Abstract
Dry outer onion leaves resulting from the industrial processing of onions are currently discarded as agricultural waste, although some studies have demonstrated that onion peel has beneficial biological effects. Considering the traditional applications of onion peel, the present study evaluated comparatively the chemical and biological characteristics of four types of onion peel extracts, utilizing methanol, ethanol, acetone, or ethyl acetate as the extracting solvent. The extracts were analyzed for their chemical composition, antioxidant potential, and antimicrobial activity. The chemical analysis by UHPLC-DAD-ESI/MS identified 23 compounds in the extracts, belonging to phenolic acids and flavonoids. Quercetin was the main compound in all extracts, ranging in concentrations from 14.91 mg/g DW in the ethanol extract to 48.53 mg/g DW in the methanol extract. The highest contents of total phenols and flavonoids were found in the acetone extract, and the methanol extract demonstrated the highest antioxidant activity in DPPH and ABTS assays. The antimicrobial potential of the extracts was screened using a microdilution method against a spectrum of gastrointestinal microorganisms. The results revealed that all four extracts have significant antimicrobial potential against the tested microorganisms, of which the ethanol extract demonstrated the highest antimicrobial potency.
1. Introduction
Onion (Allium cepa L.) is the second most cultivated vegetable crop globally, with an annual world production of around 107 million tonnes (including shallots) in 2021 [1]. The industrial processing of onion generates a large amount of agricultural waste, mostly composed of dry peels, which is incinerated or disposed of in landfills, increasing environmental pressures. The increasing global volumes of plant-residue agricultural waste that result from the rise in global crop food production is of ongoing concern, due to the potential negative impacts on the environment [2]. This generated waste may adversely affect and hinder global environmental sustainability efforts [3]. It is, therefore, acknowledged today that efforts should be made towards turning the agricultural crop waste generated during agriculture production and processing into useful resources and products that can benefit mankind. Onion peel waste has been studied as a source of dietary fiber and bioactive compounds for the food industry [4], as a source of valuable biomolecules [5], as an organic fertilizer [6], and as a renewable energy source [7,8].
A potential use of crop waste involves ‘mining’ the residual biomass for plant-derived compounds. Plant tissues are well known to contain a rich and diverse array of biologically active compounds, and, thus, have the potential to be used as source raw materials for the extraction of valuable phytochemicals [9,10]. Various agricultural by-products have, indeed, been identified as potential sources for beneficial phytochemicals having a range of protective functions, including antioxidant and antimicrobial activities [11]. The bioactive products of agro-industrial residues are used today for various applications, including in cosmetics and functional foods [12,13,14].
Due to potential health risks considered to be associated with some synthetic antioxidants [15], there is increasing interest in utilizing natural plant-derived antioxidants extracted from crop tissues. In this context, onion peel waste is one of the most promising sources of natural antioxidants, mainly due to the large number of abundant biologically active compounds it contains, and the worldwide availability and low price of the raw material [16]. Numerous studies have identified flavonoids, flavanols, phenolics, anthocyanins, tannins, ferulic acid, and vanillic acid in onion peel extracts [17]. The most common compound with antioxidant properties extracted from onion peels is the flavonoid quercetin, which occurs in a free aglycon form, or in the form of mono- and diglycosides [18]. The chemical composition of onion peel is highly dependent on the onion variety and cultivar, with red peels generally having better antioxidant activity than yellow and white peels [19]. This is in accord with results for other plant species, which reported as well genotypic variability in the chemical profile of biologically active compounds in plant tissues [20,21,22,23]. The choice of extraction technique and solvents can also influence the concentrations of phytomolecules in the extracts [24]. Mechanical processing of the peel prior to the extraction can also significantly affect significantly the content of bioactive natural compounds in the obtained extracts [25].
Considering that onion peel bio-waste may have significant economic potential, several studies have examined its possible use as a source of pharmacological and antioxidant compounds [26,27]. It was reported that dried onion peel powder and ethanolic extract improved the overall antioxidant status of plasma in aged rats, while decreasing the concentration of thiobarbituric acid reactive substances (TBARS) in liver tissue and of 8-isoprostane in brain tissue [28]. However, supplementing the diet of young women in Korea with pills made of 60% aqueous ethanol onion peel extract did not affect the plasma antioxidative vitamins or erythrocyte antioxidative parameters significantly, but did improve the lipid profile in plasma, indicating the cardioprotective effect of onion peel [29]. Onion peel extracts also possess antimicrobial, antiobesity, antidiabetic, anticancer, and anti-inflammatory activities [18]. Furthermore, onion peel teas are known to be used in traditional medicine and home-made remedies in various regions of the world for the treatment of a range of medical conditions, including gastrointestinal disorders [30].
The present study, therefore, focused on the antimicrobial and antioxidant activity of onion peel extracts, with the goal to evaluate the potential of this bio-waste as a source of natural bioactive compounds. The hypothesis guiding the work-plan was that onion peel extracts have antioxidant activity and antimicrobial activity against gastrointestinal microorganisms, which are affected by the type of solvent used for extraction. To evaluate this hypothesis, dried onion peels were extracted with methanol, ethanol, acetone, or ethyl acetate, and the produced extracts were analyzed comparatively for their antioxidant potential, chemical composition, and microbiological activity against nine species of gastrointestinal microorganisms, including eight bacterial species and one fungal species. For the microbiological testing, two strains were tested per species, with one taken from laboratory collections (ATCC) and the other an isolate from human stool samples.
2. Materials and Methods
2.1. Plant Material
The experiments were conducted with peels of a brown-skinned onion (Allium cepa L.) of the variety Elenka F1 (Cora Seeds, Cesena, Italy), which is a long-day, spring–summer variety that matures in November.
2.2. Microbial Strains
For the antimicrobial activity testing, the efficacy of the extracts was evaluated against reference strains from the ATCC (American Type Culture Collection) collection. The reference strains included two Gram (+) bacteria: Staphylococcus aureus (ATCC 6538), Enterococcus faecalis (ATCC 19433); six Gram (−) bacteria: Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 9027), Proteus mirabilis (ATCC 12453), Klebsiella aerogenes (ATCC 13048), Salmonella enteritidis (ATCC 13076), Shigella sonnei (ATCC 25931), and yeast Candida albicans (ATCC 10231). The tested panel of strains also included isolates from human stools, which were obtained from the Institute of Public Health in Nis, for each of the species tested.
2.3. Onion Peel Extraction
The onion peels were extracted by four organic solvents that differ in polarity in the following order: methanol > ethanol > acetone > ethyl acetate. For the extraction, dry onion peel was pulverized in a blender, and pure solvents were poured over the pulverized plant material (with a 5 mL:1 g ratio of solvent:plant material). The flasks containing the extracted samples were closed and kept in the dark for seven days at room temperature, and the extraction solvents were then removed using a rotary evaporator (IKARV10, Staufen, Germany) and the obtained crude extracts were kept at 4 °C until analyses.
2.4. Qualitative and Quantitative Analysis by UHPLC-DAD-ESI/MS
The liquid chromatography (UHPLC or ultrahigh performance liquid chromatography) analyses were conducted with a Dionex Ultimate 3000 UHPLC+ system equipped with a diode array (DAD) detector and an LCQ Fleet ion trap mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). The chromatography was performed with a Hypersil Gold C18 column (50 × 2.1 mm, 1.9 μm) from the same producer, at 25 °C. The mobile phase consisted of (A) 0.1% formic acid in water and (B) 0.1% formic acid in methanol. Methanol and water (LC–MS purity) were purchased from Fisher Scientific Co. (Waltham, MA, USA). Formic acid of HPLC purity was purchased from Carlo Erba (Val-de-Reuil, France). The following linear gradient program, at a flow rate of 0.250 mL/min, was applied: during the first two minutes, from 10% to 30% (B), then for 4–7 min, from 35% to 40% (B), and for 8–11 min, from 60% to 90% (B), followed by an isocratic run at 90% (B) for 11–15 min, and finally, for 15–15.01 min from 90 to10% (B), followed by an isocratic run with 10% (B) until the 20th min. For qualitative analysis, the injection volume for the extract solutions was 0.5 μL, with a 10.0 mg/mL sample concentration, and for the quantitative analysis, all samples were 4 μL with a 0.2 mg/mL sample concentration. All experiments were performed in triplicates.
The absorption UV-Vis spectra were logged on a DAD-detector (with a total spectral range at 190 and 700 nm), set at four detection wavelengths, λdet., of 280 nm, 300 nm, 330 nm, and 350 nm, simultaneously (Table S1 in the Supplementary Materials). The MS analysis was performed using an LCQ 3D-ion trap mass spectrometer with electrospray ionization (ESI) in the negative ion mode. The ESI–source parameters for the negative mode were set as follows: source voltage, −4.5 kV, capillary voltage, −41 V, tube lens voltage, −95 V, capillary temperature 350 °C, and sheath and auxiliary gas (N2) flow, 32 and 8 (arbitrary units), respectively. The compounds were identified by experiments in full scan mode (MS: full range acquisition of m/z, 100–900), and tandem mass experiments of precursor ion scans (for a fragmentation study, MS/MS, with a data-dependent scan). Detected compounds with similar retention times were discriminated by additional extraction of single molecular ions from the full MS spectra in the chromatograms (Figures S4 and S5 in the Supplementary Materials). The collision-induced dissociation (CID) was set at 30 eV. Instrument control, data acquisition, and data analysis were conducted with the Xcalibur software (version 2.1, Thermo Fisher Scientific, Waltham, MA, USA). Origin 7.5 software (OriginLab Corporation, Northampton, MA, USA) was used for data processing. The assignation of the detected compounds was based on their retention times and elution order, the UV–Vis from the DAD-detector, and MS spectra with the corresponding molecular ion peaks ([M-H]−), as well as the characteristic ion fragmentation of selected peaks (MS/MS) from UHPLC chromatograms. The identification of detected compounds was also achieved by using reference standards for kaempferol, quercetin, quercetin-3-O-glucoside, isorhamnetin, taxifolin, and caffeic acid (Sigma, Burlington, MA, USA). The corresponding data were compared with the available literature. Standard stock solutions were prepared by dissolving 1–2 mg of each reference standard in methanol.
The quantification of the detected compounds was conducted using the external standard at a 350 nm detection wavelength, which is specific for two main detected compounds in the extracts, e.g., quercetin and quercetin hexoside. Standard sample solutions of quercetin and quercetin-3-O-glucoside were prepared before analysis by diluting the stock solution with methanol to the desired concentrations. For quantitative analysis, linearity, LOD (limit of detection), and LOQ (limit of quantification) were taken as the main method parameters and were evaluated following the work of Zvezdanović et al. (2021) [31] (Table S2 in the Supplementary Materials). Quercetin concentrations were calculated against a reference standard, while for quercetin-hexoside (as the second main compound in the extracts and the most probable quercetin-4′-O-glucoside), the equivalent quercetin-3-O-glucoside standard was used. Crude extract samples were dissolved in methanol to a concentration of 0.2 mg/mL. The results are expressed as mg/g DW of the extract.
2.5. Total Phenolic and Flavonoid Content
The total phenolic content (TFC) of the extracts was determined by the Folin–Ciocalteu method, with some modifications [32]. Aliquots of the extract solutions (300 µL and 1500 µL) were added to Folin–Ciocalteau reagent (1:10 ratio), then 1200 µL of sodium carbonate (7.5%) was added to each sample after 6 min in the dark. The mixtures were incubated at room temperature in the dark (2 h), and absorbance was measured at 740 nm. TPC was expressed as gallic acid equivalents per g of dry weight of the extract (GA/g DW), calculated from a gallic acid (GA) calibration curve (10–100 mg/L). All measurements were performed in triplicates.
Total flavonoid content (TFC) was measured following the method used by Woisky and Salatino [33]. Samples were prepared by mixing 600 µL of the extract solution with 2580 µL of a reaction mixture (80% C2H5OH, 10% Al(NO3)3 × 9 H2O, and 1 M C2H3KO2). The samples were then incubated at room temperature for 40 min and the absorbance was measured at 415 nm. The quercetin (Qu) calibration curve (10–100 mg/L) was used for the calculation of total flavonoid concentration, which was expressed as quercetin equivalents per g of dry weight of extract (Qu/g DW). All measurements were performed in triplicate.
2.6. DPPH Scavenging Activity
The antioxidant activity of the onion peel extracts and two selected standard compounds (vitamin C and butylated hydroxyanisole, BHA) (Sigma, Burlington, MA, USA) was evaluated by the 2,2-diphenyl-1-picrylhydrazil (DPPH) radical scavenging method by a spectrophotometric assay, which uses the stable DPPH radical as a reagent [34]. For the analysis, 300 μL of the analyzed extracts, prepared by dissolving various volumes of the crude extract in methanol at various concentrations ranging from 0.01 to 0.15 mg/mL, were added to 2700 μL of DPPH methanol solution (0.04 mg/mL). The reaction mixture was allowed to stand in the dark at room temperature for 30 min, and the absorbance of the remaining DPPH radical was determined at 517 nm. All measurements were performed in triplicates for the samples, Vitamin C, and BHA, against methanol as a blank. The DPPH-radical scavenging activity was calculated by the equation:
where A1 represents the absorbance of the samples, and A0 represents the absorbance of the initial DPPH solution. The sample concentration that caused a 50% decrease in the initial DPPH concentration was defined as the IC50 value.
Scavenging activity (%) = (A0 − A1) × 100/A0
2.7. ABTS Radical Scavenging Activity
The ABTS ((2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) radical-scavenging activity was performed following the modified method of Miller and Rice-Evans [35]. The ABTS+ (ABTS radical cation) solution was prepared by mixing 19.2 mg of ABTS with 5 mL of potassium persulfate (2.46 mM). Following storage at room temperature for 12–16 h in the dark, 1 mL of the ABTS+ solution was diluted in 100–110 mL of water to obtain an absorbance of 0.7 ± 0.02 at 734 nm. The reactive medium was made by mixing 75 μL of the extract in methanolic solution (the extract concentration was 0.1 mg/mL) or BHA solution, and 3 mL of diluted ABTS·+ solution, then incubating at 30 °C for 30 min. The absorbance was measured at 734 nm, with water as a blank. The ABTS radical scavenging activity of the extracts was calculated from a Vitamin C (Sigma, Burlington, MA, USA) calibration curve (0–2 mg/L), expressed as Vitamin C (VitC)/g of DW of the extract. The measurements were performed in triplicates.
2.8. Antimicrobial Assay
The minimum inhibitory concentration (MIC) and minimum microbicidal concentration (MMC) of the extracts were determined using the micro/well dilution method [36]. An antimicrobial assay was performed against the bacterial reference strains Staphylococcus aureus (ATCC 6538), Enterococcus faecalis (ATCC 19433), Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 9027), Proteus mirabilis (ATCC 12453), Klebsiella aerogenes (ATCC 13048), Salmonella enteritidis (ATCC 13076), Shigella sonnei (ATCC 25931), fungal strain Candida albicans (ATCC 10231), and human stool isolates of the same species.
Mueller–Hinton broth (Torlak, Belgrade, Serbia) for the bacteria and Sabouraud dextrose broth (Torlak, Belgrade, Serbia) for the yeast were inoculated with microbial cell suspensions (0.5 McFarland standard turbidity), prepared from overnight cultures of the tested strains. Inoculated broths (100 µL) were transferred to 96-well microtiter plates and the extracts, which had been dissolved in 10% aqueous dimethyl sulfoxide (DMSO), were added to the first well. Serial dilutions of the extracts were prepared in microtiter plates at concentrations ranging from 0.02 to 100.00 mg/mL. The prepared microliter plates were incubated at 37 °C for 24 h. After incubation, 20 μL of 0.5% aqueous triphenyl tetrazolium chloride (TTC) solution was added to each well of the microtiter plate to determine microbial growth. Ciprofloxacin (Sigma Aldrich, St Louis, MO, USA), at concentrations ranging from 0.01 to 100 µg/mL, was used as a positive control for antibacterial activity, while nystatin (Sigma Aldrich, St Louis, MO, USA) was used as a positive control for antifungal activity. All tests were performed in triplicate. The MIC was defined as the lowest concentration of the extracts that inhibited microbial growth. MBC was determined by streaking the broth cultures from each well without visible growth onto Mueller–Hinton agar (for bacteria) and Sabouraud dextrose agar (for yeasts) and incubating the plates at 37 °C for 24 h. The MMC was defined as the lowest concentration of those extracts that killed 99.9% of the inoculated microorganisms.
2.9. Statistical Analysis
All measurements were conducted with three replicates, and the results are presented as averages ± standard deviation (SD). The IC50 values obtained in the antioxidant assays were determined by a regression equation for the concentration of samples and the scavenging activity using OriginPro 8.0 (OriginLab, Northampton, MA, USA). The results were analyzed using one-way analysis of variance (ANOVA), followed by Tukey’s HSD test (p ≤ 0.05) using the Minitab®17 software (Minitab, LLC, State College, PA, USA)
3. Results
3.1. Chemical Composition of the Extracts
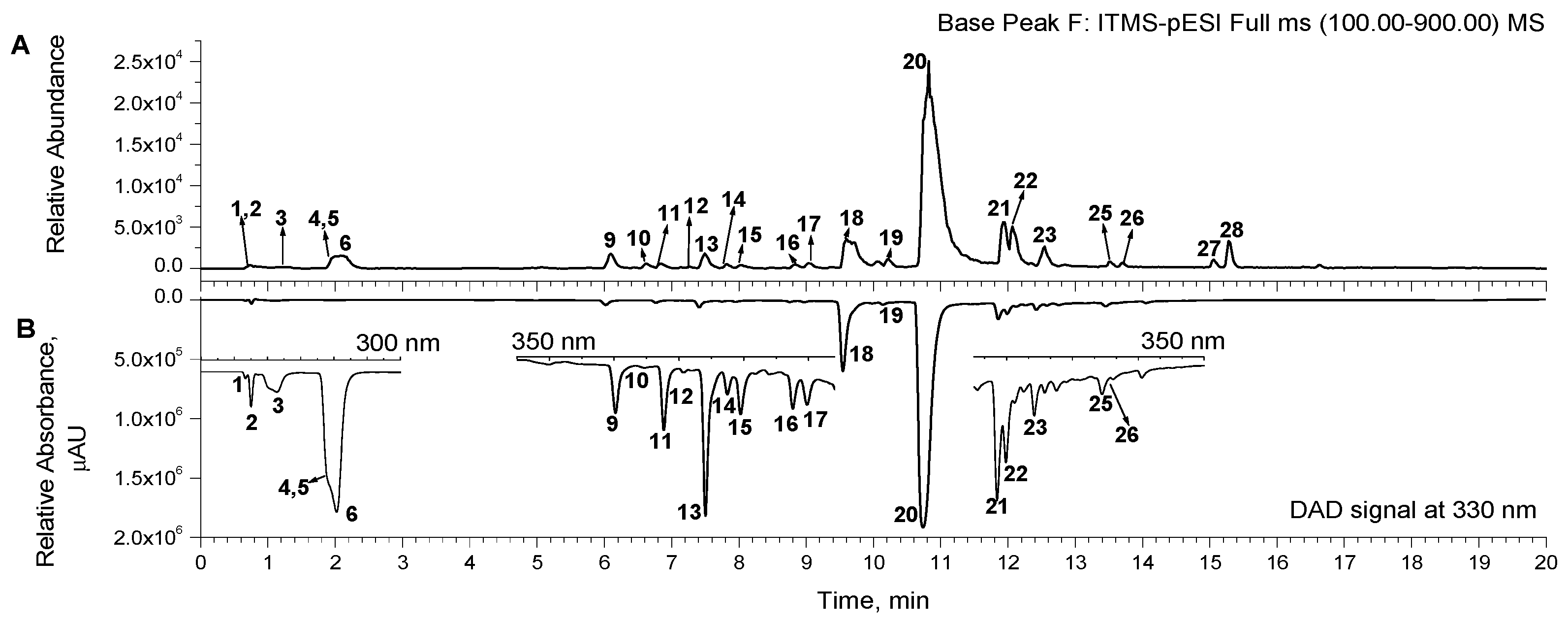
The extracts produced from the onion peel were analyzed by UHPLC-DAD-ESI/MS analysis for the identification of major components that may participate in the antioxidant and antimicrobial activity. The list of phenolic compounds detected in the extracts is presented in Table 1. The chromatogram for the methanol extract is presented in Figure 1. The corresponding chromatograms of the extracts, obtained with DAD and MS detectors, were similar in terms of the number and area of peaks (Figures S1–S3 in the Supplementary Materials).

Table 1.
Compounds detected in methanol (MeOH), ethanol (EtOH), acetone (Ac), and ethyl acetate (EA) extracts, by UHPLC-DAD-MS/MS analysis.
Figure 1.
Representative UHPLC chromatogram of methanol extract, recorded from MS signal and ranged by base peak (A) and DAD-signal at 330 nm (B). Additionally, for better visibility of the peaks, three insets show enlarged parts of the chromatogram at 300 nm (0–3 min), 350 nm (4.5–9.4 min), and 350 nm (11.5–15 min) (B). The numbers marking the peaks are in accord with the numbering of the corresponding detected compounds in Table 1.
Among the twenty-three compounds identified, phenolic acids and their glycosides (compounds 2–6, 8, and 10), and flavonoids (compounds 7, 9, 11–22, and 24) were the two major classes of compounds detected in the extracts (Table 1). Compound 1 was determined to be quinic acid, based on its MS/MS spectrum and available literature [37]. Compounds 2–5 were assigned as protocatechuic acid hexosides (Table 1). Since protocatechuic acid has two available –OH groups (in positions C-3 and C-4), the possible molecules may be protocatechuic acid 3- or 4-glucoside or galactoside. Compound 6 was assigned to protocatechuic acid [38] and two other phenolic acids (8 and 10) were assigned as caffeic acid and vanillic acid by using a reference standard and the available literature data, respectively (Table 1).
The flavonoids detected in the extracts are represented by flavonols (11, 13–22, 24), flavanones (9, 12), and flavan-3-ols (7). The UV-Vis spectra of flavonols with two characteristic bands in the ranges of 240–280 nm and 300–380 nm demonstrate their basic flavonoid structure (Table 1). Three aglycones were identified in the extracts using reference standards: quercetin, kaempferol, and isorhamnetin, the major molecular ions of which were found in the mass spectra at m/z 301, 285, and 315, respectively. The other flavonols are their mono- and diglycosides, mainly in the form of hexosides (glucosides or galactosides). Their MS/MS spectra substantiate the proposed structures, based on a characteristic fragmentation pattern involving the loss of the sugar unit(s) to the corresponding aglycone ion peak (Table 1). In the case of the discovered diglycosides (13 and 14), the loss of 2 × 162 units from the molecular ion peaks indicates that they are hexose units of quercetin and isorhamnetin diexoside, respectively. According to the elution order in the reversed-phase HPLC chromatography systems used for the common onion extracts [39,40,41], compounds 13 and 14 could be tentatively assigned as quercetin-4′,7 or quercetin-3,4′-diglucoside and isorhamnetin-3,4′-diglucoside, respectively.
Monoglycosides (hexosides) were detected in peaks 15, 16, and 18 for quercetin and in peak 19 for isorhamnetin. The quercetin hexosides (15 and 18) were tentatively assigned as quercetin-7-O-glucoside and quercetin-4′-O-glucoside, using the reference standard of quercetin-3-O-glucoside (16) and according to the elution order in the reversed-phase HPLC chromatography systems registered in a number of previous papers [39,42,43,44]. The corresponding MS/MS spectra were also consistent with the available literature [39,40]. According to the MS/MS spectrum, compound 24 was assigned as rhamnocitrin, 7-methyl ether, a derivative of kaempferol [41].
Two flavanones (9, 12) were identified as dihydroisorhamnetin and taxifolin (dihydroquercetin), based on their UV-Vis spectra (maximum at 296 and 292, with a shoulder at 330 nm) and the corresponding mass spectra (Table 1). In addition, the identification of taxifolin was confirmed using the reference standard, and compound 7 was determined as (+) catechol using literature data and the elution position in the chromatogram [31].
The chromatograms of the extracts (Figure 1) show that quercetin (peak 20) and quercetin hexoside (peak 18) have the largest peak areas, indicating that these two compounds are present at the highest concentrations in all extracts. Given that the absorption UV-Vis and mass spectra of all quercetin hexosides were basically similar, and that only component 18, tentatively identified as quercetin-4′-O-glucoside, was present in a higher concentration in the extracts, the corresponding quantitative analysis of quercetin-4′-O-glucoside was performed using a quercetin-3-O-glucoside equivalent.
Based on the quantitative analysis of these compounds in the studied extracts (Table 2), the methanol extract contained the highest amounts of quercetin and quercetin hexoside (48.54 ± 7.96 mg/gDW and 6.23 ± 1.43 mg/g DW, respectively). A slightly lower concentration of quercetin (38.48 ± 4.68 mg/g DW) was observed in the acetone extract, but it did not statistically differ from that of the methanol extract. The concentration of quercitin in the ethyl acetate extract was 29.01 ± 0.71 mg/g DW, with no statistical difference between the acetone and ethyl acetate extracts (Table 2). The concentration of quercetin hexoside was not significantly different between the ethanol, acetone, and ethyl acetate extracts.

Table 2.
Concentrations of quercetin and quercetin-hexoside in methanol, ethanol, acetone, and ethyl acetate extracts, as determined by UHPLC-DAD-MS/MS analysis.
3.2. Antioxidant Activity
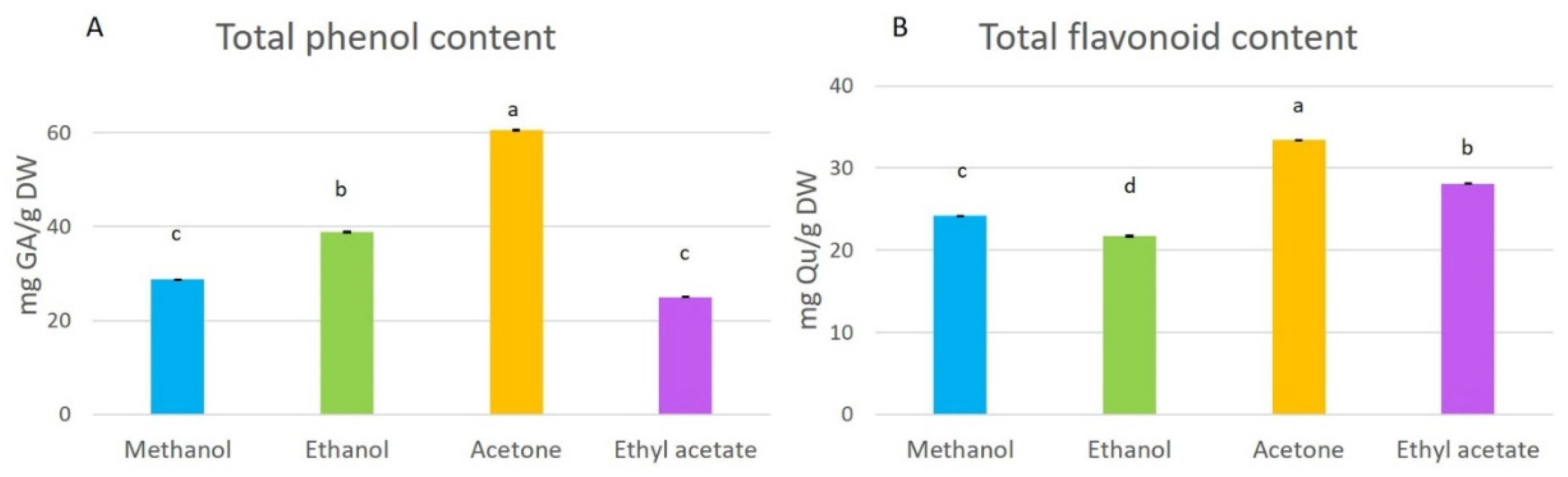
Total phenol and flavonoid contents in the extracts were affected significantly (p < 0.05) by the solvent used for the extraction (Figure 2). Total phenol content (TPC) ranged from 25.02 to 60.58 mg GA/g DW. The acetone extract had the highest content of phenolic compounds, followed by the ethanol and methanol extracts, and the ethyl acetate extract contained the lowest content of total phenols. The highest total flavonoid level was also identified in the acetone extract (Figure 2), and lower levels were detected in the ethyl acetate, methanol, and ethanol extracts. The order of total flavonoid levels in the extracts was acetone > ethyl acetate > methanol > ethanol extracts.
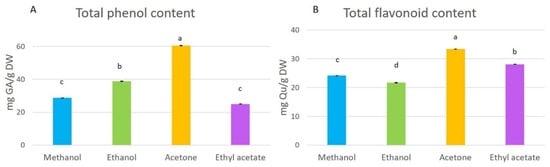
Figure 2.
Total phenol (A) and flavonoid (B) contents in methanol, ethanol, acetone, and ethyl-acetate extracts. Presented results are means ± SD. Different letters above the means represent significant difference by Tukey HSD post hoc test (p < 0.05).
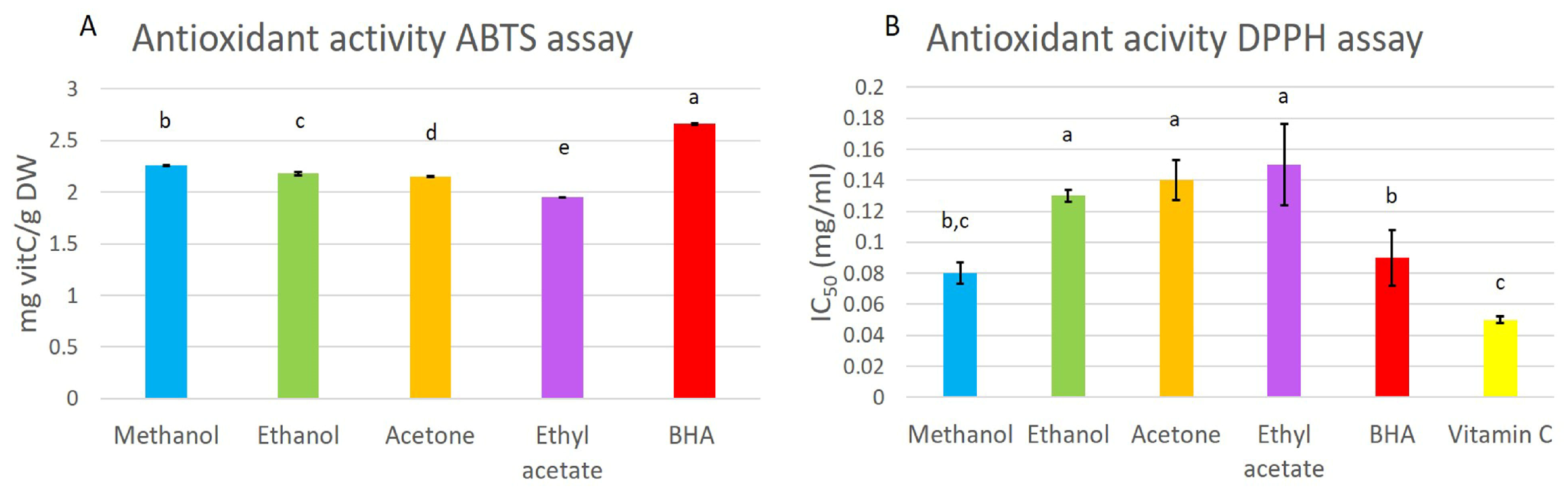
The antioxidant activity of the extracts was determined by color reduction of the DPPH and ABTS solutions used for testing the radical scavenging potential. The methanol extract exhibited similar DPPH activity to the positive control, BHA, indicating a high applicable potential (Figure 3). The lowest value was found for the positive control, vitamin C (0.05 mg/mL), followed by the methanol extract and the positive control, BHA (0.08 mg/mL and 0.09 mg/mL, respectively). The ethanol, acetone, and ethyl acetate extracts had a higher DPPH activity, with no statistical difference between extracts.
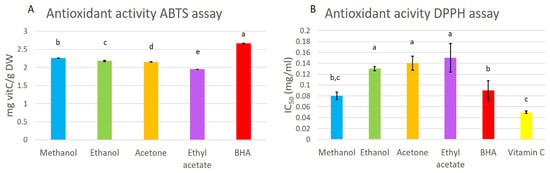
Figure 3.
Antioxidant activity, measured by the ABTS (A) and DPPH (B) methods in methanol, ethanol, acetone, and ethyl-acetate extracts. The antioxidant activity was compared to that of butylated hydroxyanisole (BHA) and vitamin C as standards. Presented results are means ± SD. Different letters above the means represent significant difference following Tukey HSD post hoc test (p < 0.05).
The obtained values from the ABTS antioxidant activity assay ranged from 1.95 to 2.26 mg vitC/g DW (Figure 3). The highest activity was noted for the methanol extract and the lowest for the ethyl-acetate extract.
3.3. Antimicrobial Activity
In order to explore whether traditional use of dry onion peel might contribute to the treatment of gastrointestinal disorders caused by microorganisms, the extracts were tested against a spectrum of microorganisms of the commensal microflora of the gastrointestinal tract, but whose overgrowth may cause disorders. The tested panel included a total of 18 strains, corresponding to 9 species (8 bacterial species, and 1 fungal species). Two strains were tested per species, one from a laboratory collection (ATCC) and a corresponding strain taken from human stool samples.
All the onion peel extracts tested demonstrated inhibition of the tested strains, at the concentration range 0.78–50.00 mg/mL (Table 3). The ethanol extract exhibited the highest antimicrobial potential, followed by the methanolic and ethyl acetate extracts, while the acetone extract showed the lowest efficiency against the tested microbes (Table 3).

Table 3.
Antimicrobial activity (MIC/MMC in mg/mL) of four types of onion peel extracts (methanol, ethanol, acetone, and ethyl-acetate extracts), and reference antimicrobials (Ciprofloxacin, Nystatin) (MIC/MMC in µg/mL) against 9 ATCC and 9 isolated microbial strains.
The ethanol extract was active at the concentrations 0.78 to 12.50 mg/mL. The most susceptible strain to this extract was Proteus mirabilis, while Escherichia coli showed the highest resistance. The methanol extract was the second most active extract, with inhibitory action ranging from 1.56 to 25.00 mg/mL. The activity of this extract was similar to that of the ethanol extract, exhibiting the highest inhibition of isolates from stool samples (Proteus mirabilis, Pseudomonas aeruginosa, and Klebsiella aerogenes) (Table 3). The most resistant strains were Shigella sonnei and Escherichia coli. The acetone extract showed an inhibitory effect in the range of 1.56–25.00 mg/mL, but some strains (Klebsiella aerogenes, Salmonella enteritidis, Shigella sonnei, and Enterococcus faecalis) showed resistance to the tested range of concentrations. This extract was the most effective against Staphylococcus aureus (ATCC strain), inhibiting its growth at 1.56 mg/mL. The ethyl acetate extract showed antimicrobial activity in the range of 1.56–50.00 mg/mL, with the highest potential against the yeast strains Candida albicans and Proteus mirabilis. This extract was the only one that showed different activity in relation to cell-wall structure: the yeasts were the most susceptible (MIC = 1.56 mg/mL), followed by Gram-positive strains (MIC = 3.13 mg/mL), while Gram-negative strains generally showed higher resistance (MIC = 1.56–50.00 mg/mL) (Table 3). Considering the origin of the strains, in most cases, the isolates from the stools showed higher susceptibility to the extracts in comparison to the ATCC strains. In the tested panel of strains, Proteus mirabilis showed the highest sensitivity, while Escherichia coli was the most resistant strain.
4. Discussion
Onion peel waste is a valuable source of various compounds with different biological activities that can potentially be used in the pharmaceutical and food industries. The amount of phytochemicals in the waste is affected by various factors, the most influential of which is the onion variety from which the waste is obtained [45]. In this study, we focused on chemical and biological characteristics of waste from the onion variety Elenika F1, which is characterized by a unique dark bronze color of the outer skins, which are present in large numbers around the bulb. Four different types of solvents (methanol, ethanol, acetone, and ethyl acetate) were used to extract compounds from the onion waste, in order to determine which of these is the most suitable for obtaining extracts with high antioxidant and antimicrobial activities.
The outer layers of the onion are known to be rich in phenolic compounds, including polyphenolic flavonoids, which are more abundant in the outer layer than in the inner and middle layers of the bulb [27]. In the studied onion peel extracts from the Elenika variety, phenolics were detected in the range of 25.02 to 60.58 mg GA/g DW, and total flavonoid content was in the range of 21.71 to 33.41 mg Qu/g DW. Similar levels of total phenolics and flavonoids in onion peel extracts were reported for other onion varieties [4,46]. Acetone extraction resulted in the highest levels of total phenols and flavonoids, which is in agreement with the results of Skerget et al. (2009), that identified higher levels of total phenols in extracts obtained with pure acetone, compared to ethanol extracts [47]. However, Kim et al. (2013) found that methanol was better than ethanol and acetone for the extraction of total phenols and individual flavonoids from a yellow onion variety [48]. These differences can be explained by the different chemical compositions of the various onion peels, which consist of a complex mixture of compounds that are selectively soluble in solvents of different polarities. Thus, while ethanol is a preferable solvent for the extraction of polyphenols, methanol is a good solvent for the extraction of low-molecular-weight polyphenols, and the higher-molecular-weight flavonols are best extracted with acetone [49]. Ethyl-acetate was identified as a good solvent for the extraction of polyphenols of intermediate polarity [50]. Accordingly, total phenol content was found to be higher in the more polar fractions (methanol and ethanol) of a methanol extract from onion peels than in the fractions with less polar solvents (ethyl acetate, chloroform, and n-hexane) [51]. This is consistent with our results, as the use of ethyl acetate for extraction yielded the lowest total phenol levels. In contrast, when comparing the extraction efficiency of a series of solvents (water, methanol, ethyl acetate, and n-butanol ethanol), Khalili et al. (2022) reported that ethyl acetate was the best solvent for the extraction of total phenols and flavonoids [52]. In our study, only the content of total flavonoids was higher in the ethyl acetate extract compared to the methanol and ethanol extracts (Figure 2).
UHPLC-DAD-ESI/MS analysis of the extracts revealed that the aglycon of flavonoid quercetin and quercetin hexoside were the predominant constituents of all extracts, while quercetin diglycosides were less abundant (Table 2). Onion peels of other cultivars were also found to contain quercetin and its glycosides as the main phytochemical constituents, in free, bound, or esterified forms [19,24]. Onion peel contains a high concentration of quercetin, probably due to a sun-induced deglucosidation of its glucosides [53,54]. Among the flavonoids, isorhamnetin, its mono- and diglycosides, and kaempferol were also found in all extracts, as well as protocatechuic acid and its hexosides, as the main phenolic acids. These compounds were previously detected in onion peel but at significantly lower concentrations compared to quercetin and its glycosides [27,55,56]. Protocatechuic acid is otherwise regarded as a degradation product of quercetin, which is formed during the decomposition of quercetin through the action of peroxidase [57]. The extracts contained smaller amounts of quinic acid, caffeic acid, vanillic acid, and taxifolin, which were all previously detected in onion peels [58,59,60].
The antioxidant activity of the extracts was evaluated using two in vitro antioxidant tests, DPPH and ABTS, which are based on a single electron and/or hydrogen atom transfer (SET/HAT) mechanism [61]. The methanolic extract exhibited the best antioxidant activity in both tests, followed by the ethanol, acetone, and ethyl acetate extracts in the ABTS assay, while in the DPPH assay, the IC50 values of the other extracts did not differ statistically (Figure 3). Quercetin, which has been shown to have better antioxidant power than its mono- and di-glycosides due to the presence of free OH groups on the ring, is the main carrier of the antioxidant potential of onion peel [4,62]. Scavenging of free radicals, chelation of metal ions, and inhibition of oxidase enzymes are considered the main mechanisms of the antioxidant activity of quercetin [63]. The highest concentration of quercetin and quercetin hexoside was detected in the tested methanolic extract. However, the ethanol extract that had the lowest detected concentration of quercetin had a higher antioxidant capacity in the ABTS test compared to the acetone and ethyl acetate extracts. This indicates that other compounds can also contribute to the antioxidant capacity of the extract. Ramos et al. (2006) reported that the oxidative products of quercetin in yellow onion peel acted as better antioxidants than quercetin [64]. It can be noted that the antioxidant activity levels obtained for the extracts in this study by both antioxidant assays are not correlated with the total contents of phenols and flavonoids. Antioxidant activities of onion peel extracts were shown before to highly depend on total phenol and flavonoid contents [65], although there are exceptions which indicate that the antioxidant property of each individual compound, rather than total phenol and flavonoid contents, is the key factor determining the antioxidant potential of extracts [46,48]. Fredotović et al. demonstrated that yellow onion peel extract inhibited DPPH radicals at a higher level than red onion extract, which had higher concentrations of quercetin 4-monoglucoside, quercetin aglycone, quercetin 3,4-diglucoside, kaempferol, and isorhamnetin [66]. Quercetin, vanillic acid, kaempferol, and p-hydroxybezoic acid were identified in onion skins as compounds that most affect the antioxidant activity of onion peel [46].
Onion peel displays antimicrobial properties, which have been reported in several studies, although it is still not completely clear which component from the peel contributes the most to the antimicrobial activity [67,68]. Also, it has been reported that Gram-positive bacteria (Bacillus cereus, Staphylococcus aureus, Microcroccus luteus, and Listeria monocytogenes) were more sensitive to the action of the antimicrobial compounds found in onion peel, compared to Gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa) [52,69,70]. In our study, such a clear correlation was observed only for the ethyl acetate extract (Table 3). The ethanol extract had the best antimicrobial activity, with MIC values in the range of 0.78 to 12.50 mg/mL. MIC values of extracts obtained by sonication-assisted extraction of various cultivars of onion peel (dark red, red, pink, and white) were slightly lower compared to the values we identified for Staphylococcus aureus, Salmonella typhimurium, and Pseudomonas aeruginosa [65]. The yellow onion peel had a better antimicrobial activity than the red onion peel, with a very low MIC value for Staphylococcus aureus (7.8 and 62.5 μg/mL), and slightly higher MIC values of 500 μg/mL and 2000 μg/mL for Enterococcus faecalis and Escerichia coli, respectively [66]. In our study, the strains of Escherichia coli were the most resistant to the action of the extracts, which is in agreement with previous findings [71]. The difference in MIC values of the various extracts can be explained by the different chemical composition of onion peels from various cultivars, the applied extraction methods, and the analytical method used for determining the antibacterial activity. To the best of our knowledge, antimicrobial activity against Proteus mirabilis, Klebsiella aerogenes, Salmonella enteritidis, and Shigella sonnei has not been determined so far. Candida albicans was sensitive to the action of all extracts, with MIC values ranging from 1.56 to 12.05 mg/mL. Similar MIC values were obtained in a study by Mounir et al. (2023), which showed that yellow onion peel extract can limit skin infection with Candida albicans in vivo. [60].
5. Conclusions
The results indicate that dry onion peel can be utilized as a good raw material for the extraction of compounds with important biological effects. The type of solvent used for the extraction had a significant impact on the biological activity of the extract. The methanol extract, which had the highest concentration of quercetin, demonstrated the highest antioxidant activity, while the ethanol extract exhibited the highest antimicrobial potential against a spectrum of microorganisms from the gastrointestinal tract. Additionally, the present manuscript is first to present results of the antimicrobial activity of onion peel extracts against a panel of enteropathogenic microorganisms, including ATCC strains and isolates from human stool. The results of the antimicrobial activity of onion peel extracts support the potential of onion peel as a source of compounds that can be used to reduce the level of gastrointestinal problems caused by the tested microorganisms.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy14030453/s1. Figures S1–S3: Representative UHPLC chromatograms of ethanol, acetone and ethyl acetate extracts, recorded from MS-signal ranged by base peak, and DAD-signal at 330 nm. Figures S4 and S5: UHPLC-MS chromatograms with extracted molecular ion peaks for compounds with similar retention times, No. 12–15. Table S1: Supportive data for the UHPLC-DAD-ESI/MS analyses. Table S2: UHPLC—Quantification method of quercetin and quercetin-hexoside.
Author Contributions
Conceptualization, N.J., N.B. and T.M.-K.; methodology, J.Z., N.B., J.M. and T.M.-K.; writing—N.J. and N.B.; formal analysis, Z.S.-R. and N.S.; data curation, J.Z., N.B., J.M., T.M.-K. and N.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Education, Science, and Technological Development of the Republic of Serbia (Grant Nos. 451-03-47/2023-01/200124; 451-03-47/2023-01/200133 and 451-03-47/2023-01/200113), and by funds from the Volcnai Center, Ministry of Agriculture, Israel.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAOSTAT, Food and Agriculture Organization of the United Nations. Statistics Division. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 1 November 2023).
- Maji, S.; Dwivedi, D.H.; Singh, N.; Kishor, S.; Gond, M. Agricultural Waste: Its Impact on Environment and Management Approaches. In Emerging Eco-friendly Green Technologies for Wastewater Treatment, Microorganisms for Sustainability; Springer: Berlin/Heidelberg, Germany, 2020; Volume 18, pp. 329–351. [Google Scholar]
- Ugwuoke, C.U.; Monwuba, N.; Onu, F.M.; Shimave, A.G.; Okonkwo, E.N.; Opurum, C.C. Impact of Agricultural Waste on Sustainable Environment and Health of Rural Women. Civ. Environ. Res. 2018, 10, 9. [Google Scholar]
- Benítez, V.; Mollá, E.; Martín-Cabrejas, M.A.; Aguilera, Y.; López-Andréu, F.J.; Cools, K.; Terry, L.A.; Esteban, R.M. Characterization of industrial onion wastes (Allium cepa L.): Dietary fibre and bioactive compounds. Plant Foods Hum. Nutr. 2011, 66, 48–57. [Google Scholar] [CrossRef]
- Sagar, N.A.; Kumar, Y.; Singh, R.; Nickhil, C.; Kumar, D.; Sharma, P.; Pandey, H.O.; Bhoj, S.; Tarafdar, A. Onion waste based-biorefinery for sustainable generation of value-added products. Bioresour. Technol. 2022, 362, 127870. [Google Scholar] [CrossRef]
- Mallek, S.B.; Prather, T.S.; Stapleton, J.J. Interaction effects of Allium spp. residues, concentrations and soil temperature on seed germination of four weedy plant species. Appl. Soil Ecol. 2007, 37, 233–239. [Google Scholar] [CrossRef]
- Romano, R.T.; Zhang, R. Anaerobic digestion of onion residuals using a mesophilic Anaerobic Phased Solids Digester. Biomass Bioenergy 2011, 35, 4174–4179. [Google Scholar] [CrossRef]
- Segundo, R.F.; De La Cruz-Noriega, M.; Milly Otiniano, N.; Benites, S.M.; Esparza, M.; Nazario-Naveda, R. Use of Onion Waste as Fuel for the Generation of Bioelectricity. Molecules 2022, 27, 625. [Google Scholar] [CrossRef]
- Kumar, A.P.N.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.K.S. Major phytochemicals: Recent advances in health benefits and extraction method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef]
- Mihajilov-Krstev, T.; Jovanović, B.; Zlatković, B.; Matejić, J.; Vitorović, J.; Cvetković, V.; Ilić, B.; Đorđević, L.; Joković, N.; Miladinović, D.; et al. Phytochemistry, toxicology and therapeutic value of Petasites hybridus subsp. Ochroleucus (Common Butterbur) from the Balkans. Plants 2020, 9, 700. [Google Scholar] [CrossRef] [PubMed]
- Kasapidou, E.; Sossidou, E.; Mitlianga, P. Fruit and vegetable co-products as functional feed ingredients in farm animal nutrition for improved product quality. Agriculture 2015, 5, 1020–1034. [Google Scholar] [CrossRef]
- Rodrigues, F.; Nunes, M.A.; Alves, R.C.; Oliveira, M.B.P. Applications of recovered bioactive compounds in cosmetics and other products. In Handbook of Coffee Processing By-Products; Elsevier: Amsterdam, The Netherlands, 2017; pp. 195–220. [Google Scholar]
- Ngwasiri, P.N.; Ambindei, W.A.; Adanmengwi, V.A.; Ngwi, P.; Mah, A.T.; Ngangmou, N.T.; Fonmboh, D.J.; Ngwabie, N.M.; Ngassoum, M.B.; Aba, E.R. A Review Paper on Agro-food Waste and Food by-Product Valorization into Value Added Products for Application in the Food Industry: Opportunities and Challenges for Cameroon Bioeconomy. J. Biotechnol. Bioresour. Technol. 2022, 8, 32–61. [Google Scholar] [CrossRef]
- Oleszek, M.; Kowalska, I.; Bertuzzi, T.; Oleszek, W. Phytochemicals derived from agricultural residues and their valuable properties and applications. Molecules 2023, 28, 342. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed]
- Bains, A.; Sridhar, K.; Singh, B.N.; Kuhad, R.C.; Chawla, P.; Sharma, M. Valorization of onion peel waste: From trash to treasure. Chemosphere 2023, 343, 140178. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Barbhai, M.D.; Hasan, M.; Punia, S.; Dhumal, S.; Rais, N.; Chandran, D.; Pandiselvam, R.; Kothakota, A.; Tomar, M. Onion (Allium cepa L.) peels: A review on bioactive compounds and biomedical activities. Biomed. Pharmacother. 2022, 146, 112498. [Google Scholar] [CrossRef] [PubMed]
- Stoica, F.; Rațu, R.N.; Veleșcu, I.D.; Stănciuc, N.; Râpeanu, G. A comprehensive review on bioactive compounds, health benefits, and potential food applications of onion (Allium cepa L.) skin waste. Trends Food Sci. Technol. 2023, 141, 104173. [Google Scholar] [CrossRef]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Antioxidative phenolic constituents of skins of onion varieties and their activities. J. Funct. Foods 2013, 5, 1191–1203. [Google Scholar] [CrossRef]
- Gorelick, J.; Iraqi, R.H.; Bernstein, N. Ecdysteroid content and therapeutic activity in elicited spinach accessions. Plants 2020, 9, 727. [Google Scholar] [CrossRef]
- Shiponi, S.; Bernstein, N. Response of medical cannabis (Cannabis sativa L.) genotypes to P supply under long photoperiod: Functional phenotyping and the ionome. Ind. Crops Prod. 2021, 161, 113154. [Google Scholar] [CrossRef]
- Danziger, N.; Bernstein, N. Plant architecture manipulation increases cannabinoid standardization in ‘drug-type’ medical cannabis. Ind. Crops Prod. 2021, 167, 113528. [Google Scholar] [CrossRef]
- Saloner, A.; Bernstein, N. Effect of Potassium (K) supply on cannabinoids, terpenoids and plant function in medical cannabis. Agronomy 2022, 12, 1242. [Google Scholar] [CrossRef]
- Kumar, M.; Barbhai, M.D.; Hasan, M.; Dhumal, S.; Singh, S.; Pandiselvam, R.; Rais, N.; Natta, S.; Senapathy, M.; Sinha, N. Onion (Allium cepa L.) peel: A review on the extraction of bioactive compounds, its antioxidant potential, and its application as a functional food ingredient. J. Food Sci. 2022, 87, 4289–4311. [Google Scholar] [CrossRef]
- AbouZid, S.F.; Elsherbeiny, G.M. Increase in flavonoids content in red onion peel by mechanical shredding. J. Med. Plants Res. 2008, 82, 258–260. [Google Scholar]
- Ly, T.N.; Hazama, C.; Shimoyamada, M.; Ando, H.; Kato, K.; Yamauchi, R. Antioxidative compounds from the outer scales of onion. J. Agric. Food Chem. 2005, 53, 8183–8189. [Google Scholar] [CrossRef] [PubMed]
- Prakash, D.; Singh, B.N.; Upadhyay, G. Antioxidant and free radical scavenging activities of phenols from onion (Allium cepa). Food Chem. 2007, 102, 1389–1393. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Kim, M.K. Onion flesh and onion peel enhance antioxidant status in aged rats. J. Nutr. Sci. Vitaminol. 2007, 53, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cha, Y.J.; Lee, K.H.; Park, E. Effect of onion peel extract supplementation on the lipid profile and antioxidative status of healthy young women: A randomized, placebo-controlled, double-blind, crossover trial. Nutr. Res. Pract. 2013, 7, 373–379. [Google Scholar] [CrossRef]
- Ayanniyi, R.; Olumoh-Abdul, H.; Ojuade, F.; Akintola, J. Evaluation of Anti-Inflammatory and Gastroprotective Activity of Aqueous Peel Extract of Allium cepa. J. Res. Pharm. 2022, 26, 734–741. [Google Scholar] [CrossRef]
- Zvezdanović, J. UHPLC–DAD–ESI–MS/MS characterization of St. John’s wort infusions from Serbia origin. Chem. Pap. 2022, 76, 1329–1347. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Woisky, R.G.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.A. Factors influencing the antioxidant activity determined by the ABTS radical cation assay. Free Radic. Res. 1997, 26, 195–199. [Google Scholar] [CrossRef]
- M07-A9; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Approved Standard—Ninety Edition 2012. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; Volume 32.
- Masike, K.; Mhlongo, M.I.; Mudau, S.P.; Nobela, O.; Ncube, E.N.; Tugizimana, F.; George, M.J.; Madala, N.E. Highlighting mass spectrometric fragmentation differences and similarities between hydroxycinnamoyl-quinic acids and hydroxycinnamoyl-isocitric acids. Chem. Cent. J. 2017, 11, 29. [Google Scholar] [CrossRef]
- Zimmermann, B.F.; Walch, S.G.; Tinzoh, L.N.; Stühlinger, W.; Lachenmeier, D.W. Rapid UHPLC determination of polyphenols in aqueous infusions of Salvia officinalis L. (sage tea). J. Chromatogr. B 2011, 879, 2459–2464. [Google Scholar] [CrossRef]
- Grzelak-Błaszczyk, K.; Milala, J.; Kołodziejczyk, K.; Sójka, M.; Czarnecki, A.; Kosmala, M.; Klewicki, R.; Fotschki, B.; Jurgoński, A.; Juśkiewicz, J. Protocatechuic acid and quercetin glucosides in onions attenuate changes induced by high fat diet in rats. Food Funct. 2020, 11, 3585–3597. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Price, W.E.; Ashton, J.; Tapsell, L.C.; Johnson, S. Identification and characterization of phenolic compounds in hydromethanolic extracts of sorghum wholegrains by LC-ESI-MSn. Food Chem. 2016, 211, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Chernonosov, A.A.; Karpova, E.A.; Lyakh, E.M. Identification of phenolic compounds in Myricaria bracteata leaves by high-performance liquid chromatography with a diode array detector and liquid chromatography with tandem mass spectrometry. Rev. Bras. Farmacogn. 2017, 27, 576–579. [Google Scholar] [CrossRef]
- Kramer, C.M.; Prata, R.T.; Willits, M.G.; De Luca, V.; Steffens, J.C.; Graser, G. Cloning and regiospecificity studies of two flavonoid glucosyltransferases from Allium cepa. Phytochemistry 2003, 64, 1069–1076. [Google Scholar] [CrossRef]
- Yoo, K.S.; Lee, E.J.; Patil, B.S. Quantification of Quercetin Glycosides in 6 Onion Cultivars and Comparisons of Hydrolysis-HPLC and Spectrophotometric Methods in Measuring Total Quercetin Concentrations. J. Food Sci. 2010, 75, C160–C165. [Google Scholar] [CrossRef] [PubMed]
- Sesink, A.L.A.; O’Leary, K.A.; Hollman, P.C.H. Quercetin Glucuronides but Not Glucosides Are Present in Human Plasma after Consumption of Quercetin-3-Glucoside or Quercetin-4′-Glucoside. J. Nutr. 2001, 131, 1938–1941. [Google Scholar] [CrossRef] [PubMed]
- Beesk, N.; Perner, H.; Schwarz, D.; George, E.; Kroh, L.W.; Rohn, S. Distribution of quercetin-3, 4′-O-diglucoside, quercetin-4′-O-monoglucoside, and quercetin in different parts of the onion bulb (Allium cepa L.) influenced by genotype. Food Chem. 2010, 122, 566–571. [Google Scholar] [CrossRef]
- Burri, S.C.; Ekholm, A.; Håkansson, Å.; Tornberg, E.; Rumpunen, K. Antioxidant capacity and major phenol compounds of horticultural plant materials not usually used. J. Funct. Foods 2017, 38, 119–127. [Google Scholar] [CrossRef]
- Škerget, M.; Majhenič, L.; Bezjak, M.; Knez, Ž. Antioxidant, radical scavenging and antimicrobial activities of red onion (Allium cepa L) skin and edible part extracts. Chem. Biochem. Eng. Q. 2009, 23, 435–444. [Google Scholar]
- Kim, J.; Kim, J.S.; Park, E. Antioxidative and antigenotoxic effects of onion peel extracts in non-cellular and cellular systems. Food Sci. Biotechnol. 2013, 22, 1–8. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Singh, B.N.; Singh, B.; Singh, R.; Prakash, D.; Singh, D.; Sarma, B.; Upadhyay, G.; Singh, H. Polyphenolics from various extracts/fractions of red onion (Allium cepa) peel with potent antioxidant and antimutagenic activities. Food Chem. Toxicol. 2009, 47, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Nile, A.S.; Keum, Y.S.; Sharma, K. Utilization of quercetin and quercetin glycosides from onion (Allium cepa L.) solid waste as an antioxidant, urease and xanthine oxidase inhibitors. Food Chem. 2017, 235, 119–126. [Google Scholar] [CrossRef]
- Khalili, S.; Saeidi Asl, M.R.; Khavarpour, M.; Vahdat, S.M.; Mohammadi, M. Comparative study on the effect of extraction solvent on total phenol, flavonoid content, antioxidant and antimicrobial properties of red onion (Allium cepa). J. Food Meas. Charact. 2022, 16, 3578–3588. [Google Scholar] [CrossRef]
- Takahama, U.; Hirota, S. Deglucosidation of quercetin glucosides to the aglycone and formation of antifungal agents by peroxidase-dependent oxidation of quercetin on browning of onion scales. Plant Cell Physiol. 2000, 41, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Higashio, H.; Hirokane, H.; Sato, F.; Tokuda, S.; Uragami, A. Effect of UV irradiation after the harvest on the content of flavonoid in vegetables. In V International Postharvest Symposium 682; ISHS: Korbeek-Lo, Belgium, 2004; pp. 1007–1012. [Google Scholar]
- Campone, L.; Celano, R.; Piccinelli, A.L.; Pagano, I.; Carabetta, S.; Di Sanzo, R.; Russo, M.; Ibañez, E.; Cifuentes, A.; Rastrelli, L. Response surface methodology to optimize supercritical carbon dioxide/co-solvent extraction of brown onion skin by-product as source of nutraceutical compounds. Food Chem. 2018, 269, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Piechowiak, T.; Grzelak-Błaszczyk, K.; Bonikowski, R.; Balawejder, M. Optimization of extraction process of antioxidant compounds from yellow onion skin and their use in functional bread production. LWT 2020, 117, 108614. [Google Scholar] [CrossRef]
- Khiari, Z.; Makris, D.P. Stability and transformation of major flavonols in onion (Allium cepa) solid wastes. J. Food Sci. Technol. 2012, 49, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Sukor, N.; Selvam, V.P.; Jusoh, R.; Kamarudin, N.; Abd Rahim, S. Intensified DES mediated ultrasound extraction of tannic acid from onion peel. J. Food Eng. 2021, 296, 110437. [Google Scholar] [CrossRef]
- Chia, P.W.; Lim, B.S.; Tan, K.C.; Yong, F.S.J.; Kan, S.Y. Water extract of onion peel for the synthesis of bisindolylmethanes. J. King Saud Univ. Sci. 2019, 31, 642–647. [Google Scholar] [CrossRef]
- Mounir, R.; Alshareef, W.A.; El Gebaly, E.A.; El-Haddad, A.E.; Ahmed, A.M.S.; Mohamed, O.G.; Enan, E.T.; Mosallam, S.; Tripathi, A.; Selim, H.M.R.M. Unlocking the Power of Onion Peel Extracts: Antimicrobial and Anti-Inflammatory Effects Improve Wound Healing through Repressing Notch-1/NLRP3/Caspase-1 Signaling. Pharmaceuticals 2023, 16, 1379. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Celano, R.; Docimo, T.; Piccinelli, A.L.; Gazzerro, P.; Tucci, M.; Di Sanzo, R.; Carabetta, S.; Campone, L.; Russo, M.; Rastrelli, L. Onion peel: Turning a food waste into a resource. Antioxidants 2021, 10, 304. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Smith, B.G.; O’Connor, C.J.; Melton, L.D. Effect of raw and cooked onion dietary fibre on the antioxidant activity of ascorbic acid and quercetin. Food Chem. 2008, 111, 580–585. [Google Scholar] [CrossRef]
- Ramos, F.A.; Takaishi, Y.; Shirotori, M.; Kawaguchi, Y.; Tsuchiya, K.; Shibata, H.; Higuti, T.; Tadokoro, T.; Takeuchi, M. Antibacterial and antioxidant activities of quercetin oxidation products from yellow onion (Allium cepa) skin. J. Agric. Food Chem. 2006, 54, 3551–3557. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S. Antimicrobial assessment of polyphenolic extracts from onion (Allium cepa L.) skin of fifteen cultivars by sonication-assisted extraction method. Heliyon 2020, 6, e05478. [Google Scholar] [CrossRef]
- Fredotović, Ž.; Puizina, J.; Nazlić, M.; Maravić, A.; Ljubenkov, I.; Soldo, B.; Vuko, E.; Bajić, D. Phytochemical Characterization and Screening of Antioxidant, Antimicrobial and Antiproliferative Properties of Allium× cornutum Clementi and Two Varieties of Allium cepa L. Peel Extracts. Plants 2021, 10, 832. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.A.; Kim, K.T.; Nah, S.Y.; Chung, M.-S.; Cho, S.; Paik, H.D. Antimicrobial and antioxidative effects of onion peel extracted by the subcritical water. Food Sci. Biotechnol. 2011, 20, 543–548. [Google Scholar] [CrossRef]
- Kim, W.J.; Lee, K.A.; Kim, K.T.; Chung, M.S.; Cho, S.W.; Paik, H.D. Antimicrobial effects of onion (Allium cepa L.) peel extracts produced via subcritical water extraction against Bacillus cereus strains as compared with ethanolic and hot water extraction. Food Sci. Biotechnol. 2011, 20, 1101–1106. [Google Scholar] [CrossRef]
- Santas, J.; Almajano, M.P.; Carbó, R. Antimicrobial and antioxidant activity of crude onion (Allium cepa, L.) extracts. Int. J. Food Sci. Technol. 2010, 45, 403–409. [Google Scholar] [CrossRef]
- Moosazad, S.; Ghajarbeigi, P.; Mahmoudi, R.; Shahsavari, S.; Vahidi, R.; Soltani, A. Antibacterial and antioxidant properties of colorant extracted from red onion skin. J. Chem. Health Risks 2019, 9, 235–243. [Google Scholar]
- Loredana, L.; Giuseppina, A.; Filomena, N.; Florinda, F.; Marisa, D.M.; Donatella, A. Biochemical, antioxidant properties and antimicrobial activity of different onion varieties in the Mediterranean area. J. Food Meas. Charact. 2019, 13, 1232–1241. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).