Abstract
Global climate change is increasing the frequency and intensity of drought and salt stress worldwide, with profound impacts on tree growth and survival. However, the response of plant hydraulic transport and carbon balance to combined drought and salt stress remains unclear. This study investigated the leaf physiological traits, stem xylem hydraulic traits, and nonstructural carbohydrate concentration of Robinia pseudoacacia seedlings under normal irrigation treatment (CK, freshwater at 80–100% FC); salt stress treatment (SS, 0.3% soil salinity with freshwater); drought stress treatment (DS, withholding irrigation); and combined drought and salt treatments (SDS, 0.3% soil salinity withholding irrigation). Our results showed that the leaf physiological traits responded differently to different treatments. DS and SDS treatment significantly decreased leaf water potential and stomatal conductance, while SS treatment did not. DS treatment increased stomatal density but decreased stomatal area to adapt to water deficit, while SS and SDS treatment decreased stomatal length or width. In terms of xylem hydraulic traits, SS, DS and SDS significantly decreased xylem specific hydraulic conductivity by 47%, 42% and 49%, while percent loss of conductivity (PLC) significantly increased by 81% and 62% in DS and SDS, but the PLC of SS was not increased significantly. Additionally, net photosynthetic rate and transpiration rate significantly decreased in SS, DS and SDS, while leaf water use efficiency significantly increased. The chlorophyll content index and maximum light quantum efficiency of photosystem II were also decreased. For nonstructural carbohydrate, the soluble sugars, starch and total non-structural carbohydrate were significantly decreased in DS in specific tissues, showing reductions of 42%, 68%, and 56% in leaves, 69%, 61%, and 62% in stem, and 30%, 59%, and 57% in root. Our findings provide evidence that salt addition alleviated drought stress by improving hydraulic traits and carbohydrate reserves, which is expected to contribute to predicting future vegetation dynamics under climate change.
1. Introduction
Climate change has led to more severe and prolonged extreme drought events, resulting in increased forest mortality globally [1,2]. Global warming, which leads to an increasing vapor pressure deficit, intensifies the evaporative demand and water requirements of plants [3], accelerates salt accumulation in the soil, aggravating soil salinization [4]. Additionally, the rise in sea levels intensifies flooding and saltwater intrusion, posing significant threats to coastal ecosystems and contributing to the expansion of ‘ghost forests’ and abandoned farmland [5]. Drought is frequently accompanied by salt stress in coastal, arid and semi-arid areas [6]. Therefore, understanding how plants respond to drought and salt stress plays an important role in predicting plant growth and sustainability in a changing climate.
Over the past decade, a large body of research has proposed two main mechanisms of drought-induced tree mortality: hydraulic failure and carbon starvation [1,7]. The hydraulic failure hypothesis suggests that high xylem tension caused by soil drying and/or high atmospheric evaporative demand during drought leads to cavitation and impedes water transport, drying out tissues and ultimately causing cell death [8]. Carbon starvation occurs when limited carbohydrate supply rate impairs the maintenance of carbon-dependent metabolic, defense or hydraulic functions [9,10]. Non-structural carbon (NSC) reserves indicate the balance of carbohydrate supply and utilization, usually as soluble sugars and starch, providing substrates for primary and secondary metabolism [11]. Initially, under drought stress, plants close stomata to prevent water loss while maintaining leaf water potential, but this decreases photosynthesis and carbon assimilation [12]. Acclimation and repair of embolized xylem also consume stored carbohydrates [13,14]. Carbon starvation occurs when NSC from photosynthesis cannot sustain respiration under drought [15].
The physiological impact of salinity stress on plants shares similarities with drought stress but is characterized by greater complexity. Salt stress disrupts osmotic balance and causes ionic toxicity due to the excessive accumulation of Na+ and Cl−. This hinders the absorption of other essential ions for plant growth [16]. In progressively saline environments, the osmotic imbalance between soil water and root water reduces the soil-root water potential gradient results in limiting water uptake [17]. The decline in hydraulic conductivity and stomatal conductance, along with reduced water potential in leaves, leads to impaired xylem vessel hydraulic function [18]. The closure of stomata in plants is widely recognized to decrease the rate of photosynthesis, consequently leading to diminished plant growth [19]. In addition, salinity also leads to Na+ accumulation in leaves at toxic concentrations, resulting in metabolic dysfunction and reduced rates of carbon assimilation [20]. NSC availability depends on the balance of carbon supply and utilization. If respiration exceeds photosynthesis, excessive depletion of NSC may occur, leading to carbon starvation in a salt environment [21]. While the physiological mechanisms underlying tree mortality in response to individual droughts or salt stress have been investigated, the mechanisms involved in tree mortality under combined droughts and salt stress remain poorly understood.
Indeed, the physiological and metabolic responses of plants to combined drought and salt stress are complex and not simply a superposition of the individual effects. The interactions between these stressors can involve mutual feedback and synergistic processes, leading to unique plant responses [22,23]. Drought can decrease soil water potential, while salt stress can reduce osmotic potential via ion accumulation, both imposing limitations on plant water uptake [16,24]. Although Na+ is generally acknowledged as phytotoxic, it has also been shown to beneficially influence drought adaptation in some plant species [25,26]. Multiple studies have found salinity and drought stress have an additive effect on dry matter accumulation and chlorophyll content [27]. Leaf water potential and photosynthetic recovery capacity were significantly diminished under combined stress [28]. Plant growth was further impeded by leaf tissue dehydration and excessive Na+ and Cl− accumulation under combined stress [29]. Conversely, Li et al. [30] indicated water deficit and salinity stress decreased plant water status, leaf gas exchange and fruit growth, but increased fruit quality. He et al. [31] found moderate NaCl effectively alleviated deleterious impacts on growth and leaf morphological structure, significantly improving photosynthetic capacity and leaf relative water content. However, there is still no consistent conclusion on the plant responses to combined salt and drought stress. Previous studies mainly focused on salinity or drought impacts on leaf water relationship, yield, and quality [32,33], while few studied synergistic changes in hydraulic limitation and carbohydrates reserves associated with plant mortality.
Robinia pseudoacacia, Ailanthus altissima, Populus tomentosa, Fraxinus chinensis, Salix matsudana and Pinus tabuliformis are the main tree species for afforestation in warm temperate zones [34]. Robinia pseudoacacia is a fast-growing species with high survival rates. It has well-developed vertical and horizontal root systems, including root nodules. It also has a good capacity for carbon and nitrogen fixation. Robinia pseudoacacia demonstrates strong environmental adaptability, drought resistance, salt tolerance, and barren resistance. It has strong regeneration ability and root suckering. As such, it is an important timber species and is also a good tree species for water and soil conservation and saline-alkali soil improvement. It plays a crucial role in the processes of ecological protection, environmental improvement and hydrological regulation [35,36].
The purpose of this study was to investigate the physiological processes of R. pseudoacacia seedlings under drought stress, salt stress and their combination. The main objectives were: (1) to assess the impacts on plant hydraulic function by measuring leaf water potential, stomatal conductance, leaf stomatal anatomical properties and stem xylem hydraulic conductivity; and (2) to determine the effects on carbon reserves by examining photosynthetic rate, transpiration rate, chlorophyll content, maximum light quantum efficiency of photosystem II, and nonstructural carbohydrate concentration. We hypothesized that (1) drought and salt stress would decrease plant hydraulic function by decreasing leaf water potential, stomatal conductance and stem xylem hydraulic conductivity and reduce carbon supply by decreasing photosynthetic rate, transpiration rate, chlorophyll content, maximum light quantum efficiency of photosystem II, and nonstructural carbohydrate concentration; and (2) their combined effects on hydraulic transport and carbohydrate depletion would be more severe than for single stress.
2. Materials and Methods
2.1. Plant Material and Experimental Design
The pot experiment was conducted in a glasshouse at Northwest A&F University. Topsoil (0–30 cm) was collected from the local cultivation layer and dried naturally. It was then crushed and passed through a 5 mm sieve. The collected soil was thoroughly mixed with sand and peat soil at 3:1:1 (v/v) to minimize the effects of irrigation and avoid soil compaction. Fertilizer Osmocote (NPK 15:10:12 + 2 MgO, plus micro elements) was mixed thoroughly at 2.5 kg m−3 and 40 plastic pots (30 cm height, 30 cm top diameter, 23.5 cm bottom diameter) were filled with 16 kg of soil. The field water holding capacity (FC) of the test soil was 0.25 g g−1. We obtained 2-year-old Robinia pseudoacacia seedlings from a local nursery, and transplanted them into pots on 23 April 2022, and managed normally until the seedlings developed new stems and fully expanded leaves. on 2 September 2022. A total of 20 seedings were subjected to salt irrigation until the soil salinity reached 0.3%, which is considered the salinity tolerance threshold for Robinia pseudoacacia [37,38]. The irrigation schedule for salt stress was as follows: 1 L 100 mM NaCl on 2 September 2022; 1 L 150 mM NaCl on 6 September 2022; 1 L 200 mM NaCl on 12 September 2022; and 1 L 250 mM NaCl on 17 September 2022. The other 20 seedlings were irrigated freshwater during this period. All seedlings (n = 40) started the experiment on 24 September 2022 and ended it on 23 October 2022. At the end of the experiment, the leaves treated with drought stress all fell off, and there were not fully expanded and mature leaves used to determine the physiological indexes.
A randomized block design established four treatments with 10 replications each: normal irrigation treatment (CK, freshwater at 80–100% FC); salt stress treatment (SS, 0.3% soil salinity with freshwater); drought stress treatment (DS, withholding irrigation from 24 September); and combined drought and salt treatments (SDS, 0.3% soil salinity withholding irrigation).
2.2. Measurements
2.2.1. Soil Water Content and Conductivity
Gravimetric soil water content (SWC, g g−1) was determined from 17:00 to 18:00 using an electronic balance (Langge R30-A01, Langge Technology, Beijing, China) with a precision of 0.1 g. Six replicates per treatment were taken. The relative soil water content (%) was calculated as SWC divided by field water holding capacity (g g−1).
Soil electrical conductivity (EC1:5, dS m−1) was measured using a DDS-307A conductivity meter (Shanghai Yidian, Shanghai, China). The measurements were conducted with 4 replicates according to the soil: water ratio of 1:5 [39].
The SWC and EC1:5 were determined on September 24, October 1, October 9, October 16 and October 23.
2.2.2. Leaf Physiological Traits
Leaf water potential was measured weekly on mature, healthy and fully expanded upper leaves of trees. The predawn (Ѱpd) and midday (Ѱmd) water potentials were measured at 5:00–6:00 and 12:00–14:00 on sunny days, respectively. Leaves were wrapped in foil and equilibrated with the xylem water potential for 1 h before the measurement. Then, leaves were collected by cutting them with sharp shears and quickly wrapping them in a wet towel in an insulated box to bring them back to the laboratory for determination using a 1505D pressure chamber (PMS Instruments, Albany, OR, USA). The mean of four leaves was calculated to for Ѱpd and Ѱmd of each treatment.
Gas exchange of mature, fully expanded leaves for each treatment was also determined from 9:00 to 12:00 on a clear day, using a Li-6800 portable photosynthesis system (Li-Cor, Lincoln, NE, USA). Li-6800 was adjusted to a fixed CO2 concentration (400 μmol mol−1) and a light quantum flux density of 1000 µmol m−2 s−1. Net photosynthetic rate (A, µmol m−2 s−1), stomatal conductance (Gs, mol m−2 s−1) and transpiration rate (Tr, mmol m−2 s−1) were recorded. Leaf water use efficiency (WUEL) was calculated, expressed as A/Tr.
The chlorophyll content index (SPAD value) was determined on the same leaves used for measuring gas exchange with a SPAD-502 Plus (Soil Plant Analysis Development, Minolta, Tokyo, Japan). Chlorophyll fluorescence parameters were measured using the Fluor Cam Plantscreen system (PlantScreen, PSI, Drásov, Czech Republic). Leaves were dark-adapted for 30 min, and then put into the photo-acclimatization chamber to measure the maximum light quantum efficiency (Fv/Fm).
For measuring stomatal morphological traits, six fully expanded leaves were randomly selected for each treatment on 16 October 2022. Leaf samples (2 × 2 mm) were cut from the middle section of each leaf and fixed in 2.5% (v/v) glutaraldehyde phosphate buffer (0.1 mol L−1, pH = 7.0). The leaf samples were rinsed four times with 0.1 M PBS buffer and then dehydrated with 10%, 30%, 50%, 70%, 80%, 90%, and 100% ethanol solutions. Next, the leaf samples were carefully coated with a layer of gold in a high-vacuum evaporation unit. Finally, stomatal morphological features were observed and photographed using a Quanta 200 scanning electron microscope (Fei Corp, Hillsboro, OR, USA). Eight to ten images were analyzed for each leaf, and stomatal density was observed at 1000× lens, and stomatal area, stomatal length, and stomatal width were observed at 20,000× lens.
2.2.3. Stem Xylem Hydraulic Traits
At the end of the experiment, branches were excised underwater in pre-dawn hours and transported to the lab for hydraulic conductivity measurements. Under water, stems were cut to 30 cm in length, and initial hydraulic conductivity (Ki) was measured at 3 kPa water pressure using a low-pressure flow meter [40]. Then, the stem segments were flushed with 150 kPa water pressure for 10–20 min to remove embolisms, and the maximum hydraulic conductivity of the xylem (Kmax) was determined. Basal stem diameter was measured using a vernier caliper to calculate sapwood area (As). The maximum xylem-specific hydraulic conductivity (Ks) was calculated as Kmax divided by As. The percentage loss of xylem hydraulic conductivity (PLC, %) was calculated as (1-Ki/Kmax) × 100%.
2.2.4. Nonstructural Carbohydrates (NSC)
The root, stem and leaf samples were collected and oven-dried at the end of the experiment The leaves of DS treatment are collected from withered and fallen leaves. Samples were dried at 105 °C for 30 min and then at 70 °C to a constant weight. Dried samples were ground to a fine powder using a FW-100D universal crusher (Beijing Kewei, Beijing, China) and passed through a 0.15 mm fine sieve. The total Non-structural carbohydrates (NSC) were extracted and quantified using the modified anthrone method [41,42]. Total NSC was calculated as the sum of soluble sugar and starch concentrations. Extracts were analyzed using a UV-2600A spectrophotometer (UNICO, Princeton, NJ, USA), measuring absorbance at 620 nm.
2.3. Statistical Analysis
A one-way analysis of variance (ANOVA) was conducted to evaluate differences in parameters among treatments. Pairwise comparisons were made using the least significant difference (LSD) test at the p = 0.05 significance level. All statistical analyses and correlation analysis were performed using SPSS 25.0 (IBM, Armonk, NY, USA). Graphs were generated in Origin 2024 (OriginLab Corp., Northampton, MA, USA).
3. Results
3.1. Relative Soil Water Content and Soil Conductivity
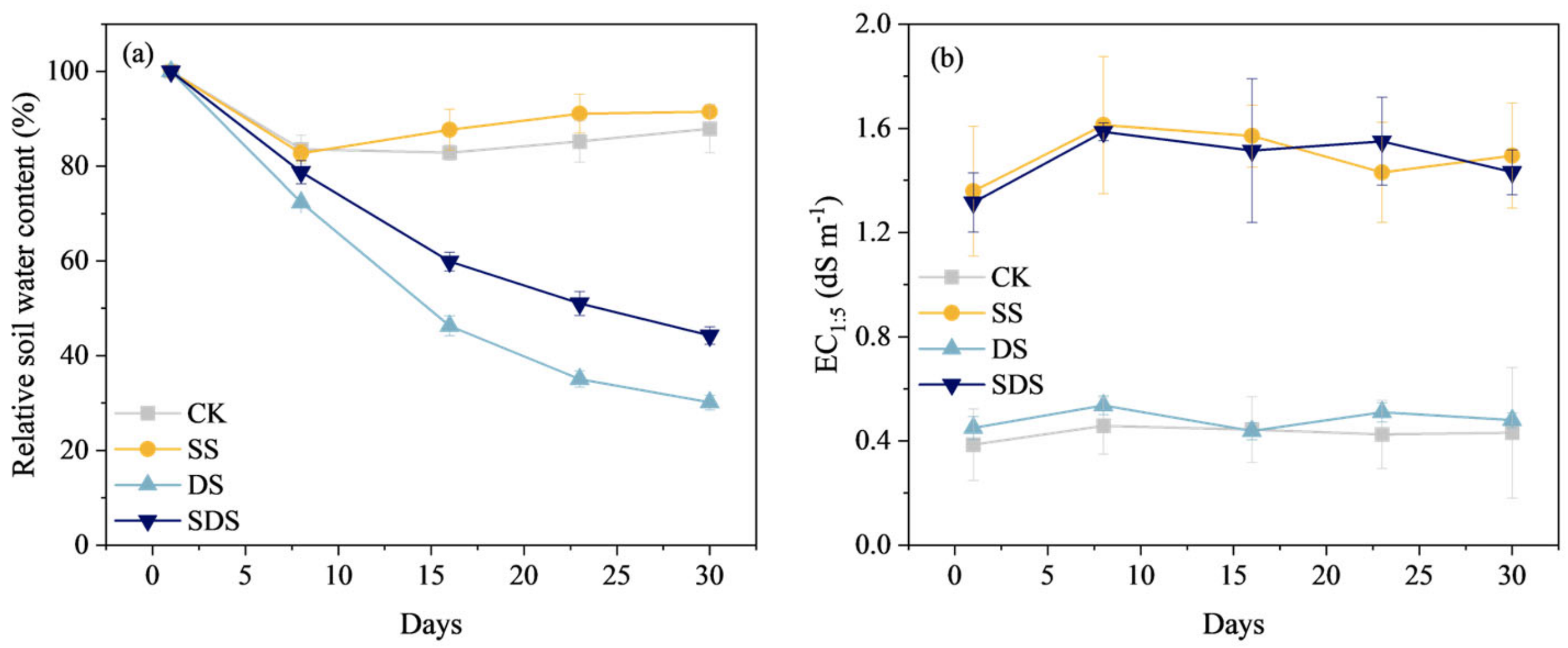
The relative soil water content of the DS and SDS treatments continuously decreased after the start of the experiment (Figure 1). By the end of the experiment (day 30), the relative soil water content of DS treatment decreased to 30.2% FC, while that of SDS treatment was at 44.3% FC, which was 14.1% higher than DS treatment. The CK and SS treatments were fully irrigated throughout the experiment, and the evolution of relative soil water content varied in the range of 82.7–91.5% FC (Figure 1a).
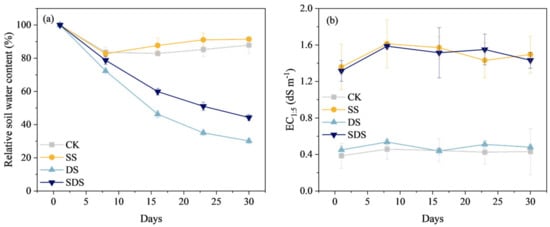
Figure 1.
Changes in relative soil water content (a) and electrical conductivity EC1:5 (b) throughout the experiment. CK: normal freshwater irrigation treatment at 80–100% FC; SS: salt stress treatment, 0.3% soil salinity with freshwater irrigation at 80–100% FC; DS: drought stress treatment, withholding irrigation; SDS: combined drought and salt treatments, 0.3% soil salinity withholding irrigation.
The evolution of EC1:5 for CK and DS treatments ranged from 0.39 to 0.54 dS m−1, while that of SS and SDS treatments ranged from 1.32 to 1.61 dS m−1. The salt-irrigated soils showed a conductivity increase of 0.93 to 1.08 dS m−1 relative to non-salt soils (Figure 1b).
3.2. Leaf Water Potential, Gas Exchange Parameters, Chlorophyll Fluorescence Characteristics and Stomatal Morphological Traits
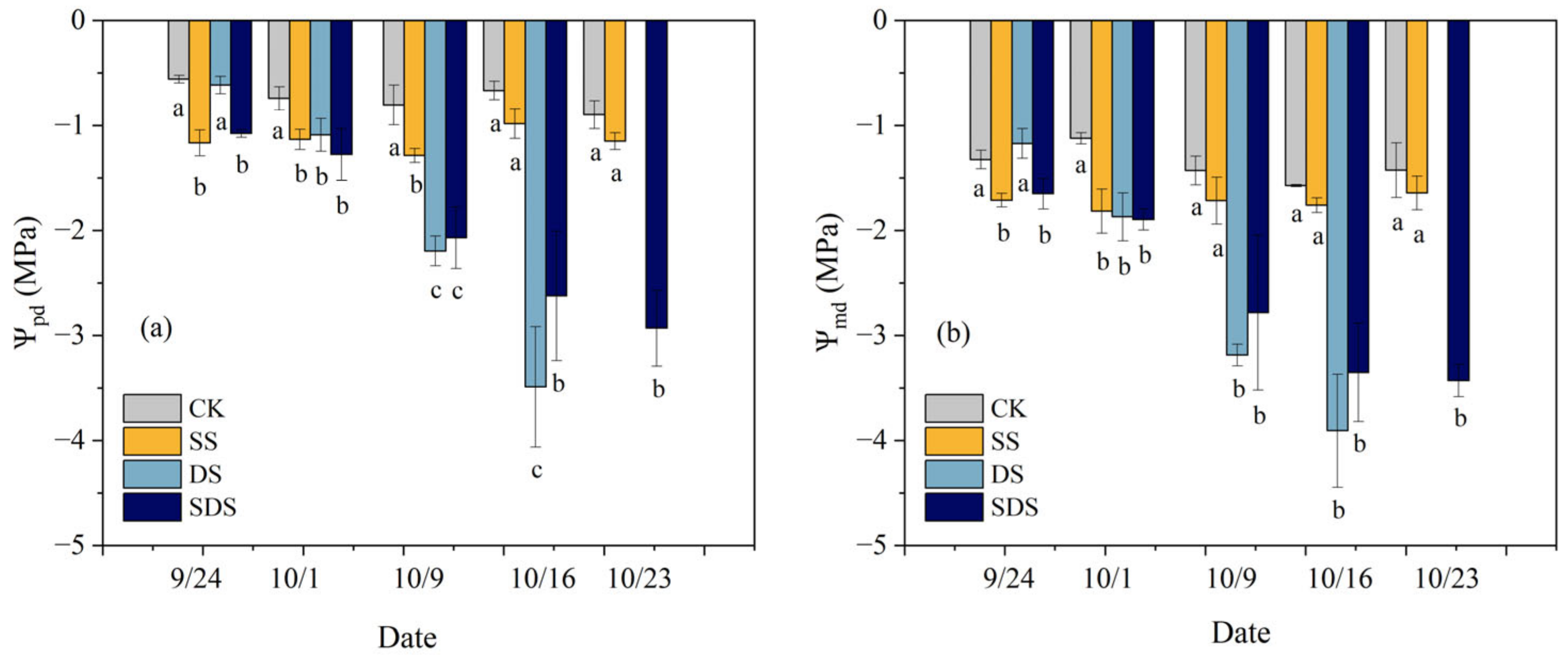
The leaf water potential was sensitive to SS, DS and SDS treatment (Figure 2). On day 1 (September 24), the Ѱpd and Ѱmd of plants significantly reduced by 108%, 92% and 29%, 25% in SS and SDS. On day 8 (October 1), the Ѱpd and Ѱmd significantly reduced by 52%, 46%, 72% and 62%, 67%, 69% in SS, DS and SDS. On day 16 (October 9), the Ѱpd significantly reduced by 60% in SS, while the Ѱmd was not significantly different. In contrast, the Ѱpd and Ѱmd showed greater reductions of 173%, 157% and 123%, 95% in DS and SDS. By day 23 (October 16), the Ѱpd and Ѱmd were significantly reduced by 292% and 113% in SDS, while reductions in DS (421% and 148%) were greater. On day 30 (October 23), there were no mature leaves remaining in the DS to determine water potential, while Ѱpd and Ѱmd in SDS were significantly decreased by 227% and 141%, respectively.
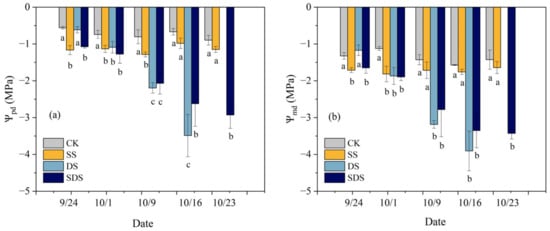
Figure 2.
Changes in predawn: Ѱpd (a)and midday leaf water potential: Ѱmd (b) and throughout the experiment. The different letters below the columns represent significant differences between treatments (p < 0.05). CK: normal freshwater irrigation treatment at 80–100% FC; SS: salt stress treatment, 0.3% soil salinity with freshwater irrigation at 80–100% FC; DS: drought stress treatment, withholding irrigation; SDS: combined drought and salt treatments, 0.3% soil salinity withholding irrigation.
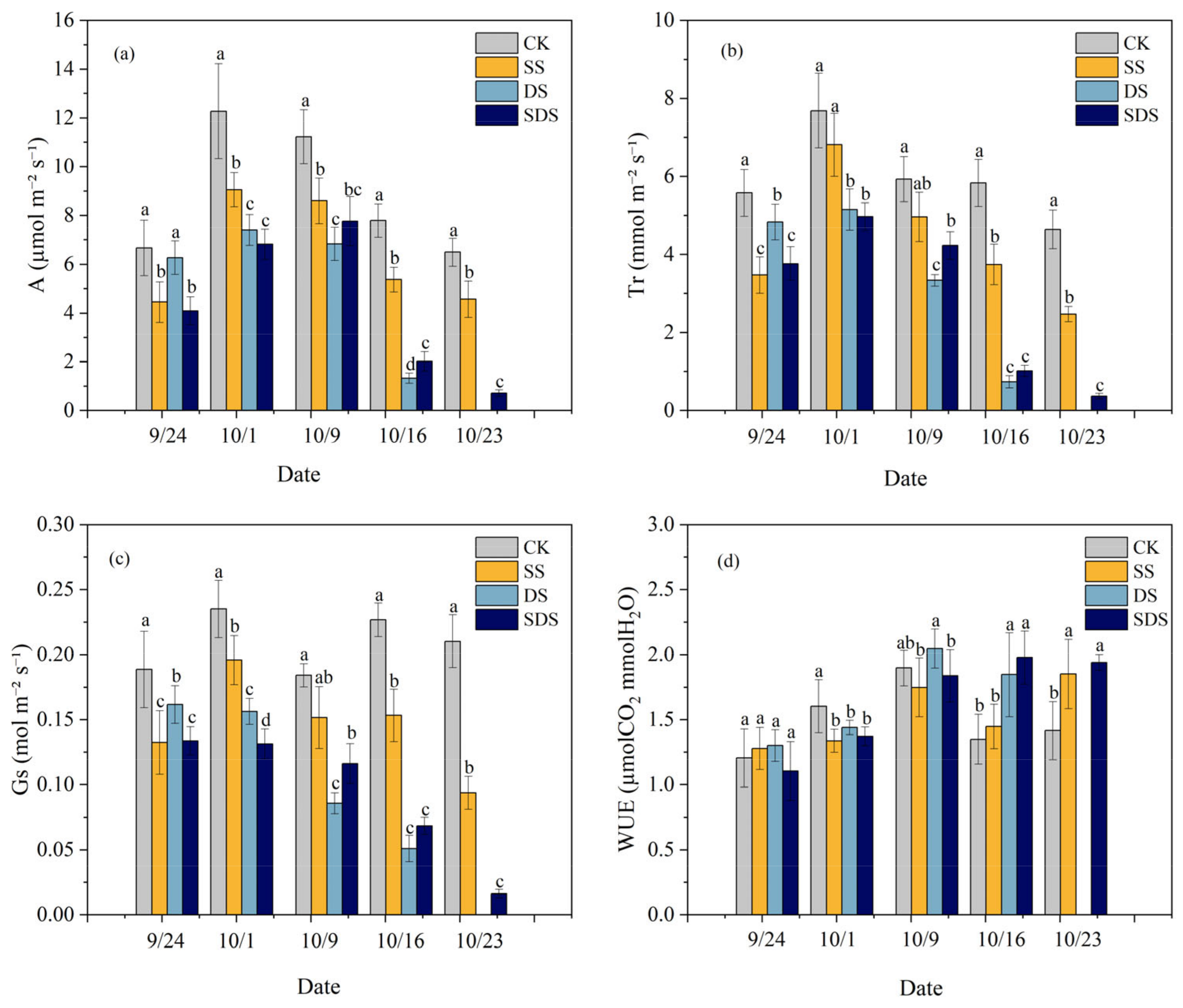
The changes in leaf net photosynthetic rate (A), transpiration rate (Tr), stomatal conductance (Gs) and leaf water use efficiency (WUEL) throughout the experiment are shown in Figure 3. At the beginning of the experiment (September 24), A, Tr and Gs were significantly reduced 33%, 38%, 30% and 39%, 33%, 29% in SS and SDS, but not significantly different in DS. On day 8 (October 1), A and Gs were significantly decreased by 26% and 17% in SS, while Tr decreased but was not significant. A, Tr and Gs showed greater reductions by 40%, 33%, 34% and 44%, 35%, 44% in DS and SDS. By day 16 (October 9), A had significantly decreased by 23% in SS, while Gs and Tr had decreased but were not significant. A, Tr and Gs significantly decreased in DS and SDS by 39%, 44%, 53% and 31%, 29%, 37%, respectively. On day 23 (October 16), A, Tr and Gs in DS and SDS were significantly lower than those in SS by 75%, 81%, 67% and 63%, 73% and 55%, respectively. By day 30 (October 23), A, Tr and Gs in SS and SDS treatments were significantly lower than that in CK treatment.
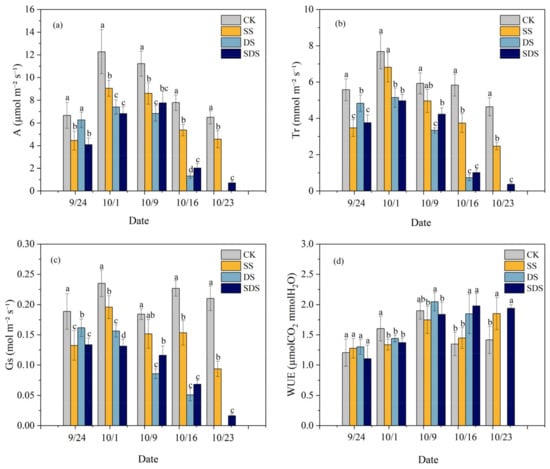
Figure 3.
Net photosynthetic rate: A (a); transpiration rate: Tr (b); stomatal conductance: Gs (c); and leaf water use efficiency: WUEL (d) throughout the experiment. The different letters above the columns represent significant differences between treatments (p < 0.05). CK: normal freshwater irrigation treatment at 80–100% FC; SS: salt stress treatment, 0.3% soil salinity with freshwater irrigation at 80–100% FC; DS: drought stress treatment, withholding irrigation; SDS: combined drought and salt treatments, 0.3% soil salinity withholding irrigation.
On day 1 (September 24), the WUEL was not significantly different among treatments, while on day 8 (October 1) it was significantly decreased by 17%, 10%, and 14% in SS, DS and SDS compared to CK treatment. As stress time increased, WUEL was significantly increased by 37% and 47% in DS and SDS than CK treatment on day 23 (October 16), while not significantly increasing in SS. By day 30 (October 23), WUEL had increased significantly by 31% and 37% in the SS and SDS compared to the CK treatment.
The chlorophyll content index (SPAD value) and maximum light quantum efficiency (Fv/Fm) among different treatments were shown in Table 1 and Table 2. On day 8 (October 1), there was no significant difference in SPAD among treatments. However, Fv/Fm was significantly lower in SDS than CK, SS and DS by 4%, 3% and 3%, respectively. As stress time increased, both SPAD and Fv/Fm were significantly decreased in SS, DS and SDS compared to CK. On the day 23 (October 16), SPAD and Fv/Fm were significantly lower in SS, DS and SDS than CK by 18%, 56%, 42% and 8%, 24% and 16%, respectively. SPAD and Fv/Fm were also significantly lower in DS than SS and SDS treatments by 46%, 17% and 24%, 9%. On the final day of the experiment (October 23), SPAD and Fv/Fm were significantly lower in SS and SDS than CK by 23%, 51% and 16%, 40%, respectively.

Table 1.
The chlorophyll content index (SPAD value) among different treatments. The different letters after the numbers represent significant differences between treatments among each row (p < 0.05). The “/” represents no fully expanded and mature leaves used to determine in DS treatment.

Table 2.
The maximum light quantum efficiency (Fv/Fm) among different treatments. The different letters after the numbers represent significant differences between treatments among each row (p < 0.05). The “/” represents no fully expanded and mature leaves used to determine in DS treatment.
3.3. Stomatal Morphological Traits
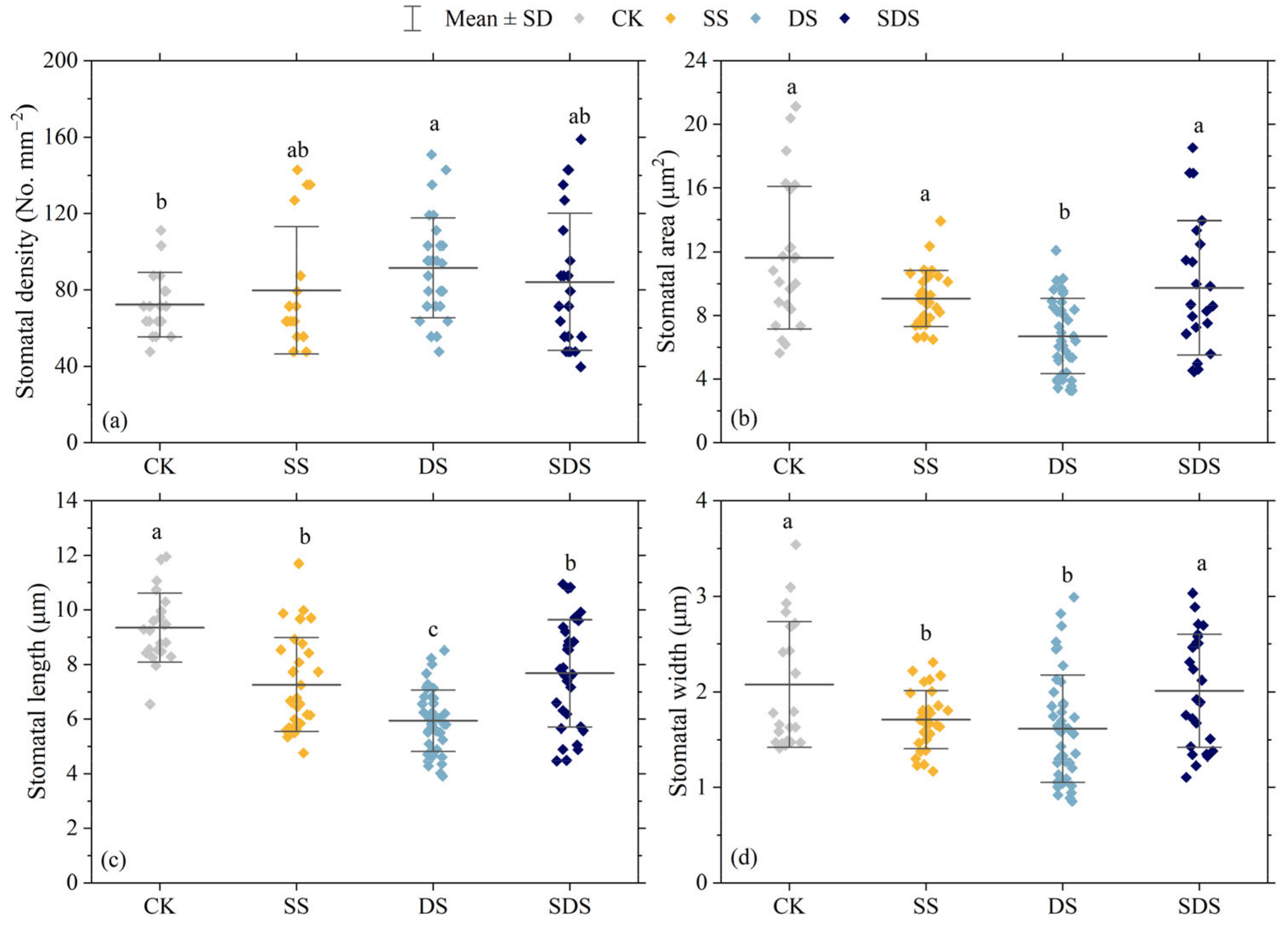
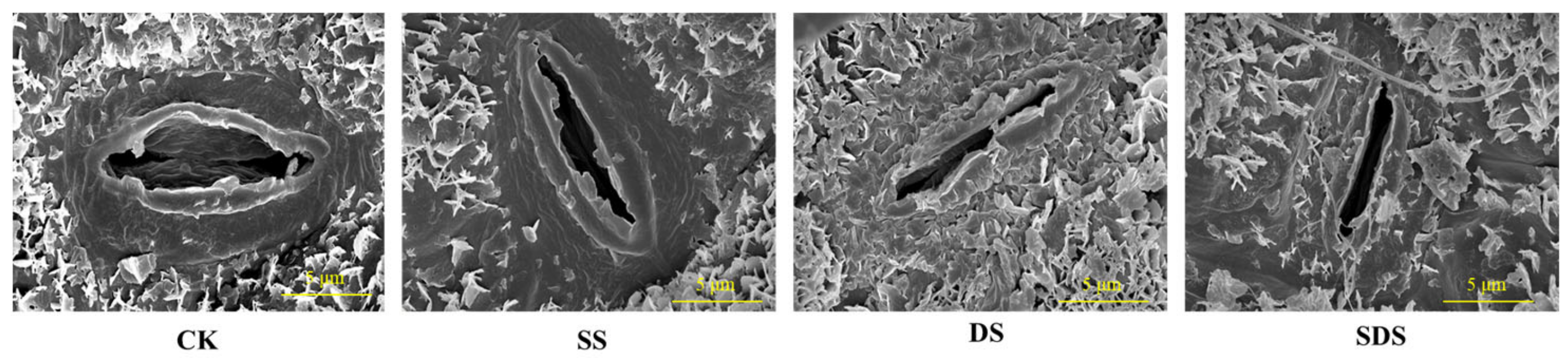
The effects of different treatments on stomatal density, stomatal area, stomatal length and stomatal width are shown in Figure 4 and Figure 5. Stomatal density was significantly increased by 27% in DS, and increased by 10% and 16% in SS and SDS, but not significantly. The stomatal area in the DS treatment was significantly decreased by 42%, 26% and 31% compared to the CK, SS and SDS treatment, respectively. Stomatal length was significantly decreased in SS, DS and SDS by 22%, 37% and 18%, respectively. Stomatal width significantly decreased by 18% and 22% in SS and DS. In summary, the stomata adapted to DS by decreasing their length, width, and area while increasing their density.
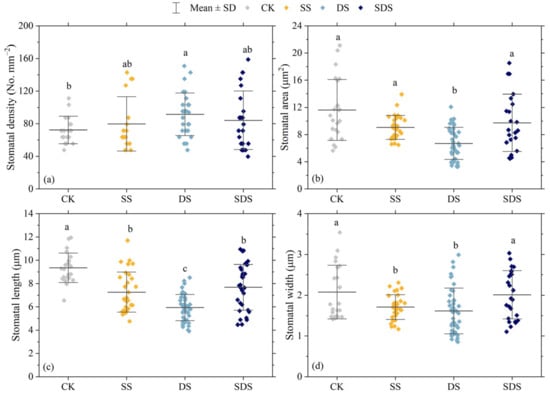
Figure 4.
Effect of different treatments on stomatal density (a); stomatal area (b); stomatal length (c) and stomatal width (d). The square dots represent stomatal parameters of each leaf sample. The different letters represent significant differences between treatments (p < 0.05). CK: normal freshwater irrigation treatment at 80–100% FC; SS: salt stress treatment, 0.3% soil salinity with freshwater irrigation at 80–100% FC; DS: drought stress treatment, withholding irrigation; SDS: combined drought and salt treatments, 0.3% soil salinity withholding irrigation.
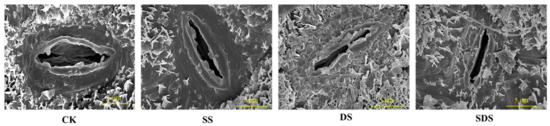
Figure 5.
Changes in the morphological traits of stomata under different treatments observed with scanning electron microscopy. Scale bar = 5 μm. CK: normal freshwater irrigation treatment at 80–100% FC; SS: salt stress treatment, 0.3% soil salinity with freshwater irrigation at 80–100% FC; DS: drought stress treatment, withholding irrigation; SDS: combined drought and salt treatments, 0.3% soil salinity withholding irrigation.
3.4. Stem Xylem Hydraulic Traits
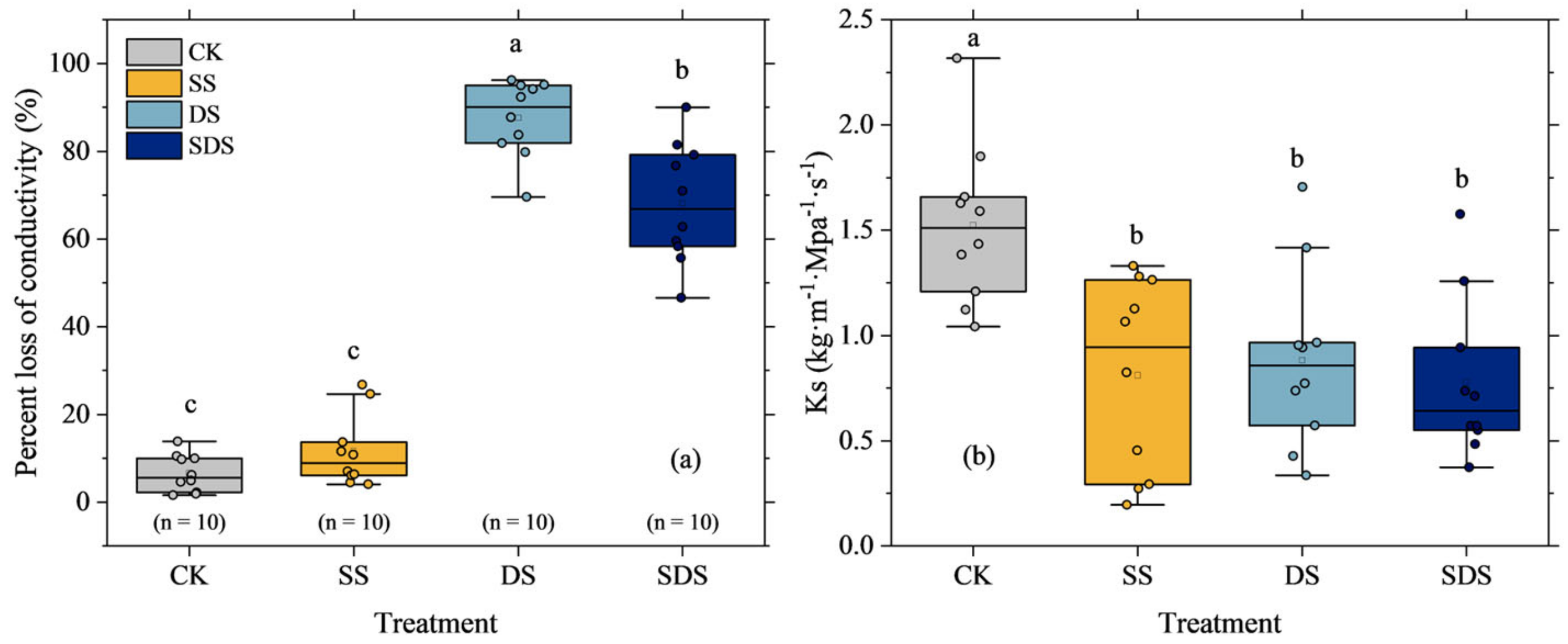
The mean percentage loss of conductivity (PLC) values of CK and SS treatments were 6.5 ± 4.3% and 11.6 ± 7.1%, respectively, with no significant difference between them. While the mean PLC values of DS and SDS treatments were significantly higher at 87.6 ± 8.7% and 68.1 ± 13.7%, respectively (Figure 6a). Furthermore, PLC significantly increased by 81% and 62% in DS and SDS, respectively. The maximum xylem-specific hydraulic conductivity (Ks) differed among treatments (Figure 6b). Ks significantly decreased by 47%, 42% and 49% in SS, DS and SDS, but there were no significant differences among them.
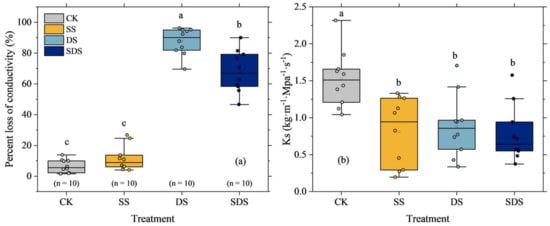
Figure 6.
The percentage loss of xylem hydraulic conductivity (a) and the maximum xylem specific hydraulic conductivity (b). The round solid dots represent hydraulic traits of each tree stem sample. Boxes represent the interquartile range (IQR), the bars within the boxes indicate median values, and whiskers extend to the maximum and minimum data values within 1.5 × IQR. The different letter on the column indicated there were significant differences (p < 0.05) among various treatments according to LSD. CK: normal freshwater irrigation treatment at 80–100% FC; SS: salt stress treatment, 0.3% soil salinity with freshwater irrigation at 80–100% FC; DS: drought stress treatment, withholding irrigation; SDS: combined drought and salt treatments, 0.3% soil salinity withholding irrigation.
3.5. Soluble Sugars, Starch and Total Nonstructural Carbohydrates
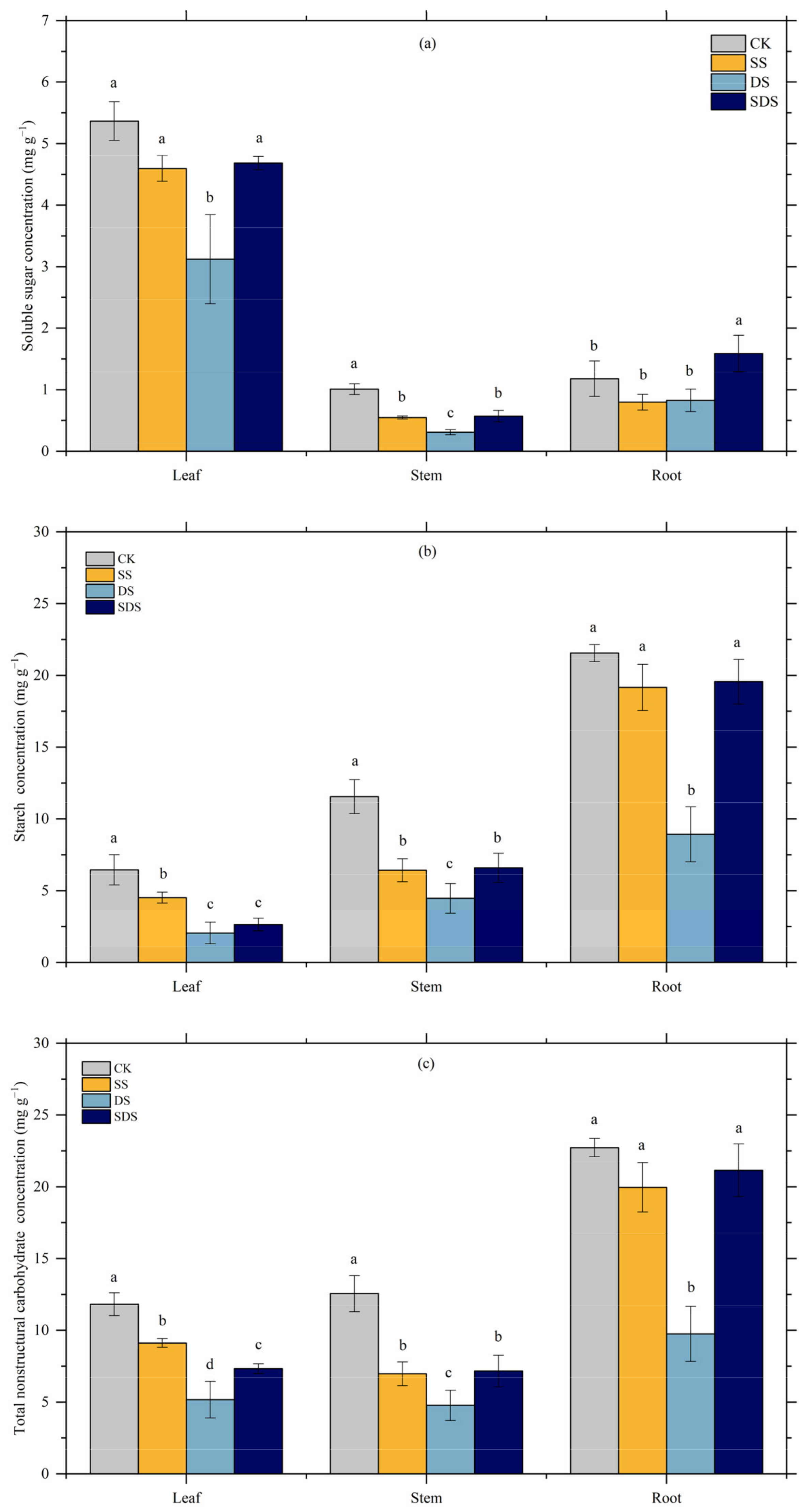
The effects of different treatments on soluble sugar, starch, and total nonstructural carbohydrates (NSC) varied between plant organs (Figure 7). In leaves, soluble sugar concentration, but was significantly decreased by 42% in DS, while not significantly changed in SS and SDS. Starch concentration showed a significant reduction of 30% in SS, and greater significant decreases of 68% and 59% in DS and SDS, respectively. In stems, soluble sugar was significantly decreased by 46% and 44% in SS and SDS, with a greater reduction of 69% in DS. Starch was reduced significantly by 44% and 43% in SS and SDS, and more so by 61% in DS. In roots, soluble sugar in SDS increased significantly by 41%, while starch in DS decreased significantly by 59%, 53% and 54% compared to CK, SS and SDS.
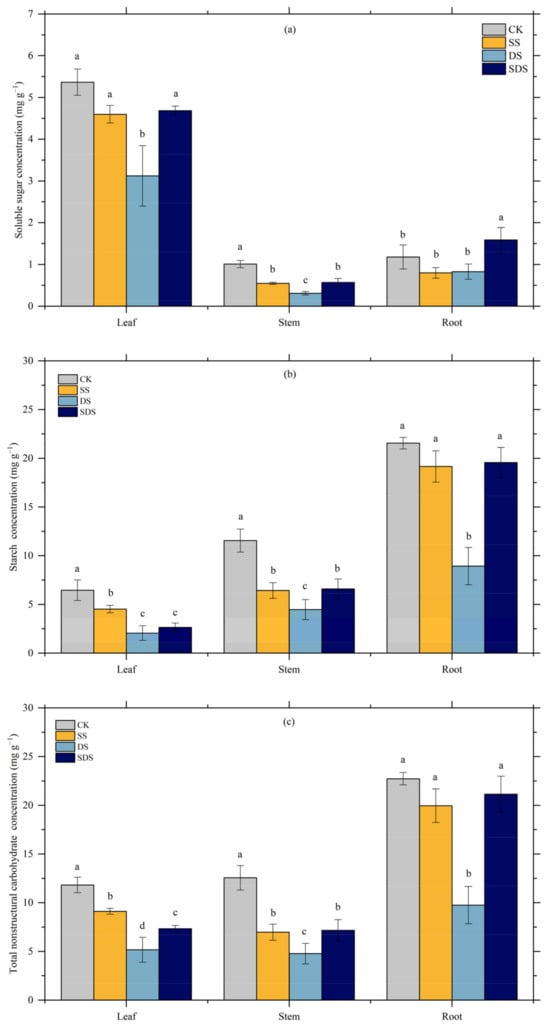
Figure 7.
Effect of different treatments on soluble sugar (a), starch (b), total NSC (c) concentration in different organs. The different letter on the column indicated there were significant differences (p < 0.05) among various treatments according to LSD. Values are means ± SD (n = 3). CK: normal freshwater irrigation treatment at 80–100% FC; SS: salt stress treatment, 0.3% soil salinity with freshwater irrigation at 80–100% FC; DS: drought stress treatment, withholding irrigation; SDS: combined drought and salt treatments, 0.3% soil salinity withholding irrigation.
Total NSC was affected by SS, DS and SDS in leaves and stem. In roots, it was the lowest due to decreased soluble sugar and starch. Total NSC in DS was significantly decreased by 56%, 62% and 57% in leaves, stems and roots, respectively.
3.6. Correlation Analysis of Physiological Characteristics
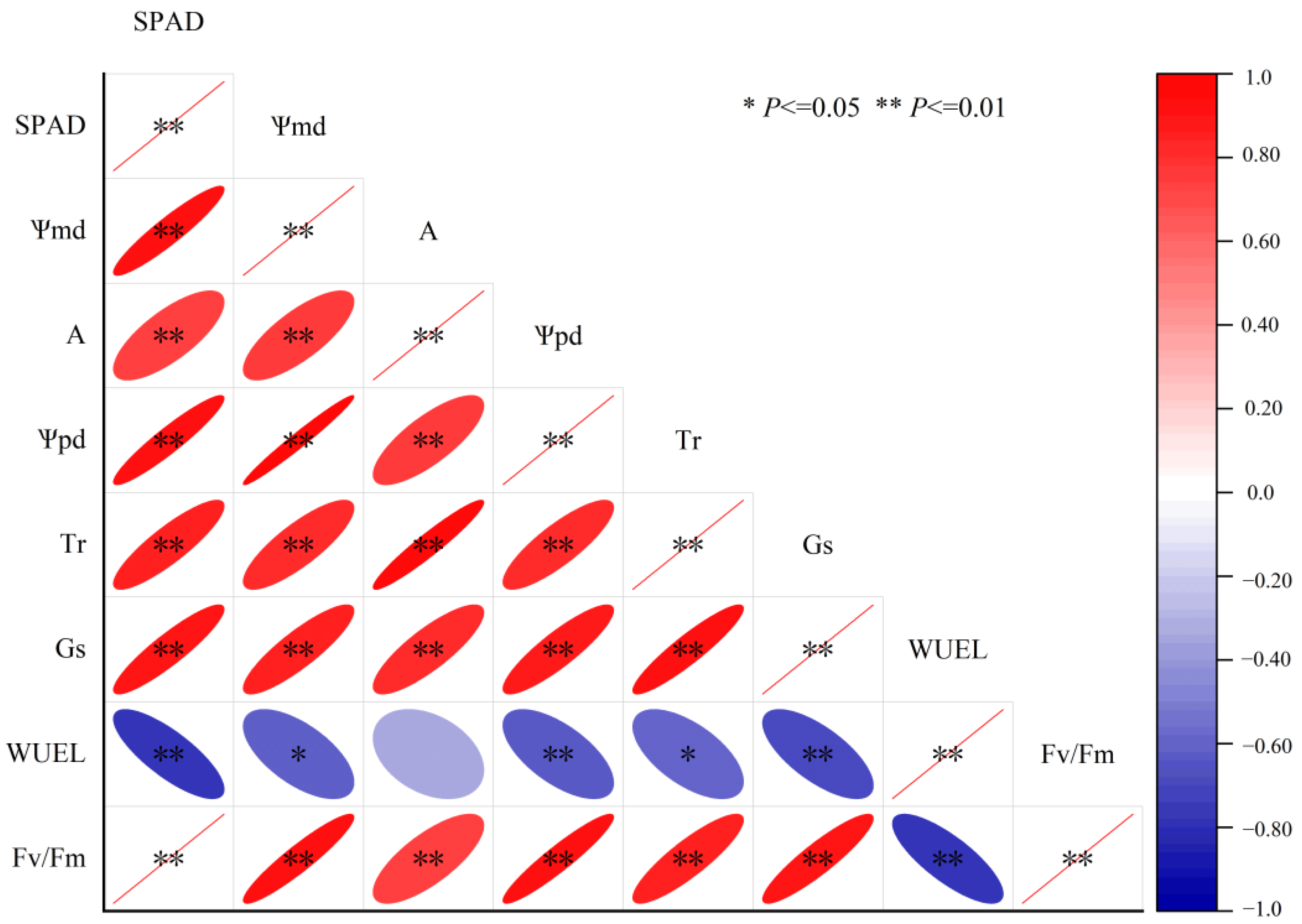
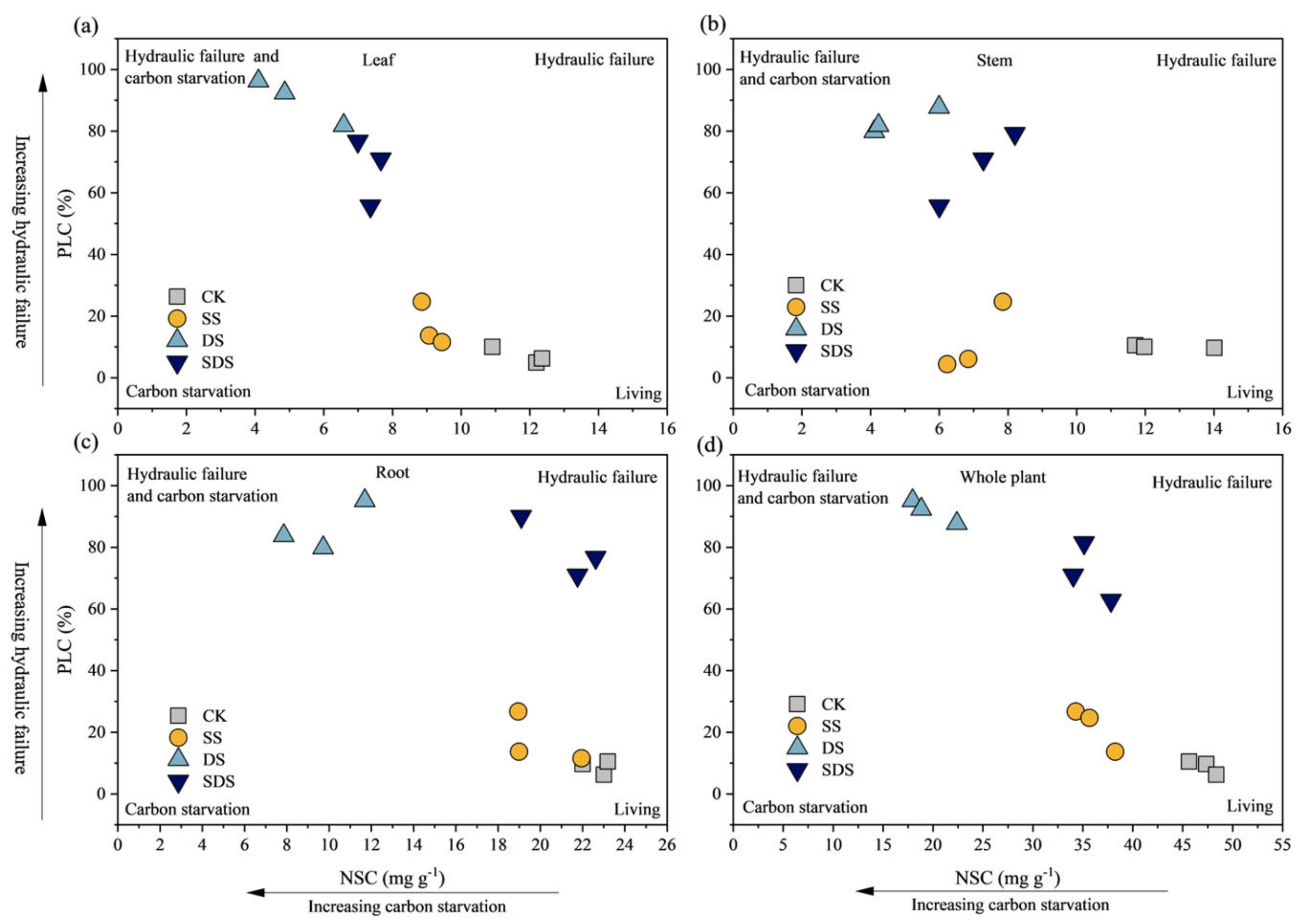
Significant positive correlations occurred between SPAD, Ѱpd, Ѱmd, A, Tr and Gs, as shown in Figure 8. WUEL was negatively significantly correlated with SPAD, Ѱpd, Ѱmd, Fv/Fm, Tr and Gs, but showed no linear relationship with A. Fv/Fm was positively significantly correlated with Ѱpd, Ѱmd, A, Tr and Gs, while negatively significantly correlated with WUEL. According to Figure 9, the risk of hydraulic failure and carbon starvation was lower for CK and SS treatment than for the DS and SDS. Moreover, the DS treatment was more likely to have a mortality risk than the SDS treatment.
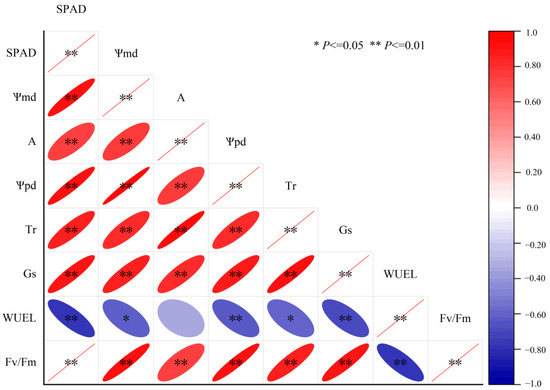
Figure 8.
Correlation analysis of the key physiological characteristics. SPAD: the chlorophyll content index; Ѱmd: midday leaf water potential; A: net photosynthetic rate; Ѱpd: predawn leaf water potential; Gs: stomatal conductance; Tr: transpiration rate; WUEL: leaf water use efficiency; Fv/Fm: maximum light quantum efficiency.
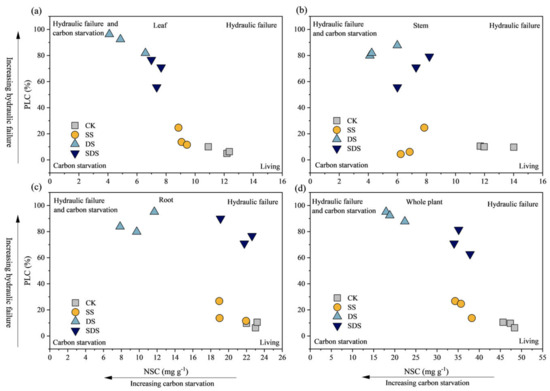
Figure 9.
The mortality risk with hydraulic failure and carbon starvation in leaf (a); in stem (b); in root (c) and in whole plant (d). The higher the PLC value, the greater the risk of tree mortality due to hydraulic failure. The smaller the NSC value, the greater the risk of tree mortality due to carbon starvation. CK: normal freshwater irrigation treatment at 80–100% FC; SS: salt stress treatment, 0.3% soil salinity with freshwater irrigation at 80–100% FC; DS: drought stress treatment, withholding irrigation; SDS: combined drought and salt treatments, 0.3% soil salinity withholding irrigation.
4. Discussion
4.1. Drought and Salt Stress Decreased Hydraulic Function by Decreasing Leaf Water Potential and Stomatal Conductance
In this study, drought and salt stress, along with their combination, decreased leaf water potential and stomatal conductance, and modified stomatal morphology. Xylem hydraulic specific conductivity was also significantly decreased, while percentage loss of conductivity (PLC) significantly increased.
The decreased leaf water potential and stomatal conductance observed are consistent with plants regulating stomatal closure to optimize water use and maintain osmotic pressure under drought and salt stresses [43,44,45]. Stomatal closure is regulated by a combination of a chemical signal (ABA) and a hydraulic signal [32]. ABA content increases under stress conditions, while salt stress also induces ABA production by elevating osmotic pressure [46,47]. Combined water and salt stresses likely caused a more rapid decline in leaf water potential due to the additive effects of water deficit and salt accumulation [48]. This decrease then triggered stomatal sensitivity to ABA at lower water potentials [49]. However, in our study, leaf water potential did not decline most severely under SDS treatment. This could be because stomatal closure induced by irrigation NaCl under SDS treatment reduces water consumption, maintaining a higher soil water content under the SDS treatment than the DS treatment (Figure 1a). This suggests that the addition of salt had an alleviating effect on drought stress by mediating stomatal conductance and soil water content. The retention of more available water sources likely helped offset the lowering of water potential expected from combined osmotic and water deficits.
Our results showed notable reductions in stomatal conductance occurred following all stressed treatments, most pronounced in the DS and SDS treatments. Stomata conductance is determined by the coordination of the maximum stomatal conductance and the actual stomata aperture [49]. Some studies have shown that stomatal density increased under soil water stress conditions [50], but more severe deficits led to a reduction [51]. In our study, stomatal density was significantly increased in the DS only. In addition, plants can adjust stomatal size in response to stress. Stomatal area was significantly reduced in the DS treatment, while stomatal length was significantly reduced in the SS, DS and SDS treatments, and stomatal width was significantly reduced in the SS and DS treatments. In agreement with the findings of others, stomatal density decreased when subjected to water and salt stress, and maximum stomatal conductance also decreased due to a decrease in stomatal length and width [52]. Furthermore, water stress has a more pronounced effect on stomatal opening and spatial distribution compared to stomatal number, as stomatal opening is positively correlated with stomatal conductance [53]. Gas exchange is influenced by the water status of plants, and stomatal closure can limit water transport and reduce soil-to-leaf hydraulic conductance, thereby preventing an excessive reduction in leaf water potential or catastrophic cavitation in the xylem [54]. In our study, DS, SS and SDS treatment all significantly decreased stem maximum xylem-specific hydraulic conductivity (Figure 6b), while there was no significant difference in PLC between the SS and CK treatment, and the PLC of DS treatment was significantly higher than that of SDS treatment (Figure 6a). These results indicate that drought treatments had more negative effects on hydraulic function than combined drought and salt stress. Leaf water potential is closely related to the loss of hydraulic conductivity [55], and in our study, the predawn and midday leaf water potentials were not significantly decreased in the SS treatment, while the DS treatment showed a significant decrease compared to the SDS treatment (Figure 2). These findings align with the observed effects on xylem conductivity loss.
4.2. Drought and Salt Stress Decreased Carbon Assimilation by Affecting Stomatal Limiting and Non-Stomatal Limiting Factors
In summary, drought and salt stress, along with their combination, not only decreased stomatal conductance (Gs), photosynthesis rate (A), and transpiration rate (Tr) but also reduced chlorophyll content (SPAD) and the maximum light quantum efficiency of photosystem II (Fv/Fm), while increasing leaf water use efficiency (WUEL). These stressors can lead to both stomatal and non-stomatal limitations, resulting in a reduction in non-structural carbohydrate supply.
The impact of water deficit and high salt conditions on carbon assimilation is complex [56]. The reduction in Gs inhibited the diffusion of CO2 into leaves, depleting substrates for photosynthesis and further decreasing A [57]. Leaf water use efficiency (WUEL), which is determined by both A and Tr, is also affected by changes in Gs. In our study, WUEL of SS treatment showed a non-significant increase of 7%, while WUEL of DS and SDS treatments exhibited significant increases of 37% and 47%, respectively (Figure 3d). The response of WUEL to salt and drought stress depends on the intensity and duration of the stress [32]. In our study, salt stress alone did not significantly affect WUEL, and the lack of significant difference between DS and SDS treatments suggests that drought plays a dominant role when combined with salt stress.
As the severity and duration of stress increase, photosynthesis is primarily affected by non-stomatal limitations [50], as well as other processes related to photosynthesis, such as photochemical processes, including photosystem PSII [58]. In our study, chlorophyll content and the maximum light quantum efficiency of photosystem II (Fv/Fm) were affected by the combined effects of drought and salt stress, as well as their individual effects. The chlorophyll content and Fv/Fm decreased significantly with increasing drought severity and salt stress duration, with the DS treatment showing the most severe decrease. The water stress oxidizes PSII proteins and sufficiently disrupts the electronic excitation processes, resulting in the disruption of PSII, thereby decreasing Fv/Fm, ФPSII, and Jmax [59]. A decrease in chlorophyll due to salt stress reduced light energy uptake, affecting photochemical reactions, dissipation and fluorescence emission [60]. The significant decline in Fv/Fm suggests damage to the PSII reaction center and inhibition of the photochemical conversion efficiency of PSI [61]. Ultimately, our study demonstrated that drought and salt stress influenced the photochemical process, inhibited CO2 fixation and disturbed the synthesis of sucrose and starch.
Photosynthesis is the main driver of tree productivity and is essential for carbon assimilation, as it provides the primary source of carbohydrates [62]. Non-structural carbon (NSC), which includes soluble sugars and starch, plays a crucial role in plant growth and survival under environmental stresses [63]. In our study, different organs showed varying responses in terms of soluble sugars, starch and NSC under drought stress, salt stress and their combination. The DS treatment resulted in the lowest levels of soluble sugars, starch and NSC in leaves, stems and roots. Under drought stress, stored NSC can be mobilized to support metabolic activity when carbon assimilation is insufficient to meet demand [64]. The soluble sugar content was higher in comparison to stems and roots, while starch and NSC were higher in roots compared to leaves and stems. Starch in the leaves may potentially be decomposed into soluble sugars to support plant metabolism, while stress does not seem to affect the starch content in the roots.
In conclusion, this study demonstrates that drought and salt stresses negatively influence carbon assimilation in R. pseudoacacia by impairing both stomatal gas exchange and photochemical reactions. It is important to note that the effects of drought and salt stress on carbon assimilation can vary depending on factors such as plant species, the severity and duration of the stress, and other environmental conditions.
4.3. Salt Addition Mitigates the Negative Effects of Drought Stress
The results of our study indicate that the addition of salt mitigated the negative effects of drought stress on plants (Figure 9). Under drought stress alone, there was a decrease in non-structural carbohydrates (NSC) and an increase in percent loss of conductivity (PLC). However, when drought stress was combined with salt stress, the levels of NSC were higher and PLC were lower compared to drought stress alone. This suggests that salt addition reduced the risk of hydraulic failure and carbon starvation. Reduced irrigation, as seen in drought stress, leads to a decrease in soil water potential, while salt stress contributes to limited plant water utilization due to high ion accumulation and decreased soil osmotic potential [24]. When water and salt stresses are combined, the hydraulic resistance in roots, stems, and leaves increases, reducing the water transport capacity of the plant [65]. However, some studies have shown opposite results, indicating combined water and salt stresses can enhance drought tolerance, including improved water-carbon balance and osmoregulation [25,66]. In our study, the combined drought and salinity stress treatments exhibited significantly higher Ѱpd, Ѱmd, and Gs compared to single drought stress. This resulted in less loss of xylem hydraulic conductivity and a reduced risk of hydraulic failure in trees. By maintaining cell turgor under combined stresses, osmoregulation could have helped sustain water uptake compared to drought alone [67,68]. In particular, sodium (Na+) serves as an important inorganic osmolyte, accumulating in tissues to offset the osmotic effects of drought when present at non-toxic levels [26]. However, excessive Na+ accumulation can disrupt ions distribution in cells and lead to ionic toxicity [69].
Hydraulic failure, accompanied by carbon starvation due to canopy loss, can occur when there is a decline in hydraulic function [70]. Water stress causes leaf abscission, reducing carbon fixation and forcing plants to use stored carbon for metabolic activities [71]. Both ion toxicity and reduction in freshwater availability of salt stress could lead to stomatal closure and decreased CO2 uptake, ultimately leading to declining tree growth [72]. The higher leaf water potential (Ψpd, Ψmd) and stomatal conductance (Gs) observed in SDS versus DS implied less impairment of xylem hydraulic function under combined stresses. This likely reduced the risk of hydraulic failure and associated carbon starvation through the depletion of NSC reserves [73]. Additionally, DS exhibited more pronounced decreases in Ψpd, Ψmd and Gs, along with increased PLC, coinciding with greater utilization versus assimilation of NSCs. Nonetheless, interactions between drought and salinity are complex, varying across genotypes, stress intensities and durations [66]. Moreover, carbon starvation does not entirely preclude hydraulic failure, as these processes can promote each other under severe stress [74]. Our study provided initial insights using young seedlings under single salinity levels. Further work is needed to validate these mechanisms under field conditions, incorporating a range of salinity concentrations and mature trees. Variation in plant attributes should also be considered to better understand combined drought and salt stress responses.
5. Conclusions
This study investigated the effects of drought, salt stress and their combination on leaf physiology, xylem hydraulics and carbon reserves of Robinia pseudoacacia seedlings. Salt stress alone did not significantly reduce leaf water potential or impact carbon reserves. Drought stress and combined salt and drought stress significantly decreased leaf water potential, xylem hydraulic conductivity and depleted carbon stores, with drought stress having more pronounced effects. However, salt addition mitigated the negative impacts of drought by maintaining hydraulic function and carbon reserves. These findings demonstrate the potential for salt priming to alleviate drought stress in R. pseudoacacia seedlings. As climate change is expected to increase the frequency and intensity of droughts and salinization worldwide, further research on carbon dynamics and water balance in mature trees under field conditions would help to assess how multiple factors may affect drought tolerance. At the ecosystem scale, landscape practices that promote water preservation and control salt accumulation are recommended. Sustainable irrigation techniques and revegetation of drought- and salt-tolerant species can help forests adapt to new environments while continuing to provide carbon sequestration services. Understanding the physiological response of trees under stress conditions could help mitigate the risk of vulnerable forest degradation in the future.
Author Contributions
Conceptualization, Y.F., M.L. and B.S.; methodology, Y.F., X.W. and G.D.; Investigation: Y.F., J.W. and M.Y.; Resources: G.D. and M.L.; Data curation: Y.F., M.L. and B.S.; Formal analysis: Y.F., J.W. and M.Y.; Validation: X.W. and G.D.; Visualization: Y.F. and H.L.; Writing—original draft: Y.F.; Writing—review and editing: J.W., M.Y., X.W., B.S., H.L., M.L. and G.D.; Supervision: B.S. and M.L.; Funding acquisition: B.S. and H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Key Research and Development Program of China (2022YFE0100300), the Natural Science Foundation of Shandong Province (ZR2020QD119), the Major Scientific and Technological Innovation Projects of Shandong Key R & D Plan (2019JZZY010710), National Natural Science Foundation of China (42101018), and the Natural Science and Engineering Research Council of Canada (NSERC).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Adams, H.D.; Zeppel, M.; Anderegg, W.; Hartmann, H.; Mcdowell, N.G. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat. Ecol. Evol. 2017, 1, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.F.; Feng, S.Y.; Huo, Z.L.; Ji, Q.Y. Effects of deficit irrigation with saline water on soil water-salt distribution and water use efficiency of maize for seed production in arid Northwest China. Agric. Water Manag. 2019, 212, 424–432. [Google Scholar] [CrossRef]
- Hopkinson, C.S.; Lugo, A.E.; Alber, M.; Covich, A.P.; Van Bloem, S.J. Forecasting effects of sea-level rise and windstorms on coastal and inland ecosystems. Front. Ecol. Environ. 2008, 6, 255–263. [Google Scholar] [CrossRef][Green Version]
- Zelm, E.V.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Mcdowell, N.G.; Beerling, D.J.; Breshears, D.; Fisher, R.A.; Raffa, K.F.; Stitt, M. The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends Ecol. Evol. 2011, 26, 523–532. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Cochard, H. Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiol. 2009, 149, 575–584. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? N. Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- McDowell, N.G.; Sapes, G.; Pivovaroff, A.; Adams, H.D.; Allen, C.D.; Anderegg, W.R.L.; Arend, M.; Breshears, D.D.; Brodribb, T.; Choat, B.; et al. Mechanisms of woody-plant mortality under rising drought, CO2 and vapour pressure deficit. Nat. Rev. Earth Environ. 2022, 3, 294–308. [Google Scholar] [CrossRef]
- Hartmann, H.; Trumbore, S. Understanding the roles of nonstructural carbohydrates in forest trees—From what we can measure to what we want to know. N. Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef]
- Plaut, J.A.; Wadsworth, W.D.; Pangle, R.; Yepez, E.A.; McDowell, N.G.; Pockman, W.T. Reduced transpiration response to precipitation pulses precedes mortality in a pinon-juniper woodland subject to prolonged drought. N. Phytol. 2013, 200, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Salleo, S.; Trifilo, P.; Esposito, S.; Nardini, A.; Lo Gullo, M.A. Starch-to-sugar conversion in wood parenchyma of field-growing Laurus nobilis plants: A component of the signal pathway for embolism repair? Funct. Plant Biol. 2009, 36, 815–825. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Liu, H.; Qi, Y.; Liu, F.; Zhu, X. Patterns in nonstructural carbohydrate contents at the tree organ level in response to drought duration. Glob. Change Biol. 2020, 26, 3627–3638. [Google Scholar] [CrossRef] [PubMed]
- Guneralp, B.; Gertner, G. Feedback loop dominance analysis of two tree mortality models: Relationship between behavior. Tree Physiol. 2007, 27, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Boursiac, Y.; Chen, S.; Luu, D.T.; Sorieul, M.; van den Dries, N.; Maurel, C. Early effects of salinity on water transport in Arabidopsis roots. Molecular and cellular features of aquaporin expression. Plant Physiol. 2005, 139, 790–805. [Google Scholar] [CrossRef] [PubMed]
- Stavridou, E.; Hastings, A.; Webster, R.J.; Robson, P. The impact of soil salinity on the yield, composition and physiology of the bioenergy grass Miscanthus×giganteus. GCB Bioenergy 2017, 9, 92–104. [Google Scholar] [CrossRef]
- Desantis, L.R.G.; Bhotika, S.; Williams, K.; Putz, F.E. Sea-level rise and drought interactions accelerate forest decline on the Gulf Coast of Florida, USA. Glob. Change Biol. 2007, 13, 2349–2360. [Google Scholar] [CrossRef]
- Negrao, S.; Schmockel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef]
- Zhang, P.; McDowell, N.G.; Zhou, X.; Wang, W.; Leff, R.T.; Pivovaroff, A.L.; Zhang, H.; Chow, P.S.; Ward, N.D.; Indivero, J.; et al. Declining carbohydrate content of Sitka-spruce treesdying from seawater exposure. Plant Physiol. 2021, 185, 1682–1696. [Google Scholar] [CrossRef] [PubMed]
- Bartels, D.; Sunkar, R. Drought and salt tolerance in plants. Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Schaefer, R.B.; Piggott, J.J. Advancing understanding and prediction in multiple stressor research through a mechanistic basis for null models. Glob. Change Biol. 2018, 24, 1817–1826. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Glenn, E.P.; Nelson, S.G.; Ambrose, B.; Martinez, R.; Soliz, D.; Pabendinskas, V.; Hultine, K. Comparison of salinity tolerance of three Atriplex spp. in well-watered and drying soils. Environ. Exp. Bot. 2012, 83, 62–72. [Google Scholar] [CrossRef]
- Katuwal, K.B.; Xiao, B.; Jespersen, D. Physiological responses and tolerance mechanisms of seashore paspalum and centipedegrass exposed to osmotic and iso-osmotic salt stresses. J. Plant Physiol. 2020, 248, 153154. [Google Scholar] [CrossRef] [PubMed]
- Angon, P.B.; Tahjib-Ul-Arif, M.; Samin, S.I.; Habiba, U.; Hossain, M.A.; Brestic, M. How Do Plants Respond to Combined Drought and Salinity Stress?-A Systematic Review. Plants 2022, 11, 2884. [Google Scholar] [CrossRef]
- Perez-Perez, J.G.; Syvertsen, J.P.; Botia, P.; Garcia-Sanchez, F. Leaf water relations and net gas exchange responses of salinized Carrizo citrange seedlings during drought stress and recovery. Ann. Bot. 2007, 100, 335–345. [Google Scholar] [CrossRef]
- Sahin, U.; Ekinci, M.; Ors, S.; Turan, M.; Yildiz, S.; Yildirim, E. Effects of individual and combined effects of salinity and drought on physiological, nutritional and biochemical properties of cabbage (Brassica oleracea var. capitata). Sci. Hortic. 2018, 240, 196–204. [Google Scholar] [CrossRef]
- Li, H.; Hou, X.; Du, T. Responses of tomato fruit water balance and xylem hydraulic property of pedicel and calyx to water deficit and salinity stress. Environ. Exp. Bot. 2023, 206, 105195. [Google Scholar] [CrossRef]
- He, F.-L.; Bao, A.-K.; Wang, S.-M.; Jin, H.-X. NaCl stimulates growth and alleviates drought stress in the salt-secreting xerophyte Reaumuria soongorica. Environ. Exp. Bot. 2019, 162, 433–443. [Google Scholar] [CrossRef]
- Xue, F.; Liu, W.; Cao, H.; Song, L.; Ji, S.; Tong, L.; Ding, R. Stomatal conductance of tomato leaves is regulated by both abscisic acid and leaf water potential under combined water and salt stress. Physiol. Plant 2021, 172, 2070–2078. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Gu, S.; Kang, S.; Du, T.; Tong, L.; Wood, J.D.; Ding, R. Mild water and salt stress improve water use efficiency by decreasing stomatal conductance via osmotic adjustment in field maize. Sci. Total Environ. 2022, 805, 150364. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.B.; Yang, H.; Jiang, J. Leaf N and P resorption and stoichiometry characteristics of main tree species in the plain afforestation area of Beijing. J. Beijing For. Univ. 2022, 44, 8–15. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, M.; Li, Q.; Liu, X.; Song, H.; Peng, X.; Wang, H.; Yang, N.; Fan, P.; Wang, R.; et al. Effects of defoliation modalities on plant growth, leaf traits, and carbohydrate allocation in Amorpha fruticosa L. and Robinia pseudoacacia L. seedlings. Ann. For. Sci. 2020, 77, 53. [Google Scholar] [CrossRef]
- Fang, J.Y.; Wang, Z.H.; Tang, Z.Y. Atlas of Woody Plants in China; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar] [CrossRef]
- Mao, P.; Zhang, Y.; Cao, B.; Guo, L.; Shao, H.; Cao, Z.; Jiang, Q.; Wang, X. Effects of salt stress on eco-physiological characteristics in Robinia pseudoacacia based on salt-soil rhizosphere. Sci. Total Environ. 2016, 568, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Pang, Y.; Cao, B.; Fan, Z.; Mao, P.; Li, Z.; Liu, W.; Li, P. Robinia pseudoacacia decline and fine root dynamics in a plantation chronosequence in the Yellow River Delta, China. For. Sci. 2022, 68, 425–433. [Google Scholar] [CrossRef]
- Sonmez, S.; Buyuktas, D.; Okturen, F.; Citak, S. Assessment of different soil to water ratios (1:1, 1:2.5, 1:5) in soil salinity studies. Geoderma 2008, 144, 361–369. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, L.; Zhang, S.; Cai, J.; Tyree, M.T. Water relations of Robinia pseudoacacia L.: Do vessels cavitate and refill diurnally or are R-shaped curves invalid in Robinia? Plant Cell Environ. 2014, 37, 2667–2678. [Google Scholar] [CrossRef]
- Hansen, J.; Moller, I. Percolation of starch and soluble carbohydrates from plant tissue for quantitative determination with anthrone. Anal. Biochem. 1975, 68, 87–94. [Google Scholar] [CrossRef]
- Mitchell, P.J.; O’Grady, A.P.; Tissue, D.T.; White, D.A.; Ottenschlaeger, M.L.; Pinkard, E.A. Drought response strategies define the relative contributions of hydraulic dysfunction and carbohydrate depletion during tree mortality. N. Phytol. 2013, 197, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Bi, M.H.; Nie, Z.F.; Jiang, H.; Liu, X.D.; Fang, X.W.; Brodribb, T.J. Evolution of stomatal closure to optimize water-use efficiency in response to dehydration in ferns and seed plants. N. Phytol. 2021, 230, 2001–2010. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; Holbrook, N.M. Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiol. 2003, 132, 2166–2173. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.S.; Andersen, M.N.; Liu, F. Biochar Mitigates Salinity Stress in Potato. J. Agron. Crop Sci. 2015, 201, 368–378. [Google Scholar] [CrossRef]
- Martorell, S.; Diaz-Espejo, A.; Tomas, M.; Pou, A.; El Aou-Ouad, H.; Escalona, J.M.; Vadell, J.; Ribas-Carbo, M.; Flexas, J.; Medrano, H. Differences in water-use-efficiency between two Vitis vinifera cultivars (Grenache and Tempranillo) explained by the combined response of stomata to hydraulic and chemical signals during water stress. Agric. Water Manag. 2015, 156, 1–9. [Google Scholar] [CrossRef]
- Hussain, M.; Farooq, S.; Hasan, W.; Ul-Allah, S.; Tanveer, M.; Farooq, M.; Nawaz, A. Drought stress in sunflower: Physiological effects and its management through breeding and agronomic alternatives. Agric. Water Manag. 2018, 201, 152–166. [Google Scholar] [CrossRef]
- Wilkinson, S.; Davies, W.J. Drought, ozone, ABA and ethylene: New insights from cell to plant to community. Plant Cell Environ. 2010, 33, 510–525. [Google Scholar] [CrossRef]
- Lawson, T.; Matthews, J. Guard Cell Metabolism and Stomatal Function. Annu. Rev. Plant Biol. 2020, 71, 273–302. [Google Scholar] [CrossRef]
- Fraser, L.H.; Greenall, A.; Carlyle, C.; Turkington, R.; Friedman, C.R. Adaptive phenotypic plasticity of Pseudoroegneria spicata: Response of stomatal density, leaf area and biomass to changes in water supply and increased temperature. Ann. Bot. 2009, 103, 769–775. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef]
- Sun, Y.; Yan, F.; Cui, X.; Liu, F. Plasticity in stomatal size and density of potato leaves under different irrigation and phosphorus regimes. J. Plant Physiol. 2014, 171, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Gao, X.; Li, C.; He, H.; Siddique, K.H.M.; Zhao, X. Elevated CO2 concentration regulate the stomatal traits of oilseed rape to alleviate the impact of water deficit on physiological properties. Environ. Exp. Bot. 2023, 211, 105355. [Google Scholar] [CrossRef]
- Stiller, V. Soil salinity and drought alter wood density and vulnerability to xylem cavitation of baldcypress (Taxodium distichum (L.) Rich.) seedlings. Environ. Exp. Bot. 2009, 67, 164–171. [Google Scholar] [CrossRef]
- Choat, B.; Brodribb, T.J.; Brodersen, C.R.; Duursma, R.A.; Lopez, R.; Medlyn, B.E. Triggers of tree mortality under drought. Nature 2018, 558, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Magnani, F. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ. 2005, 28, 834–849. [Google Scholar] [CrossRef]
- Manzi, O.J.L.; Bellifa, M.; Ziegler, C.; Mihle, L.; Levionnois, S.; Burban, B.; Leroy, C.; Coste, S.; Stahl, C. Drought stress recovery of hydraulic and photochemical processes in Neotropical tree saplings. Tree Physiol. 2022, 42, 114–129. [Google Scholar] [CrossRef]
- Gu, L.; Han, J.; Wood, J.D.; Chang, C.Y.Y.; Sun, Y. Sun-induced Chl fluorescence and its importance for biophysical modeling of photosynthesis based on light reactions. N. Phytol. 2019, 223, 1179–1191. [Google Scholar] [CrossRef]
- Abdelkader, A.F.; Aronsson, H.; Sundqvist, C. High salt stress in wheat leaves causes retardation of chlorophyll accumulation due to a limited rate of protochlorophyllide formation. Physiol. Plant. 2007, 130, 157–166. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, L.; Wang, L.; Guo, S. Bottle gourd rootstock-grafting promotes photosynthesis by regulating the stomata and non-stomata performances in leaves of watermelon seedlings under NaCl stress. J. Plant Physiol. 2015, 186–187, 50–58. [Google Scholar] [CrossRef]
- Flexas, J.; Carriqui, M. Photosynthesis and photosynthetic efficiencies along the terrestrial plant’s phylogeny: Lessons for improving crop photosynthesis. Plant J. 2020, 101, 964–978. [Google Scholar] [CrossRef]
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Nonstructural Carbon in Woody Plants. Annu. Rev. Plant Biol. 2014, 65, 667–687. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.G. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol. 2011, 155, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.; Ahmed, M.A.; Cai, G.; Zarebanadkauki, M.; Carminati, A. Coupled effects of soil drying and salinity on soil-plant hydraulics. Plant Physiol. 2022, 190, 1228–1241. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic adjustment and energy limitations to plant growth in saline soil. N. Phytol. 2020, 225, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.P.; Kinet, J.M.; Bajji, M.; Lutts, S. NaCl alleviates polyethylene glycol-induced water stress in the halophyte species Atriplex halimus L. J. Exp. Bot. 2005, 56, 2421–2431. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Gilliham, M. Salinity tolerance of crops—What is the cost? N. Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, P.; Zhang, H.; Grossiord, C.; Pennington, S.C.; Norwood, M.J.; Li, W.; Pivovaroff, A.L.; Fernández-de-Uña, L.; Leff, R.; et al. Severe declines in hydraulic capacity and associated carbon starvation drive mortality in seawater exposed Sitka-spruce (Picea sitchensis) trees. Environ. Res. Commun. 2022, 4, 035005. [Google Scholar] [CrossRef]
- D’Andrea, E.; Rezaie, N.; Battistelli, A.; Gavrichkova, O.; Kuhlmann, I.; Matteucci, G.; Moscatello, S.; Proietti, S.; Scartazza, A.; Trumbore, S.; et al. Winter’s bite: Beech trees survive complete defoliation due to spring late-frost damage by mobilizing old C reserves. N. Phytol. 2019, 224, 625–631. [Google Scholar] [CrossRef]
- Wang, W.; McDowell, N.G.; Ward, N.D.; Indivero, J.; Gunn, C.; Bailey, V.L. Constrained tree growth and gas exchange of seawater-exposed forests in the Pacific Northwest, USA. J. Ecol. 2019, 107, 2541–2552. [Google Scholar] [CrossRef]
- McDowell, N.G.; Sevanto, S. The mechanisms of carbon starvation: How, when, or does it even occur at all? N. Phytol. 2010, 186, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Sevanto, S.; McDowell, N.G.; Dickman, L.T.; Pangle, R.; Pockman, W.T. How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant Cell Environ. 2014, 37, 153–161. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).