammonium. Am. Soc. Plant Biol. 2020, 183, 25–26. [Google Scholar] [CrossRef]
- Silva, P.A.; Cosme, V.S.; Rodrigues, K.C.B.; Detmann, K.S.C.; Leo, F.M.; Cunha, R.L.a. Drought tolerance in two oil palm hybrids as related to adjustments in carbon metabolism and vegetative growth. Acta Physiol. Plant. 2017, 39, 58. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, L.; Sun, H.; Zhang, Y.; Liu, X.; Wang, N.; Chen, J.; Zhang, K.; Bai, Z.; Wang, G. The responses of lateral roots and root hairs to nitrogen stress in cotton based on daily root measurements. J. Agron. Crop Sci. 2022, 208, 89–105. [Google Scholar] [CrossRef]
- Trachsel, S.; Kaeppler, S.; Brown, K.; Lynch, J. Maize root growth angles become steeper under low N conditions. Field Crops Res. 2013, 140, 18–31. [Google Scholar] [CrossRef]
- Zhan, A.; Lynch, J.P. Reduced frequency of lateral root branching improves N capture from low-N soils in maize. J. Exp. Bot. 2015, 66, 2055–2065. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De la Peña, M.; Ruiz-Romero, R.; Castro-Arza, L.I.; Romero, H.M. Changes in the Root Architecture of Oil Palm Seedlings in Response to Nitrogen Starvation. Agronomy 2024, 14, 409. https://doi.org/10.3390/agronomy14030409
De la Peña M, Ruiz-Romero R, Castro-Arza LI, Romero HM. Changes in the Root Architecture of Oil Palm Seedlings in Response to Nitrogen Starvation. Agronomy. 2024; 14(3):409. https://doi.org/10.3390/agronomy14030409
Chicago/Turabian StyleDe la Peña, Marlon, Rodrigo Ruiz-Romero, Laura Isabel Castro-Arza, and Hernán Mauricio Romero. 2024. "Changes in the Root Architecture of Oil Palm Seedlings in Response to Nitrogen Starvation" Agronomy 14, no. 3: 409. https://doi.org/10.3390/agronomy14030409
APA StyleDe la Peña, M., Ruiz-Romero, R., Castro-Arza, L. I., & Romero, H. M. (2024). Changes in the Root Architecture of Oil Palm Seedlings in Response to Nitrogen Starvation. Agronomy, 14(3), 409. https://doi.org/10.3390/agronomy14030409




