Diversity as a Plant Breeding Objective
Abstract
1. Introduction
2. Messages from Ecology
3. Messages from Medicine
4. Breeding Strategies to Increase Diversity
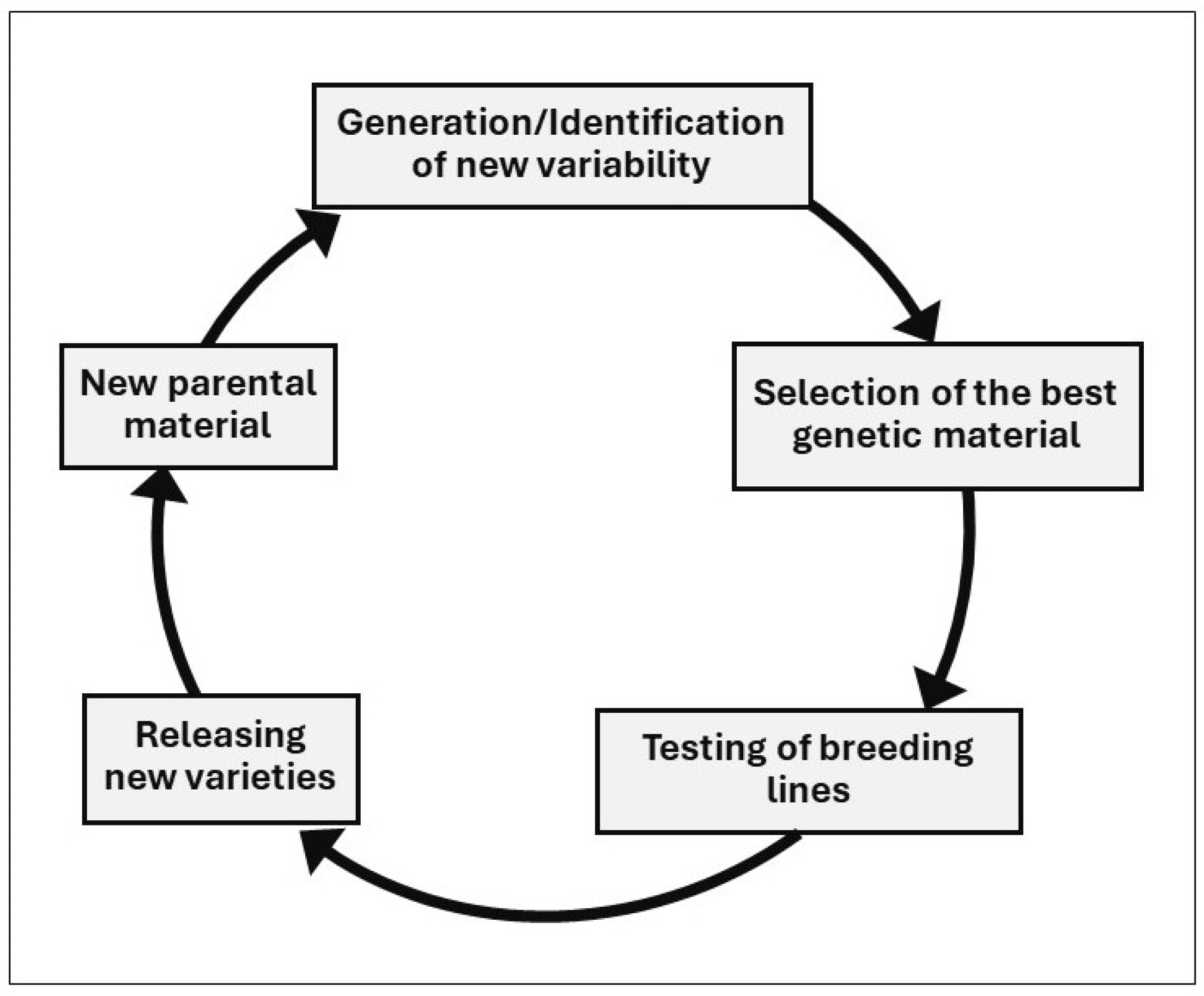
4.1. Plant Breeding
- Generating genetic variability; this includes the selection of parents, making crosses, choice of type and number of crosses, induced mutation, introduction of accessions from germplasm banks, or material from other breeding programs or from farmers;
- Selection of the best genetic material within the genetic variability created or acquired in stage one. This stage lasts a number of cropping seasons (5 to 10) depending on the crop and on the methodologies used;
- Testing of breeding lines; this includes comparisons between existing cultivars and the breeding lines emerging from stage 2 using the appropriate experimental designs and statistical analysis to conduct such comparisons. This stage lasts at least three cropping seasons, and it is followed by the variety registration, the seed production, and, eventually, by farmers’ adoption.
4.2. Participatory Plant Breeding
- Objectives and type of final product (known as product profile) are discussed and decided in collaboration with the clients—generally, but not exclusively, the farmers;
- The program is conducted in farmers’ fields and therefore is decentralized, i.e., conducted in the target environment(s) rather than in the research station(s);
- The clients participate in all the most important decisions, including the choice of parents to be used for crossing and in the selection stage.
4.3. Evolutionary Plant Breeding
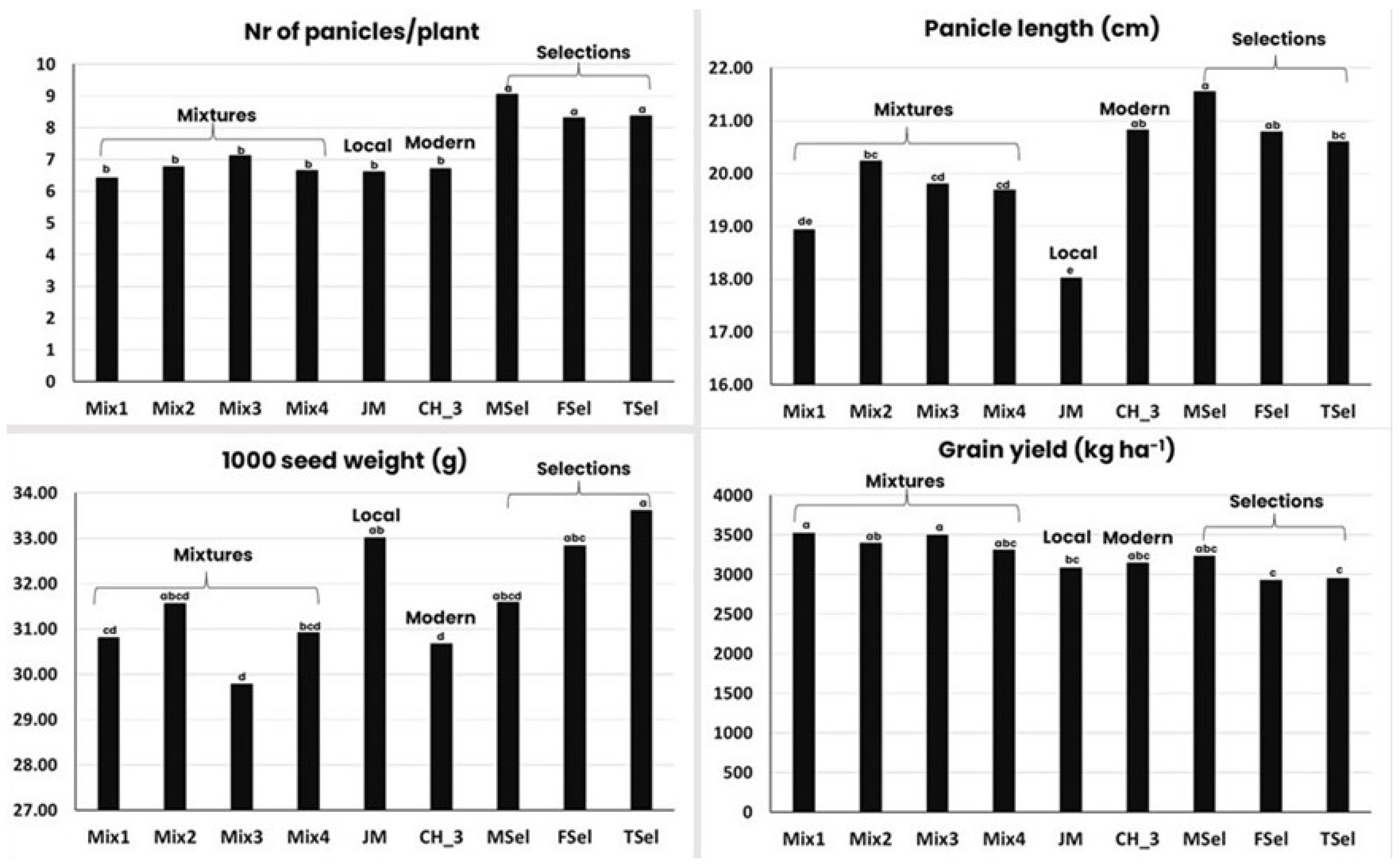
- They evolve, becoming more and more productive. The first indication of a progressive evolution towards a higher yield came from work on barley and lima bean [84], followed by experiments with barley [78,85,86,87]. A recent study on barley shows that EPs are as productive, in terms of grain yield, as commercial varieties under low input conditions [88]. This has been confirmed in a number of studies [87,89,90,91,92,93,94,95,96], indicating a higher resilience of populations and mixtures as expected from ecological studies;
- EPs, and to a lesser extent mixtures, have a more stable yield over time than uniform varieties, but not over space, i.e., they become specifically adapted to a given location. The original paper on the superior stability of populations over mixtures and of mixtures over pure lines was published in 1961 [91]; the paper reported the results of an experiment with lima beans in which stability was measured as the consistency of ranks. The results showed that while the pure lines were successful in many environments (high frequency of low ranks) and unsuccessful in many others (high frequency of high ranks), the mixtures and the populations were intermediate in any one environment. The higher stability of mixtures and populations has been confirmed in oats [97], wheat [90,93,98,99,100], and barley [88,92,101];
- EPs and mixtures evolve, becoming more and more resistant to diseases. This effect of populations and mixtures has been by far the most extensively studied by both breeders and pathologists [92,102,103,104,105,106,107,108,109,110]. The most important mechanism to explain the reduction in disease severity in mixtures and EPs is the dilution of inoculum that occurs due to the distance between plants of the same genotype [104]. However, there is also a large variation in the efficacy of mixtures in reducing disease incidence;
- The ability of EPs and mixtures to control weeds, a particularly important issue in organic farming where options to control weeds are limited, has not been studied as extensively as disease resistance. One hypothesis [111] claimed that communities with more diversity are more resistant to invasive species than communities with less diversity. A study conducted with a perennial species showed that the weed biomass decreased by 32% from a community consisting of a single genotype (thus corresponding to a uniform variety) to a community consisting of 12 different genotypes [112]. This result not only confirms the hypothesis above but also extends it from diversity between species to diversity within species, which is particularly relevant for mixtures and EPs.
4.4. Combining Participation with Evolutionary Plant Breeding
- The selected spikes are threshed individually, and the seed of each spike is planted separately in a row. This can be carried out by breeders on stations or, preferably, by farmers in their fields. If, because of technical problems, the head rows are planted on stations, they should only be multiplied, and selection should be delayed until the following year;
- If the head rows are planted in farmers’ fields, they should be planted under the same conditions (for example, reduced irrigation, shallow soil, or heavy weed infestation) in which they were selected in order to continue the selection;
- The seed collected on the selected rows can be handled in three different ways (Figure 2, paths 3, 4, and 5).
4.4.1. Spike Selection to Feed a PPB Program (Figure 2, Paths 2–3)
4.4.2. Spike Selection to Create Sub-Populations (Figure 2 Paths 2–4 or 2–5)
5. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2023. In Urbanization, Agrifood Systems Transformation and Healthy Diets across the Rural–Urban Continuum; FAO: Rome, Italy, 2023. [Google Scholar] [CrossRef]
- Kumar, J.; Choudhary, A.K.; Gupta, D.S.; Kumar, S. Towards Exploitation of Adaptive Traits for Climate-Resilient Smart Pulses. Int. J. Mol. Sci. 2019, 20, 2971. [Google Scholar] [CrossRef]
- Razzaq, A.; Kaur, P.; Akhter, N.; Wani, S.H.; Saleem, F. Next-Generation Breeding Strategies for Climate-Ready Crops. Front. Plant Sci. 2021, 12, 620420. [Google Scholar] [CrossRef]
- Varotto, S.; Tani, E.; Abraham, E.; Krugman, T.; Kapazoglou, A.; Melzer, R.; Radanović, A.; Miladinović, D. Epigenetics: Possible applications in climate-smart crop breeding. J. Exp. Bot. 2020, 71, 5223–5236. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.R.; Skea, J.; Slade, R.; van Diemen, R.; Haughey, E.; Malley, J.; Pathak, M.; Portugal Pereira, J. Technical Summary. In Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Shukla, P.R., Skea, J., Calvo Buendia, E., Masson-Delmotte, V., Pörtner, H.-O., Roberts, D.C., Zhai, P., Slade, R., Connors, S., van Diemen, R., et al., Eds.; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar] [CrossRef]
- United Nations Framework Convention on Climate Change. Climate Change: Impacts, Vulnerabilities and Adaptation in Developing Countries; United Nations: New York, NY, USA, 2007; Available online: https://unfccc.int/resource/docs/publications/impacts.pdf (accessed on 28 December 2023).
- Kambach, S.; Sabatini, F.M.; Attorre, F.; Biurrun, I.; Boenisch, G.; Bonari, G.; Čarni, A.; Carranza, M.L.; Chiarucci, A.; Chytrý, M.; et al. Climate-trait relationships exhibit strong habitat specificity in plant communities across Europe. Nat. Commun. 2023, 14, 712. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, K.; Matloob, A.; Aslam, F.; Florentine, S.K. Weeds in a Changing Climate: Vulnerabilities, Consequences, and Implications for Future Weed Management. Front. Plant Sci. 2017, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Copernicus: September 2023—Unprecedented Temperature Anomalies. 2023 on Track to be the Warmest Year on Record. Available online: https://climate.copernicus.eu/copernicus-september-2023-unprecedented-temperature-anomalies (accessed on 15 November 2023).
- Langridge, P.; Braun, H.; Hulke, B.; Ober, E.; Prasanna, B.M. Breeding crops for climate resilience. Theor. Appl. Genet. 2021, 134, 1607–1611. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Reynolds, M.; Xu, Y. Climate change challenges plant breeding. Curr. Opin. Plant Biol. 2022, 70, 102308. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, S.; Chapman, S.; Mahop, M.T.; Deva, C.; Masamba, K.; Mwamahonje, A. Exploring assumptions in crop breeding for climate resilience: Opportunities and principles for integrating climate model projections. Clim. Chang. 2021, 164, 38. [Google Scholar] [CrossRef]
- Manners, R.; van Etten, J. Are agricultural researchers working on the right crops to enable food and nutrition security under future climates? Glob. Environ. Chang. 2018, 53, 182–194. [Google Scholar] [CrossRef]
- Fess, T.L.; Kotcon, J.B.; Benedito, V.A. Crop Breeding for Low Input Agriculture: A Sustainable Response to Feed a Growing World Population. Sustainability 2011, 3, 1742–1772. [Google Scholar] [CrossRef]
- Brumlop, S.; Reichenbecher, W.; Tappeser, B.; Finckh, M.R. What is the SMARTest way to breed plants and increase agrobiodiversity? Euphytica 2013, 194, 53–66. [Google Scholar] [CrossRef]
- Frankel, O.H. The development and maintenance of superior genetic stocks. Heredity 1950, 4, 89–102. [Google Scholar] [CrossRef]
- Keneni, G.; Bekele, E.; Imtiaz, M.; Dagne, K. Genetic Vulnerability of Modern Crop Cultivars: Causes, Mechanism and Remedies. Int. J. Plant Res. 2012, 2, 69–79. [Google Scholar] [CrossRef]
- Louwaars, N.P. Plant breeding and diversity: A troubled relationship? Euphytica 2018, 214, 114. [Google Scholar] [CrossRef]
- Hufford, M.B.; Berny Mier y Teran, J.C.; Gepts, P. Crop Biodiversity: An Unfinished Magnum Opus of Nature. Annu. Rev. Plant Biol. 2019, 70, 727–751. [Google Scholar] [CrossRef]
- Gepts, P. Biocultural diversity and crop improvement. Emerg. Top. Life Sci. 2023, 7, 151–196. [Google Scholar] [CrossRef]
- Barot, S.; Allard, V.; Cantarel, A.; Enjalbert, J.; Gauffreteau, A.; Goldringer, I.; Lata, J.-C.; Le Roux, X.; Niboyet, A.; Porcher, E. Designing mixtures of varieties for multifunctional agriculture with the help of ecology: A review. Agron. Sustain. Dev. 2017, 37, 13. [Google Scholar] [CrossRef]
- Burger, J.R.; Allen, C.D.; Brown, J.H.; Burnside, W.R.; Davidson, A.D.; Fristoe, T.S.; Hamilton, M.J.; Mercado-Silva, N.; Nekola, J.C.; Okie, J.G.; et al. The Macroecology of Sustainability. PLoS Biol. 2012, 10, e1001345. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D. The ecological consequences of changes in biodiversity: A search for general principles. Ecology 1999, 80, 1455–1474. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Wright, J.P.; Cadotte, M.W.; Carroll, I.T.; Hector, A.; Srivastava, D.S.; Loreau, M.; Weis, J.J. Impacts of plant diversity on biomass production increase through time because of species complementarity. Proc. Natl. Acad. Sci. USA 2007, 104, 18123–181288. [Google Scholar] [CrossRef]
- Gross, K.; Cardinale, B.J.; Fox, J.W.; Gonzalez, A.; Loreau, M.; Polley, H.W.; Reich, P.B.; van Ruijven, J. Species richness and the temporal stability of biomass production: A new analysis of recent biodiversity experiments. Am. Nat. 2013, 183, 1–12. [Google Scholar] [CrossRef]
- Renard, D.; Tilman, D. National food production stabilized by crop diversity. Nature 2019, 571, 257–260. [Google Scholar] [CrossRef]
- McCann, K.S. The diversity–stability debate. Nature 2000, 404, 228–233. [Google Scholar] [CrossRef] [PubMed]
- May, R.M. Stability and Complexity in Model Ecosystems; Princeton University Press: Princeton, NJ, USA, 2001. [Google Scholar] [CrossRef]
- Tamburini, G.; Bommarco, R.; Wanger, C.T.; Kremen, C.; van der Heijden, M.G.A.; Liebman, M.; Hallin, S. Agricultural diversification promotes multiple ecosystem services without compromising yield. Sci. Adv. 2020, 6, eaba1715. [Google Scholar] [CrossRef] [PubMed]
- MacLaren, C.; Mead, A.; van Balen, D.; Claessens, L.; Etana, A.; de Haan, J.; Haagsma, W.; Jäck, O.; Keller, T.; Labuschagne, J.; et al. Long-term evidence for ecological intensification as a pathway to sustainable agriculture. Nat. Sustain. 2022, 5, 770–779. [Google Scholar] [CrossRef]
- Nelson, K.S.; Burchfield, E.K. Defining features of diverse and productive agricultural systems: An archetype analysis of U.S. agricultural counties. Front. Sustain. Food Syst. 2023, 7, 1081079. Available online: https://api.semanticscholar.org/CorpusID:257301561 (accessed on 28 December 2023). [CrossRef]
- Balvanera, P.; Pfisterer, A.B.; Buchmann, N.; He, J.-S.; Nakashizuka, T.; Raffaelli, D.; Schmid, B. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol. Lett. 2006, 9, 1146–1156. [Google Scholar] [CrossRef]
- Cardinale, B.; Srivastava, D.; Duffy, E.; Justin, J.; Wright, A.L. Downing, Mahesh Sankaran & Claire Jouseau. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 2006, 443, 989–992. [Google Scholar] [CrossRef]
- Stachowicz, J.J.; Bruno, J.F.; Duffy, J.E. Understanding the Effects of Marine Biodiversity on Communities and Ecosystems. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 739–766. [Google Scholar] [CrossRef]
- von Hertzen, L.; Hanski, I.; Haahtela, T. Natural immunity: Biodiversity loss and inflammatory diseases are two global megatrends that might be related. EMBO Rep. 2011, 12, 1089–1093. [Google Scholar] [CrossRef]
- Khamsi, R. A gut feeling about immunity. Nat. Med. 2015, 21, 674–676. [Google Scholar] [CrossRef]
- Sibhatu, K.T.; Qaim, N. Review: Meta-analysis of the association between production diversity, diets, and nutrition in smallholder farm households. Food Policy 2018, 77, 1–18. [Google Scholar] [CrossRef]
- Jones, A.D. Critical review of the emerging research evidence on agricultural biodiversity, diet diversity, and nutritional status in low- and middle-income countries. Nutr. Rev. 2017, 75, 769–782. [Google Scholar] [CrossRef]
- Mulmi, P.; Masters, W.A.; Ghosh, S.; Namirembe, G.; Rajbhandary, R.; Manohar, S.; Shrestha, S.; West, K.P.; Webb, P. Household food production is positively associated with dietary diversity and intake of nutrient-dense foods for older preschool children in poorer families: Results from a nationally-representative survey in Nepal. PLoS ONE 2017, 12, e0186765. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiota and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Carter, M.M.; Olm, M.R.; Merrill, B.D.; Dahan, D.; Tripathi, S.; Spencer, S.P.; Yu, F.B.; Jain, S.; Neff, N.; Jha, A.R.; et al. Ultra-deep sequencing of Hadza hunter-gatherers recovers vanishing gut microbes. Cell 2023, 186, 3111–3124.e13. [Google Scholar] [CrossRef]
- Ritz, N.L.; Brocka, M.; Butler, M.I.; Cowan, C.S.M.; Barrera-Bugueño, C.; Turkington, C.J.R.; Draper, L.A.; Bastiaanssen, T.F.S.; Turpin, V.; Morales, L.; et al. Social anxiety disorder-associated gut microbiota increases social fear. Proc. Natl. Acad. Sci. USA 2024, 121, e2308706120. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yin, F.; Guo, Z.; Li, R.; Sun, W.; Wang, Y.; Geng, Y.; Sun, C.; Sun, D. Association between gut microbiota and glioblastoma: A Mendelian randomization study. Front. Genet. 2024, 14, 1308263. [Google Scholar] [CrossRef]
- Galván-Peña, S.; Zhu, Y.; Hanna, B.S.; Mathis, D.; Benoist, C. A dynamic atlas of immunocyte migration from the gut. Sci. Immunol. 2024, 9, eadi0672. [Google Scholar] [CrossRef] [PubMed]
- Heiman, M.L.; Greenway, F.L. A healthy gastrointestinal microbiome is dependent on dietary diversity. Mol. Metab. 2016, 5, 317–320. [Google Scholar] [CrossRef]
- Ceccarelli, S. Nurturing diversity in our guts and on our farms to reduce health risks and increase food system resilience. In Bioversity International, Agrobiodiversity Index Report: Risk and Resilience; Bioversity International: Rome, Italy, 2019; pp. 107–113. [Google Scholar]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet Comm. 2019, 393, 447–492. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.; Goldman, I.; Ortiz, R. Pursuing the Potential of Heirloom Cultivars to Improve Adaptation, Nutritional, and Culinary Features of Food Crops. Agronomy 2019, 9, 441. [Google Scholar] [CrossRef]
- Dinu, M.; Whittaker, A.; Pagliai, G.; Benedettelli, S.; Sofi, F. Ancient wheat species and human health: Biochemical and clinical implications. J. Nutr. Biochem. 2018, 52, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gotti, R.; Amadesi, E.; Fiori, J.; Bosi, S.; Bregola, V.; Marotti, I.; Dinelli, G. Differentiation of modern and ancient varieties of common wheat by quantitative capillary electrophoretic profile of phenolic acids. J. Chromatogr. A 2018, 1532, 208–215. [Google Scholar] [CrossRef]
- Sofi, F.; Dinu, M.; Pagliai, G.; Cei, L.; Sacchi, G.; Benedettelli, D.; Stefani, G.; Gagliardi, E.; Tosi, P.; Bocci, R.; et al. Health and Nutrition Studies Related to Cereal Biodiversity: A Participatory Multi-Actor Literature Review Approach. Nutrients 2018, 10, 1207. [Google Scholar] [CrossRef]
- Medina-Lozano, I.; Díaz, A. Nutritional Value and Phytochemical Content of Crop Landraces and Traditional Varieties. In Landraces; Elkelish, A., Ed.; IntechOpen: Rijeka, Croatia, 2021; pp. 1–22. [Google Scholar] [CrossRef]
- Kumar, N.; Harris, J.; Rawat, R. If They Grow It, Will They Eat and Grow? Evidence from Zambia on Agricultural Diversity and Child Undernutrition. J. Dev. Stud. 2015, 51, 1060–1077. [Google Scholar] [CrossRef]
- Saaka, M.; Osman, S.M.; Hoeschle-Zeledon, I. Relationship between agricultural biodiversity and dietary diversity of children aged 6–36 months in rural areas of Northern Ghana. Food Nutr. Res. 2017, 61, 1391668. [Google Scholar] [CrossRef]
- Luna-González, D.V.; Sørensen, M. Higher agrobiodiversity is associated with improved dietary diversity, but not child anthropometric status, of Mayan Achí people of Guatemala. Public Health Nutr. 2018, 21, 2128–2141. [Google Scholar] [CrossRef]
- Tollefson, J. Why deforestation and extinctions make pandemics more likely. Nature 2020, 584, 175. [Google Scholar] [CrossRef]
- Schnell, F.W. A synoptic study of the methods and categories of plant breeding. Z. Pflanzenzüchtung 1982, 89, 1–18. [Google Scholar]
- Galluzzi, G.; Seyoum, A.; Halewood, M.; López Noriega, I.; Welch, E.W. The Role of Genetic Resources in Breeding for Climate Change: The Case of Public Breeding Programmes in Eighteen Developing Countries. Plants 2020, 9, 1129. [Google Scholar] [CrossRef]
- Ceccarelli, S. Main stages of a plant breeding programme. In Plant Breeding and Farmer Participation; Ceccarelli, S., Guimaraes, E.P., Weltzien, E., Eds.; FAO: Rome, Italy, 2009; pp. 63–74. [Google Scholar]
- Venuprasad, R.; Lafitte, H.R.; Atlin, G.N. Response to Direct Selection for Grain Yield under Drought Stress in Rice. Crop Sci. 2007, 47, 285–293. [Google Scholar] [CrossRef]
- Barah, B.C.; Binswanger, H.P.; Rana, B.S.; Rao, N.G.P. The use of risk aversion in plant breeding: Concept and application. Euphytica 1981, 30, 451–458. [Google Scholar] [CrossRef]
- Lin, C.S.; Binns, M.R. A method of analyzing cultivar × location × year experiments: A new stability parameter. Theor. Appl. Genet. 1988, 76, 425–430. [Google Scholar] [CrossRef]
- Evans, L.T. Crop Evolution, Adaptation and Yield; Cambridge University Press: New York, NY, USA, 1993. [Google Scholar]
- Baranski, M.R. The Globalization of Wheat: A Critical History of the Green Revolution; University of Pittsburgh Press: Pittsburgh, PA, USA, 2022; p. 247. [Google Scholar]
- Ceccarelli, S. Wide Adaptation. How Wide? Euphytica 1989, 40, 197–205. [Google Scholar] [CrossRef]
- Hammer, K.; Knüpffer, H.; Xhuveli, L.; Perrino, P. Estimating genetic erosion in landraces—Two case studies. Genet. Resour. Crop Evol. 1996, 43, 329–336. [Google Scholar] [CrossRef]
- Eriksson, D. The evolving EU regulatory framework for precision breeding. Theor. Appl. Genet. 2019, 132, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Yadav, A.; Ahmad, R.; Dwivedi, U.N.; Yadav, K. CRISPR-Based Genome Editing for Nutrient Enrichment in Crops: A Promising Approach Toward Global Food Security. Front. Genet. 2022, 13, 932859. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, S.; Grando, S.; Bailey, E.; Amri, A.; El Felah, M. Farmer Participation in Barley Breeding in Syria, Morocco and Tunisia. Euphytica 2001, 122, 521–536. [Google Scholar] [CrossRef]
- Bellon, M.R.; Morris, M.L. Linking Global and Local Approaches to Agricultural Technology Development: The Role of Participatory Plant Breeding Research in the CGIAR; Economic Working Paper 02–03; International Maize and Wheat Improvement Center: El Batan, Mexico, 2003. [Google Scholar]
- Colley, M.R.; Dawson, J.C.; McCluskey, C.; Myers, J.R.; Tracy, W.F.; Lammerts van Bueren, E.T. Exploring the emergence of participatory plant breeding in countries of the Global North—A review. J. Agric. Sci. 2021, 159, 320–338. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Grando, S.; Tutwiler, R.; Baha, J.; Martini, A.M. A Methodological Study on Participatory Barley Breeding. I. Selection Phase. Euphytica 2000, 111, 91–104. [Google Scholar] [CrossRef]
- Ceccarelli, S. Efficiency of plant breeding. Crop Sci. 2015, 55, 87–97. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Grando, S. Participatory Plant Breeding: Who did it, who does it and where? Exp. Agric. 2020, 56, 1–11. [Google Scholar] [CrossRef]
- Shelton, A.C.; Tracy, W.F. Recurrent Selection and Participatory Plant Breeding for Improvement of Two Organic Open-Pollinated Sweet Corn (Zea mays L.) Populations. Sustainability 2015, 7, 5139–5152. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Grando, S. Organic agriculture and evolutionary populations to merge mitigation and adaptation strategies to fight climate change. South Sustain. 2020, 1, e002. [Google Scholar] [CrossRef]
- Descheemaeker, K.; Ronner, E.; Ollenburger, M.; Franke, A.; Klapwijk, C.; Falconnier, G.N.; Wichern, J.; Giller, K. Which Options Fit Best? Operationalizing The Socio-Ecological Niche Concept. Exp. Agric. 2019, 55, 169–190. [Google Scholar] [CrossRef]
- Suneson, C.A. An Evolutionary Plant Breeding Method. Agron. J. 1956, 48, 188–191. [Google Scholar] [CrossRef]
- Harlan, H.V.; Martini, M.L. A composite hybrid mixture. J. Am. Soc. Agron. 1929, 21, 487–490. [Google Scholar] [CrossRef]
- Wolfe, M.S.; Ceccarelli, S. The need to use more diversity in cereal cropping requires more descriptive precision. J. Sci. Food Agric. 2020, 100, 4119–4123. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, S.; Grando, S. Evolutionary Plant Breeding with an Introduction to Participatory Plant Breeding; Mimesis Edizioni Srl: Milano, Italy, 2022; 175p, Available online: https://archive.org/details/evolutionary-plant-breeding (accessed on 28 December 2023).
- Allard, R.W.; Jain, S.K. Population Studies in Predominantly Self-Pollinated Species. II. Analysis of Quantitative Genetic Changes in a Bulk-Hybrid Population of Barley. Evolution 1962, 16, 90–101. [Google Scholar] [CrossRef]
- Rhoné, B.; Vitalis, R.; Goldringer, I.; Bonnin, I. Evolution of flowering time in experimental wheat populations: A comprehensive approach to detect genetic signatures of natural selection. Evolution 2010, 64, 2110–2125. [Google Scholar] [CrossRef] [PubMed]
- Allard, R.W.; Hansche, P.E. Some parameters of population variability and their implications in plant breeding. Adv. Agron. 1964, 16, 281–325. [Google Scholar] [CrossRef]
- Patel, J.D.; Reinbergs, E.; Mather, D.E.; Choo, T.M.; Sterling, J.D.E. Natural selection in a double-haploid mixture and a composite cross of barley. Crop Sci. 1987, 27, 474–479. [Google Scholar] [CrossRef]
- Rasmusson, D.C.; Beard, B.H.; Johnson, F.K. Effect of Natural Selection on Performance of a Barley Population. Crop Sci. 1967, 7, 543. [Google Scholar] [CrossRef]
- Soliman, K.M.; Allard, R.W. Grain Yield of Composite Cross Populations of Barley: Effects of Natural Selection. Crop Sci. 1991, 31, 705–708. [Google Scholar] [CrossRef]
- Raggi, L.; Ciancaleoni, S.; Torricelli, R.; Terzi, V.; Ceccarelli, S.; Negri, V. Evolutionary breeding for sustainable agriculture: Selection and multi-environmental evaluation of barley populations and lines. Field Crops Res. 2017, 204, 76–88. [Google Scholar] [CrossRef]
- Goldringer, I.; Enjalbert, J.; David, J.; Paillard, S.; Pham, J.; Brabant, P. Dynamic management of genetic resources: A 13-year experiment on wheat. In Broadening the Genetic Base of Crop Production; Cooper, H., Spillane, C., Hodgkin, T., Eds.; CABI: Wallingford, UK, 2001; pp. 245–260. [Google Scholar]
- Baresel, J.P.; Bülow, L.; Finckh, M.R.; Frese, L.; Knapp, S.; Schmidhalter, U.; Weedon, O.D. Performance and evolutionary adaptation of heterogeneous wheat populations. Euphytica 2022, 218, 137. [Google Scholar] [CrossRef]
- Allard, R.W. Relationship Between Genetic Diversity and Consistency of Performance in Different Environments. Crop Sci. 1961, 1, 127–133. [Google Scholar] [CrossRef]
- Kiær, L.P.; Skovgaard, I.M.; Østergård, H. Effects of inter-varietal diversity, biotic stresses and environmental productivity on grain yield of spring barley variety mixtures. Euphytica 2012, 185, 123–138. [Google Scholar] [CrossRef]
- Brumlop, S.; Pfeiffer, T.; Finckh, M. Evolutionary Effects on Morphology and Agronomic Performance of Three Winter Wheat Composite Cross Populations Maintained for Six Years under Organic and Conventional Conditions. Org. Farming 2017, 3, 34–50. [Google Scholar] [CrossRef]
- Weedon, O.D.; Finckh, M.R. Heterogeneous Winter Wheat Populations Differ in Yield Stability Depending on their Genetic Background and Management System. Sustainability 2019, 11, 6172. [Google Scholar] [CrossRef]
- Weedon, O.D.; Finckh, M.R. Response of Wheat Composite Cross Populations to Disease and Climate Variation Over 13 Generations. Front. Agric. Sci. Eng. 2021, 8, 400–415. [Google Scholar]
- Weedon, O.D.; Brumlop, S.; Haak, A.; Baresel, J.P.; Borgen, A.; Döring, T.; Goldringer, I.; Lammerts van Bueren, E.; Messmer, M.M.; Mikó, P.; et al. High Buffering Potential of Winter Wheat Composite Cross Populations to Rapidly Changing Environmental Conditions. Agronomy 2023, 13, 1662. [Google Scholar] [CrossRef]
- Frey, K.J.; Maldonado, U. Relative Productivity of Homogeneous and Heterogeneous Oat Cultivars in Optimum and Suboptimum Environments. Crop Sci. 1967, 7, 532–535. [Google Scholar] [CrossRef]
- Dubin, H.J.; Wolfe, M.S. Comparative behavior of three wheat cultivars and their mixture in India, Nepal and Pakistan. Field Crops Res. 1994, 39, 71–83. [Google Scholar] [CrossRef]
- Merrick, L.F.; Lyon, S.R.; Balow, K.A.; Murphy, K.M.; Jones, S.S.; Carter, A.H. Utilization of evolutionary plant breeding increases stability and adaptation of winter wheat across diverse precipitation zones. Sustainability 2020, 12, 9728. [Google Scholar] [CrossRef]
- van Frank, G.; Rivière, P.; Pin, S.; Baltassat, R.; Berthellot, J.-F.; Caizergues, F.; Dalmasso, C.; Gascuel, J.-S.; Hyacinthe, A.; Mercier, F.; et al. Genetic Diversity and Stability of Performance of Wheat Population Varieties Developed by Participatory Breeding. Sustainability 2020, 12, 384. [Google Scholar] [CrossRef]
- Salimi, M.; Razavi, K.C.; Amiri, M.N.; Esmaeili, M.; Khorramdel, S.; Moghani, H.; Grando, S.; Ceccarelli, S. Stability of Agronomic Traits of Barley Evolutionary Populations under Drought Conditions in Iran. Agronomy 2023, 13, 1931. [Google Scholar] [CrossRef]
- Simmonds, N.W. Variability in Crop Plants, Its Use and Conservation. Biol. Rev. 1962, 37, 422–465. [Google Scholar] [CrossRef]
- Smithson, J.B.; Lenné, J.M. Varietal mixtures: A viable strategy for sustainable productivity in subsistence agriculture. Ann. Appl. Biol. 1996, 128, 127–158. [Google Scholar] [CrossRef]
- Mundt, C.C. Use of multiline cultivars and cultivar mixtures for disease management. Annu. Rev. Phytopathol. 2002, 40, 381–410. [Google Scholar] [CrossRef]
- Jackson, L.F.; Webster, R.K.; Allard, R.W.; Kahler, A.L. Genetic Analysis of Changes in Scald Resistance in Barley Composite Cross V. Phytopathology 1982, 72, 1069–1072. [Google Scholar] [CrossRef]
- Allard, R.W. The Genetics of Host-Pathogen Coevolution: Implications for Genetic Resource Conservation. J. Hered. 1990, 81, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, K.M.; Barrett, J.A. Evolution of mildew resistance in a hybrid bulk population of barley. Heredity 1991, 67, 247–256. [Google Scholar] [CrossRef]
- McDonald, B.A.; Allard, R.W.; Webster, R.K. Responses of Two, Three, and Four Component Barley Mixtures to a Variable Pathogen Population. Crop Sci. 1988, 28, 447–452. [Google Scholar] [CrossRef]
- Finckh, M.R.; Gacek, E.; Goyeau, H.; Lannou, C.; Merz, U.; Mundt, C.C.; Munk, L.; Nadziak, L.; Newton, A.C.; de Vallavieille-Popec, C.; et al. Cereal variety and species mixtures in practice, with emphasis on disease resistance. Agron. EDP Sci. 2000, 20, 813–837. [Google Scholar] [CrossRef]
- Finckh, M.R.; Wolfe, M. Diversification strategies. In The Epidemiology of Plant Diseases; Cooke, B., Jones, D., Kaye, B., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 269–307. [Google Scholar] [CrossRef]
- Elton, C.S. The Ecology of Invasions by Animals and Plants; Methuen: London, UK, 1958. [Google Scholar]
- Crutsinger, G.M.; Souza, L.; Sanders, N.J. Intraspecific diversity and dominant genotypes resist plant invasions. Ecol. Lett. 2008, 11, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Lemerle, D.; Gill, G.S.; Murphy, C.E.; Walker, S.R.; Cousens, R.D.; Mokhtari, S.; Peltzer, S.J.; Coleman, R.; Luckett, D.J. Genetic improvement and agronomy for enhanced wheat competitiveness with weeds. Aust. J. Agric. Res. 2001, 52, 527–548. [Google Scholar] [CrossRef]
- Bertholdsson, N.O. Early vigour and allelopathy—Two useful traits for enhanced barley and wheat competitiveness against weeds. Weed Res. 2005, 45, 94–102. [Google Scholar] [CrossRef]
- Lazzaro, M.; Costanzo, A.; Farag, D.H.; Bàrberi, P. Grain yield and competitive ability against weeds in modern and heritage common wheat cultivars are differently influenced by sowing density. Ital. J. Agron. 2017, 12, 343–349. [Google Scholar] [CrossRef]
- Hoad, S.P.; Bertholdsson, N.O.; Neuhoff, D.; Köpke, U. Approaches to Breed for Improved Weed Suppression in Organically Grown Cereals. In Organic Crop Breeding; Lammerts van Bueren, E., Myers, J.R., Eds.; Wiley-Blackwell: Chichester, UK; Ames, IA, USA, 2012; pp. 61–76. [Google Scholar] [CrossRef]
- Hockett, E.A.; Eslick, R.F.; Qualset, C.O.; Dubbs, A.L.; Stewart, V.R. Effects of Natural Selection in Advanced Generations of Barley Composite Cross II. Crop Sci. 1983, 23, 752–756. [Google Scholar] [CrossRef]
- Goldringer, I.; Enjalbert, J.; Raquin, A.-L.; Brabant, P. Strong selection in wheat populations during ten generations of dynamic management. Genet. Sel. Evol. 2001, 33 (Suppl. S1), S441–S463. [Google Scholar] [CrossRef]
- Knapp, S.; Döring, T.F.; Jones, H.E.; Snape, J.; Wingen, L.U.; Wolfe, M.S.; Leverington-Waite, M.; Griffiths, S. Natural Selection Towards Wild-Type in Composite Cross Populations of Winter Wheat. Front. Plant Sci. 2020, 10, 1757. [Google Scholar] [CrossRef]
- Wright, A.J.; de Kroon, H.; Visser, E.J.W.; Buchmann, T.; Ebeling, A.; Eisenhauer, N.; Fischer, C.; Hildebrandt, A.; Ravenek, J.; Roscher, C.; et al. Plants are less negatively affected by flooding when growing in species-rich plant communities. New Phytol. 2017, 213, 645–656. [Google Scholar] [CrossRef]
- Wolfe, M.S.; Brändle, U.; Koller, B.; Limpert, E.; McDermott, J.M.; Müller, K.; Schaffner, D. Barley mildew in Europe: Population biology and host resistance. Euphytica 1992, 63, 125–139. [Google Scholar] [CrossRef]
- Winge, T. Seed Legislation in Europe and Crop Genetic Diversity. In Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer: Cham, Switzerland, 2015; Volume 15. [Google Scholar]
- Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32014D0150 (accessed on 29 November 2023).
- Available online: https://food.ec.europa.eu/system/files/2022-04/prm_temp-exp_pop-exp_en.pdf (accessed on 28 January 2024).
- Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32018R0848 (accessed on 29 November 2023).
- Ceccarelli, S. Plant Breeding with Farmers—A Technical Manual; ICARDA: Aleppo, Syria, 2012; pp. xi + 126. [Google Scholar]
- Neupane, S.P.; Joshi, B.K.; Ayer, D.K.; Ghimire, K.H.; Gauchan, D.; Karkee, A.; Jarvis, D.J.; Mengistu, D.K.; Grando, S.; Ceccarelli, S. Farmers’ Preferences and Agronomic Evaluation of Dynamic Mixtures of Rice and Bean in Nepal. Diversity 2023, 15, 660. [Google Scholar] [CrossRef]
- Cobb, J.N.; Juma, R.U.; Biswas, P.S.; Arbelaez, J.D.; Rutkoski, J.; Atlin, G.; Hagen, T.; Quinn, M.; Ng, E.H. Enhancing the rate of genetic gain in public-sector plant breeding programs: Lessons from the breeder’s equation. Theor. Appl. Genet. 2019, 132, 627–645. [Google Scholar] [CrossRef] [PubMed]
- Fadda, C.; Mengistu, D.J.; Kidane, Y.G.; Dell’Acqua, M.; Pè, M.E.; Van Etten, J. Integrating Conventional and Participatory Crop Improvement for Smallholder Agriculture Using the Seeds for Needs Approach: A Review. Front. Plant Sci. 2020, 11, 559515. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, P.; Zou, C.; Lu, Y.; Xie, C.; Zhang, X.; Prasanna, B.M.; Olsen, M.S. Enhancing genetic gain in the era of molecular breeding. J. Exp. Bot. 2017, 68, 2641–2666. [Google Scholar] [CrossRef]
- De Souza, A.P.; Burgess, S.J.; Doran, L.; Hansen, J.; Manukyan, L.; Maryn, N.; Gotarkar, D.; Leonelli, L.; Niyogi, K.K.; Long, S.P. Soybean photosynthesis and crop yield are improved by accelerating recovery from photoprotection. Science 2022, 377, 851–854. [Google Scholar] [CrossRef]
- Kromdijk, J.; Głowacka, K.; Leonelli, L.; Gabilly, S.T.; Iwai, M.; Niyogi, K.K.; Long, S.P. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 2016, 354, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Li, X.; Lu, Z.; Zhang, H.; Ye, X.; Zhou, Y.; Li, J.; Yan, Y.; Pei, H.; Duan, F.; et al. A transcriptional regulator that boosts grain yields and shortens the growth duration of rice. Science 2022, 377, eabi8455. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, S.; Yu, L.; Xiao, Y.; Zhang, S.; Wang, X.; Xu, Y.; Yu, H.; Li, Y.; Yang, J.; et al. RNA demethylation increases the yield and biomass of rice and potato plants in field trials. Nat. Biotechnol. 2021, 39, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lawit, S.J.; Weers, B.; Sun, J.; Mongar, N.; Van Hemert, J.; Melo, R.; Meng, X.; Rupe, M.; Clapp, J.; et al. Overexpression of zmm28 increases maize grain yield in the field. Proc. Natl. Acad. Sci. USA 2019, 116, 23850–23858. [Google Scholar] [CrossRef]
- Khaipho-Burch, M.; Cooper, M.; Crossa, J.; de Leon, N.; Holland, J.; Lewis, R.; McCouch, S.; Murray, S.C.; Rabbi, I.; Ronald, P.; et al. Scale up trials to validate modified crops’ benefits. Nature 2023, 621, 470–473. [Google Scholar] [CrossRef]
- Fischer, K.; Rock, J.S. The scientific narrative around new food technologies needs to change. Nat. Rev. Bioeng. 2023, 1, 786–787. [Google Scholar] [CrossRef]
- Robinson, R.A. Return to Resistance—Breeding Crops to Reduce Pesticide Dependence; agAccess: Davis, CA, USA, 1996; 480p, ISBN 0-932857-17-5. [Google Scholar]
- Wolfe, M.S. Crop strength through diversity. Nature 2000, 406, 681–682. [Google Scholar] [CrossRef]
- Ceccarelli, S. GMO, Organic Agriculture and Breeding for Sustainability. Sustainability 2014, 6, 4273–4286. [Google Scholar] [CrossRef]
- Vencill, W.; Nichols, R.; Webster, T.; Soteres, J.; Mallory-Smith, C.; Burgos, N.; Johnson, W.G.; McClelland, M. Herbicide Resistance: Toward an Understanding of Resistance Development and the Impact of Herbicide-Resistant Crops. Weed Sci. 2012, 60, 2–30. [Google Scholar] [CrossRef]
- Wolfe, M.S.; Finckh, M.R. Diversity of host resistance within the crop: Effects on host, pathogen and disease. In Plant Resistance to Fungal Diseases; Hartleb, H., Heitefuss, R., Hoppe, H.H., Eds.; G. Fischer Verlag: Jena, Germany, 1997; pp. 378–400. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceccarelli, S.; Grando, S. Diversity as a Plant Breeding Objective. Agronomy 2024, 14, 550. https://doi.org/10.3390/agronomy14030550
Ceccarelli S, Grando S. Diversity as a Plant Breeding Objective. Agronomy. 2024; 14(3):550. https://doi.org/10.3390/agronomy14030550
Chicago/Turabian StyleCeccarelli, Salvatore, and Stefania Grando. 2024. "Diversity as a Plant Breeding Objective" Agronomy 14, no. 3: 550. https://doi.org/10.3390/agronomy14030550
APA StyleCeccarelli, S., & Grando, S. (2024). Diversity as a Plant Breeding Objective. Agronomy, 14(3), 550. https://doi.org/10.3390/agronomy14030550








