Abstract
It is crucial to comprehend how fertilization and cultivation management alter the composition of dissolved organic carbon (DOC) and microbial communities to regulate the biogeochemical cycling of soil nutrients and mitigate adverse impacts on soil–water quality. Based on 15 years of long-term field trials conducted in purple soil on farmland with a slope of 15° in southwestern China, the following five treatments were examined: CK (no fertilizer was applied), T1 (NPK plus organic manure and downslope cultivation), T2 (NPK and downslope cultivation), T3 (1.5-fold NPK and downslope cultivation), and T4 (NPK and contour cultivation). Soil samples were obtained from summer maize at two soil depths (0–10 and 10–20 cm) and from rhizospheric soil, and the changes in the DOC content, UV–visible (UV–Vis) absorptivity, and phospholipid fatty acids (PLFAs) were assessed. Our results revealed a significant change in the DOC content following fertilization, especially in T1, as it was 136.0%, 179.4%, and 132.2% higher, respectively, than that in CK at the 0–10 and 10–20 cm depths and rhizospheric soil. Fertilization decreased the UV–Vis absorptivity variables of DOC (i.e., SUVA254, SUVA260, SUVA400, SUVA465, SUVA665, and C:C ratio) and raised the E4:E6 ratio (fulvic acid to humic acid in DOC), regardless of T2 and T3 at the 10–20 cm depth and in the rhizospheric soil compared with those in CK, respectively. Fertilization significantly increased the total PLFA content and selected microbial groups relative to CK. Among the treatments, T1 significantly increased the total PLFA content by 50.6%, 59.0%, and 46.2%, respectively, relative to CK, at the 0–10 and 10–20 cm depths and in the rhizospheric soil. The microbial community structure in contour cultivation (T4) was significantly greater than in downslope cultivation (T2). Random forest analysis (RFA) revealed that SOC and DOC were likely the primary variables for regulating the total PLFAs in the examined soil. Partial least squares path modeling (PLS-PM) further indicated that the DOC content and the ratio of E4:E6 among DOC compositions had greater effects on the soil microbial community structure in the examined soil. These observations suggested that long-term fertilization and cultivation management are effective approaches to regulating the soil microbial community structure by altering the composition of DOC in sloping farmland.
1. Introduction
Dissolved organic carbon (DOC) commonly passes in solution through a pore size membrane of 0.45 μm. It is a highly reactive and labile form of organic carbon that influences the biogeochemical cycle in aquatic and terrestrial environments [1,2]. It mainly originates from allochthonous (i.e., soil organic matter and plant litter), autochthonous (i.e., microbial metabolites), and anthropogenic (i.e., mineral and organic fertilization, tillage systems, and wastewater) sources in an agroecosystem [3,4,5], thereby serving as an essential indicator of soil quality. Despite constituting less than 0.25% of the total organic carbon [6,7], DOC exerts a substantial influence on the regulation of soil nutrient availability and transformation in arable soil [3] and serves as a C substrate for microorganisms within the soil [8]. Hence, it is imperative to comprehensively understand the quantity and quality of DOC in the soil in relation and its absorptivity variables in response to fertilization and cultivation management in sloping farmland to better explore soil biogeochemistry in intensive agricultural environments from the regional to the global scale.
Due to the inherent spectroscopic properties of DOC’s composition [9], quantitative and qualitative information on DOC is frequently sought by using ultraviolet–visible (UV–Vis) absorptivity to assess variations in the quality of DOC [10,11,12]. For instance, the content of colored compounds in DOC can be determined using the specific values of absorbance (SUVA) measured at wavelengths of 254, 260, 400, 465, and 665 (SUVA254, SUVA260, SUVA400, SUVA465, and SUVA665) [12], whereas SUVA254 and SUVA260 reflect the DOC contents through aromaticity [13,14]. The proportion of colored humic compounds relative to uncolored nonhumic compounds in DOC is represented by the C:C (SUVA400/DOC content) ratio [9,10], and the proportion of fulvic acid relative to humic acid in DOC is represented by the E4:E6 (SUVA400/SUVA665) ratio [14]. Regardless of the source, DOM can either enhance or inhibit the mobility of metals in soils, depending on their origin or the specific properties of agronomic soil [5]. Compared with SOC, DOC is more sensitive to the agricultural practice used, including the fertilization strategy [2,15]. Numerous biotic and abiotic factors, including soil properties, climate, crop types, and agricultural management practices (e.g., tillage, liming, fertilization, and crop residue management), control the fate of DOM in agronomic soil [3,16,17]. The results reported on the fate of DOC in agricultural soils are often debatable concerning the effect of fertilization. For example, the impact of the application of long-term mineral fertilization (i.e., N and P) on the DOC content in arable agricultural fields is not finite and was found to have either decreasing or nonsignificant effects [2,3,18,19]. However, a significant increase in DOC content following organic amendment is due to the inclusion of soluble elements in organic manures [16,18]. Regardless of the fertilization system, the variations in DOC content among different ecosystems are also substantial. Whether in the topsoil or subsoil, the DOC resulting from other soil management practices acts differently in different environments [12]. However, rhizospheric soil may be regulated by plant species and soil properties (i.e., SOC and microbial biomass C), which can primarily be associated with the large C flux due to root exudation [7,20,21]. However, owing to spatiotemporal variations and variations in the standard methods, the results of previous DOC studies on arable agricultural land are difficult to compare [7,22,23]. To better comprehend the fate and pathways of soil DOC in the agroecosystem, it is imperative to identify the variations in DOC content and the specific value of UV–Vis absorptivity in the bulk soil of the top- and subsoil depths and rhizospheric soil following fertilization and cultivation management (downslope vs. contour) on sloping farmland.
In agricultural systems, soil microbes are essential for regulating soil nutrients (mainly through C cycling) and biogeochemical processes [24]. The alteration of the microbial community structure can significantly impact crop productivity and soil health and quality [25,26], and there are several ways to quantify this structure in agricultural soil [27]. To gain insights into the variations in the microbial composition in arable soils, the phospholipid fatty acid (PLFA) analysis technique is widely applied [28] since different organisms contain various PLFA functional groups (i.e., fungi, Gram-positive (G+) and Gram-negative (G−) bacteria, actinomycetes, and eukaryotes) [29,30]. Thus, interpreting variations in PLFAs delivers a quantitative picture of microbial composition, which can vary with different fertilization practices, soil types, and climate origins [31,32]. Prior investigation demonstrated that agricultural management practices, particularly the application of synthetic fertilizer and organic amendments, have direct and indirect effects on microbial compositions in diverse habitats for soil biota [33,34,35]. For instance, the addition of NPK fertilizer coupled with organic manure leads to a rapid increase in the abundance of PLFA groups in soil microbial communities (e.g., fungi, bacteria, actinomycetes) relative to mineral fertilizer alone and control soils [31,36,37]. However, the application of mineral fertilizers has been reported to have positive [34], negative [38,39], or no effects on soil microbial communities [35]. Despite this, the exact mechanisms underlying such changes in and their effects on soil PLFA analysis under long-term fertilization and cultivation management to improve soil quality in purple soil are disputed, as surface runoff from fertilized sloping farmlands has become a major concern for environmental pollution [40]. Moreover, soil microorganisms inherent in the bulk soil of different soil layers and rhizosphere rely on the characteristics and properties of microbial processes (i.e., microbial biomass N and C) due to the confined input of fertilization practices (mostly fresh organic substrates) that mainly derive from preferential flow paths or adhere to rhizodeposition [41,42]. Hence, the rhizosphere is a proposed resource-rich hotspot, where soil properties differ from their corresponding bulk soils through the active roots releasing organic compounds that stimulate microbial activities [43,44]. Although the effects of different agricultural practices on microbial populations either in the soil depths or rhizosphere soil have been separately analyzed, understanding the comprehensively characterized bulk soil samples from top- and subsoils and rhizosphere soil will provide new prospects for the ecology and functioning of microbial community structure [42,45], which could be essential for the production of crops and soil health.
Sloping farmland with purple soil is the most important agricultural land in Southwest China [46], with a slope gradient ranging from 6° to 25° that covers up to 6 million ha of farmland, accounting for 78.7% of the total farmland of the Three Gorges Reservoir Area (TGRA) [47,48]. Implementing fertilization and cultivation management are the most common effective practices in sloping farmland in Southwest China, which reduce the losses of soil nutrients prone to soil erosion and help to stimulate the growth and activity of microorganisms [49]. The benefits of fertilization practices in sloping farmland can replenish and sustain nutrient levels in the soil [50] and stimulate microbial activity by supplying the necessary nutrients for enzyme production and function [51], which can help promote soil health and contribute to sustainable agricultural practices. In addition, cultivation practices have also been acknowledged as an effective measure for retaining water and soil erosion, declining land degradation, and promoting the sustainability of sloping farmland in China [51]. In opposed to traditional cultivation (downslope cultivation), contour cultivation is a more effective strategy by diverting flow direction to alter runoff velocity [46], reducing soil nutrient loss [52], and accelerating the microbial-mediated ecological processes by maintaining nutrient-rich topsoil, which helps to overcome nutrient limitations in sloping farmland [47]. However, the mechanisms of contour cultivation that govern DOC composition to boost soil microbes are still not understood in intense cultivation at a regional or global scale. Therefore, addressing the changes in quantity and quality of DOC and microbial community structure under different fertilization and cultivation management is essential to accurately assess the biogeochemical cycling of DOC, water quality, environmental conservation, and agriculture sustainability of sloping farmland soil. In the present study, a long-term field experiment conducted since 2008 in sloping farmland with five different fertilization and cultivation management procedures was carried out to determine changes in DOC quantity and its related UV–visible absorptivity and microbial community structures in a representative purple soil of southwestern China. We tested the following hypotheses: whether long-term fertilization and cultivation management could change the (1) microbial community structure, (2) DOC quantity and its related UV–Vis absorptivity, and (3) the relationships between total PLFA and DOC indices in the bulk soil of top- and subsoils and rhizosphere. The aims of this study were to (1) evaluate changes in microbial communities to different fertilization and cultivation management practices; (2) determine the DOC content and its related UV–Vis absorptivity under different fertilization and cultivation managements in the bulk soil of top- and subsoil depths and rhizosphere soil; and (3) examine the linkages between different DOC indices, related soil properties, and PLFA analysis.
2. Materials and Methods
2.1. Site Description
The study site (106° 24′ 20″ E, 29° 48′ 42″ N), which was founded in 2008, is located at the Southwest University of Soil and Water Conservation Science Park in a district of Beibei, Chongqing Municipality City, Southwest China [49]. The climate of the monitoring site is characterized by a typical subtropical monsoon environment, with an average annual rainfall pattern of 1100 mm, with approximately 75% occurring between May and September. The yearly mean temperature and relative humidity recorded at the site are 18.3 °C and 80.5%, respectively, with a recorded 1270 h of sunshine and 334 days of frost-free period. The overlying soil at the study site is classified as purple soil according to the Chinese soil taxonomic classification, corresponding to the Entisols in the United States Department of Agriculture (USDA) Taxonomy System. The average initial physicochemical characteristics of the topsoil (0–20 cm depth) when the experiment was established were soil pH (1:2.5, soil/water suspension), 8.16; soil organic carbon (SOC), 5.08 g kg−1; total nitrogen (TN), 0.76 g kg−1; total phosphorus (TP), 0.68 g kg−1; ammonium–nitrogen (NH4+-N), 24.19; nitrate–nitrogen (NO3−-N), 19.51 mg kg−1; available phosphorus (Olsen-P), 18.29 mg kg−1; and available potassium (AP) 71.39 mg kg−1 [49]. The dominant vegetation plant community in this area is mainly evergreen broadleaved forest, including Masson pine (Pinus massoniana Lamb.), white oak (Quercus fabric Hance.), and cypress (Cupressus funebris Endl.). The local practice, i.e., the winter wheat and summer maize system, was conducted in the sloping land before the installation of the experiment.
2.2. Experimental Design
An investigation was made for a total of fifteen plots (8 m in length and 4 m in width) constructed on the 15° sloping farmland in 2008. These plots were separated by a concrete ridge with a 40–50 cm width, the spacing between ridges was 40 cm, and the ridge height was approximately 5–8 cm [49]. A total of five treatments were set up for this experiment (three replicates for each), including CK (blank, no fertilizer applied), T1 (a combination of NPK fertilizer plus organic manure and downslope cultivation), T2 (NPK fertilizer and downslope cultivation), T3 (1.5-fold NPK fertilizer and downslope cultivation), and T4 (NPK fertilizer and contour cultivation). For each fertilized plot, winter wheat was rotated annually with summer maize. Since 2008, winter wheat (Yumai 7) was sown in November with row spacing of 30 cm × 30 cm, and harvesting took place in mid-May of the subsequent year. However, for summer maize, the maize seed (cv. Zhongnuo 309) was first sown in March in flat cropland in the Soil and Water Conservation Science Park of Southwest University and then transplanted (with a 40 cm × 150 cm plant spacing density) in mid-April, and then harvesting took place in early August of the same year until the subsequent growing season of winter wheat.
All fertilizers were broadcast to winter wheat as a basal dose before sowing winter wheat in early November and topdressing in late January or early February of the following year. For summer maize, fertilizer was consistently applied as a basal dose in early April and topdressing in early May. The mineral fertilizers, i.e., N, P, and K, were urea, calcium superphosphate, and potassium chloride with standard input rates of 46.4% N, 12% P2O5, and 60% K2O, respectively. The organic fertilizer was derived mainly from swine manure with organic C, N, P2O5, and K2O of 4.31, 0.24, 0.17, and 0.21% on a dried basis, respectively. A detailed description of the annual input of each fertilizer applied to wheat and maize crops is included in Table 1. During the experimental (15-year) periods, no irrigation management was conducted since rainfed management was based on local conventional farming practices that relied solely on natural rainfall.

Table 1.
The mean annual rate and duration of different nutrient content and organic manure used for applied treatments.
2.3. Soil Sampling and Chemical Analysis
In August 2022, soil samples from the three replicates of each treatment after the maize harvest were obtained from each profile at the bulk surface depth increments (0–10 and 10–20 cm) using an auger with a 2.5 cm diameter. To obtain a single composite sample, five randomized soil cores were collected from each soil layer at regular intervals along an “S” shape design in each plot, and then the soil samples were meticulously mixed and sealed in a plastic container. While considering rhizosphere soil, the respective three whole maize plants were dug out from each plot, and then the closely adhering soil was separated from the root by brushing off the root and was defined as rhizosphere soil. These samples were immediately stored in ice boxes and brought to the laboratory. All samples were sieved in the laboratory through a 2 mm sieve to remove all visible stones and plant residues before storage and further analysis. Briefly, each sample was divided into three parts: the first and second parts were immediately placed frozen at 4 °C and −80 °C, respectively, for the analysis of microbial biomass properties and phospholipid fatty acids (PLFAs), while the third part was air-dried and subjected to analysis of the soil properties.
A compound electrode (PE-10, Sartorius, Germany) was employed to determine the pH value of soil at the soil/water ratio of 1:2.5 [49]. The soil organic carbon (SOC) content was obtained by digestion with K2Cr2O7-H2SO4, according to the procedure of Nelson and Sommers [53]. Extractable organic carbon (EOC) was evaluated using 0.5 M K2SO4 extractable solution without adding chloroform fumigation, following the procedure of Schaeffer et al. [54]. Additionally, the total nitrogen (TN) content was analyzed following the method of Kjeldahl digestion [55]. The contents of soil NO3−-N and NH4+-N were evaluated using a soil extraction with 2 mol L−1 KCl and were determined through a flow injection analyzer [56]. The Olsen-P method was used to assess available P (AP) via the NaHCO3 extraction method in a colorimetric assay [57]. For the determination of microbial biomass carbon (MBC) and nitrogen (MBN), the chloroform fumigation–extraction method was adopted to calculate the sample by the differences between fumigated and unfumigated subsamples using a coefficient factor of 0.45 for MBC and 0.54 for MBN, respectively [58].
2.4. Determination of DOC Content and UV–Vis Absorption Measurements
The DOC (mg L−1 C m−1) was extracted with distilled water. Briefly, 10 g of fresh samples were extracted with 20 mL of distilled water (v/w = 2:1) by shaking for 30 min (25 °C, 250 rpm), followed by centrifugation for 10 min (4000 rpm). The supernatant was filtered through a 0.45 µm filter membrane. The extract was acidified and then analyzed for DOC by a TOC analyzer (TOC-V Shimadzu, Tokyo, Japan). We used an Aqualog® absorption spectrometer (Horiba, Japan) to determine the specific absorbance values at wavelengths 254, 260, 400, 465, and 665 nm. The UV absorbance coefficients of DOC were measured by the Aqualog® absorption spectrometer (Horiba, Japan) in a 10 mm quartz cuvette with a 1 nm interval at a constant temperature of 20 °C. Milli-Q® water was used as a blank during the scanning. The absorption spectrometer was equipped with a 150 W ozone-free xenon lamp and the UV–vis scanning range is 230–800 nm.
Following a previous study by Liang et al. [12], several specific UV absorbance (mg L−1 C m−1) variables, including SUVA254, SUVA260, SUVA400, SUVA465, and SUVA665, C:C (mg L−1 C m−1), and the E4:E6 ratio, were used to characterize the quality of DOC. The UV–Vis absorption variables were measured in a 10 mm quartz cuvette at room temperature (20 °C) within a 230–800 nm spectrum. MQW representing Milli-Q® water was used as a reference. The absorption coefficient aλ (m−1), specific values of specific UV absorbance at wavelengths of SUVA254, SUVA260, SUVA400, SUVA465, and SUVA665, and the C:C ratio and the ratio of E4:E6 were calculated by the following formulas:
where aλ represents the measured absorbance of the DOC sample at a specific wavelength λ (nm) and ƖL denotes the path length of the cuvette (1 m). aλ indicates the measured values of the absorbance coefficient at 250, 254, 260, 365, 400, 465, and 665 nm. CDOC indicates DOC concentration.
aλ = 2.303Aλ/ƖL,
SUVA = aλ/CDOC,
C:C = aλ400/CDOC,
E4:E6 = aλ465/aλ665,
2.5. Soil Microbial Community Structure
We measured the analysis of PLFA to perform soil microbial community structure [59]. The modified Bligh and Dyer techniques were adopted to extract the PLFAs [60]. A mixture of chloroform, methanol, and citrate buffer solutions (volume ratio 1:2:0·8) was extracted from 4 g of freeze-dried soil and filtered and evaporated under a stream of N2 gas. Chloroform, acetone, and methanol were used to elute the extracted fatty acids after separating from neutral lipids and glycolipids using a silica acid column (Supelco Inc., Bellefonte, PA, USA). Following the inclusion of an internal standard nonadecanoic acid methyl ester (19:0), the polar lipids were methylated to form fatty acid methyl esters (FAMEs), which were then identified and analyzed using gas chromatography and the Automated Sherlock MIDI microbial identification platforms (V.4.5, MIDI, Newark, DE, USA). The sum of each PLFA biomarker for the selected microbial groups was expressed in units of nanomoles per gram (nmol g−1). The biomarkers of PLFAs for the selected groups to analyze changes in microbial community composition and the calculation of Gram-positive/negative bacteria (G+/G−), fungi/bacteria (F/B), monosaturated to saturated fatty acids (mono/sat), and cyclopropyl fatty acids to monoenoic precursor (cy/pre) ratios are shown in Table S1.
According to Zhong et al. [34], the functional diversity of Shannon (H’), evenness (E’), and Simpson (D) indices from PLFA were calculated using the following equations:
where Pi indicates the activity of each fatty acid to the total sum of activities on all substrates, and S indicates the total number of tested fatty acids in the community [61].
2.6. Statistical Analyses
We used one-way ANOVA to evaluate the effect of fertilization and cultivation management on each variable in the examined soil. The least significant difference (LSD) between the treatment means was applied to test a statistically significant difference at the p < 0.05 probability level. Two-way ANOVAs were performed to identify the effects of fertilizer treatments, soil depths, rhizosphere, and their interactions on the tested variables. The above statistical tests for ANOVA and charts were carried out using IBM SPSS v20.0 (IBM, Chicago, IL, USA) and Origin Pro 9.0 (http://www.OriginLab.com/, accessed on 3 June 2023) software. Principal component analysis (PCA) was adopted to assess the significance of the treatment effect and specify the loadings of each PLFA biomarker in the sample depths and rhizosphere soil. To determine the relative importance of different soil indices (%IncMSE) on the total PLFAs, random forest analysis (RFA) was performed using the “R” package ‘Vegan’. We further applied partial least squares path modeling (PLS-PM) to build the general hypothetical pathways by which soil DOC and its specific value of UV–visible variables contribute to microbial community structures using the “inner plot” function in the “pls pm” package with R version 4.1.3 software.
3. Results
3.1. Soil Chemical and Biological Properties
Two-way ANOVA showed that pH, SOC, EOC, TN, NO3−-N, NH4+-N, and AP contents were significantly affected by fertilization, rhizosphere, and their interaction (p < 0.05); however, this phenomenon was not found by soil depth and their interaction with fertilization except for SOC and EOC (Table 2). In all applied treatments, soil pH ranged from 7.0 to 7.7, where soil pH under different fertilization management practices decreased compared with CK. Although fertilization management significantly increased SOC, EOC, TN, NO3−-N, NH4+-N, and AP contents compared with CK (Table 2), they were notably higher under T1 and T3 in the examined soil, with the content in the rhizosphere soil being higher than that in the bulk soil at two different (0–10 cm and 10–20 cm) depths. In addition, the content of MBC was also significantly affected by the main and interactive effects of fertilization, soil depths, and rhizosphere (excluding the interactive effect of fertilization and soil depths); however, the MBN content did not differ significantly due to the main and interactive effects of fertilization and soil depths (excluding the main fertilization effect). Among the applied treatments, fertilization management considerably (p < 0.05) increased the contents of MBC and MBN relative to the CK (Table 2). The T1 treatment had significantly higher MBC and MBN contents than the T2, T3, and T4 treatments. However, the difference observed in MBC and MBN contents between T2, T3, and T4 treatment was insignificant except for 10–20 cm depths.

Table 2.
Results of two-way ANOVAs showing the effects of fertilizer and cultivation treatments, soil depths (0–10 and 10–20 cm), rhizosphere, and their interactions on the soil chemical and biological properties.
3.2. DOC and Specific Value of UV–Vis Absorption Measurements
Fertilization, soil depths, and rhizosphere had significant main and interactive effects on the DOC and UV–Vis absorptivity variables except for soil depth and their interaction with fertilization (p < 0.05; Table 3). However, SUVA254, SUVA260, SUVA465, SUVA665, and the C:C were unaffected by the interaction between fertilization and soil depth or either alone (Table 3). Fertilization and cultivation management, except for T4 in 0–10 cm and rhizosphere soil, significantly (p < 0.05) altered DOC content compared to CK in the examined soil (Table 3). Among the four fertilization treatments, the highest DOC value (12.3, 9.8 and 13.8 mg C L−1) was presented in T1, followed by T3 (9.0, 7.1 and 10.3 mg C L−1), T2 (7.4, 5.5 and 8.1 mg C L−1) and T4 (4.6, 4.9 and 6.2 mg C L−1) in the 0–10 cm and 10–20 cm depths and rhizosphere soil, respectively. In addition, the variation observed in DOC content between the T2, T3, and T4 treatments was also significant, with the highest value recorded in T3 at depths of 0–10 cm and in the rhizosphere soil.

Table 3.
Results of two-way ANOVAs showing the effects of fertilizer and cultivation treatments, soil depths (0–10 and 10–20 cm), rhizosphere, and their interactions on the content of dissolved organic carbon (DOC) and quality indices.
In all the examined soils, long-term fertilization and cultivation management caused a significant decline in the specific values of SUVA254, SUVA260, SUVA400, SUVA465, SUVA665, and the C:C ratio compared with CK (Table 3). However, E4:E6 showed a significantly (p < 0.05) higher value in T2 and T3 at the 10–20 cm depth and in T2 in rhizosphere soil than in CK. Moreover, for the cultivation treatments, the mean values of SUVA254, SUVA260, SUVA400, SUVA465, C:C, and E4:E6 in the contour cultivation (T4) were significantly larger than those in the downslope cultivation (T2) at the 0–10 cm depth, and no differences were found at the 10–20 cm depth. For rhizosphere soil, the difference observed in C:C was significantly higher in T4 than in T2 and vice versa for the E4:E6 ratio.
3.3. Soil Microbial Community Structure
As presented in Table 4, fertilization, soil depths, and rhizosphere exerted significant main and interactive effects on the total PLFA and selected microbial biomarkers groups of PLFAs (i.e., G− bacteria, G+ bacteria, fungi, AMF, and actinomycetes) (p < 0.05; Table 4). The total PLFA content ranged from 20.8 to 42.2 nmol g−1 at 0–10 cm, from 17.2 to 42.1 nmol g−1 at 10–20 cm, and from 29.9 to 55.5 nmol g−1 in rhizosphere soil and was significantly increased (p < 0.05) by fertilization management compared with CK. Among the treatments, T1 exhibited the highest increase in the total PLFAs, approximately 50.6%, 59.0%, and 46.2% higher than the CK in the 0–10 cm, 10–20 cm, and rhizosphere soils, respectively.

Table 4.
Results of two-way ANOVAs showing the effects of fertilizer and cultivation treatments, soil depths (0–10 and 10–20 cm), rhizosphere, and their interactions on the total phospholipid fatty acid (PLFA) content and their representing various microbial biomarker groups (nmol g−1 soil).
Long-term fertilization and cultivation management (T1, T2, T3, and T4) also significantly (p < 0.05) altered the selected microbial biomarkers groups of PLFAs in the same way as total PLFAs relative to the CK in the examined soil (Table 4). Among the fertilization treatments, the amounts of bacteria, G+ bacteria, fungi, AMF, and actinomycete groups were found to be significantly higher in the T1 treatment with downslope cultivation, followed by T4 with contour cultivation, while it was the least in the T2 treatment (Table 4). However, T4 with contour cultivation exhibited a significantly higher content of G− bacteria, followed by T1 treatment, while the G− bacteria was not statistically different between T2 and T3 treatments except for 10–20 cm soil depth (Table 4). Furthermore, fertilization, soil depths, and rhizosphere also had compartment main and interactive effects on the ratios of F/B and cy/pre (p < 0.05; Figure 1A,D). Compared with CK, fertilization treatments reduced the ratios of F/B, cy/pre, and mono/sat in the study soil, except for the cy/pre ratio in T1 and T3 in rhizosphere soil (Figure 1A,C,D). However, the value of the G+/G− ratio increased in T1 compared with that in the T2, T3, T4, and CK treatments, reaching values of 1.28, 2.08, and 1.65 in the 0–10, 10–20 cm, and rhizosphere soils, respectively (Figure 1B), while no significant differences in the G+/G− ratio were found between T2, T3, T4, and CK.
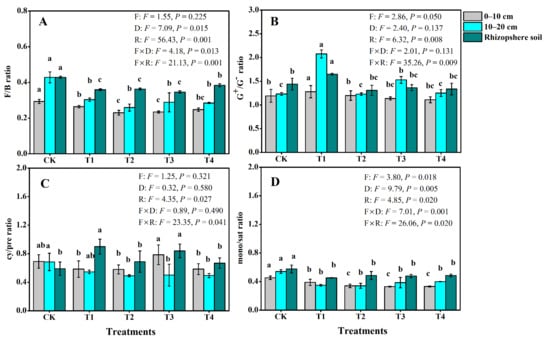
Figure 1.
Responses of the ratios of G+ to G− (A), fungi to bacteria (B), cy/pre (C), and mono/sat (D) to long-term fertilization management in the bulk soil of 0–10 cm, 10–20 cm depths, and rhizosphere soil. Different lowercase letters mean significant differences (p < 0.05) among the different treatments within the different soil. The ‘F’, ‘D’ ‘R’, ‘F × D’, and ‘F × D’ indicate the individual and interaction effects of fertilizer and cultivation treatments, soil depths, and rhizosphere (p < 0.05). Abbreviations: G+, Gram-positive; G−, Gram-negative bacteria; cy/pre: the ratio of cyclopropyl to precursor PLFAs; mono/sat, total monounsaturated fatty acids to total saturated fatty acids; CK, blank, no fertilizer applied; T1, a combination of NPK fertilizer plus organic manure and downslope cultivation; T2, NPK fertilizer and downslope cultivation; T3, 1.5-fold NPK fertilizer and downslope cultivation; T4, NPK fertilizer and contour cultivation.
3.4. Functional Microbial Diversity Indices
Fertilization, soil depth, rhizosphere, and their interaction exerted a significant main and interactive effect on the functional microbial diversity indices for PLFAs, including the Shannon (H’), evenness (E’), and Simpson (D) indices (Table 5, p < 0.05). However, the interactive effect of soil depth and fertilization did not affect the H’ and E’ indices. Among the applied treatments, the H’, E’, and D indices among the T1, T2, T3, and CK treatments were not significantly different but increased in the T3 and T4 treatments relative to the CK (Table 5). Correspondingly, the T1, T2, T3, and T4 treatments significantly increased the D index compared with CK for rhizosphere soil.

Table 5.
Results of two-way ANOVAs explain the effects of fertilizer and cultivation treatments, soil depths (0–10 and 10–20 cm), rhizosphere, and their interactions on the functional diversity indices of microbial communities calculated from PLFA.
3.5. Relationships between Microbial Communities, DOC Indices, and Soil Nutrient Properties
The RFA model was utilized to assess the relative importance of the main predictor variables contributing to total PLFAs (Figure 2). At the 0–10 cm depth, SOC (16.7%), MBC (13.6%), DOC/AP (12.3%), and AP (11.7%) based on mean predictor importance (MPI) scores were found to be the most relatively important factors of total PLFAs (Figure 2a). Correspondingly, for the 10–20 cm depth, DOC (13.7%) followed by SOC (13.1%), AP (12.8%), and MBC (9.9%) were the main variables contributing to the relative importance of total PLFAs (Figure 2b). Following rhizosphere soil, SOC, MBN, DOC/AP, and AP were the main relative importance factors, explaining 15.9, 12.5, 11.5, and 11.3% of the MPI score in the total PLFAs, respectively (Figure 2c).
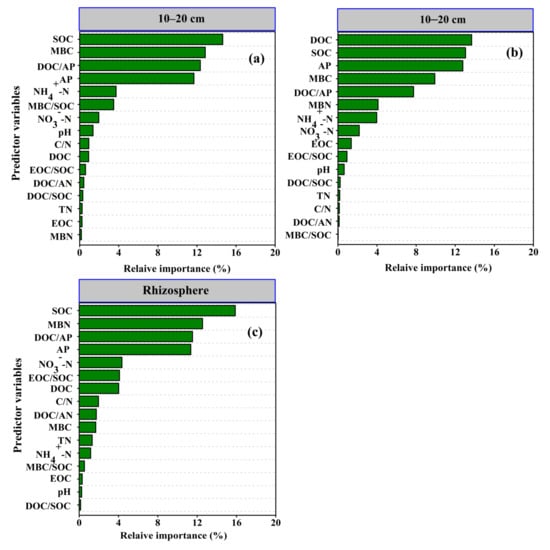
Figure 2.
Random forest analysis (RFA) of the relative importance of the soil nutrients and nutrient stoichiometry on total PLFAs for 0–10 cm (a), 10–20 cm depth (b), and rhizosphere soil (c). SOC, soil organic carbon; EOC, exchangeable organic carbon; TN, total nitrogen; AN, available nitrogen; NH4+-N, ammonium–nitrogen; NO3−-N, nitrate–nitrogen; AP, available phosphorus; MBC, microbial biomass carbon; MBN, microbial biomass nitrogen. C/N, the ratio of soil organic carbon to total nitrogen; DOC/SOC: the ratio of dissolved organic carbon to soil organic carbon; EOC/SOC, the ratio of extractable organic carbon to soil organic carbon; MBC/SOC, the ratio of microbial biomass carbon to soil organic carbon; DOC/AN, the ratio of DOC to AN; DOC/AP, the ratio of DOC to AP.
The PLS-PM analysis was further conducted to assess the direct and indirect effects of soil DOC content and its UV–Vis absorption variables on the total PLFAs in the examined soil (Figure 3). We found that the goodness-of-fit (GoF) values are greater than 0.5, indicating that the fit met the significance criteria (Figure 3A,C,E). The soil DOC contents showed a positive total effect on total PLFA, with a value of 0.498, 0.698, and 0.729 in the 0–10, 10–20 cm depths, and rhizosphere soil, respectively (Figure 3B,D,F). The ratio of E4:E6 was also shown as the most critical factor impacting a positive total effect on total PLFA at the 0–10 cm depths (0.365) and rhizosphere soil (0.572), respectively (Figure 3B,F), while showing negative total effect in the 10–20 cm depths (−0.046) (Figure 3D). The SUVA400 also showed positive total effects on total PLFAs in rhizosphere soil (0.388) (Figure 3F) and adverse effects on total PLFAs in the 0–10 and 10–20 cm depths (−1.525, −0.208), respectively (Figure 3B,D). However, UV–Vis absorption variables (SUVA254, SUVA260, SUVA465, SUVA4665, C:C, and E4:E6) negatively affected total PLFAs in the 0–10 and 10–20 cm depths and rhizosphere soil.
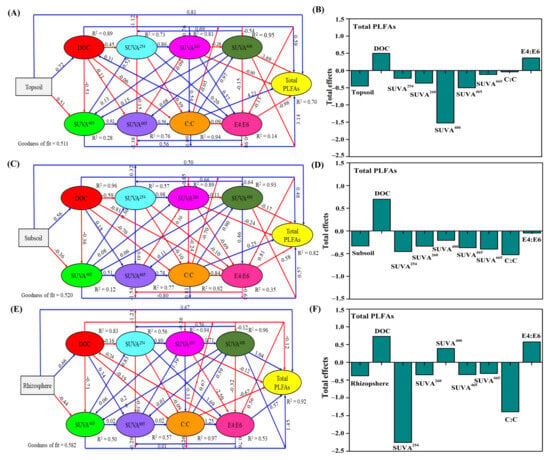
Figure 3.
The partial least squares path modeling (PLS-PM) disentangling major pathways of the influences of DOC content and its UV–Vis absorption variables on the total PLFAs for the 0–10 cm (A,B), 10–20 cm depths (C,D) and rhizosphere soil (E,F). The red and blue arrows indicate negative and positive flows of causal relationships (p < 0.05), respectively. The numbers on the arrows represent important normalized path coefficients, and the R2 is the coefficient of determination for each endogenous variable. The goodness of fit (GoF) is calculated as the geometric mean of the average communality and the average R2 value, which assess the overall prediction performance of the model. The total effects are shown as the sum of both direct and indirect effects. PLFA, phospholipid fatty acids; DOC, dissolve organic carbon; Abs254, the absorbance coefficient of 254 nm (m−1); Abs260, the absorbance coefficient of 260 nm (m−1); Abs400, the absorbance coefficient of 400 nm (m−1); Abs465, the absorbance coefficient of 465 nm (m−1); Abs665, the absorbance coefficient of 665 nm (m−1); SUAV254, the ratio of Abs254 to DOC concentration (L mg−1 m−1); SUVA260, the ratio of Abs260 to DOC concentration (L mg−1 m−1); SUVA400, the ratio of Abs400 to DOC concentration (L mg C−1 m−1); SUVA465, the ratio of Abs465 to DOC concentration (L mg C−1 m−1); SUVA665, the ratio of Abs665 to DOC concentration (L mg C−1 m−1); C:C ratio, the ratio of Abs400 to DOC concentration (L mg C −1 m−1); E4:E6 ratio, the ratio of Abs465 to Abs665.
4. Discussion
4.1. Effect of Fertilization and Cultivation Management on the Quantity and Quality of DOC
Our study revealed a significant change in DOC content following fertilization and cultivation management over the CK in all the examined soils, particularly in T1 (Table 3), which might be due to the presence of soluble material in the applied organic amendments [18,62] because organic amendments directly supply dissolved organic matter with available nutrients through organic matter decomposition and subsequently release higher DOC in the soil [63]. This result is in line with other recent studies [15,64] demonstrating that DOC content increases with the combined application of synthetic fertilizers and organic manure in arable soils, which provide higher microbial activity and SOC accumulation (Table 2) and could subsequently produce high DOC production [7,65]. A positive and significant correlation between the contents of DOC, SOC, and the DOC/SOC (Table S2) also implied that soil organic matter was a decisive variable for the production of DOC in the study soil. Following cultivation management, downslope cultivation techniques involving cultivation parallel to the contour lines of the slope also significantly influence DOC content (Table 3) by reducing nutrients and DOC losses and promoting better water infiltration and root development, subsequently providing favorable conditions for microbial activity, as moisture availability supports the decomposition of organic matter, leading to higher DOC content compared to contour cultivation [37,49]. Ma et al. [37] demonstrated that cultivation in sloping cropland is a key approach to erasing nutrient losses, resulting in considerable variations between cultivated and uncultivated areas. Among the treatments, a higher rhizosphere effect on DOC content than bulk soil of 0–10 cm and 10–20 cm depths (Table 3) was possibly due to the release of a large carbon flux near the root exudates, as a result promoting more microbial activity (MBC and MBN; Table 2) and various PLFA microbial groups (Table 4) in the rhizosphere soil, which in turn play a vital role in breaking organic matter during root turnover and lead to the release of more DOC in the rhizosphere soil [7,66]. Likewise, the DOC content also increased in the topsoil increment compared to the subsurface soil among the applied treatments (Table 3). This depth-dependent gradient is in line with other recent studies [6,17,67] demonstrating that decreasing DOC content in lower layers may be a result of the decomposition of crop residues or a decline in DOM content and fluxes in soil solution with increasing soil depths, which most probably depending on fertilization practices and soil textures [6,17].
According to the DOC quality indices, fertilization and cultivation management decreased the absorption values of SUVA254, SUVA260, SUVA400, SUVA465, SUVA665, and C:C and raised the ratio of E4:E6 under T2 and T3 at 10–20 cm depths and T3 in rhizosphere soil, respectively, compared with CK (Table 3), indicating that fertilization and cultivation management altered the optical properties of DOC differently and that E4:E6 was a good substitution for temporal change in assessing the effects of applied fertilizer treatments to the study soil, which reduced the aromaticity of DOC and made the DOC more reachable to microbial populations, thus enhancing the quality of DOC [2,8]. However, a decline in the values of UV–Vis absorptivity variables except the E4:E6 ratio indicates the presence of a more stable and recalcitrant chemical structure (i.e., lignin-derived DOM), which could be due to the soil deposition and severe soil erosion in the sloping farmland, which could preferentially absorb on the mineral surfaces [5,13]. In general, the high ratio of E4:E6 was used to assess the lower ratio of humic to fulvic acid and the higher molecular size of humic substances in the DOC [2,9]. A reduced value of SUVA254 and C:C ratio indicates a reduction in the aromaticity of DOC and a decreased proportion of colored humic compounds in the DOC [2,10]. In the present study, the average E4:E6 ratio among the treatments ranged from 1.3 to 3.8, which was lower than the value of 6 to 7.8 in a phosphorus addition experiment in Northeast China [2]. This result indicated that synthetic fertilizer with downslope cultivation decreased the degree of humification in the DOC (low aromaticity for humic acids), likely due to dominant darker-colored humic substances rather than fulvic acids, which infers some difficulty in the production of DOC and transportation processes operating within the sloping land, leading DOC to be more accessible to microbial activity in the subsurface and rhizosphere soil, respectively [2,7]. These observations imply that the lower molecular weight of humic acid and decreasing DOC aromaticity after fertilization management (i.e., mineral fertilizer) with downslope cultivation is a better strategy to enhance water quality by accelerating the microbial-mediated ecological processes in the sloping farmland and modifying hydrological routing in the aquatic ecosystem.
4.2. Effect of Fertilization and Cultivation Management on Soil Microbial Community Structure
Generally, it can be seen that the rhizosphere soil of all applied tested treatments had higher levels of both PLFA microbial groups and functional diversity irrespective of the 0–10 and 10–20 cm depths (Table 4 and Table 5). This might be attributed to the higher bioavailability of soil-borne nutrients and a root-induced shift in the microbial biomass properties (MBC and MBN; Table 2), which actively increase root growth and release organic compounds in the form of metabolites by rhizodeposition, thereby promoting the development of the microbial compositions in rhizosphere soil [43,44]. However, the magnitude of microbial community structure was less comparable between 0–10 cm and 10–20 cm. The less pronounced difference in the studied soil depths might be due to the less detected variation in SOC content (Table 2) and soil moisture, which may contribute to the differentiation of microbial communities within soil depth [68,69]. Following fertilization management, total PLFA content was significantly (p < 0.05) increased compared with CK (Table 4), indicating that applied fertilization could change the microbial community structure, as PC1 and PC2 analysis separated fertilization treatments from CK (Figure S1). Indeed, these differences were mainly highest in the T1 treatment, suggesting that the adaptation of synthetic fertilizer and organic manure greatly sustains the total PLFA content with an adequate supply of soil nutrients and microbial biomass properties (Table 2), which provides potential substrate, energy, and root exudates as a C source for microbial growth [34,70]. In addition, we also observed that the total PLFA content in contour cultivation (T4) was significantly higher than that in downslope cultivation (T2) because contour cultivation involves surface roughness or plowing to form contour ridges to disperse flow direction to alter runoff velocity [46], reducing soil nutrient loss [52], and building up microbial diversity indices (Table 5) compared with downslope cultivation, which helps to stimulate soil ecosystem resilience and functionality. According to the RFA analysis, SOC and DOC were found to be the main predictor variables responsible for variations in total PLFAs in the examined soil (Figure 2), suggesting that labile and recalcitrant organic C are the main limiting energy sources for microbial proliferation [36,71]. Furthermore, fertilization management significantly influenced the selected group of PLFA biomarkers in the examined soil (Table 4). In the study soils, regardless of fertilization practices, bacterial PLFAs dominated the microbial community (Table 4). This might be attributed to the alkaline soil reaction (pH 8.2), along with high soil moisture in the sloping farmland, where bacteria could be adapted to higher decomposition rates and lead to a lower F/B ratio (Figure 1A), as they constitute the initial group of soil microbes responsible for assimilating a majority of the readily available organic substrates (i.e., DOC) upon their inclusion in the soil [34,70]. It is also worth noting that the content of total bacteria was significantly correlated with DOC content (Table S3), suggesting that specific fatty acids found in bacteria are associated with DOC content [71,72].
Among bacteria, G+ and G− were also significantly increased by the applied fertilization treatments, mostly benefited by T1 in the examined soils (Table 4), suggesting that G− prefers to use C soon after the addition of organic manure (i.e., source of plant-derived labile C) and then benefits slow-growing microorganisms, such as G+, which utilize C from complex sources (i.e., recalcitrant substrates) [31,70]. Indeed, fertilization treatments stimulated G+ more than G−, leading to a significant increase in the G+/G− ratio in T1 compared to the other treatments (Figure 1B), indicating that the environment in T1 favors the shift in microbial structure to a more copiotrophic community when more organic matter derived from soluble organic C (i.e., DOC) is available, which may help to proliferate G+ bacteria in substrate-poor environments [38,70]. However, the decreased ratio of EOC/SOC in T1 (Table S4) indicated that G+ bacteria would have advantages as a result of changes in the availability of EOC when transitioning from a nutrient-poor to a nutrient-rich environment during the decomposition of organic or straw materials [26]. In bacteria, the ratio of cy/pre and mono/sat has been proposed as a sensitive indicator of nutritional and physiological stress conditions [72,73]. We found a substantial increase in cy/pre and mono/sat ratio following T1 and T3 in the rhizosphere soil only (Figure 1C), indicating that microbes better sustain the availability of C, N, and P in the rhizosphere soil and adopt well to intensified environmental pressures by boosting synthetic fertilizer and organic manure plus downslope cultivation strategies [74,75]. We further observed that the ratio of mono/sat among the applied treatments was less than 1.0 and decreased under the fertilization practices relative to the CK (Figure 1D), suggesting that fertilization practices encouraged the growth of autonomous and obligate anaerobes in the purple soil, which can better sustain the stability of agroecosystems in the sloping farmland. Our values affirm the value of the mono/sat ratio given by Chen et al. [70] in purple soil receiving long-term synthetic fertilizer and organic manure. Furthermore, following fertilization practices used by microorganisms also increases fungi and AMF groups, notably in T1, since fungi are principally accountable for the mineralization and breakdown of organic residues in soil, serving as a vital food source for other microbes, which can help to promote soil aggregate formation, thus benefiting the long-term sustainability of agroecosystems and soil quality [76].
4.3. Relationship between Soil Microbial Communities and DOC Indices
To assess the link between DOC indices and total PLFAs across the fertilization practices in the study soil, the result of PLS-PM analysis showed that the changes in quantity and quality of DOC directly or indirectly determined the total PLFA (Figure 3), which might regulate the microbial community structure. In terms of total effect, a key driver of total PLFA is DOC content, which directly affects microbial community structure in all the examined soil (Figure 3B,D,F), while the E4:E6 ratio was the second main variation to determine the total PLFA in the 0–10 cm and rhizosphere soil, respectively (Figure 3B,F). These findings corroborate our third hypothesis by highlighting the important role of the DOC composition in regulating microbial community structure. Our hypothesis was partly proven by previous studies [33,72,77], indicating that agricultural management would alter microbial communities, increase the quantity of DOC, and change its aromaticity because DOC is an essential substrate for microorganisms [33]. These results were mainly attributed to the higher readily decomposable compounds in DOC (i.e., organic acids and carbohydrates) and recalcitrant insoluble materials utilized primarily by soil bacteria and fungi after adding mineral fertilizer and organic amendments to the soil [33,72]. Furthermore, an increase in the E4:E6 ratio following fertilizer practices indicates a lower proportion of humic acid to fulvic acid in DOC [2,9]. The great contribution of the E4:E6 ratio in influencing the shift in the microbial community structure might be explained in several ways. First, fulvic and humic acids are organic chemicals that originate from the degradation of plant and microbial residues through root exudates; these root exudates released by plants may directly influence the microbial community structure in the rhizosphere soil [7]. Second, a change in the E4:E6 ratio following fertilization practices could affect the type of organic substrate available to microbes, which could potentially favor certain microbial groups by decreasing the degree of humification in the DOC and making DOC more accessible to microbial activity in the rhizosphere soil, which could, in turn, affect the characteristics of DOC and plant–microbe interactions [2,78]. Although variations in soil microbial community composition are related to many soil variables following different fertilization practices, the present study highlights that changes in quantity and quality of DOC regulated changes in microbial community structure. Our findings convey helpful evidence for controlling the reduction in DOC quantity and sustaining microbial community structure and imply that the addition of NPK fertilizer coupled with organic amendments plus contour cultivation could be an effective strategy to improve the quality of purple soil in sloping farmlands and minimize the adverse effects of poor water quality.
5. Conclusions
In summary, DOC content in top and subsoil depths (0–10 and 10–20 cm) and the rhizosphere significantly increased following 15 years of long-term fertilization and cultivation management in purple soil sloping farmland in Southwest China. The content of DOC in the examined soil followed the sequence of T1 > T3 > T2 > T4 > CK. However, in contrast to our hypothesis, fertilization management caused a reduction in SUVA254, SUVA260, SUVA400, SUVA465, SUVA665, and the C:C ratio and increased the ratio of E4:E6, regardless of T2 and T3, at the 10–20 cm depth and in the rhizospheric soil. Moreover, the content of total PLFA contents and selected microbial groups (i.e., bacteria, G−, G+, Fungi, AMF, and actinomycetes) also significantly increased in response to fertilization management, where soil amended with mineral fertilizer combined with organic fertilizer plus downslope cultivation by T1 treatment alter substantially the microbial community structure compared with other fertilizer treatments. When comparing the downslope and contour cultivation treatments, we observed that the microbial community structure and functional diversity under contour cultivation (T4) were considerably higher than those under downslope cultivation (T2). Further, DOC content and its UV–Vis absorptivity variables, such as the E4:E6 ratio, had apparent effects on the total PLFAs, suggesting that they were the important factors among DOC composition in regulating soil microbial community structure in the purple soil. Our results can provide a better understanding of the importance of balanced fertilization with N, P, and K coupled with organic amendment plus contour cultivation in promoting the soil microbial community structure through its indirect influence on the soil biogeochemical cycle of carbon, thus enhancing crop productivity and overall soil health. Notably, this finding can contribute to developing an effective nutrient management system toward sustainability while ensuring the delivery of soil ecosystem services in the intense cultivation of sloping farmland for local farmers and similar conditions globally.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy14030426/s1, Figure S1: Plots of first two principal component (PC1 and PC2) groups in five treatments and three soils (A), and plot of two principal components among individual PLFAs in the 0–10 cm (B), 10–20 cm depth (C), and rhizosphere soil (D). Numbers (1–3) following the treatment indicates the soil of 0–10 cm, 10–20 cm depth, and rhizosphere soil. CK, blank, no fertilizer applied; T1, a combination of NPK fertilizer plus organic manure and downslope cultivation; T2, NPK fertilizer and downslope cultivation; T3, 1.5-fold NPK fertilizer and downslope cultivation; T4, NPK fertilizer and contour cultivation; Table S1: PLFA biomarker data used to analyze microbial community composition and stress indicators; Table S2: Pearson correlation coefficients (r) between various DOC indices and soil variables determined in two soil depths and rhizosphere soil [79,80]; Table S3. Pearson’s correlations between the soil PLFA (microbial groups) and soil variables across all samples; Table S4. The nutrient stoichiometry of the 0–10 cm, 10–20 cm, and rhizosphere soil under different long-term fertilization management.
Author Contributions
Conceptualization, A.K. and B.H.; methodology, A.K. and G.Z.; software, A.K.; validation, A.K. and B.H.; data curation, A.K.; writing—original draft preparation, A.K. writing—review and editing, B.H. and T.L.; supervision, B.H.; funding acquisition, B.H.; Resources and project administration, B.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (Grant No. U20A20326, 42107347), the Fundamental Research Funds for the Central Universities (Grant No. SWU-KT22060), the Natural Science Foundation of Chongqing, China (CSTB2022NSCQ-MSX0385), and the State Cultivation Base of Eco-agriculture for Southwest Mountainous Land, Southwest University.
Data Availability Statement
Data will be made available on reasonable request.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Zsolnay, A. Dissolved organic matter: Artefacts, definitions, and functions. Geoderma 2003, 113, 187–209. [Google Scholar] [CrossRef]
- Mao, R.; Li, S.Y.; Zhang, X.H.; Wang, X.W.; Song, C.C. Effect of long-term phosphorus addition on the quantity and quality of dissolved organic carbon in a freshwater wetland of Northeast China. Sci. Total Environ. 2017, 586, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Rosa, E.; Debska, B. Seasonal changes in the content of dissolved organic matter in arable soils. J. Soils Sediments 2017, 18, 2703–2714. [Google Scholar] [CrossRef]
- Roth, V.N.; Lange, M.; Simon, C.; Hertkorn, N.; Bucher, S.; Goodall, T.; Griffiths, R.I.; Mellado-Vázquez, P.G.; Mommer, L.; Oram, N.J.; et al. Persistence of dissolved organic matter explained by molecular changes during its passage through soil. Nature Geosci. 2019, 12, 755–761. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, C.; Pan, S.; Shang, J.; Wang, X. Responses of molecular composition and biodegradation of dissolved organic matter to erosion in topsoil versus subsoil in a Mollisol agricultural ecosystem. Agric. Ecosyst. Environ. 2023, 354, 108569. [Google Scholar] [CrossRef]
- Kalbitz, K.; Solinger, S.; Park, J.H.; Michalzik, B.; Matzner, E. Controls on the dynamics of dissolved organic matter in soils: A review. Soil Sci. 2000, 165, 277–304. [Google Scholar] [CrossRef]
- Bolan, N.S.; Adriano, D.C.; Kunhikrishnan, A.; James, T.; McDowell, R.; Senesi, N. Dissolved organic matter: Biogeochemistry, dynamics, and environmental significance in soils. Adv. Agron. 2011, 110, 1–75. [Google Scholar]
- Strack, M.; Zuback, Y.; McCarter, C.; Price, J. Changes in dissolved organic carbon quality in soils and discharge 10 years after peatland restoration. J. Hydrol. 2015, 527, 345–354. [Google Scholar] [CrossRef]
- Li, T.; Liang, C.; He, B.; Li, S. Effects of cornstalk mulching and rainfall intensity on the quantity and quality of dissolved organic carbon in runoff: A field rainfall simulation at the plot scale. Land Degrad. Dev. 2023, 34, 3920–3931. [Google Scholar] [CrossRef]
- Wallage, Z.E.; Holden, J.; Mcdonald, A.T. Drain blocking: An effective treatment for reducing dissolved organic carbon loss and water discolouration in a drained peatland. Sci. Total Environ. 2006, 367, 811–821. [Google Scholar] [CrossRef]
- Ma, Y.; Li, S. Spatial and temporal comparisons of dissolved organic matter in river systems of the Three Gorges Reservoir region using fluorescence and UV–Visible spectroscopy. Environ. Res. 2020, 189, 109925. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Bi, Y.; He, B.; Feng, M.; Xi, P.; Li, T. Changes in quantity and quality of dissolved organic carbon in purple soil: Roles of land use and soil depth. Land Degrad. Develop. 2023, 34, 327–337. [Google Scholar] [CrossRef]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Tech. 2003, 37, 4702–4708. [Google Scholar] [CrossRef]
- Liang, K.; Li, T.; He, B.; Qian, T. Dynamics of dissolved organic carbon in runoff discharge under different rainfall patterns in a representative agricultural catchment. J. Hydrol. 2023, 617, 129079. [Google Scholar] [CrossRef]
- Pan, H.; Shi, L.; Liu, X.; Lei, H.; Yu, J.; Yang, G. Characteristics of Soil DOM and Its Effect on the Transformation of Potentially Toxic Elements (PTE) Forms under Organic Fertilizer Return Conditions. Agronomy 2023, 13, 630. [Google Scholar] [CrossRef]
- Long, G.; Jiang, Y.; Sun, B. Seasonal and inter-annual variation of leaching of dissolved organic carbon and nitrogen under long-term manure application in an acidic clay soil in subtropical China. Soil Till. Res. 2015, 146, 270–278. [Google Scholar] [CrossRef]
- Romero, C.M.; Engel, R.E.; D’Andrilli, J.; Chen, C.; Zabinski, C.; Miller, P.R.; Wallander, R. Bulk optical characterization of dissolved organic matter from semiarid wheat-based cropping systems. Geoderma 2017, 306, 40–49. [Google Scholar] [CrossRef]
- Chantigny, M.H. Dissolved and water-extractable organic matter in soils: A review on the influence of land use and management practices. Geoderma 2003, 113, 357–380. [Google Scholar] [CrossRef]
- Ding, Y.D.; Song, C.C.; Chen, G.J.; Zhang, X.H.; Mao, R. Effects of long-term nitrogen addition on dissolved organic matter characteristics in a temperate wetland of Northeast China. Ecotoxicol. Environ. Safety 2021, 226, 112822. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of phosphatase enzymes in soil. In Phosphorus in Action. Soil Biology; Bünemann, E., Oberson, A., Frossard, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 26, pp. 215–243. [Google Scholar]
- Fraser, T.D.; Lynch, D.H.; Gaiero, J.; Khosla, K.; Dunfield, K.E. Quantification of bacterial non-specific acid (phoC) and alkaline (phoD) phosphatase genes in bulk and rhizosphere soil from organically managed soybean fields. Appl. Soil Ecol. 2017, 111, 48–56. [Google Scholar] [CrossRef]
- Embacher, A.; Zsolnay, A.; Gattinger, A.; Munch, J.C. The dynamics of water extractable organic matter (WEOM) in common arable topsoils: I. Quantity, quality and function over a three year period. Geoderma 2007, 139, 11–22. [Google Scholar] [CrossRef]
- Li, T.; Li, S.; Liang, C. The immediate effects of downslope cornstalk mulch (DCM) on sediment yield, runoff and runoff associated dissolved carbon loss in a representative hillslope, Southwestern China. Catena 2019, 175, 9–17. [Google Scholar] [CrossRef]
- Böhme, L.; Langer, U.; Böhme, F. Microbial biomass, enzyme activities and microbial community structure in two European long-term field experiments. Agric. Ecosys. Environ. 2005, 109, 141–152. [Google Scholar] [CrossRef]
- Dangi, S.; Gao, S.; Duan, Y.; Wang, D. Soil microbial community structure affected by biochar and fertilizer sources. Appl. Soil Ecol. 2019, 150, 103452. [Google Scholar] [CrossRef]
- Liu, X.; Peng, C.; Zhang, W.; Li, S.; An, T.; Xu, Y.; Ge, Z.; Xie, N.; Wang, J. Subsoiling tillage with straw incorporation improves soil microbial community characteristics in the whole cultivated layers: A one-year study. Soil Till. Res. 2022, 215, 105188. [Google Scholar] [CrossRef]
- Kennedy, A.C.; Smith, K.L. Soil microbial diversity and the sustainability of agricultural soils. Plant Soil 1995, 170, 75–86. [Google Scholar] [CrossRef]
- Drenovsky, R.E.; Elliott, G.N.; Graham, K.J.; Scow, K.M. Comparison of phospholipid fatty acid (PLFA) and total soil fatty acid methyl esters (TSFAME) for characterizing soil microbial communities. Soil Biol. Biochem. 2004, 36, 1793–1800. [Google Scholar] [CrossRef]
- Frostegård, A.; Bååth, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil. Soils 1996, 22, 59–65. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M.; Gunapala, N.; Graham, K.J. Determinants of soil microbial communities: Effects of agricultural management, season, and soil type on phospholipids fatty acid profiles. Microb. Ecol. 1998, 36, 1–12. [Google Scholar] [CrossRef]
- Esperschütz, J.; Gattinger, A.; Mäder, P.; Schloter, M.; Fließbach, A. Response of soil microbial biomass and community structures to conventional and organic farming systems under identical crop rotations. FEMS Microbiol. Ecol. 2007, 61, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; He, Z.; Wilson, M.J.; Campbell, C.D. Microbial biomass and community structure in a sequence of soils with increasing fertility and changing land use. Microb. Ecol. 2000, 40, 223–237. [Google Scholar] [CrossRef]
- Marschner, P.; Kandeler, E.; Marschner, B. Structure and function of the soil microbial community in a long-term fertilizer experiment. Soil Biol. Biochem. 2003, 35, 453–461. [Google Scholar] [CrossRef]
- Zhong, W.; Gu, T.; Wang, W.; Zhang, B.; Lin, X.; Huang, Q.; Shen, W. The effects of mineral fertilizer and organic manure on soil microbial community and diversity. Plant Soil 2010, 326, 511–522. [Google Scholar] [CrossRef]
- Williams, A.; Börjesson, G.; Hedlund, K. The effects of 55 years of different inorganic fertiliser regimes on soil properties and microbial community composition. Soil Biol. Biochem. 2013, 67, 41–46. [Google Scholar] [CrossRef]
- Lazcano, C.; Gómez-Brandón, M.; Revilla, P.; Domínguez, J. Short-term effects of organic and inorganic fertilizers on soil microbial community structure and function. Biol. Fertil. Soils. 2012, 49, 723–733. [Google Scholar] [CrossRef]
- Ma, X.; Liu, M.; Li, Z. Shifts in microbial biomass and community composition in subtropical paddy soils under a gradient of manure amendment. Biol. Fertil. Soils. 2016, 52, 775–787. [Google Scholar] [CrossRef]
- Wei, M.; Hu, G.; Wang, H.; Bai, E.; Lou, Y.; Zhang, A.; Zhuge, Y. 35 years of manure and chemical fertilizer application alters soil microbial community composition in a Fluvo-aquic soil in Northern China. Eur. J. Soil Biol. 2017, 82, 27–34. [Google Scholar] [CrossRef]
- Samaddar, S.; Chatterjee, P.; Truu, J.; Anandham, R.; Kim, S.; Sa, T. Long-term phosphorus limitation changes the bacterial community structure and functioning in paddy soils. Appl. Soil Ecol. 2019, 134, 111–115. [Google Scholar] [CrossRef]
- Zhang, B.; He, H.; Ding, X.; Zhang, X.; Zhang, X.; Yang, X.; Filley, T.R. Soil microbial community dynamics over a maize (Zea mays L.) growing season under conventional- and no-tillage practices in a rainfed agroecosystem. Soil Till. Res. 2012, 124, 153–160. [Google Scholar] [CrossRef]
- Stone, M.M.; DeForest, J.L.; Plante, A.F. Changes in extracellular enzyme activity and microbial community structure with soil depth at the Luquillo Critical Zone Observatory. Soil Biol. Biochem. 2014, 75, 237–247. [Google Scholar] [CrossRef]
- Herre, M.; Heitkötter, J.; Heinze, S.; Rethemeyer, J.; Preusser, S.; Kandeler, E.; Marschner, B. Differences in organic matter properties and microbial activity between bulk and rhizosphere soil from the top- and subsoils of three forest stands. Geoderma 2022, 409, 115589. [Google Scholar] [CrossRef]
- Söderberg, K.H.; Probanza, A.; Jumpponen, A.; Bååth, E. The microbial community in the rhizosphere determined by community-level physiological profiles (CLPP) and direct soil– and cfu–PLFA techniques. Appl. Soil Ecol. 2004, 25, 135–145. [Google Scholar] [CrossRef]
- Ai, C.; Liang, G.; Sun, J.; Wang, X.; Zhou, W. Responses of extracellular enzyme activities and microbial community in both the rhizosphere and bulk soil to long-term fertilization practices in a fluvo-aquic soil. Geoderma 2012, 173–174, 330–338. [Google Scholar] [CrossRef]
- Schmidt, J.E.; Kent, A.D.; Brisson, V.L.; Gaudin, A.C.M. Agricultural management and plant selection interactively affect rhizosphere microbial community structure and nitrogen cycling. Microbiome 2019, 7, 146. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Li, L.; Chen, S.; Shi, Y.; Xu, M.; Zhang, Q. Straw returning on sloping farmland reduces the soil and water loss via surface flow but increases the nitrogen loss via interflow. Agric. Ecosyst. Environ. 2022, 339, 108154. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Q. Adoption and continued use of contour cultivation in the highlands of southwest China. Ecol. Econom. 2013, 91, 28–37. [Google Scholar] [CrossRef]
- Ouyang, W.; Li, Z.; Liu, J.; Guo, J.; Fang, F.; Xiao, Y.; Lu, L. Inventory of apparent nitrogen and phosphorus balance and risk of potential pollution in typical sloping cropland of purple soil in China—A case study in the Three Gorges Reservoir region. Ecol. Eng. 2017, 106, 620–628. [Google Scholar] [CrossRef]
- Khan, A.; Zhang, G.; Li, T.; He, B. Fertilization and cultivation management promotes soil phosphorus availability by enhancing soil P-cycling enzymes and the phosphatase encoding genes in bulk and rhizosphere soil of a maize crop in sloping farmland. Ecotoxicol. Environ. Safety 2023, 264, 115441. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Li, T.; He, B. Runoff-related nutrient loss affected by fertilization and cultivation in sloping croplands: An 11-year observation under natural rainfall. Agric. Ecosyst. Environ. 2021, 319, 107549. [Google Scholar] [CrossRef]
- Li, H.; Shi, D. Spatio-temporal variation in soil erosion on sloping farmland based on the integrated valuation of ecosystem services and trade-offs model: A case study of Chongqing, southwest China. Catena 2024, 236, 107693. [Google Scholar] [CrossRef]
- Quinton, J.; Catt, J. The effects of minimal cultivation and contour cultivation on surface runoff, soil loss and crop yield in the long-term Woburn erosion reference experiment on Sandy soil at Woburn, England. Soil Use Manag. 2004, 20, 343–349. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Page, A.L., Ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Schaeffer, S.M.; Sharp, E.; Schimel, J.P.; Welker, J.M. Soil plant N processes in a High Arctic ecosystem, NW Greenland are altered by long term experimental warming and higher rainfall. Glob. Chang Biol. 2013, 19, 3529–3539. [Google Scholar] [CrossRef]
- ISSCAS Institute of Soil Sciences, Chinese Academy of Sciences. Physical and Chemical Analysis Methods of Soils; Shanghai Science Technology Press: Shanghai, China, 1978. (In Chinese) [Google Scholar]
- Keeney, D.R.; Nelson, D.W. Nitrogen in organic forms. In Methods of Soil Analysis. Part 2. Agronomy, No. 9; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 643–698. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanbe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; Circ No 939; USDA: Washington, DC, USA, 1954. [Google Scholar]
- Vance, E.D.; Brookes, A.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Gómez Brandón, M.; Lores, M.; Domínguez, J. A new combination of extraction and derivatization methods that reduces the complexity and preparation time in determining phospholipid fatty acids in solid environmental samples. Bioresour. Technol. 2010, 101, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Canad. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Stumpe, B.; Marschner, B. Dissolved organic carbon from sewage sludge and manure can affect estrogen sorption and mineralization in soils. Environm. Pollut. 2010, 158, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhu, J.; Fu, Q.; Chen, J.; Hu, H.; Huang, Q. Structure and biodegradability of dissolved organic matter from Ultisol treated with long-term fertilizations. J. Soils Sedim. 2018, 18, 1865–1875. [Google Scholar] [CrossRef]
- Singh, S.; Dutta, S.; Inamdar, S. Land application of poultry manure and its influence on spectrofluorometric characteristics of dissolved organic matter. Agric. Ecosyst. Environ. 2014, 193, 25–36. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.F.; Li, D.M.; Xu, C.X.; Wu, M.; Liu, M.; Li, P.F.; Li, G.L.; Zhang, T.L.; Li, Z.P. Variation of soil dissolved organic carbon under long-term different fertilizations and its correlation with maize yields. J. Soils Sediments 2020, 20, 2761–2770. [Google Scholar] [CrossRef]
- Zhao, X.; Tian, P.; Sun, Z.; Liu, S.; Wang, Q.; Zeng, Z. Rhizosphere effects on soil organic carbon processes in terrestrial ecosystems: A meta-analysis. Geoderma 2022, 412, 115739. [Google Scholar] [CrossRef]
- Liu, E.; Yan, C.; Mei, X.; Zhang, Y.; Fan, T. Long-Term Effect of Manure and Fertilizer on Soil Organic Carbon Pools in Dryland Farming in Northwest China. PLoS ONE 2013, 8, e56536. [Google Scholar] [CrossRef]
- Fierer, N.; Schimel, J.P.; Holden, P.A. Variations in microbial community composition through two soil depth profiles. Soil Biol. Biochem. 2003, 35, 167–176. [Google Scholar]
- Börjesson, G.; Menichetti, L.; Kirchmann, H.; Kätterer, T. Soil microbial community structure affected by 53 years of nitrogen fertilisation and different organic amendments. Biol. Fertil. Soils 2012, 48, 245–257. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Zhou, W.; Guo, S.; Zhu, R.; Qin, Y.; Sun, J. Responses of crop yields, soil enzymatic activities, and microbial communities to different long-term organic materials applied with chemical fertilizer in purple soil. Eur. J. Soil Biol. 2021, 105, 103319. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, W.; Bol, R.; Xiao, Q.; Wu, L.; Zhang, W. Microbial regulation of net N mineralisation is driven by C, N, P content and stoichiometry. Eur. J. Soil Sci. 2022, 73, e13257. [Google Scholar] [CrossRef]
- Bossio, D.A.; Fleck, J.A.; Scow, K.M.; Fujii, R. Alteration of soil microbial communities and water quality in restored wetlands. Soil Biol. Biochem. 2006, 38, 1223–1233. [Google Scholar] [CrossRef]
- Wixon, D.L.; Balser, T.C. Toward conceptual clarity: PLFA in warmed soils. Soil Biol. Biochem. 2013, 57, 769–774. [Google Scholar] [CrossRef]
- Chaudhary, D.R.; Gautam, R.K.; Ghosh, A.; Chikara, J.; Jha, B. Effect of Nitrogen Management on Soil Microbial Community and Enzymatic Activities in Jatropha curcas L. Plantation. Clean Soil Air Water 2015, 43, 1058–1065. [Google Scholar] [CrossRef]
- Azziz, G.; Frade, C.; Igual, J.M.; del Pino, A.; Lezama, F.; Valverde, Á. Legume Overseeding and P Fertilization Increases Microbial Activity and Decreases the Relative Abundance of AM Fungi in Pampas Natural Pastures. Microorganisms 2023, 11, 1383. [Google Scholar] [CrossRef]
- Romaniuk, R.; Giuffré, L.; Costantini, A.; Nannipieri, P. Assessment of soil microbial diversity measurements as indicators of soil functioning in organic and conventional horticulture systems. Ecol. Indic. 2011, 11, 1345–1353. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, J.; Han, X.; Zou, W.; Chen, X.; Lu, X.; Feng, Y. Labile organic carbon fractions drive soil microbial communities after long-term fertilization. Glob. Ecol. Conserv. 2021, 32, e01867. [Google Scholar] [CrossRef]
- Fong, S.S.; Mohamed, M. Chemical characterization of humic substances occurring in the peats of Sarawak, Malaysia. Org. Geochem. 2007, 38, 967–976. [Google Scholar] [CrossRef]
- Kieft, T.L.; Wilch, E.; Oconnor, K.; Ringelberg, D.B.; White, D.C. Survival and phospholipid fatty acid profiles of surface and subsurface bacteria in natural sediment microcosms. App. Environ. Microb. 1997, 63, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Olsson, P.A. Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. FEMS Microbiol. Ecol. 1999, 29, 303–310. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).