Insights from Fertilization and Cultivation Management for Interpreting the Variations in the Quantity and Quality of Dissolved Organic Carbon and Microbial Community Structure on Purple Soil Sloping Farmland in Southwest China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Soil Sampling and Chemical Analysis
2.4. Determination of DOC Content and UV–Vis Absorption Measurements
2.5. Soil Microbial Community Structure
2.6. Statistical Analyses
3. Results
3.1. Soil Chemical and Biological Properties
3.2. DOC and Specific Value of UV–Vis Absorption Measurements
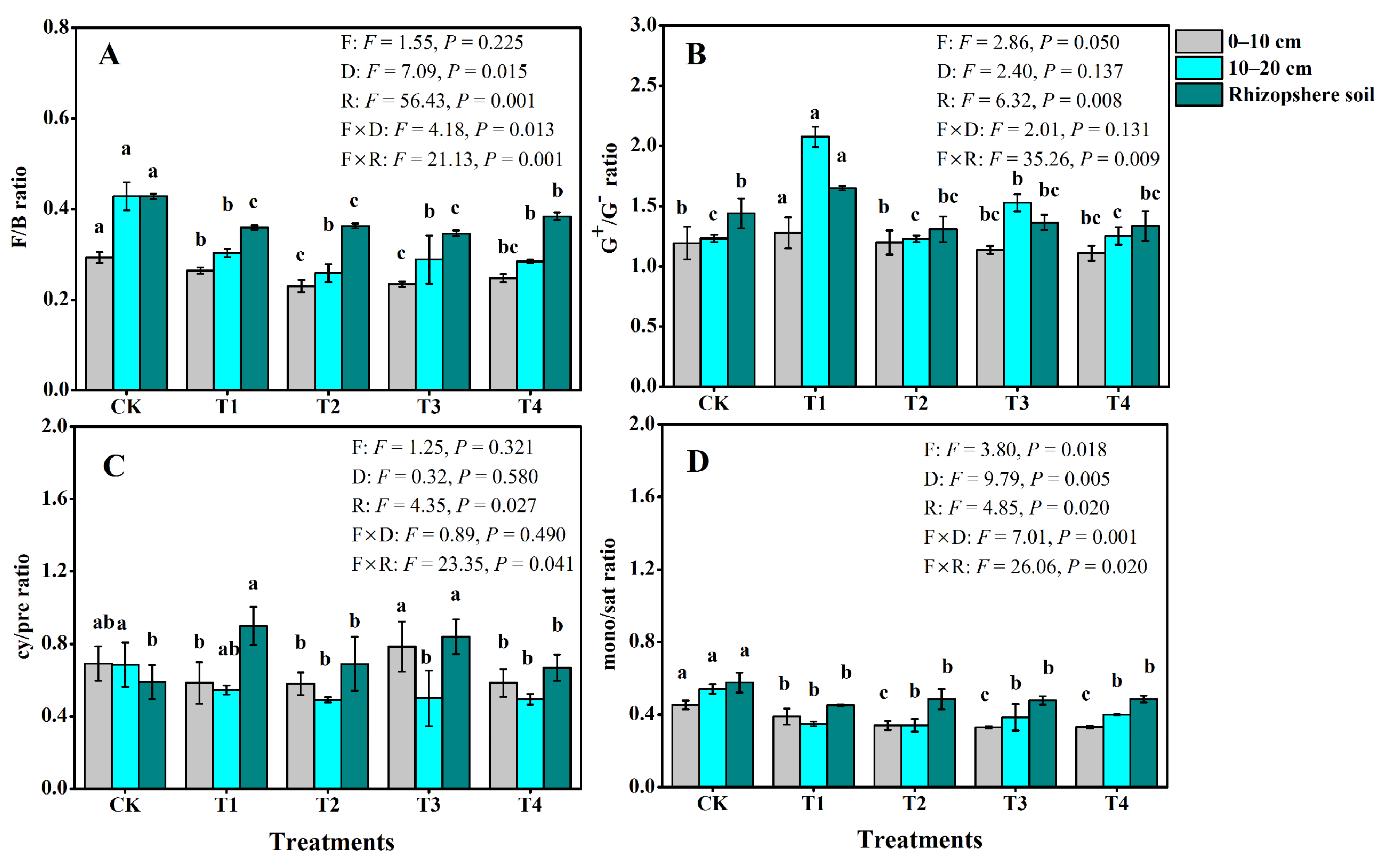
3.3. Soil Microbial Community Structure
3.4. Functional Microbial Diversity Indices
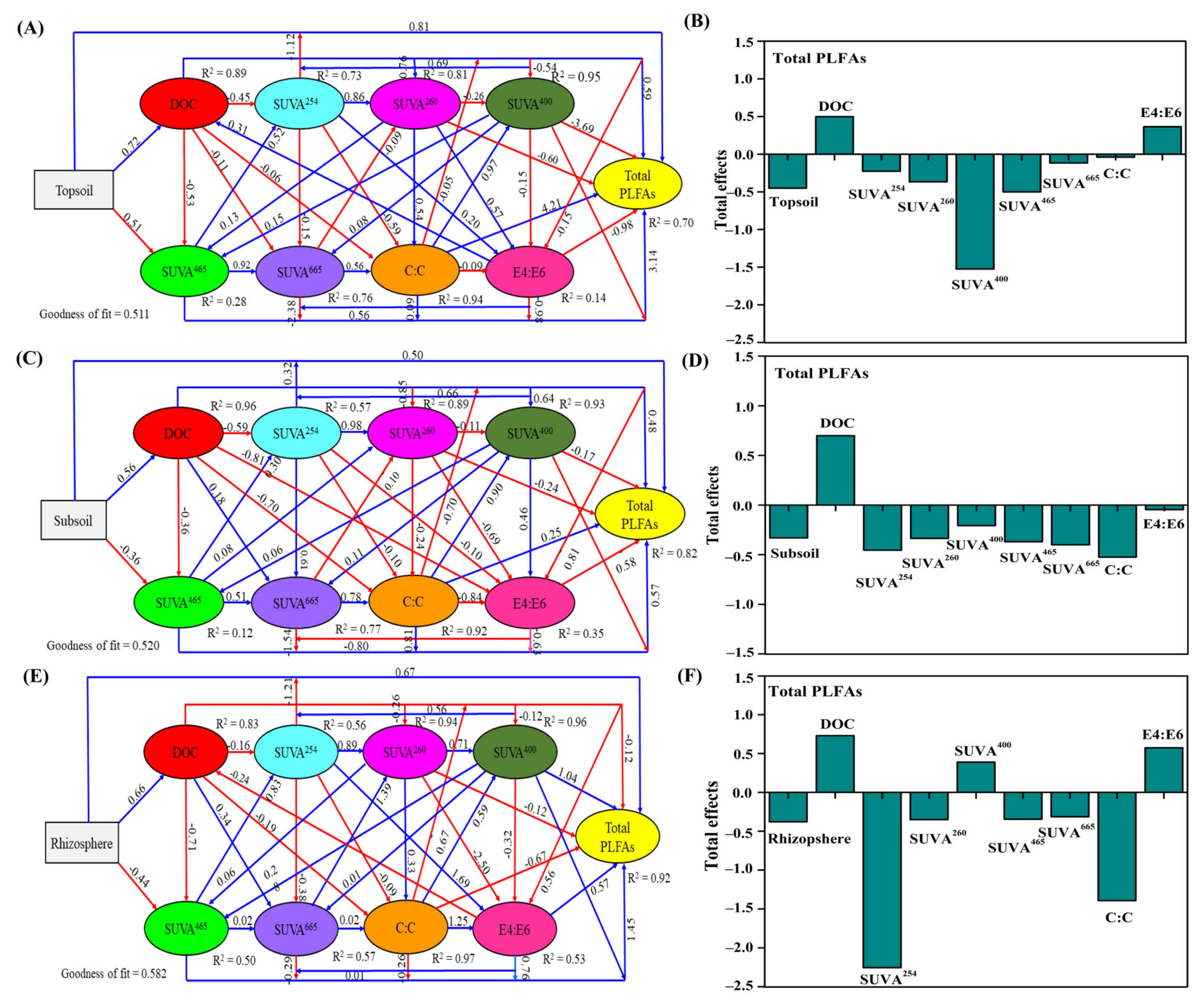
3.5. Relationships between Microbial Communities, DOC Indices, and Soil Nutrient Properties
4. Discussion
4.1. Effect of Fertilization and Cultivation Management on the Quantity and Quality of DOC
4.2. Effect of Fertilization and Cultivation Management on Soil Microbial Community Structure
4.3. Relationship between Soil Microbial Communities and DOC Indices
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zsolnay, A. Dissolved organic matter: Artefacts, definitions, and functions. Geoderma 2003, 113, 187–209. [Google Scholar] [CrossRef]
- Mao, R.; Li, S.Y.; Zhang, X.H.; Wang, X.W.; Song, C.C. Effect of long-term phosphorus addition on the quantity and quality of dissolved organic carbon in a freshwater wetland of Northeast China. Sci. Total Environ. 2017, 586, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Rosa, E.; Debska, B. Seasonal changes in the content of dissolved organic matter in arable soils. J. Soils Sediments 2017, 18, 2703–2714. [Google Scholar] [CrossRef]
- Roth, V.N.; Lange, M.; Simon, C.; Hertkorn, N.; Bucher, S.; Goodall, T.; Griffiths, R.I.; Mellado-Vázquez, P.G.; Mommer, L.; Oram, N.J.; et al. Persistence of dissolved organic matter explained by molecular changes during its passage through soil. Nature Geosci. 2019, 12, 755–761. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, C.; Pan, S.; Shang, J.; Wang, X. Responses of molecular composition and biodegradation of dissolved organic matter to erosion in topsoil versus subsoil in a Mollisol agricultural ecosystem. Agric. Ecosyst. Environ. 2023, 354, 108569. [Google Scholar] [CrossRef]
- Kalbitz, K.; Solinger, S.; Park, J.H.; Michalzik, B.; Matzner, E. Controls on the dynamics of dissolved organic matter in soils: A review. Soil Sci. 2000, 165, 277–304. [Google Scholar] [CrossRef]
- Bolan, N.S.; Adriano, D.C.; Kunhikrishnan, A.; James, T.; McDowell, R.; Senesi, N. Dissolved organic matter: Biogeochemistry, dynamics, and environmental significance in soils. Adv. Agron. 2011, 110, 1–75. [Google Scholar]
- Strack, M.; Zuback, Y.; McCarter, C.; Price, J. Changes in dissolved organic carbon quality in soils and discharge 10 years after peatland restoration. J. Hydrol. 2015, 527, 345–354. [Google Scholar] [CrossRef]
- Li, T.; Liang, C.; He, B.; Li, S. Effects of cornstalk mulching and rainfall intensity on the quantity and quality of dissolved organic carbon in runoff: A field rainfall simulation at the plot scale. Land Degrad. Dev. 2023, 34, 3920–3931. [Google Scholar] [CrossRef]
- Wallage, Z.E.; Holden, J.; Mcdonald, A.T. Drain blocking: An effective treatment for reducing dissolved organic carbon loss and water discolouration in a drained peatland. Sci. Total Environ. 2006, 367, 811–821. [Google Scholar] [CrossRef]
- Ma, Y.; Li, S. Spatial and temporal comparisons of dissolved organic matter in river systems of the Three Gorges Reservoir region using fluorescence and UV–Visible spectroscopy. Environ. Res. 2020, 189, 109925. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Bi, Y.; He, B.; Feng, M.; Xi, P.; Li, T. Changes in quantity and quality of dissolved organic carbon in purple soil: Roles of land use and soil depth. Land Degrad. Develop. 2023, 34, 327–337. [Google Scholar] [CrossRef]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Tech. 2003, 37, 4702–4708. [Google Scholar] [CrossRef]
- Liang, K.; Li, T.; He, B.; Qian, T. Dynamics of dissolved organic carbon in runoff discharge under different rainfall patterns in a representative agricultural catchment. J. Hydrol. 2023, 617, 129079. [Google Scholar] [CrossRef]
- Pan, H.; Shi, L.; Liu, X.; Lei, H.; Yu, J.; Yang, G. Characteristics of Soil DOM and Its Effect on the Transformation of Potentially Toxic Elements (PTE) Forms under Organic Fertilizer Return Conditions. Agronomy 2023, 13, 630. [Google Scholar] [CrossRef]
- Long, G.; Jiang, Y.; Sun, B. Seasonal and inter-annual variation of leaching of dissolved organic carbon and nitrogen under long-term manure application in an acidic clay soil in subtropical China. Soil Till. Res. 2015, 146, 270–278. [Google Scholar] [CrossRef]
- Romero, C.M.; Engel, R.E.; D’Andrilli, J.; Chen, C.; Zabinski, C.; Miller, P.R.; Wallander, R. Bulk optical characterization of dissolved organic matter from semiarid wheat-based cropping systems. Geoderma 2017, 306, 40–49. [Google Scholar] [CrossRef]
- Chantigny, M.H. Dissolved and water-extractable organic matter in soils: A review on the influence of land use and management practices. Geoderma 2003, 113, 357–380. [Google Scholar] [CrossRef]
- Ding, Y.D.; Song, C.C.; Chen, G.J.; Zhang, X.H.; Mao, R. Effects of long-term nitrogen addition on dissolved organic matter characteristics in a temperate wetland of Northeast China. Ecotoxicol. Environ. Safety 2021, 226, 112822. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of phosphatase enzymes in soil. In Phosphorus in Action. Soil Biology; Bünemann, E., Oberson, A., Frossard, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 26, pp. 215–243. [Google Scholar]
- Fraser, T.D.; Lynch, D.H.; Gaiero, J.; Khosla, K.; Dunfield, K.E. Quantification of bacterial non-specific acid (phoC) and alkaline (phoD) phosphatase genes in bulk and rhizosphere soil from organically managed soybean fields. Appl. Soil Ecol. 2017, 111, 48–56. [Google Scholar] [CrossRef]
- Embacher, A.; Zsolnay, A.; Gattinger, A.; Munch, J.C. The dynamics of water extractable organic matter (WEOM) in common arable topsoils: I. Quantity, quality and function over a three year period. Geoderma 2007, 139, 11–22. [Google Scholar] [CrossRef]
- Li, T.; Li, S.; Liang, C. The immediate effects of downslope cornstalk mulch (DCM) on sediment yield, runoff and runoff associated dissolved carbon loss in a representative hillslope, Southwestern China. Catena 2019, 175, 9–17. [Google Scholar] [CrossRef]
- Böhme, L.; Langer, U.; Böhme, F. Microbial biomass, enzyme activities and microbial community structure in two European long-term field experiments. Agric. Ecosys. Environ. 2005, 109, 141–152. [Google Scholar] [CrossRef]
- Dangi, S.; Gao, S.; Duan, Y.; Wang, D. Soil microbial community structure affected by biochar and fertilizer sources. Appl. Soil Ecol. 2019, 150, 103452. [Google Scholar] [CrossRef]
- Liu, X.; Peng, C.; Zhang, W.; Li, S.; An, T.; Xu, Y.; Ge, Z.; Xie, N.; Wang, J. Subsoiling tillage with straw incorporation improves soil microbial community characteristics in the whole cultivated layers: A one-year study. Soil Till. Res. 2022, 215, 105188. [Google Scholar] [CrossRef]
- Kennedy, A.C.; Smith, K.L. Soil microbial diversity and the sustainability of agricultural soils. Plant Soil 1995, 170, 75–86. [Google Scholar] [CrossRef]
- Drenovsky, R.E.; Elliott, G.N.; Graham, K.J.; Scow, K.M. Comparison of phospholipid fatty acid (PLFA) and total soil fatty acid methyl esters (TSFAME) for characterizing soil microbial communities. Soil Biol. Biochem. 2004, 36, 1793–1800. [Google Scholar] [CrossRef]
- Frostegård, A.; Bååth, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil. Soils 1996, 22, 59–65. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M.; Gunapala, N.; Graham, K.J. Determinants of soil microbial communities: Effects of agricultural management, season, and soil type on phospholipids fatty acid profiles. Microb. Ecol. 1998, 36, 1–12. [Google Scholar] [CrossRef]
- Esperschütz, J.; Gattinger, A.; Mäder, P.; Schloter, M.; Fließbach, A. Response of soil microbial biomass and community structures to conventional and organic farming systems under identical crop rotations. FEMS Microbiol. Ecol. 2007, 61, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; He, Z.; Wilson, M.J.; Campbell, C.D. Microbial biomass and community structure in a sequence of soils with increasing fertility and changing land use. Microb. Ecol. 2000, 40, 223–237. [Google Scholar] [CrossRef]
- Marschner, P.; Kandeler, E.; Marschner, B. Structure and function of the soil microbial community in a long-term fertilizer experiment. Soil Biol. Biochem. 2003, 35, 453–461. [Google Scholar] [CrossRef]
- Zhong, W.; Gu, T.; Wang, W.; Zhang, B.; Lin, X.; Huang, Q.; Shen, W. The effects of mineral fertilizer and organic manure on soil microbial community and diversity. Plant Soil 2010, 326, 511–522. [Google Scholar] [CrossRef]
- Williams, A.; Börjesson, G.; Hedlund, K. The effects of 55 years of different inorganic fertiliser regimes on soil properties and microbial community composition. Soil Biol. Biochem. 2013, 67, 41–46. [Google Scholar] [CrossRef]
- Lazcano, C.; Gómez-Brandón, M.; Revilla, P.; Domínguez, J. Short-term effects of organic and inorganic fertilizers on soil microbial community structure and function. Biol. Fertil. Soils. 2012, 49, 723–733. [Google Scholar] [CrossRef]
- Ma, X.; Liu, M.; Li, Z. Shifts in microbial biomass and community composition in subtropical paddy soils under a gradient of manure amendment. Biol. Fertil. Soils. 2016, 52, 775–787. [Google Scholar] [CrossRef]
- Wei, M.; Hu, G.; Wang, H.; Bai, E.; Lou, Y.; Zhang, A.; Zhuge, Y. 35 years of manure and chemical fertilizer application alters soil microbial community composition in a Fluvo-aquic soil in Northern China. Eur. J. Soil Biol. 2017, 82, 27–34. [Google Scholar] [CrossRef]
- Samaddar, S.; Chatterjee, P.; Truu, J.; Anandham, R.; Kim, S.; Sa, T. Long-term phosphorus limitation changes the bacterial community structure and functioning in paddy soils. Appl. Soil Ecol. 2019, 134, 111–115. [Google Scholar] [CrossRef]
- Zhang, B.; He, H.; Ding, X.; Zhang, X.; Zhang, X.; Yang, X.; Filley, T.R. Soil microbial community dynamics over a maize (Zea mays L.) growing season under conventional- and no-tillage practices in a rainfed agroecosystem. Soil Till. Res. 2012, 124, 153–160. [Google Scholar] [CrossRef]
- Stone, M.M.; DeForest, J.L.; Plante, A.F. Changes in extracellular enzyme activity and microbial community structure with soil depth at the Luquillo Critical Zone Observatory. Soil Biol. Biochem. 2014, 75, 237–247. [Google Scholar] [CrossRef]
- Herre, M.; Heitkötter, J.; Heinze, S.; Rethemeyer, J.; Preusser, S.; Kandeler, E.; Marschner, B. Differences in organic matter properties and microbial activity between bulk and rhizosphere soil from the top- and subsoils of three forest stands. Geoderma 2022, 409, 115589. [Google Scholar] [CrossRef]
- Söderberg, K.H.; Probanza, A.; Jumpponen, A.; Bååth, E. The microbial community in the rhizosphere determined by community-level physiological profiles (CLPP) and direct soil– and cfu–PLFA techniques. Appl. Soil Ecol. 2004, 25, 135–145. [Google Scholar] [CrossRef]
- Ai, C.; Liang, G.; Sun, J.; Wang, X.; Zhou, W. Responses of extracellular enzyme activities and microbial community in both the rhizosphere and bulk soil to long-term fertilization practices in a fluvo-aquic soil. Geoderma 2012, 173–174, 330–338. [Google Scholar] [CrossRef]
- Schmidt, J.E.; Kent, A.D.; Brisson, V.L.; Gaudin, A.C.M. Agricultural management and plant selection interactively affect rhizosphere microbial community structure and nitrogen cycling. Microbiome 2019, 7, 146. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Li, L.; Chen, S.; Shi, Y.; Xu, M.; Zhang, Q. Straw returning on sloping farmland reduces the soil and water loss via surface flow but increases the nitrogen loss via interflow. Agric. Ecosyst. Environ. 2022, 339, 108154. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Q. Adoption and continued use of contour cultivation in the highlands of southwest China. Ecol. Econom. 2013, 91, 28–37. [Google Scholar] [CrossRef]
- Ouyang, W.; Li, Z.; Liu, J.; Guo, J.; Fang, F.; Xiao, Y.; Lu, L. Inventory of apparent nitrogen and phosphorus balance and risk of potential pollution in typical sloping cropland of purple soil in China—A case study in the Three Gorges Reservoir region. Ecol. Eng. 2017, 106, 620–628. [Google Scholar] [CrossRef]
- Khan, A.; Zhang, G.; Li, T.; He, B. Fertilization and cultivation management promotes soil phosphorus availability by enhancing soil P-cycling enzymes and the phosphatase encoding genes in bulk and rhizosphere soil of a maize crop in sloping farmland. Ecotoxicol. Environ. Safety 2023, 264, 115441. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Li, T.; He, B. Runoff-related nutrient loss affected by fertilization and cultivation in sloping croplands: An 11-year observation under natural rainfall. Agric. Ecosyst. Environ. 2021, 319, 107549. [Google Scholar] [CrossRef]
- Li, H.; Shi, D. Spatio-temporal variation in soil erosion on sloping farmland based on the integrated valuation of ecosystem services and trade-offs model: A case study of Chongqing, southwest China. Catena 2024, 236, 107693. [Google Scholar] [CrossRef]
- Quinton, J.; Catt, J. The effects of minimal cultivation and contour cultivation on surface runoff, soil loss and crop yield in the long-term Woburn erosion reference experiment on Sandy soil at Woburn, England. Soil Use Manag. 2004, 20, 343–349. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Page, A.L., Ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Schaeffer, S.M.; Sharp, E.; Schimel, J.P.; Welker, J.M. Soil plant N processes in a High Arctic ecosystem, NW Greenland are altered by long term experimental warming and higher rainfall. Glob. Chang Biol. 2013, 19, 3529–3539. [Google Scholar] [CrossRef]
- ISSCAS Institute of Soil Sciences, Chinese Academy of Sciences. Physical and Chemical Analysis Methods of Soils; Shanghai Science Technology Press: Shanghai, China, 1978. (In Chinese) [Google Scholar]
- Keeney, D.R.; Nelson, D.W. Nitrogen in organic forms. In Methods of Soil Analysis. Part 2. Agronomy, No. 9; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 643–698. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanbe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; Circ No 939; USDA: Washington, DC, USA, 1954. [Google Scholar]
- Vance, E.D.; Brookes, A.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Gómez Brandón, M.; Lores, M.; Domínguez, J. A new combination of extraction and derivatization methods that reduces the complexity and preparation time in determining phospholipid fatty acids in solid environmental samples. Bioresour. Technol. 2010, 101, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Canad. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Stumpe, B.; Marschner, B. Dissolved organic carbon from sewage sludge and manure can affect estrogen sorption and mineralization in soils. Environm. Pollut. 2010, 158, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhu, J.; Fu, Q.; Chen, J.; Hu, H.; Huang, Q. Structure and biodegradability of dissolved organic matter from Ultisol treated with long-term fertilizations. J. Soils Sedim. 2018, 18, 1865–1875. [Google Scholar] [CrossRef]
- Singh, S.; Dutta, S.; Inamdar, S. Land application of poultry manure and its influence on spectrofluorometric characteristics of dissolved organic matter. Agric. Ecosyst. Environ. 2014, 193, 25–36. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.F.; Li, D.M.; Xu, C.X.; Wu, M.; Liu, M.; Li, P.F.; Li, G.L.; Zhang, T.L.; Li, Z.P. Variation of soil dissolved organic carbon under long-term different fertilizations and its correlation with maize yields. J. Soils Sediments 2020, 20, 2761–2770. [Google Scholar] [CrossRef]
- Zhao, X.; Tian, P.; Sun, Z.; Liu, S.; Wang, Q.; Zeng, Z. Rhizosphere effects on soil organic carbon processes in terrestrial ecosystems: A meta-analysis. Geoderma 2022, 412, 115739. [Google Scholar] [CrossRef]
- Liu, E.; Yan, C.; Mei, X.; Zhang, Y.; Fan, T. Long-Term Effect of Manure and Fertilizer on Soil Organic Carbon Pools in Dryland Farming in Northwest China. PLoS ONE 2013, 8, e56536. [Google Scholar] [CrossRef]
- Fierer, N.; Schimel, J.P.; Holden, P.A. Variations in microbial community composition through two soil depth profiles. Soil Biol. Biochem. 2003, 35, 167–176. [Google Scholar]
- Börjesson, G.; Menichetti, L.; Kirchmann, H.; Kätterer, T. Soil microbial community structure affected by 53 years of nitrogen fertilisation and different organic amendments. Biol. Fertil. Soils 2012, 48, 245–257. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Zhou, W.; Guo, S.; Zhu, R.; Qin, Y.; Sun, J. Responses of crop yields, soil enzymatic activities, and microbial communities to different long-term organic materials applied with chemical fertilizer in purple soil. Eur. J. Soil Biol. 2021, 105, 103319. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, W.; Bol, R.; Xiao, Q.; Wu, L.; Zhang, W. Microbial regulation of net N mineralisation is driven by C, N, P content and stoichiometry. Eur. J. Soil Sci. 2022, 73, e13257. [Google Scholar] [CrossRef]
- Bossio, D.A.; Fleck, J.A.; Scow, K.M.; Fujii, R. Alteration of soil microbial communities and water quality in restored wetlands. Soil Biol. Biochem. 2006, 38, 1223–1233. [Google Scholar] [CrossRef]
- Wixon, D.L.; Balser, T.C. Toward conceptual clarity: PLFA in warmed soils. Soil Biol. Biochem. 2013, 57, 769–774. [Google Scholar] [CrossRef]
- Chaudhary, D.R.; Gautam, R.K.; Ghosh, A.; Chikara, J.; Jha, B. Effect of Nitrogen Management on Soil Microbial Community and Enzymatic Activities in Jatropha curcas L. Plantation. Clean Soil Air Water 2015, 43, 1058–1065. [Google Scholar] [CrossRef]
- Azziz, G.; Frade, C.; Igual, J.M.; del Pino, A.; Lezama, F.; Valverde, Á. Legume Overseeding and P Fertilization Increases Microbial Activity and Decreases the Relative Abundance of AM Fungi in Pampas Natural Pastures. Microorganisms 2023, 11, 1383. [Google Scholar] [CrossRef]
- Romaniuk, R.; Giuffré, L.; Costantini, A.; Nannipieri, P. Assessment of soil microbial diversity measurements as indicators of soil functioning in organic and conventional horticulture systems. Ecol. Indic. 2011, 11, 1345–1353. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, J.; Han, X.; Zou, W.; Chen, X.; Lu, X.; Feng, Y. Labile organic carbon fractions drive soil microbial communities after long-term fertilization. Glob. Ecol. Conserv. 2021, 32, e01867. [Google Scholar] [CrossRef]
- Fong, S.S.; Mohamed, M. Chemical characterization of humic substances occurring in the peats of Sarawak, Malaysia. Org. Geochem. 2007, 38, 967–976. [Google Scholar] [CrossRef]
- Kieft, T.L.; Wilch, E.; Oconnor, K.; Ringelberg, D.B.; White, D.C. Survival and phospholipid fatty acid profiles of surface and subsurface bacteria in natural sediment microcosms. App. Environ. Microb. 1997, 63, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Olsson, P.A. Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. FEMS Microbiol. Ecol. 1999, 29, 303–310. [Google Scholar] [CrossRef]

| Crops | Fertilizers and Applied Durations | Treatments | Nutrient Content (N:P:K) kg ha−1 | ||
|---|---|---|---|---|---|
| CF † | OM †† | Total N:P:K | |||
| Wheat | Basal fertilizer, early November | CK | 0:0:0 | 0:0:0 | 0:0:0 |
| T1 | 70:20:0 | 26:8:20 | 96:28:20 | ||
| T2 | 68:33:87 | 0:0:0 | 68:33:87 | ||
| T3 | 101:49:131 | 0:0:0 | 101:49:131 | ||
| T4 | 68:33:87 | 0:0:0 | 68:33:87 | ||
| Topdressing fertilizer, late January or early February | CK | 0:0:0 | 0:0:0 | 0:0:0 | |
| T1 | 70:0:0 | 0:0:0 | 70:0:0 | ||
| T2 | 160:0:37 | 0:0:0 | 160:0:37 | ||
| T3 | 238:0:56 | 0:0:0 | 238:0:56 | ||
| T4 | 160:0:37 | 0:0:0 | 160:0:37 | ||
| Maize | Basal fertilizer, early April | CK | 0:0:0 | 0:0:0 | 0:0:0 |
| T1 | 0:24:0 | 27:8:20 | 27:32:20 | ||
| T2 | 64:39:125 | 0:0:0 | 64:39:125 | ||
| T3 | 94:59:187 | 0:0:0 | 94:59:187 | ||
| T4 | 64:39:125 | 0:0:0 | 64:39:125 | ||
| Topdressing fertilizer, early May | CK | 0:0:0 | 0:0:0 | 0:0:0 | |
| T1 | 223:0:0 | 54:17:39 | 277:17:39 | ||
| T2 | 126:0:0 | 0:0:0 | 126:0:0 | ||
| T3 | 189:0:0 | 0:0:0 | 189:0:0 | ||
| T4 | 126:0:0 | 0:0:0 | 126:0:0 | ||
| Treatments | Soils | pH | SOC | EOC | TN | NO3−-N | NH4+-N | AP | MBC | MBN | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1:2.5) | (g kg−1) | (mg kg−1) | |||||||||||||||||
| CK | 0–10 cm | 7.5 ± 0.3 a | 4.5 ± 0.3 d | 4.1 ± 0.4 c | 0.6 ± 0.1 c | 0.6 ± 0.1 d | 1.5 ± 0.1 c | 5.0 ± 0.9 c | 46.7 ± 2.3 c | 6.7 ± 1.2 c | |||||||||
| 10–20 cm | 7.5 ± 0.2 a | 4.1 ± 0.2 d | 3.0 ± 1.1 b | 0.6 ± 0.1 a | 0.6 ± 0.2 d | 1.4 ± 0.1 c | 4.5 ± 1.7 d | 27.7 ± 2.0 d | 3.3 ± 0.6 c | ||||||||||
| Rhizosphere | 7.7 ± 0.2 a | 5.2 ± 0.3 d | 6.9 ± 0.8 a | 0.7 ± 0.1 b | 1.0 ± 0.1 d | 1.8 ± 0.1 b | 7.8 ± 0.7 d | 48.1 ± 6.8 c | 6.0 ± 0.4 c | ||||||||||
| T1 | 0–10 cm | 7.3 ± 0.1 ab | 9.13 ± 0.19 a | 3.6 ± 0.3 c | 1.0 ± 0.1 a | 2.7 ± 0.4 a | 2.7 ± 0.2 a | 31.0 ± 2.3 a | 74.0 ± 3.5 a | 9.7 ± 0.7 a | |||||||||
| 10–20 cm | 7.3 ± 0.1 ab | 8.06 ± 0.32 a | 2.6 ± 0.1 b | 0.7 ± 0.1 a | 2.6 ± 0.3 a | 2.3 ± 0.4 a | 26.5 ± 2.2 a | 51.1 ± 5.4 a | 6.7 ± 0.6 ab | ||||||||||
| Rhizosphere | 7.4 ± 0.4 ab | 8.9 ± 0.5 a | 3.9 ± 1.2 b | 0.8 ± 0.1 a | 2.9 ± 0.3a | 2.7 ± 0.3 a | 37.5 ± 1.7 a | 79.7 ± 3.5 a | 11.8 ± 1.7 a | ||||||||||
| T2 | 0–10 cm | 7.2 ± 0.1 b | 6.56 ± 0.19 c | 11.2 ± 0.2 a | 0.8 ± 0.1 b | 1.1 ± 0.3 c | 2.7 ± 0.4 a | 20.3 ± 1.3 b | 63.6 ± 2.1 b | 7.0 ± 0.8 bc | |||||||||
| 10–20 cm | 7.1 ± 0.2 b | 5.59 ± 0.37 c | 5.6 ± 1.3 a | 0.6 ± 0.1 a | 1.0 ± 0.4 c | 2.2 ± 0.3 ab | 18.2 ± 1.7 c | 43.01 ± 1.5 b | 4.5 ± 0.2 b | ||||||||||
| Rhizosphere | 7.4 ± 0.1 ab | 7.0 ± 0.6 c | 4.2 ± 0.7 b | 0.7 ± 0.1 ab | 1.4 ± 0.1 c | 2.6 ± 0.2 a | 29.6 ± 0.7 c | 67.2 ± 3.3 ab | 7.2 ± 0.4 b | ||||||||||
| T3 | 0–10 cm | 7.0 ± 0.1 c | 7.31 ± 0.81 b | 7.7 ± 1.8 b | 0.8 ± 0.1 ab | 1.8 ± 0.3 b | 1.9 ± 0.3 b | 28.6 ± 1.8 a | 64.6 ± 3.9 b | 8.7 ± 0.8 b | |||||||||
| 10–20 cm | 7.1 ± 0.1 b | 6.56 ± 0.72 b | 4.3 ± 1.1 ab | 0.6 ± 0.1 a | 1.5 ± 0.2 b | 1.7 ± 0.2 b | 24.0 ± 1.1 ab | 50.2 ± 4.5 a | 6.9 ± 0.9 a | ||||||||||
| Rhizosphere | 7.3 ± 0.1 b | 7.7 ± 0.2 b | 8.2 ± 1.3 a | 0.8 ± 0.1 a | 1.8 ± 0.1 b | 1.9 ± 0.2 b | 33.1 ± 1.4 b | 70.7 ± 5.5 ab | 9.6 ± 0.9 ab | ||||||||||
| T4 | 0–10 cm | 7.1 ± 0.1 c | 7.09 ± 0.32bc | 10.7 ± 2.1 a | 0.8 ± 0.1 b | 1.0 ± 0.6 c | 2.2 ± 0.6 ab | 23.3 ± 1.7 b | 59.3 ± 1.7 b | 8.8 ± 0.3 b | |||||||||
| 10–20 cm | 7.1 ± 0.1 b | 6.13 ± 0.21 bc | 6.7 ± 2.0 a | 0.6 ± 0.1 a | 0.9 ± 0.5 c | 2.0 ± 0.7 ab | 20.7 ± 1.2 bc | 36.0 ± 2.0 c | 6.6 ± 1.0 ab | ||||||||||
| Rhizosphere | 7.2 ± 0.1 c | 6.9 ± 0.7 c | 3.4 ± 1.3 b | 0.7 ± 0.1 ab | 1.2 ± 0.2 cd | 2.4 ± 0.1 a | 29.1 ± 1.3 c | 61.7 ± 3.7 b | 10.0 ± 0.8 ab | ||||||||||
| Factors | F | P | F | P | F | P | F | P | F | P | F | P | F | P | F | P | F | P | |
| Fertilizers | 8.2 | * | 73.4 | * | 3.2 | * | 1.7 | NS | 23.6 | * | 4.2 | * | 193.8 | * | 18.9 | * | 4.1 | * | |
| Depths | 0.02 | NS | 26.8 | * | 14.2 | * | 2.0 | NS | 8.5 | NS | 0.01 | NS | 22.7 | * | 100.2 | * | 1.42 | NS | |
| Rhizosphere | 2.8 | * | 21.41 | * | 13.5 | * | 2.6 | NS | 55.9 | * | 12.9 | * | 265.6 | * | 6.08 | * | 2.45 | NS | |
| Fertilizer × depths | 0.3 | NS | 0.5 | * | 5.5 | * | 2.9 | NS | 18.2 | * | 5.5 | * | 1.65 | NS | 0.65 | NS | 0.8 | NS | |
| Fertilizer × rhizosphere | 265.7 | * | 291.9 | * | 423.5 | * | 114.5 | NS | 126.1 | * | 113.1 | * | 758.3 | * | 945.9 | * | 512.3 | NS | |
| Treatments | Soils | DOC | SUVA254 | SUVA260 | SUVA400 | SUVA465 | SUVA665 | C:C | E4:E6 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg L−1) | L mg C−1 m−1 | ||||||||||||||||
| CK | 0–10 cm | 5.2 ± 0.7 d | 14.1 ± 4.8 a | 16.0 ± 2.5 a | 3.0 ± 0.7 a | 1.9 ± 0.6 a | 1.2 ± 0.3 a | 0.6 ± 0.2 a | 1.6 ± 0.3 ab | ||||||||
| 10–20 cm | 3.5 ± 0.4 d | 12.6 ± 1.9 a | 12.3 ± 2.0 a | 1.8 ± 0.4 a | 1.6 ± 0.8 a | 1.0 ± 0.1 a | 0.5 ± 0.2 a | 1.6 ± 0.7 b | |||||||||
| Rhizosphere | 5.9 ± 0.5 d | 13.8 ± 2.1 a | 14.2 ± 1.8 a | 2.2 ± 0.3 a | 1.3 ± 0.2 a | 0.5 ± 0.1 a | 0.4 ± 0.1 a | 2.8 ± 0.2 b | |||||||||
| T1 | 0–10 cm | 12.3 ± 1.3 a | 5.2 ± 0.8 c | 3.8 ± 0.5 c | 1.4 ± 0.1 bc | 1.1 ± 0.1 bc | 0.7 ± 0.1 b | 0.1 ± 0.01 c | 1.6 ± 0.2 ab | ||||||||
| 10–20 cm | 9.8 ± 0.4 a | 6.3 ± 0.8 b | 3.3 ± 0.2 c | 1.0 ± 0.1 b | 0.9 ± 0.2 ab | 0.4 ± 0.1 b | 0.1 ± 0.01 b | 2.1 ± 0.2 ab | |||||||||
| Rhizosphere | 13.8 ± 1.5 a | 3.8 ± 0.8 c | 3.4 ± 1.0 c | 0.8 ± 0.2 c | 0.6 ± 0.1 bc | 0.3 ± 0.1 b | 0.1 ± 0.02 c | 2.1 ± 0.16 c | |||||||||
| T2 | 0–10 cm | 7.4 ± 1.0 c | 6.7 ± 0.5 c | 6.0 ± 1.2 bc | 1.1 ± 0.1 cd | 0.8 ± 0.1 cd | 0.7 ± 0.3 b | 0.2 ± 0.03 c | 1.3 ± 0.5 b | ||||||||
| 10–20 cm | 5.5 ± 0.1 c | 7.5 ± 1.1 b | 7.2 ± 1.1 b | 1.2 ± 0.3 b | 1.0 ± 0.2 ab | 0.3 ± 0.05 c | 0.2 ± 0.1 b | 3.7 ± 1.1 a | |||||||||
| Rhizosphere | 8.1 ± 0.8 c | 7.1 ± 1.2 b | 5.4 ± 1.6 bc | 1.1 ± 0.2 bc | 0.7 ± 0.1 bc | 0.2 ± 0.03 b | 0.1 ± 0.02 c | 3.8 ± 0.6 a | |||||||||
| T3 | 0–10 cm | 9.0 ± 0.3 b | 9.0 ± 1.7 bc | 8.6 ± 1.6 b | 0.7 ± 0.2 d | 0.6 ± 0.1 d | 0.4 ± 0.03 b | 0.1 ± 0.02 c | 1. 5 ± 0.1 ab | ||||||||
| 10–20 cm | 7.1 ± 0.3 b | 6.2 ± 2.0 b | 5.3 ± 2.3 bc | 1.0 ± 0.2 b | 0.8 ± 0.1 b | 0.2 ± 0.1 c | 0.1 ± 0.03 b | 3.5 ± 1.4 a | |||||||||
| Rhizosphere | 10.3 ± 0.9 b | 4.5 ± 1.4 c | 4.5 ± 1.3 bc | 0.8 ± 0.2 c | 0.5 ± 0.17 c | 0.3 ± 0.1 b | 0.1 ± 0.03 c | 1.5 ± 0.5 d | |||||||||
| T4 | 0–10 cm | 4.6 ± 0.3 d | 11.4 ± 1.5 ab | 13.7 ± 2.1 a | 1.8 ± 0.2 b | 1.4 ± 0.3 b | 0.7 ± 0.1 b | 0.4 ± 0.07 b | 2.0 ± 0.4 a | ||||||||
| 10–20 cm | 4.9 ± 0.3 c | 8.1 ± 2.5 b | 7.3 ± 1.6 b | 1.1 ± 0.01 b | 0.6 ± 0.1 b | 0.3 ± 0.1 c | 0.2 ± 0.02 b | 2.5 ± 0.3 ab | |||||||||
| Rhizosphere | 6.2 ± 0.4 d | 7.2 ± 1.1 b | 7.0 ± 1.1 b | 1.3 ± 0.3 b | 0.9 ± 0.1 b | 0.3 ± 0.1 b | 0.2 ± 0.04 b | 2.8 ± 0.4 b | |||||||||
| Factors | F | P | F | P | F | P | F | P | F | P | F | P | F | P | F | P | |
| Fertilizers | 110.9 | * | 11.7 | * | 35.8 | * | 25.4 | * | 8.2 | * | 24.21 | * | 0.00 | * | 2.1 | * | |
| Depths | 46.5 | * | 2.2 | NS | 17.7 | * | 14.8 | * | 2.2 | NS | 31.76 | * | 0.50 | NS | 20.4 | * | |
| Rhizosphere | 39.2 | * | 24.2 | * | 29.3 | * | 19.3 | * | 16.9 | * | 8.35 | * | 38.1 | * | 14.1 | * | |
| Fertilizer × depths | 4.2 | * | 1.4 | NS | 4.7 | NS | 6.2 | * | 2.11 | NS | 1.25 | NS | 1.50 | NS | 3.91 | * | |
| Fertilizer × rhizosphere | 176.6 | * | 413.1 | * | 375.9 | * | 412.3 | * | 527.9 | * | 451.35 | * | 331.1 | * | 645.6 | * | |
| Treatments | Soils | Total PLFAs | Bacteria | G− | G+ | Fungi | AMF | Actinomycetes | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CK | 0–10 cm | 20.8 ± 0.3 d | 7.5 ± 0.1 c | 3.4 ± 0.4 c | 4.0 ± 0.1 c | 2.2 ± 0.1 d | 0.7 ± 0.1 d | 3.0 ± 0.1 d | |||||||
| 10–20 cm | 17.2 ± 0.6 b | 5.6 ± 0.1 c | 2.3 ± 0.2 d | 2.9 ± 0.2 d | 2.4 ± 0.2 e | 0.8 ± 0.1 d | 3.2 ± 0.1 d | ||||||||
| Rhizosphere | 29.9 ± 0.3 e | 9.7 ± 0.2 d | 4.1 ± 0.4 b | 5.9 ± 0.1 d | 4.2 ± 0.1 e | 1.3 ± 0.1 d | 4.7 ± 0.1 d | ||||||||
| T1 | 0–10 cm | 42.2 ± 1.1 a | 15.6 ± 0.2 a | 6.6 ± 0.7 ab | 8.4 ± 0.2 a | 4.1 ± 0.1 a | 1.5 ± 0.1 a | 6.1 ± 0.1 a | |||||||
| 10–20 cm | 42.1 ± 0.9 a | 14.8 ± 0.8 a | 4.8 ± 0.2 c | 9.9 ± 0.1 a | 4.5 ± 0.1 a | 1.6 ± 0.1 a | 6.6 ± 0.1 a | ||||||||
| Rhizosphere | 55.5 ± 0.1 a | 19.2 ± 0.2 a | 7.0 ± 0.1 a | 11.6 ± 0.1 a | 6.9 ± 0.1 a | 2.3 ± 0.1 a | 8.6 ± 0.2 a | ||||||||
| T2 | 0–10 cm | 35.6 ± 0.3 c | 13.8 ± 0.1 b | 6.1 ± 0.3 b | 7.3 ± 0.2 b | 3.2 ± 0.2 c | 1.1 ± 0.1 bc | 4.1 ± 0.1 c | |||||||
| 10–20 cm | 33.1 ± 0.9 c | 12.1 ± 0.9 b | 5.4 ± 0.2 b | 6.6 ± 0.1 c | 3.1 ± 0.1 d | 1.1 ± 0.1 c | 4.9 ± 0.1 b | ||||||||
| Rhizosphere | 42.3 ± 0.5 d | 14.9 ± 0.2 c | 6.2 ± 0.6 a | 8.1 ± 0.1 c | 5.4 ± 0.1 d | 1.7 ± 0.1 c | 5.9 ± 0.1 c | ||||||||
| T3 | 0–10 cm | 35.6 ± 0.6 c | 13.5 ± 0.2 b | 6.2 ± 0.1 b | 7.0 ± 0.2 b | 3.2 ± 0.1 c | 1.0 ± 0.1 c | 4.8 ± 0.1 b | |||||||
| 10–20 cm | 34.7 ± 1.5 c | 12.7 ± 2.4 b | 5.0 ± 0.2 c | 7.6 ± 0.2 b | 3.6 ± 0.1 c | 1.0 ± 0.1 c | 4.9 ± 0.1 b | ||||||||
| Rhizosphere | 45.9 ± 0.3 c | 16.3 ± 0.3 b | 6.7 ± 0.2 a | 9.1 ± 0.3 b | 5.7 ± 0.1 c | 1.7 ± 0.1 c | 6.4 ± 0.2 b | ||||||||
| T4 | 0–10 cm | 40.0 ± 0.7 b | 15.5 ± 0.1 a | 7.2 ± 0.1 a | 8.0 ± 0.3 a | 3.8 ± 0.2 b | 1.3 ± 0.2 ab | 4.1 ± 0.3 c | |||||||
| 10–20 cm | 37.4 ± 0.4 b | 13.9 ± 0.1 ab | 6.1 ± 0.1 a | 7.6 ± 0.4 b | 4.0 ± 0.1 b | 1.4 ± 0.1 b | 4.5 ± 0.2 c | ||||||||
| Rhizosphere | 47.7 ± 0.4 b | 16.7 ± 0.3 b | 6.9 ± 0.5 a | 9.1 ± 0.2 b | 6.4 ± 0.1 b | 2.1 ± 0.1 b | 6.6 ± 0.1 b | ||||||||
| Factors | F | P | F | P | F | P | F | P | F | P | F | P | F | P | |
| Fertilizers | 96.6 | * | 29.4 | * | 9.1 | * | 51.8 | * | 71.1 | * | 48.2 | * | 43.8 | * | |
| Depths | 64.9 | * | 28.8 | * | 23.9 | * | 24.1 | * | 15.9 | * | 1.3 | NS | 0.5 | NS | |
| Rhizosphere | 56.9 | * | 85.9 | * | 26.4 | * | 38.2 | * | 99.4 | * | 103.7 | * | 42.2 | * | |
| Fertilizer × depths | 62.01 | * | 20.4 | * | 5.78 | * | 29.1 | * | 28.4 | * | 21.61 | * | 47.84 | * | |
| Fertilizer × rhizosphere | 914.3 | * | 48.5 | * | 22.8 | * | 92.02 | * | 94.5 | * | 73. 7 | * | 146.6 | * | |
| Treatments | Soils | Shannon Index (H’) | Evenness Index (E) | Simpson Index (D) | |||
|---|---|---|---|---|---|---|---|
| CK | 0–10 cm | 3.251 ± 0.02 b | 0.930 ± 0.01 b | 0.955 ± 0.01 c | |||
| 10–20 cm | 3.177 ± 0.03 b | 0.909 ± 0.01 b | 0.949 ± 0.01 b | ||||
| Rhizosphere | 3.183 ± 0.01 c | 0.910 ± 0.01 d | 0.950 ± 0.01 b | ||||
| T1 | 0–10 cm | 3.238 ± 0.02 b | 0.926 ± 0.01 b | 0.954 ± 0.01 c | |||
| 10–20 cm | 3.204 ± 0.01 b | 0.916 ± 0.01 b | 0.951 ± 0.01 b | ||||
| Rhizosphere | 3.188 ± 0.01 bc | 0.912 ± 0.01 cd | 0.952 ± 0.01 a | ||||
| T2 | 0–10 cm | 3.261 ± 0.01 b | 0.933 ± 0.01 b | 0.957 ± 0.01 b | |||
| 10–20 cm | 3.191 ± 0.03 b | 0.913 ± 0.01 b | 0.951 ± 0.01 b | ||||
| Rhizosphere | 3.235 ± 0.01 a | 0.925 ± 0.01 a | 0.954 ± 0.01 a | ||||
| T3 | 0–10 cm | 3.262 ± 0.01 b | 0.933 ± 0.01 b | 0.956 ± 0.01 bc | |||
| 10–20 cm | 3.242 ± 0.01 b | 0.927 ± 0.01 a | 0.955 ± 0.01 a | ||||
| Rhizosphere | 3.211 ± 0.01 ab | 0.918 ± 0.01 bc | 0.953 ± 0.01 a | ||||
| T4 | 0–10 cm | 3.306 ± 0.01 a | 0.946 ± 0.01 a | 0.959 ± 0.01 a | |||
| 10–20 cm | 3.262 ± 0.01 a | 0.933 ± 0.01 a | 0.956 ± 0.01 a | ||||
| Rhizosphere | 3.228 ± 0.02 a | 0.923 ± 0.01 ab | 0.954 ± 0.01 a | ||||
| Factors | F | P | F | P | F | P | |
| Fertilizers | 16.9 | * | 17.4 | * | 16.0 | * | |
| Depths | 61.4 | * | 62.4 | * | 47.2 | * | |
| Rhizosphere | 9.9 | * | 10.1 | * | 6.9 | * | |
| Fertilizer × depths | 2.8 | NS | 2.7 | NS | 3.8 | * | |
| Fertilizer × rhizosphere | 50.2 | * | 94.8 | * | 86.4 | * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.; Li, T.; He, B.; Zhang, G. Insights from Fertilization and Cultivation Management for Interpreting the Variations in the Quantity and Quality of Dissolved Organic Carbon and Microbial Community Structure on Purple Soil Sloping Farmland in Southwest China. Agronomy 2024, 14, 426. https://doi.org/10.3390/agronomy14030426
Khan A, Li T, He B, Zhang G. Insights from Fertilization and Cultivation Management for Interpreting the Variations in the Quantity and Quality of Dissolved Organic Carbon and Microbial Community Structure on Purple Soil Sloping Farmland in Southwest China. Agronomy. 2024; 14(3):426. https://doi.org/10.3390/agronomy14030426
Chicago/Turabian StyleKhan, Asif, Tianyang Li, Binghui He, and Gaoning Zhang. 2024. "Insights from Fertilization and Cultivation Management for Interpreting the Variations in the Quantity and Quality of Dissolved Organic Carbon and Microbial Community Structure on Purple Soil Sloping Farmland in Southwest China" Agronomy 14, no. 3: 426. https://doi.org/10.3390/agronomy14030426
APA StyleKhan, A., Li, T., He, B., & Zhang, G. (2024). Insights from Fertilization and Cultivation Management for Interpreting the Variations in the Quantity and Quality of Dissolved Organic Carbon and Microbial Community Structure on Purple Soil Sloping Farmland in Southwest China. Agronomy, 14(3), 426. https://doi.org/10.3390/agronomy14030426






