Genome-Wide Association Analysis of Cowpea Mild Mottle Virus Resistance in Soybean Germplasms from Northeast China
Abstract
1. Introduction
2. Materials and Methods
2.1. CpMMV Isolate, Plant Materials and Culture Conditions
2.2. Resistance Evaluation Assay
2.3. Genome-Wide Association Study
2.4. Candidate Genes Prediction and Analysis
2.4.1. Candidate Genes Prediction and Functional Analysis
2.4.2. Haplotype Analysis
2.5. Statistical Analysis
3. Results
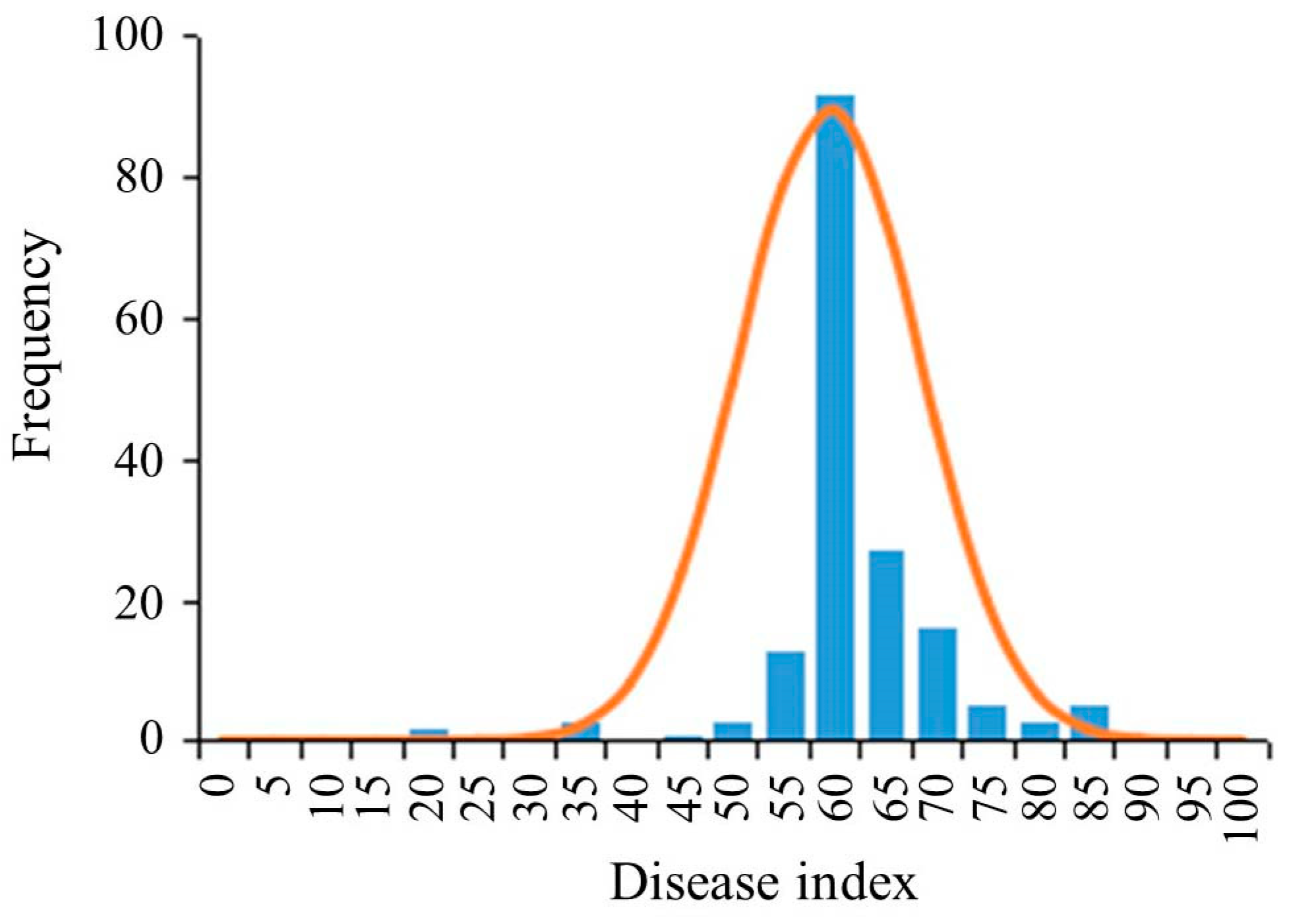
3.1. Resistance Evaluation of Soybean Germplasms
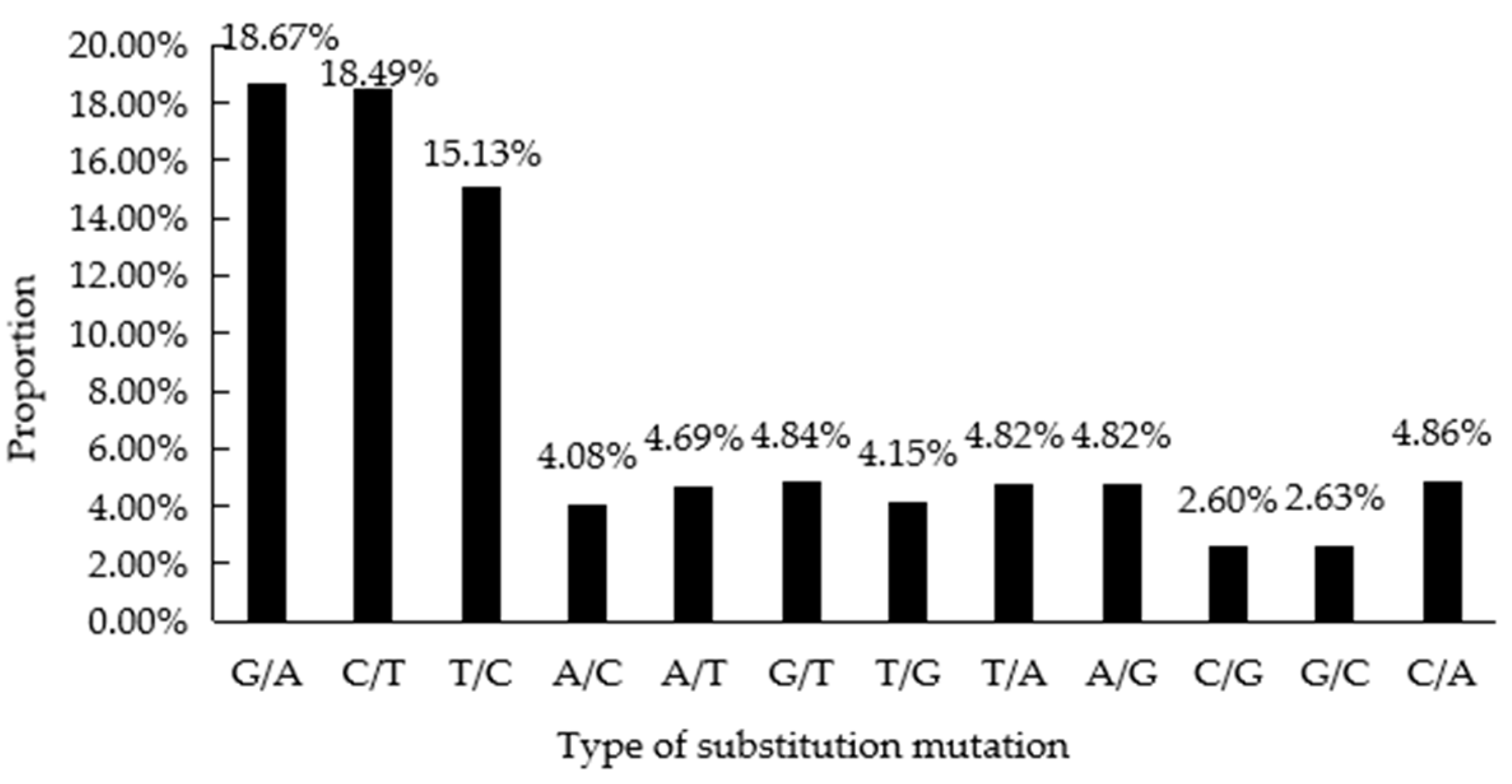
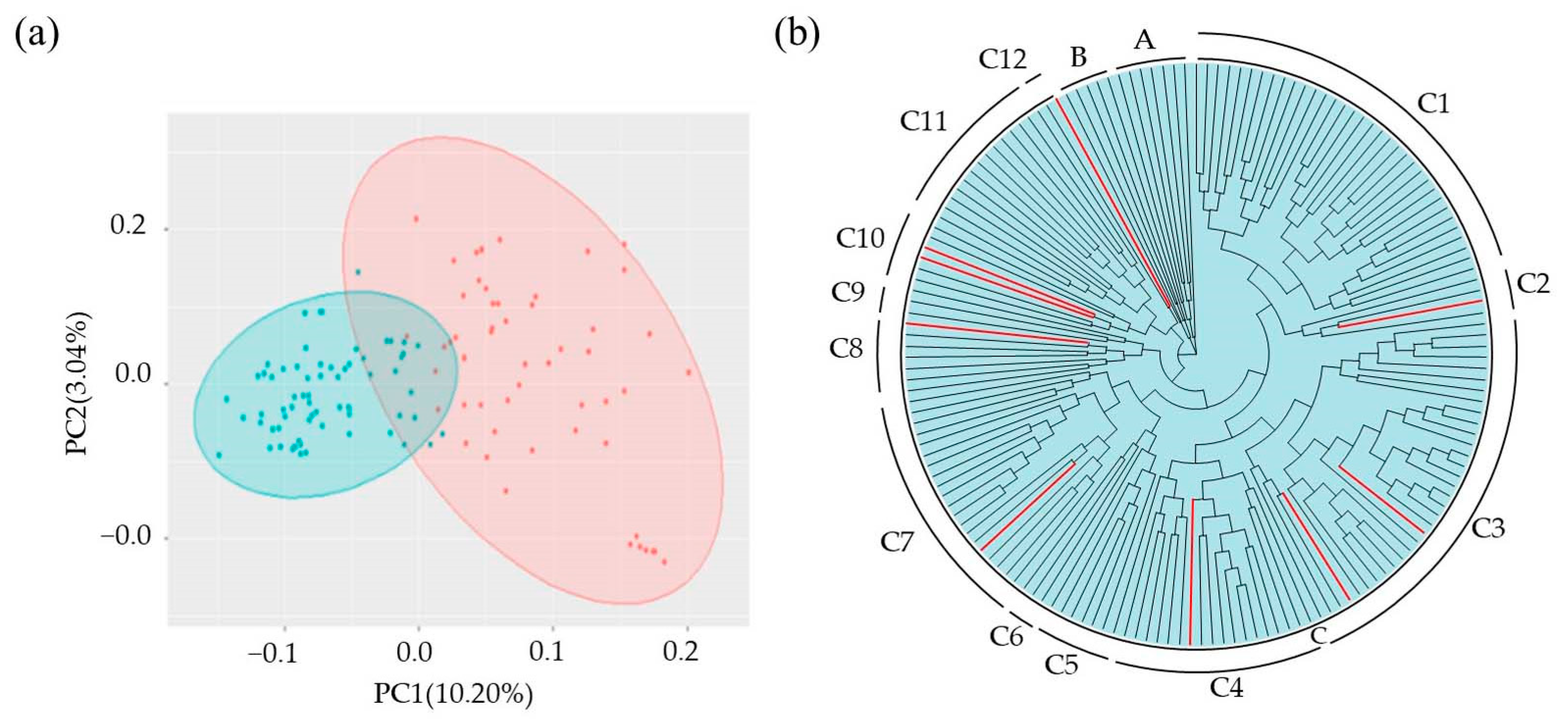
3.2. SNP Characterization and Population Structure Analyses
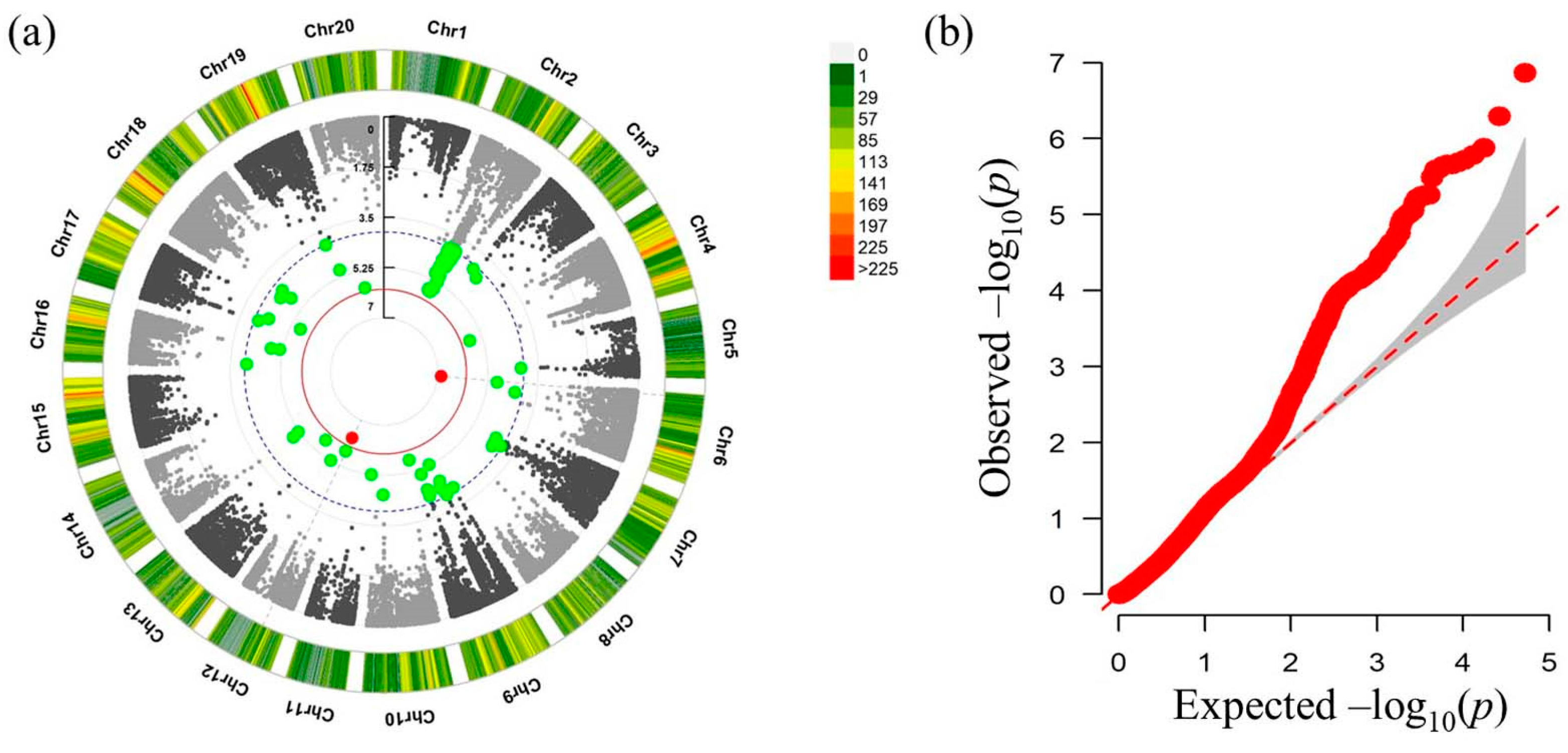
3.3. Identification of CpMMV Resistance-Associated SNPs by GWAS
3.4. Candidate Genes Prediction
3.5. Haplotype Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nadeem, M.; Chen, A.; Hong, H.; Li, D.; Li, J.; Zhao, D.; Wang, W.; Wang, X.; Qiu, L. GmMs1 encodes a kinesin-like protein essential for male fertility in soybean (Glycine max L.). J. Integr. Plant Biol. 2021, 63, 1054–1064. [Google Scholar] [CrossRef]
- Hartman, G.L.; Rupe, J.C.; Sikora, E.J.; Domier, L.L.; Davis, J.A.; Steffey, K.L. Compendium of Soybean Diseases and Pests, 5th ed.; APS Press: St. Paul, MN, USA, 2015; p. 201. [Google Scholar]
- Brunt, A.A.; Kenten, R.H. Cowpea mild mottle, a newly recognized virus infecting cowpeas (Vigna unguiculata) in Ghana. Ann. Appl. Biol. 1973, 74, 67–74. [Google Scholar] [CrossRef]
- Almeida, A.M.R.; Piuga, F.F.; Kitajima, E.W.; Gaspar, J.O.; Valentin, N.; Benato, L.C.; Marin, S.R.; Binneck, E.; De Oliveira, T.; Belintani, P. Necrose da haste da soja. ProBiota Sér Doc. 2003, 221. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/CNPSO-2010/24143/1/doc221.pdf (accessed on 25 February 2024).
- Zanardo, L.G.; Silva, F.N.; Bicalho, A.A.C.; Urquiza, G.P.C.; Lima, A.T.M.; Almeida, A.M.R.; Zerbini, F.M.; Carvalho, C.M. Molecular and biological characterization of cowpea mild mottle virus isolates infecting soybean in Brazil and evidence of recombination. Plant Pathol. 2014, 63, 456–465. [Google Scholar] [CrossRef]
- Ghorbani, S.; Shahraeen, N.; Elahinia, S. Serodiagnosis of cowpea (Vigna unguiculata) viruses in Gilan province, Iran. Iran. J. Virol. 2008, 2, 30–33. [Google Scholar] [CrossRef][Green Version]
- Tavassoli, M.; Shahraeen, N.; Ghorbani, S. Detection and some properties of cowpea mild mottle virus isolated from soybean in Iran. Pak. J. Biol. Sci. 2008, 11, 2624–2628. [Google Scholar] [CrossRef][Green Version]
- Brito, M.; Fernández-Rodríguez, T.; Garrido, M.J.; Mejías, A.; Romano, M.; Marys, E. First report of cowpea mild mottle carlavirus on yardlong bean (Vigna unguiculata subsp. sesquipedalis) in Venezuela. Viruses 2012, 4, 3804–3811. [Google Scholar] [CrossRef] [PubMed]
- Rosario, K.; Capobianco, H.; Ng, T.F.F.; Breitbart, M.; Polston, J.E. RNA viral metagenome of whiteflies leads to the discovery and characterization of a whitefly-transmitted carlavirus in North America. PLoS ONE 2014, 9, e86748. [Google Scholar] [CrossRef] [PubMed]
- Celli, M.G.; Perotto, M.C.; Merino, M.C.; Nome, C.F.; Flores, C.R.; Conci, V.C. First report of cowpea mild mottle virus in chia (Salvia hispanica). Crop Prot. 2016, 89, 1–5. [Google Scholar] [CrossRef]
- Chang, C.; Chien, L.; Tsai, C.; Lin, Y.; Cheng, Y. First report of cowpea mild mottle virus in cowpea and French bean in Taiwan. Plant Dis. 2013, 97, 1001. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Mao, C.; Jiang, C.; Zhang, H.; Chen, J.; Sun, Z. Identification of a new genetic clade of cowpea mild mottle virus and characterization of its interaction with soybean mosaic virus in co-infected soybean. Front. Microbiol. 2021, 12, 650773. [Google Scholar] [CrossRef] [PubMed]
- Arias, C.A.A.; Almeida, A.M.R.; Mituti, T.; Kitajima, E.W. Inheritance of tolerance to cowpea mild mottle virus in soybean. Crop Breed. Appl. Biotechnol. 2015, 15, 132–138. [Google Scholar] [CrossRef]
- Zanardo, L.G.; Carvalho, C.M. Cowpea mild mottle virus (Carlavirus, Betaflexiviridae): A review. Trop. Plant Pathol. 2017, 42, 417–430. [Google Scholar] [CrossRef]
- Barreto da Silva, F.; Muller, C.; Bello, V.H.; Watanabe, L.F.M.; Rossitto De Marchi, B.; Fusco, L.M.; Ribeiro-Junior, M.R.; Minozzi, G.B.; Vivan, L.M.; Tamai, M.A.; et al. Effects of cowpea mild mottle virus on soybean cultivars in Brazil. PeerJ 2020, 8, e9828. [Google Scholar] [CrossRef]
- Marubayashi, J.M.; Yuki, V.A.; Wutke, E.B. Transmission of the cowpea mild mottle virus by whitefly Bemisia tabaci biotype B for plants of beans and soy. Summa Phytopathol. 2010, 36, 158–160. [Google Scholar] [CrossRef]
- Sutrawati, M.; Hidayat, S.H.; Suastika, G.; Purnomo Wahyu Sukarno, B.; Nurmansyah, A. Seed-transmission of cowpea mild mottle virus on several varieties of soybean in Indonesia. Biodiversitas 2021, 22, 4182–4185. [Google Scholar] [CrossRef]
- Martelli, G.P.; Adams, M.J.; Kreuze, J.F.; Dolja, V.V. Family Flexiviridae: A case study in virion and genome plasticity. Ann. Rev. Phytopathol. 2007, 45, 73–100. [Google Scholar] [CrossRef]
- Menzel, W.; Winter, S.; Vetten, H.J. Complete nucleotide sequence of the type isolate of cowpea mild mottle virus from Ghana. Arch. Virol. 2010, 155, 2069–2073. [Google Scholar] [CrossRef] [PubMed]
- Lefkowitz, E.J.; Dempsey, D.M.; Hendrickson, R.C.; Orton, R.J.; Siddell, S.G.; Smith, D.B. Virus taxonomy: The database of the International Committee on Taxonomy of Viruses (ICTV). Nucleic Acids Res. 2018, 46, D708–D717. [Google Scholar] [CrossRef]
- Suryanto, A.; Kuswanto, K.; Sitompul, S.; Kasno, A. Estimation of number and genes actions of CpMMV (cowpea mild mottle virus) disease resistance genes on soybean crop. IOSR J. Agric. Vet. Sci. 2014, 7, 51–56. [Google Scholar] [CrossRef]
- Cheruku, D.; Lal, S.K.; Talukdar, A.; Mandal, B.; Singh, R. Inheritance and mapping of resistance against cowpea mild mottle virus strain D1 in soybean. Plant Breed. 2017, 136, 155–160. [Google Scholar] [CrossRef]
- Brace, R.C.; Fehr, W.R.; Graham, M. Inheritance and molecular mapping of an allele providing resistance to a cowpea mild mottle-like virus in soybean. Crop Sci. 2012, 52, 2109–2114. [Google Scholar] [CrossRef]
- Oliveira, M.A.R.D.; Carpentieri-Pípolo, V.; Nora, T.D.; Vieira, E.S.N.; Schuster, I. Rbc2, a new dominant gene for resistance of soybean to cowpea mild mottle virus: Inheritance and mapping. Crop Breed. Appl. Biotechnol. 2018, 18, 169–175. [Google Scholar] [CrossRef]
- Gómez, P.; Rodríguez-Hernández, A.M.; Moury, B.; Aranda, M.A. Genetic resistance for the sustainable control of plant virus diseases: Breeding, mechanisms and durability. Eur. J. Plant Pathol. 2009, 125, 1–22. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Y.; Wu, X.; Cheng, X.; Wu, X. Synonymous codon pattern of cowpea mild mottle virus sheds light on its host adaptation and genome evolution. Pathogens 2022, 11, 419. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Chen, Y.; Pan, Y.B.; Wang, J.; Lu, W.; Shi, A. A genome-wide association study and genomic prediction for Phakopsora pachyrhizi resistance in soybean. Front. Plant Sci. 2023, 14, 1179357. [Google Scholar] [CrossRef] [PubMed]
- Che, Z.; Zhang, S.; Pu, Y.; Yang, Y.; Liu, H.; Yang, H.; Wang, L.; Zhang, Y.; Liu, B.; Zhang, H.; et al. A novel soybean malectin-like receptor kinase-encoding gene, GmMLRK1, provides resistance to soybean mosaic virus. J. Exp. Bot. 2023, 74, 2692–2706. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Lin, C.; Lu, W.; Han, Z.; Wei, D.; Huo, X.; Li, T.; Zhang, J.; He, Y.; Chen, C.; et al. OsBLS6.2: A rice bacterial leaf streak resistance gene identified by GWAS and RNA-seq. Crop J. 2023, 11, 1862–1871. [Google Scholar] [CrossRef]
- Hull, R. Mechanical inoculation of plant viruses. Curr. Protoc. Microbiol. 2009, 13, 16B-6. [Google Scholar] [CrossRef]
- Zhi, H.; Gai, J. Performances and germplasm evaluation of quantitative resistance to soybean mosaic virus in soybeans. Sci. Agric. Sin. 2004, 3, 247–253. [Google Scholar]
- Merk, H.L.; Yarnes, S.C.; Van Deynze, A.; Tong, N.; Menda, N.; Mueller, L.A.; Mutschler, M.A.; Loewen, S.A.; Myers, J.R.; Francis, D.M. Trait diversity and potential for selection indices based on variation among regionally adapted processing tomato germplasm. J. Am. Soc. Hortic. Sci. 2012, 137, 427–437. [Google Scholar] [CrossRef]
- Zubaidah, S.; Kuswantoro, H. Foliar symptoms recovery: Developing scoring technique for assessment of soybean resistance to CpMMV (cowpea mild mottle virus). J. Biol. Res. 2016, 21, 85–89. [Google Scholar] [CrossRef]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Zhang, Z.; Ersoz, E.; Lai, C.-Q.; Todhunter, R.J.; Tiwari, H.K.; Gore, M.A.; Bradbury, P.J.; Yu, J.; Arnett, D.K.; Ordovas, J.M.; et al. Mixed linear model approach adapted for genome-wide association studies. Nat. Genet. 2010, 42, 355–360. [Google Scholar] [CrossRef]
- Turner, S. Annotated Manhattan plots and QQ plots for GWAS using R, Revisited. Nat. Prec. 2011. [Google Scholar] [CrossRef]
- Barrett, J.C. Haploview: Visualization and analysis of SNP genotype data. Cold Spring Harb. Protoc. 2009, 2009, pdb.ip71. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef]
- Qi, Z.; Guo, C.; Li, H.; Qiu, H.; Li, H.; Jong, C.; Yu, G.; Zhang, Y.; Hu, L.; Wu, X. Natural variation in fatty acid 9 is a determinant of fatty acid and protein content. Plant Biotechnol. J. 2023. [Google Scholar] [CrossRef]
- Ngou, B.P.M.; Ding, P.; Jones, J.D.G. Thirty years of resistance: Zig-zag through the plant immune system. Plant Cell 2022, 34, 1447–1478. [Google Scholar] [CrossRef]
- Diao, P.; Sun, H.; Bao, Z.; Li, W.; Niu, N.; Li, W.; Wuriyanghan, H. Expression of an antiviral gene GmRUN1 from soybean is regulated via intron-mediated enhancement (IME). Viruses 2021, 13, 2032. [Google Scholar] [CrossRef]
- Whitham, S.; Dinesh-Kumar, S.P.; Choi, D.; Hehl, R.; Corr, C.; Baker, B. The product of the tobacco mosaic virus resistance gene N: Similarity to toll and the interleukin-1 receptor. Cell 1994, 78, 1101–1115. [Google Scholar] [CrossRef]
- Wu, X.; Valli, A.; García, J.A.; Zhou, X.; Cheng, X. The tug-of-war between plants and viruses: Great progress and many remaining questions. Viruses 2019, 11, 203. [Google Scholar] [CrossRef]
- Yuan, M.; Jiang, Z.; Bi, G.; Nomura, K.; Liu, M.; Wang, Y.; Cai, B.; Zhou, J.-M.; He, S.Y.; Xin, X.F. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature 2021, 592, 105–109. [Google Scholar] [CrossRef]
- Seo, Y.S.; Rojas, M.R.; Lee, J.Y.; Lee, S.W.; Jeon, J.S.; Ronald, P.; Lucas, W.J.; Gilbertson, R.L. A viral resistance gene from common bean functions across plant families and is up-regulated in a non-virus-specific manner. Proc. Natl. Acad. Sci. USA 2006, 103, 11856–11861. [Google Scholar] [CrossRef]
- Xun, Q.; Wu, Y.; Li, H.; Chang, J.; Ou, Y.; He, K.; Gou, X.; Tax, F.E.; Li, J. Two receptor-like protein kinases, MUSTACHES and MUSTACHES-LIKE, regulate lateral root development in Arabidopsis thaliana. New Phytol. 2020, 227, 1157–1173. [Google Scholar] [CrossRef]
- Tam, V.; Patel, N.; Turcotte, M.; Bossé, Y.; Paré, G.; Meyre, D. Benefits and limitations of genome-wide association studies. Nat. Rev. Genet. 2019, 20, 467–484. [Google Scholar] [CrossRef]

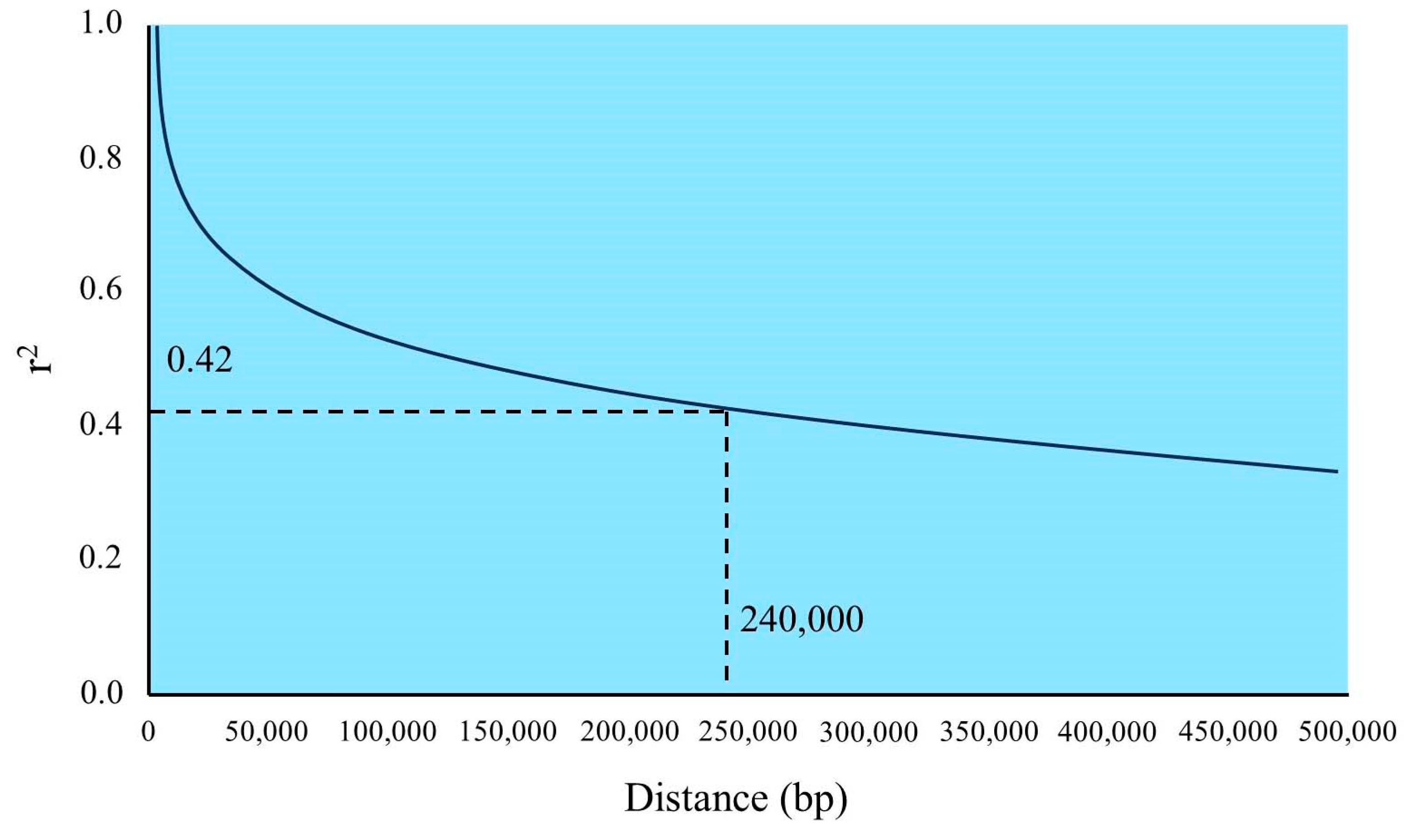
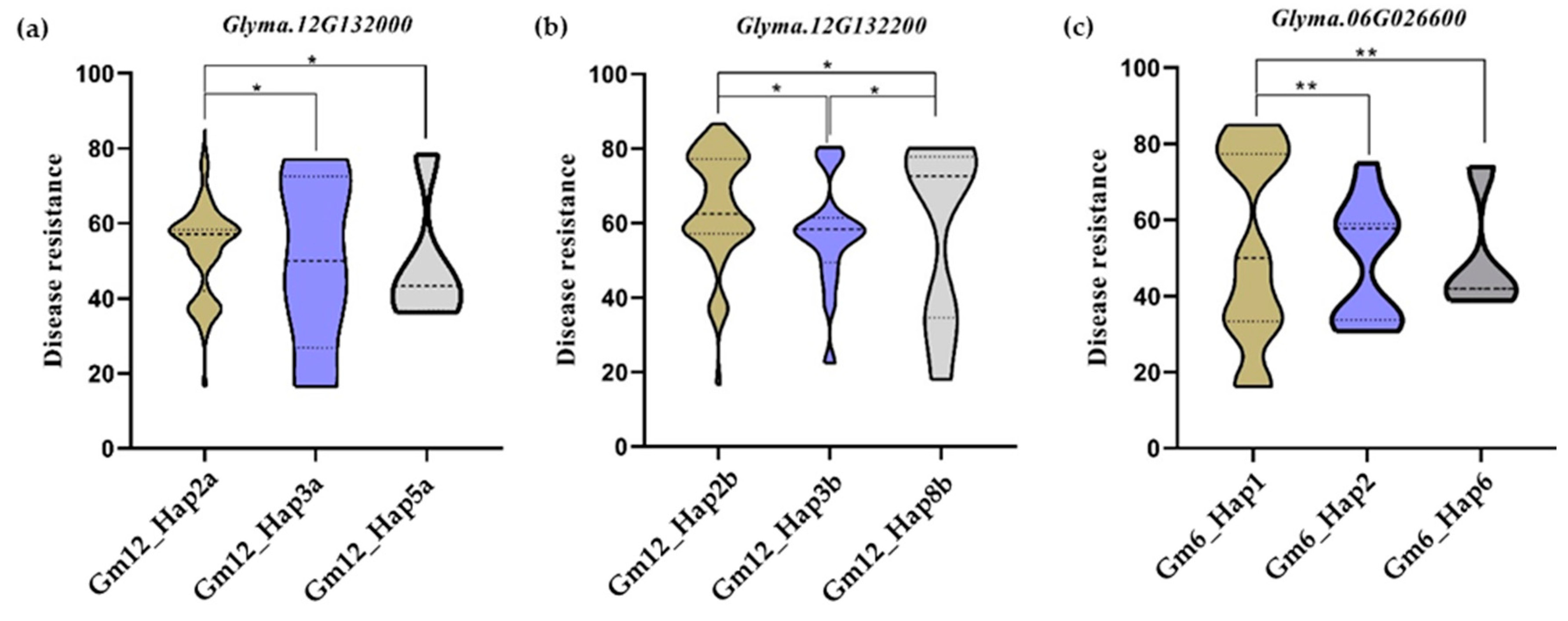
| Candidate Genes | Haplotype Number | Haplotype Names | Germplasm Number | Haplotype Ratios |
|---|---|---|---|---|
| Glyma.06G026600 | 16 | Gm6_Hap1 | 109 | 64.50% |
| Gm6_Hap2 | 11 | 6.51% | ||
| Gm6_Hap6 | 10 | 5.92% | ||
| Glyma.12G132000 | 18 | Gm12_Hap2a | 128 | 75.74% |
| Gm12_Hap3a | 13 | 7.69% | ||
| Gm12_Hap5a | 9 | 5.33% | ||
| Glyma.12G132200 | 23 | Gm12_Hap2b | 102 | 60.36% |
| Gm12_Hap3b | 21 | 12.43% | ||
| Gm12_Hap8b | 10 | 5.92% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luan, Y.; Yang, S.; Wang, Y.; Zhao, Y.; Wu, X.; Chen, Q.; Qi, Z.; Wu, X.; Ji, W.; Cheng, X. Genome-Wide Association Analysis of Cowpea Mild Mottle Virus Resistance in Soybean Germplasms from Northeast China. Agronomy 2024, 14, 489. https://doi.org/10.3390/agronomy14030489
Luan Y, Yang S, Wang Y, Zhao Y, Wu X, Chen Q, Qi Z, Wu X, Ji W, Cheng X. Genome-Wide Association Analysis of Cowpea Mild Mottle Virus Resistance in Soybean Germplasms from Northeast China. Agronomy. 2024; 14(3):489. https://doi.org/10.3390/agronomy14030489
Chicago/Turabian StyleLuan, Yameng, Siqi Yang, Yuting Wang, Yu Zhao, Xiaoyun Wu, Qingshan Chen, Zhaoming Qi, Xiaoxia Wu, Weiqin Ji, and Xiaofei Cheng. 2024. "Genome-Wide Association Analysis of Cowpea Mild Mottle Virus Resistance in Soybean Germplasms from Northeast China" Agronomy 14, no. 3: 489. https://doi.org/10.3390/agronomy14030489
APA StyleLuan, Y., Yang, S., Wang, Y., Zhao, Y., Wu, X., Chen, Q., Qi, Z., Wu, X., Ji, W., & Cheng, X. (2024). Genome-Wide Association Analysis of Cowpea Mild Mottle Virus Resistance in Soybean Germplasms from Northeast China. Agronomy, 14(3), 489. https://doi.org/10.3390/agronomy14030489







