Turning Waste into Wealth: Utilizing Trichoderma’s Solid-State Fermentation to Recycle Tea Residue for Tea Cutting Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism and Culture Conditions
2.2. Growth-Promoting Test of Different Trichoderma Strains
2.3. Optimization of SSF Parameters for NJAU 4742
2.4. Pot Experiment Design and Nursery Substrate Sampling
2.5. DNA Extraction and Illumina MiSeq Sequencing
2.6. Bioinformatic Analysis
2.7. Statistical Analysis
3. Results
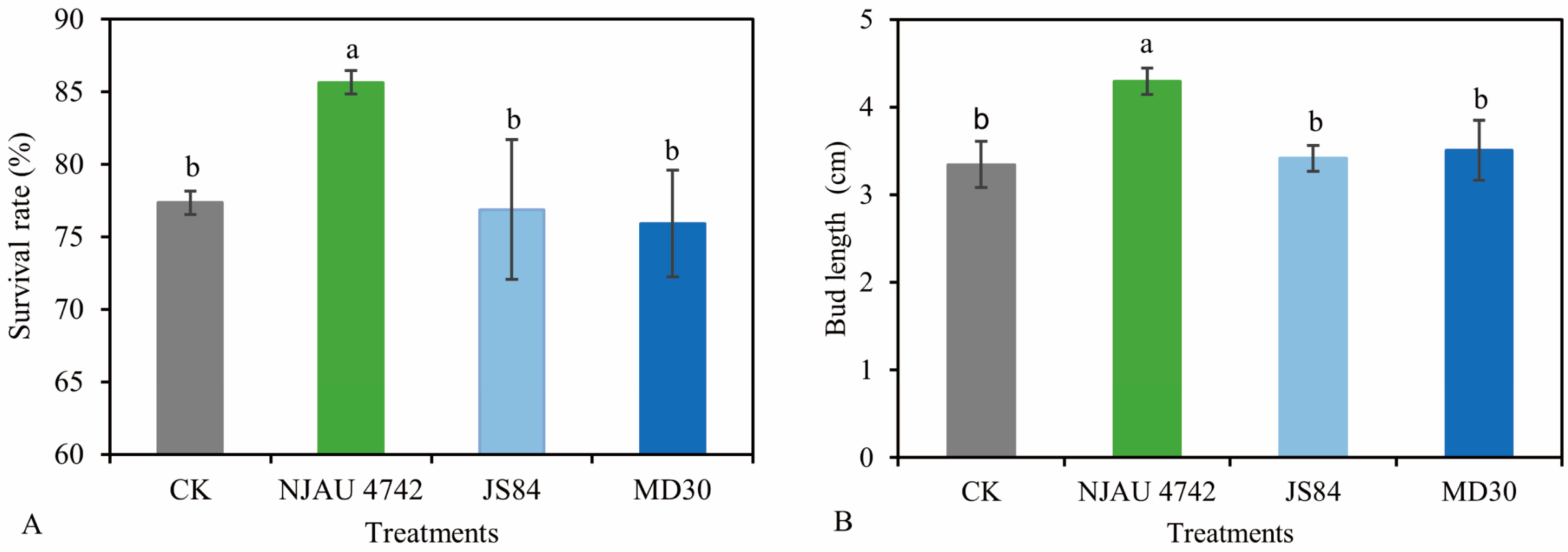
3.1. Effects of Different Trichoderma Strains on the Growth of Tea Cuttings
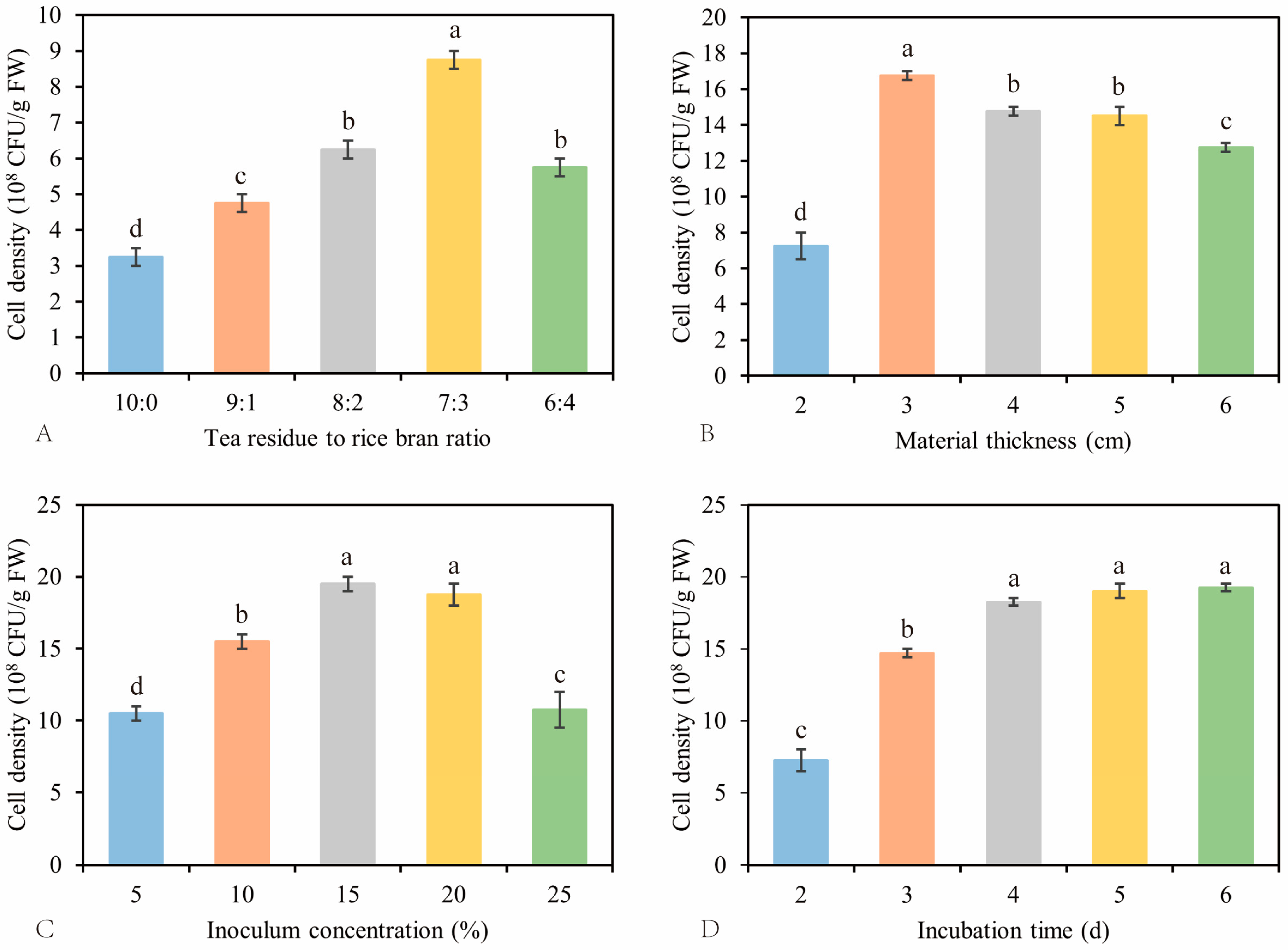
3.2. Optimized Parameters of SSF for NJAU 4742
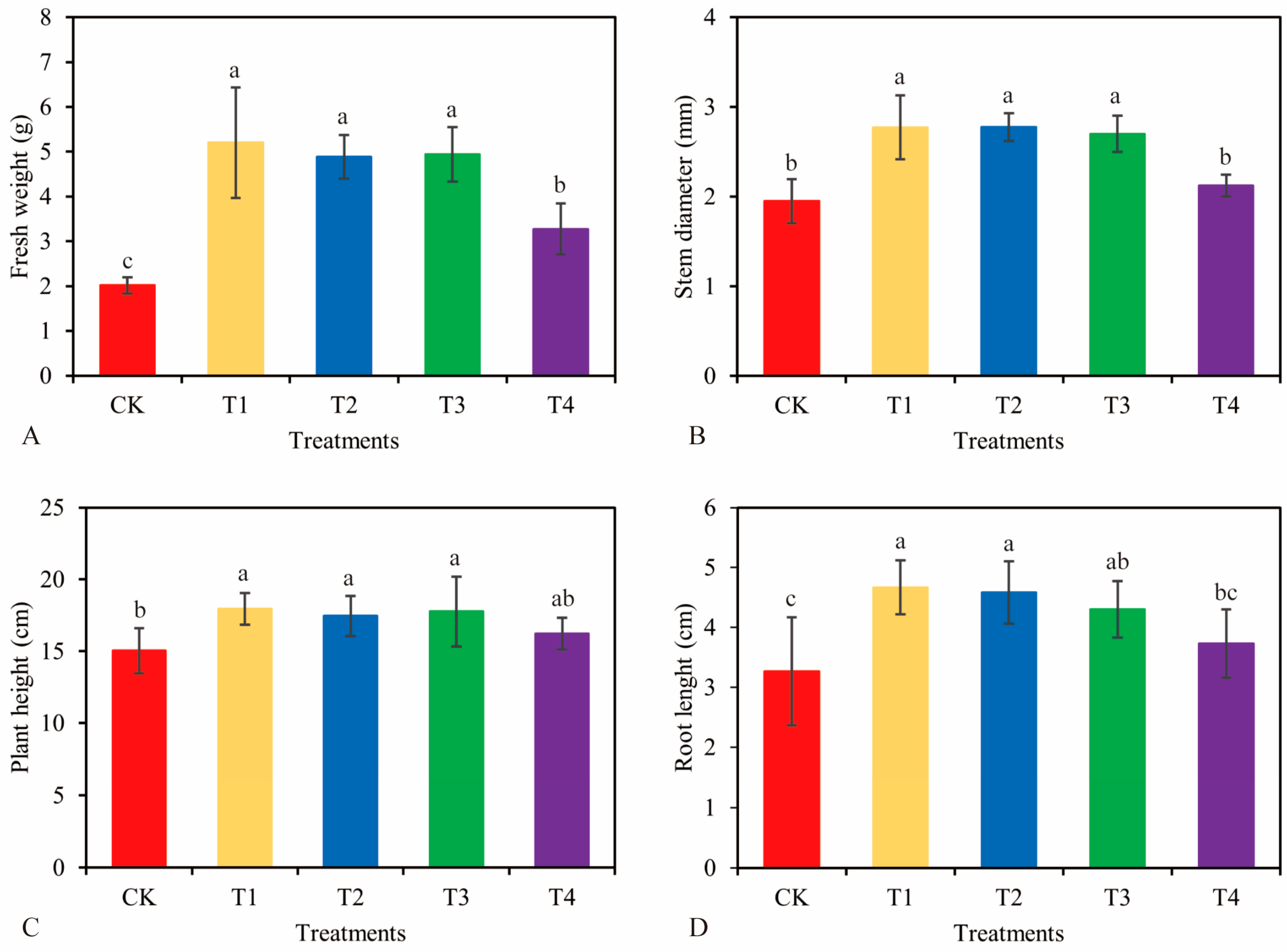
3.3. Effects of Different Concentrations of NJAU 4742 Spores on the Growth of Tea Cuttings
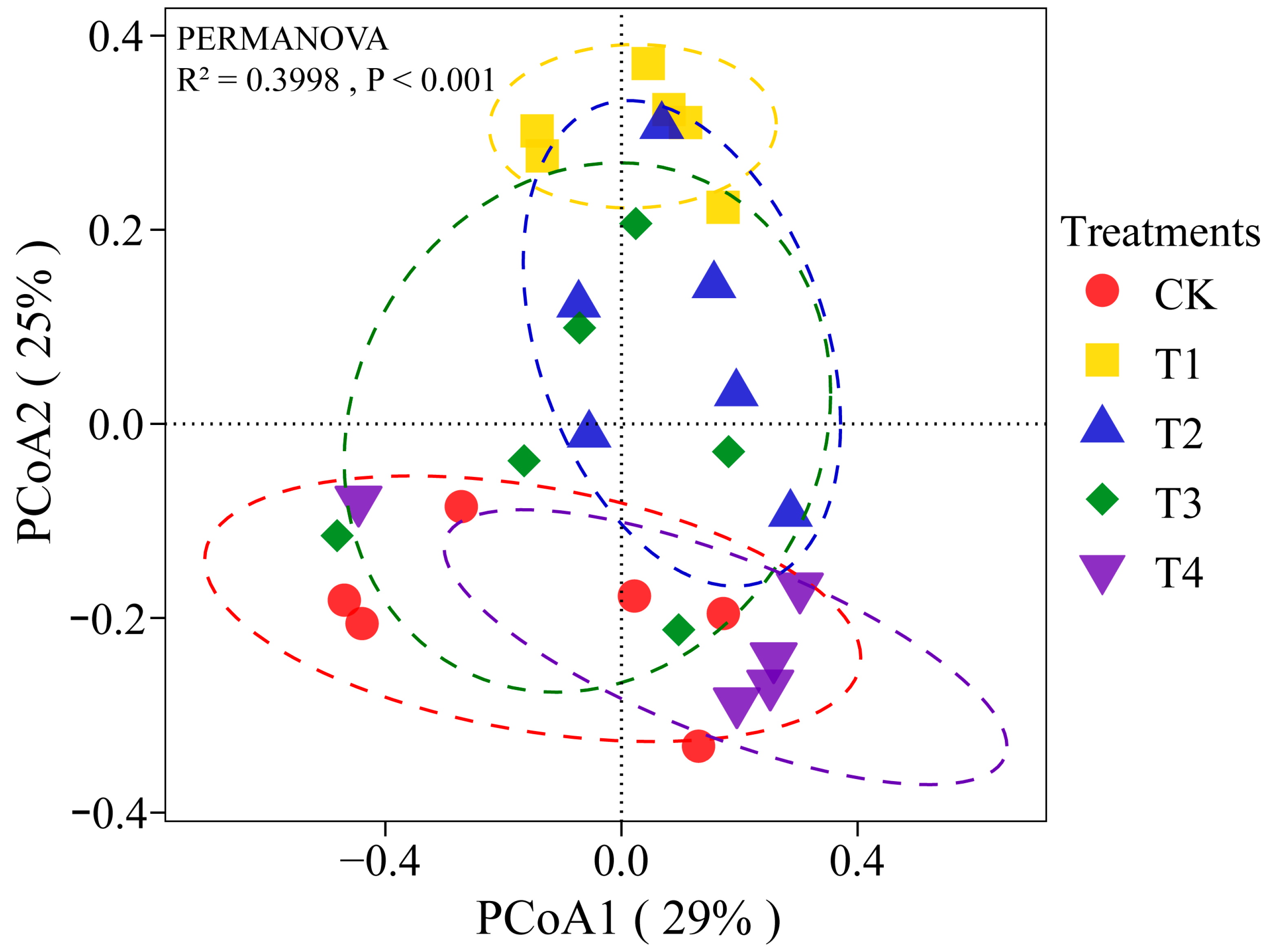
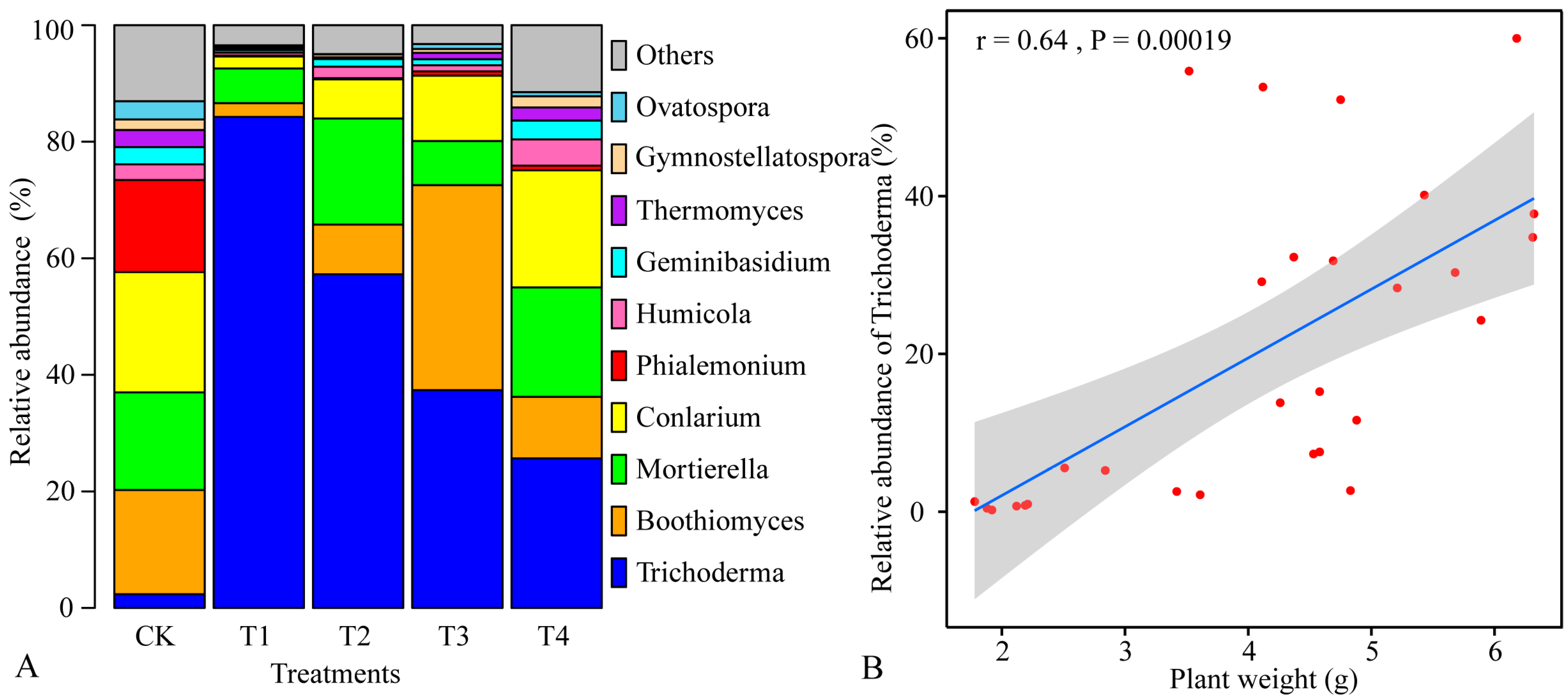
3.4. Effects of Different Concentrations of NJAU 4742 Spores on Fungal Community Composition
3.5. Relationships between Fungal Community Composition and Fresh Weight of Tea Cuttings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Matar, N.; Macadre, C.; Ammar, G.A.; Peres, A.; Collet, B.; Boustany, N.E.; Rajjou, L.; As-Sadi, F.; Dufresne, M.; Ratet, P. Identification of beneficial Lebanese Trichoderma spp. wheat endophytes. Front. Plant. Sci. 2022, 13, 1017890. [Google Scholar] [CrossRef]
- Shalaby, T.A.; Taha, N.; El-Beltagi, H.S.; El-Ramady, H. Combined application of Trichoderma harzianum and paclobutrazol to control root rot disease caused by Rhizoctonia solani of tomato seedlings. Agronomy 2022, 12, 3186. [Google Scholar] [CrossRef]
- Druzhinina, I.S.; Seidl-Seiboth, V.; Herrera-Estrella, A.; Horwitz, B.A.; Kenerley, C.M.; Monte, E.; Mukherjee, P.K.; Zeilinger, S.; Grigoriev, I.V.; Kubicek, C.P. Trichoderma: The genomics of opportunistic success. Nat. Rev. Microbiol. 2011, 9, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Hyde, K.D.; Xu, J.; Rapior, S.; Jeewon, R.; Lumyong, S.; Niego, A.G.T.; Abeywickrama, P.D.; Aluthmuhandiram, J.V.S.; Brahamanage, R.S.; Brooks, S.; et al. The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Divers. 2019, 97, 1–136. [Google Scholar] [CrossRef]
- Inbar, J.; Menendez, A.N.A.; Chet, I. Hyphal interaction between Trichoderma harzianum and Sclerotinia sclerotiorum and its role in biological control. Soil Biol. Biochem. 1996, 28, 757–763. [Google Scholar] [CrossRef]
- Schirmbock, M.; Lorito, M.; Wang, Y.L.; Hayes, C.K.; Arisan-Atac, I.; Scala, F.; Harman, G.E.; Kubicek, C.P. Parallel formation and synergism of hydrolytic enzymes and peptaibol antibiotics, molecular mechanisms involved in the antagonistic action of Trichoderma harzianum against phytopathogenic fungi. Appl. Environ. Microbiol. 1994, 60, 4364–4370. [Google Scholar] [CrossRef]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “secrets” of a multitalented biocontrol agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef]
- Bostock, R.M.; Karban, R.; Thaler, J.S.; Weyman, P.D.; Gilchrist, D. Signal interactions in induced resistance to pathogens and insect herbivores. Eur. J. Plant Pathol. 2001, 107, 103–111. [Google Scholar] [CrossRef]
- Arshad, M.; Frankenberger, W.T., Jr. Plant growth-regulating substances in the rhizosphere: Microbial production and functions. Adv. Agron. 1997, 62, 45–151. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Gallo, M.E.; Lauber, C.; Waldrop, M.P.; Zak, D.R. Extracellular enzyme activities and soil organic matter dynamics for Northern Hardwood Forests receiving simulated nitrogen deposition. Biogeochemistry 2005, 75, 201–215. [Google Scholar] [CrossRef]
- Chen, D.; Liu, X.; Li, C.; Tian, W.; Shen, Q.; Shen, B. Isolation of Bacillus amyloliquefaciens S20 and its application in control of eggplant bacterial wilt. J. Environ. Manag. 2014, 137, 120–127. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, Z.; Xiao, Y. Trichoderma biofertilizer facilitating Leymus chinensis production in different growth stages is strongly linked to dynamically altered soil microbiomes. Agric. Ecosyst. Environ. 2022, 324, 107706. [Google Scholar] [CrossRef]
- Naeimi, S.; Khosravi, V.; Varga, A.; Vágvölgyi, C.; Kredics, L. Screening of organic substrates for solid-state fermentation, viability and bioefficacy of Trichoderma harzianum AS12-2, a biocontrol Strain against Rice Sheath Blight Disease. J. Agron. 2020, 10, 1258. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess 2018, 5, 1. [Google Scholar] [CrossRef]
- Li, C.; Li, S.; Zhu, Y.; Chen, S.; Wang, X.; Deng, X.; Liu, G.; Beckers, Y.; Cai, H. Improving the Nutritional Value of Plant Protein Sources as Poultry Feed through Solid-State Fermentation with a Special Focus on Peanut Meal—Advances and Perspectives. Fermentation 2023, 9, 364. [Google Scholar] [CrossRef]
- Abdeshahian, P.; Samat, N.; Hamid, A.A.; Yusoff, W.M.W. Utilization of palm kernel cake for production of β-mannanase by Aspergillus niger FTCC 5003 in solid substrate fermentation using an aerated column bioreactor. J. Ind. Microbiol. Biotechnol. 2010, 37, 103–109. [Google Scholar] [CrossRef]
- Steudler, S.; Werner, A.; Walther, T. It Is the Mix that Matters: Substrate-Specific Enzyme Production from Filamentous Fungi and Bacteria Through Solid-State Fermentation. In Solid State Fermentation, Research and Industrial Applications; Werner, A., Steudler, S., Cheng, J.J., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 51–81. [Google Scholar] [CrossRef]
- Sánchez, A.; Artola, A.; Gea, T.; Barrena, R.; Font, X. A new paradigm for waste management of organic materials. Waste Manag. 2015, 42, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Vittone, S.; Barrena, R.; Sanchez, A.; Artola, A. Scanning agro-industrial wastes as substrates for fungal biopesticide production: Use of Beauveria bassiana and Trichoderma harzianum in solid-state fermentation. J. Environ. Manag. 2021, 295, 113113. [Google Scholar] [CrossRef]
- Karak, T.; Bhagat, R.M. Trace elements in tea leaves, made tea and tea infusion: A review. Food Res. Int. 2010, 43, 2234–2252. [Google Scholar] [CrossRef]
- Pan, S.Y.; Nie, Q.; Tai, H.C.; Song, X.L.; Tong, Y.F.; Zhang, L.J.F.; Wu, X.W.; Lin, Z.H.; Zhang, Y.Y.; Ye, D.Y.; et al. Tea and tea drinking: China’s outstanding contributions to the mankind. Chin. Med. 2022, 17, 27. [Google Scholar] [CrossRef]
- Miao, S.; Wei, Y.; Chen, J.; Wei, X. Extraction methods, physiological activities and high value applications of tea residue and its active components: A review. Crit. Rev. Food. Sci. 2023, 63, 12150–12168. [Google Scholar] [CrossRef]
- Voora, V.; Bermúdez, S.; Larrea, C. Global Market Report: Tea. International Institute for Sustainable Development (IISD). 2019. Available online: http://www.jstor.org/stable/resrep22027 (accessed on 1 December 2023).
- Debnath, B.; Haldar, D.; Purkait, M.K. Potential and sustainable utilization of tea waste: A review on present status and future trends. J. Environ. Chem. Eng. 2021, 9, 106179. [Google Scholar] [CrossRef]
- Hussain, S.; Anjali, K.P.; Hassan, S.T.; Dwivedi, P.B. Waste tea as a novel adsorbent: A review. Appl. Water Sci. 2018, 8, 165. [Google Scholar] [CrossRef]
- Negi, T.; Kumar, Y.; Sirohi, R.; Singh, S.; Tarafdar, A.; Pareek, S.; Awasthi, M.K.; Sagar, N.A. Advances in bioconversion of spent tea leaves to value-added products. Bioresour. Technol. 2022, 346, 126409. [Google Scholar] [CrossRef]
- Peng, C.; Yan, X.B.; Wang, R.T.; Lang, J.W.; Ou, Y.J.; Xue, Q.J. Promising activated carbons derived from waste tea-leaves and their application in high performance supercapacitors electrodes. Electrochim. Acta 2013, 87, 401–408. [Google Scholar] [CrossRef]
- Barathi, M.; Kumar, A.S.K.; Kodali, J.; Mittal, S.; Samhith, G.D.; Rajesh, N. Probing the Interaction between Fluoride and the Polysaccharides in Al (III)-and Zr (IV)-Modified Tea Waste by Using Diverse Analytical Characterization Techniques. ChemistrySelect 2017, 2, 10123–10135. [Google Scholar] [CrossRef]
- Tao, J.; Huo, P.; Fu, Z.; Zhang, J.; Yang, Z.; Zhang, D. Characterization and phenol adsorption performance of activated carbon prepared from tea residue by NaOH activation. Environ. Technol. 2019, 40, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Akbayrak, S.; Özçifçi, Z.; Tabak, A. Activated carbon derived from tea waste: A promising supporting material for metal nanoparticles used as catalysts in hydrolysis of ammonia borane. Biomass Bioenergy 2020, 138, 105589. [Google Scholar] [CrossRef]
- Kondo, M.; Hirano, Y.; Kita, K.; Jayanegara, A.; Yokota, H.O. Nutritive evaluation of spent green and black tea leaf silages by in vitro gas production characteristics, ruminal degradability and post-ruminal digestibility assessed with inhibitory activity of their tannins. Anim. Sci. J. 2018, 89, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, T.; Ying, Y.; Yao, X. Effects of different additives on the chemical composition and microbial diversity during composting of Camellia oleifera shell. Bioresour. Technol. 2021, 330, 124990. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Awasthi, M.K.; Wang, Y.; Xu, P. Current understanding in conversion and application of tea waste biomass: A review. Bioresour. Technol. 2021, 338, 125530. [Google Scholar] [CrossRef] [PubMed]
- Borin, G.P.; Carazzolle, M.F.; dos Santos, R.A.C.; Riaño-Pachón, D.M.; Oliveira, J.V.D.C. Gene co-expression network reveals potential new genes related to sugarcane bagasse degradation in Trichoderma reesei RUT-30. Front. Bioeng. Biotechnol. 2018, 6, 151. [Google Scholar] [CrossRef] [PubMed]
- Sijinamanoj, V.; Muthukumar, T.; Muthuraja, R.; Rayappan, K.; Karmegam, N.; Saminathan, K.; Govarthanan, M.; Kathireswari, P. Ligninolytic valorization of agricultural residues by Aspergillus nomius and Trichoderma harzianum isolated from gut and comb of Odontotermes obesus (Termitidae). Chemosphere 2021, 284, 131384. [Google Scholar] [CrossRef]
- Dos Santos Pereira, T.; Monteiro de Paula, A.; Ferrari, L.H.; da Silva, J.; Borges Pinheiro, J.; Navas Cajamarca, S.M.; Jindo, K.; Santos, M.P.; Zandonadi, D.B.; Busato, J.G. Trichoderma-Enriched Vermicompost Extracts Reduces Nematode Biotic Stress in Tomato and Bell Pepper Crops. Agronomy 2021, 11, 1655. [Google Scholar] [CrossRef]
- Sala, A.; Barrena, R.; Artola, A.; Sánchez, A. Current developments in the production of fungal biological control agents by solid-state fermentation using organic solid waste. Crit. Rev. Environ. Sci. Technol. 2019, 49, 655–694. [Google Scholar] [CrossRef]
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat. Rev. Microbiol. 2023, 21, 312–326. [Google Scholar] [CrossRef]
- He, J.; Zhu, N.; Xu, Y.; Wang, L.; Zheng, J.; Li, X. The microbial mechanisms of enhanced humification by inoculation with Phanerochaete chrysosporium and Trichoderma longibrachiatum during biogas residues composting. Bioresour. Technol. 2022, 351, 126973. [Google Scholar] [CrossRef]
- Hamrouni, R.; Regus, F.; Claeys-Bruno, M.; Farnet Da Silva, A.-M.; Orsière, T.; Laffont-Schwob, I.; Boudenne, J.-L.; Dupuy, N. Statistical Experimental Design as a New Approach to Optimize a Solid-State Fermentation Substrate for the Production of Spores and Bioactive Compounds from Trichoderma asperellum. J. Fungi 2023, 9, 1123. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, Z.; Yang, X.; Ran, W.; Shen, Q.R. Trichoderma harzianum T-E5 significantly affects cucumber root exudates and fungal community in the cucumber rhizosphere. Appl. Soil Ecol. 2013, 72, 41–48. [Google Scholar] [CrossRef]
- Liu, L.; Xu, Y.; Cao, H.; Fan, Y.; Du, K.; Bu, X.; Gao, D. Effects of Trichoderma harzianum biofertilizer on growth, yield, and quality of Bupleurum chinense. Plant Direct 2022, 6, e461. [Google Scholar] [CrossRef]
- Abdelhafez, A.A.; Eid, K.E.; El-Abeid, S.E.; Abbas, M.H.H.; Ahmed, N.; Mansour, R.R.M.E.; Zou, G.; Iqbal, J.; Fahad, S.; Elkelish, A.; et al. Application of soil biofertilizers to a clayey soil contaminated with Sclerotium rolfsii can promote production, protection and nutritive status of Phaseolus vulgaris. Chemosphere 2021, 271, 129321. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Bowles, T.M.; Acosta-Martínez, V.; Calderón, F.; Jackson, L.E. Soil enzyme activities, microbial communities, and carbon and nitrogen availability in organic agroecosystems across an intensively-managed agricultural landscape. Soil Biol. Biochem. 2014, 68, 252–262. [Google Scholar] [CrossRef]
- Hestrin, R.; Hammer, E.C.; Mueller, C.W.; Lehmann, J. Synergies between mycorrhizal fungi and soil microbial communities increase plant nitrogen acquisition. Commun. Biol. 2019, 2, 233. [Google Scholar] [CrossRef] [PubMed]
- Carrión, V.J.; Perez-Jaramillo, J.; Cordovez, V.; Tracanna, V.; De Hollander, M.; Ruiz-Buck, D.; Mendes, L.W.; van Ijcken, W.F.J.; Gomez-Exposito, R.; Elsayed, S.S.; et al. Pathogen-induced activation of disease-suppressive functions in the endophytic root microbiome. Science 2019, 366, 606–612. [Google Scholar] [CrossRef] [PubMed]
- MacLean, D.; Jones, J.D.; Studholme, D.J. Application of “next-generation” sequencing technologies to microbial genetics. Nat. Rev. Microbiol. 2009, 7, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Bellini, A.; Gilardi, G.; Idbella, M.; Zotti, M.; Pugliese, M.; Bonanomi, G.; Gullino, M.L. Trichoderma enriched compost, BCAs and potassium phosphite control Fusarium wilt of lettuce without affecting soil microbiome at genus level. Appl. Soil Ecol. 2023, 182, 104678. [Google Scholar] [CrossRef]
- Zhu, J.X.; Shang, M.N.; Li, R.; Liu, H.J.; Qiao, C.C.; Tao, C.Y.; Shen, Z.Z.; Shen, Q.R. Development and application of specific bio-organic fertilizer containing Trichoderma strain for mango orchard in tropic areas. Soil Fertil. Sci. China 2021, 3, 155–162. (In Chinese) [Google Scholar] [CrossRef]
- Zhu, J.X.; Zhang, G.; Shang, M.N.; Liu, H.J.; Shen, Z.Z.; Li, R.; Shen, Q.R. Isolation and identification of a thermostable Trichoderma strain for banana growth promotion in tropic area. Acta Microbiol. Sin. 2021, 61, 206–218. (In Chinese) [Google Scholar] [CrossRef]
- Liu, H.J.; Duan, W.D.; Liu, C.; Meng, L.X.; Li, H.X.; Li, R.; Shen, Q.R. Spore production in the solid-state fermentation of stevia residue by Trichoderma guizhouense and its effects on corn growth. J. Integr. Agric. 2021, 20, 1147–1156. [Google Scholar] [CrossRef]
- Liu, H.; Xiong, W.; Zhang, R.; Hang, X.; Wang, D.; Li, R.; Shen, Q. Continuous application of different organic additives can suppress tomato disease by inducing the healthy rhizospheric microbiota through alterations to the bulk soil microflora. Plant Soil 2018, 423, 229–240. [Google Scholar] [CrossRef]
- Li, R.; Shen, Z.; Sun, L.; Zhang, R.; Fu, L.; Deng, X.; Shen, Q. Novel soil fumigation method for suppressing cucumber Fusarium wilt disease associated with soil microflora alterations. Appl. Soil Ecol. 2016, 101, 28–36. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.S.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef]
- Cai, F.; Druzhinina, I.S. In honor of John Bissett: Authoritative guidelines on molecular identification of Trichoderma. Fungal Divers. 2021, 107, 1–69. [Google Scholar] [CrossRef]
- Liu, B.; Ji, S.; Zhang, H.; Wang, Y.; Liu, Z. Isolation of Trichoderma in the rhizosphere soil of Syringa oblata from Harbin and their biocontrol and growth promotion function. Microbiol. Res. 2020, 235, 126445. [Google Scholar] [CrossRef]
- Hao, D.; Lang, B.; Wang, Y.; Wang, X.; Liu, T.; Chen, J. Designing synthetic consortia of Trichoderma strains that improve antagonistic activities against pathogens and cucumber seedling growth. Microb. Cell Fact. 2022, 21, 234. [Google Scholar] [CrossRef]
- Sun, F.S.; Yu, G.H.; Ning, J.Y.; Zhu, X.D.; Goodman, B.A.; Wu, J. Biological removal of cadmium from biogas residues during vermicomposting, and the effect of earthworm hydrolysates on Trichoderma guizhouense sporulation. Bioresour. Technol. 2020, 312, 123635. [Google Scholar] [CrossRef]
- Xu, L.; Sun, K.; Wang, F.; Zhao, L.; Hu, J.; Ma, H.; Ding, Z. Laccase production by Trametes versicolor in solid-state fermentation using tea residues as substrate and its application in dye decolorization. J. Environ. Manag. 2020, 270, 110904. [Google Scholar] [CrossRef]
- Ghoreishi, G.; Barrena, R.; Font, X. Using green waste as substrate to produce biostimulant and biopesticide products through solid-state fermentation. Waste Manag. 2023, 159, 84–92. [Google Scholar] [CrossRef]
- Zhang, J.D.; Yang, Q. Optimization of solid-state fermentation conditions for Trichoderma harzianum using an orthogonal test. Genet. Mol. Res. 2015, 14, 1771–1781. [Google Scholar] [CrossRef]
- Zhang, C.; Khan, R.A.A.; Wei, H.; Wang, R.; Hou, J.; Liu, T. Rapid and mass production of biopesticide Trichoderma Brev T069 from cassava peels using newly established solid-state fermentation bioreactor system. J. Environ. Manag. 2022, 313, 114981. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Z.; Jiang, Q.; Roubík, H.; Xu, Q.; Cai, M.; Yang, K.; Sun, P. Fungal solid-state fermentation of crops and their by-products to obtain protein resources: The next frontier of food industry. Trends Food Sci. Technol. 2023, 138, 628–644. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Q.; Huang, Z.; Wang, Y.; Roubik, H.; Yang, K.; Cai, M.; Sun, P. Solid-State Fermentation of Soybean Meal with Edible Mushroom Mycelium to Improve Its Nutritional, Antioxidant Capacities and Physicochemical Properties. Fermentation 2023, 9, 322. [Google Scholar] [CrossRef]
- Pozo, M.J.; Zabalgogeazcoa, I.; de Aldana, B.R.V.; Martinez-Medina, A. Untapping the potential of plant mycobiomes for applications in agriculture. Curr. Opin. Plant Biol. 2021, 60, 102034. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Chen, W.; Wei, Z.; Pang, G.; Li, R.; Ran, W.; Shen, Q. Colonization of Trichoderma harzianum strain SQR-T037 on tomato roots and its relationship to plant growth, nutrient availability and soil microflora. Plant Soil 2015, 388, 337–350. [Google Scholar] [CrossRef]
- Liu, Q.; Meng, X.; Li, T.; Raza, W.; Liu, D.; Shen, Q. The growth promotion of peppers (Capsicum annuum L.) by Trichoderma guizhouense NJAU4742-based biological organic fertilizer: Possible role of increasing nutrient availabilities. Microorganisms 2020, 8, 1296. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Miao, Y.; Liu, Q.; Ma, L.; Guo, K.; Liu, D.; Ran, W.; Shen, Q. TgSWO from Trichoderma guizhouense NJAU4742 promotes growth in cucumber plants by modifying the root morphology and the cell wall architecture. Microb. Cell Fact. 2019, 18, 148. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, N.; Ventorino, V.; Woo, S.L.; Pepe, O.; De Rosa, A.; Gioia, L.; Romano, I.; Lombardi, N.; Napolitano, M.; Colla, G.; et al. Trichoderma-based biostimulants modulate rhizosphere microbial populations and improve N uptake efficiency, yield, and nutritional quality of leafy vegetables. Front. Plant Sci. 2018, 9, 743. [Google Scholar] [CrossRef]
- Stefan, L.; Hartmann, M.; Engbersen, N.; Six, J.; Schöb, C. Positive effects of crop diversity on productivity driven by changes in soil microbial composition. Front. Microbiol. 2021, 12, 660749. [Google Scholar] [CrossRef] [PubMed]
- Hang, X.N.; Meng, L.X.; Ou, Y.N.; Shao, C.; Xiong, W.; Zhang, N.; Liu, H.J.; Li, R.; Shen, Q.R.; Kowalchuk, G.A. Trichoderma-amended biofertilizer stimulates soil resident Aspergillus population for joint plant growth promotion. NPJ Biofilms Microb. 2022, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Li, J.; Li, Y.; Niu, Y.; He, T.; Liu, D.; Ding, W. Long-Term Compost Amendment Spurs Cellulose Decomposition by Driving Shifts in Fungal Community Composition and Promoting Fungal Diversity and Phylogenetic Relatedness. mBio 2022, 13, e0032322. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Xiao, Y.; Wang, Y.-F.; Liu, Z.-H.; Zhang, Y.-F.; Yang, K.-J. Trichoderma asperellum alters fungal community composition in saline-alkaline soil maize rhizospheres. Soil Sci. Soc. Am. J. 2021, 85, 1091–1104. [Google Scholar] [CrossRef]
- Zhang, F.; Huo, Y.; Cobb, A.B.; Luo, G.; Zhou, J.; Yang, G.; Wilson, G.W.T.; Zhang, Y. Trichoderma biofertilizer links to altered soil chemistry, altered microbial communities, and improved grassland biomass. Front. Microbiol. 2018, 9, 848. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Z.; Xiang, S.; Wang, X.; Zhang, J.; Bai, G.; Liu, H.; Li, R.; Shen, Q. Turning Waste into Wealth: Utilizing Trichoderma’s Solid-State Fermentation to Recycle Tea Residue for Tea Cutting Production. Agronomy 2024, 14, 526. https://doi.org/10.3390/agronomy14030526
Meng Z, Xiang S, Wang X, Zhang J, Bai G, Liu H, Li R, Shen Q. Turning Waste into Wealth: Utilizing Trichoderma’s Solid-State Fermentation to Recycle Tea Residue for Tea Cutting Production. Agronomy. 2024; 14(3):526. https://doi.org/10.3390/agronomy14030526
Chicago/Turabian StyleMeng, Zhen, Shuangshuang Xiang, Xue Wang, Jian Zhang, Guoxin Bai, Hongjun Liu, Rong Li, and Qirong Shen. 2024. "Turning Waste into Wealth: Utilizing Trichoderma’s Solid-State Fermentation to Recycle Tea Residue for Tea Cutting Production" Agronomy 14, no. 3: 526. https://doi.org/10.3390/agronomy14030526




