Ecofriendly Application of Calabrese Broccoli Stalk Waste as a Biosorbent for the Removal of Pb(II) Ions from Aqueous Media
Abstract
1. Introduction
2. Experimental
2.1. Chemical Reagents and Equipment
2.2. Preparation of Broccoli Stalks as a Natural Sorbent
2.3. Methodologies for Biomass Characterisation
2.4. Metal Removal Experiments
2.5. Sorption Isotherms
2.6. Sorption Kinetics
2.7. Thermodynamic Studies
3. Results and Discussion
3.1. Previous Studies to Select the Biomass Preparation
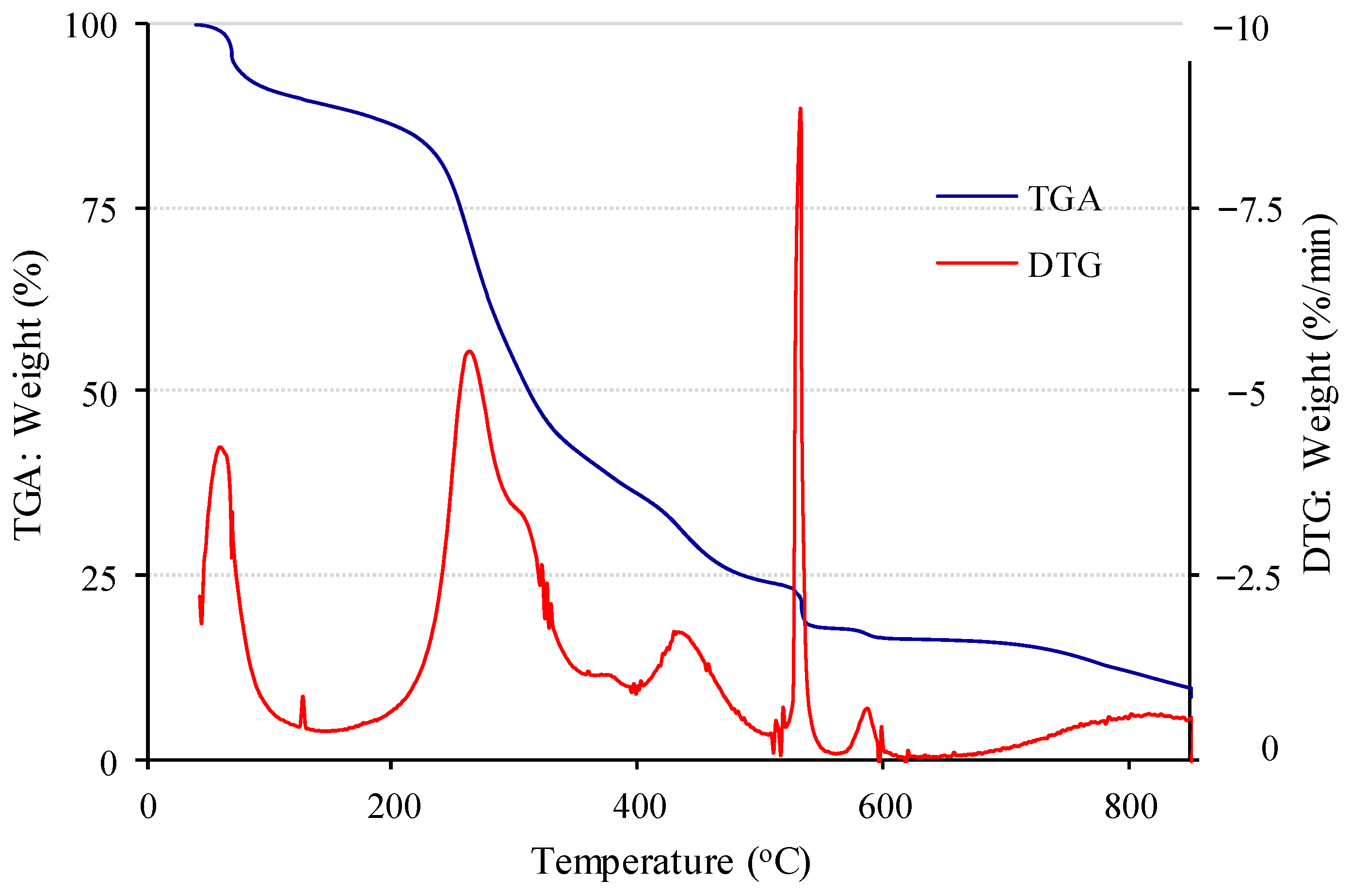
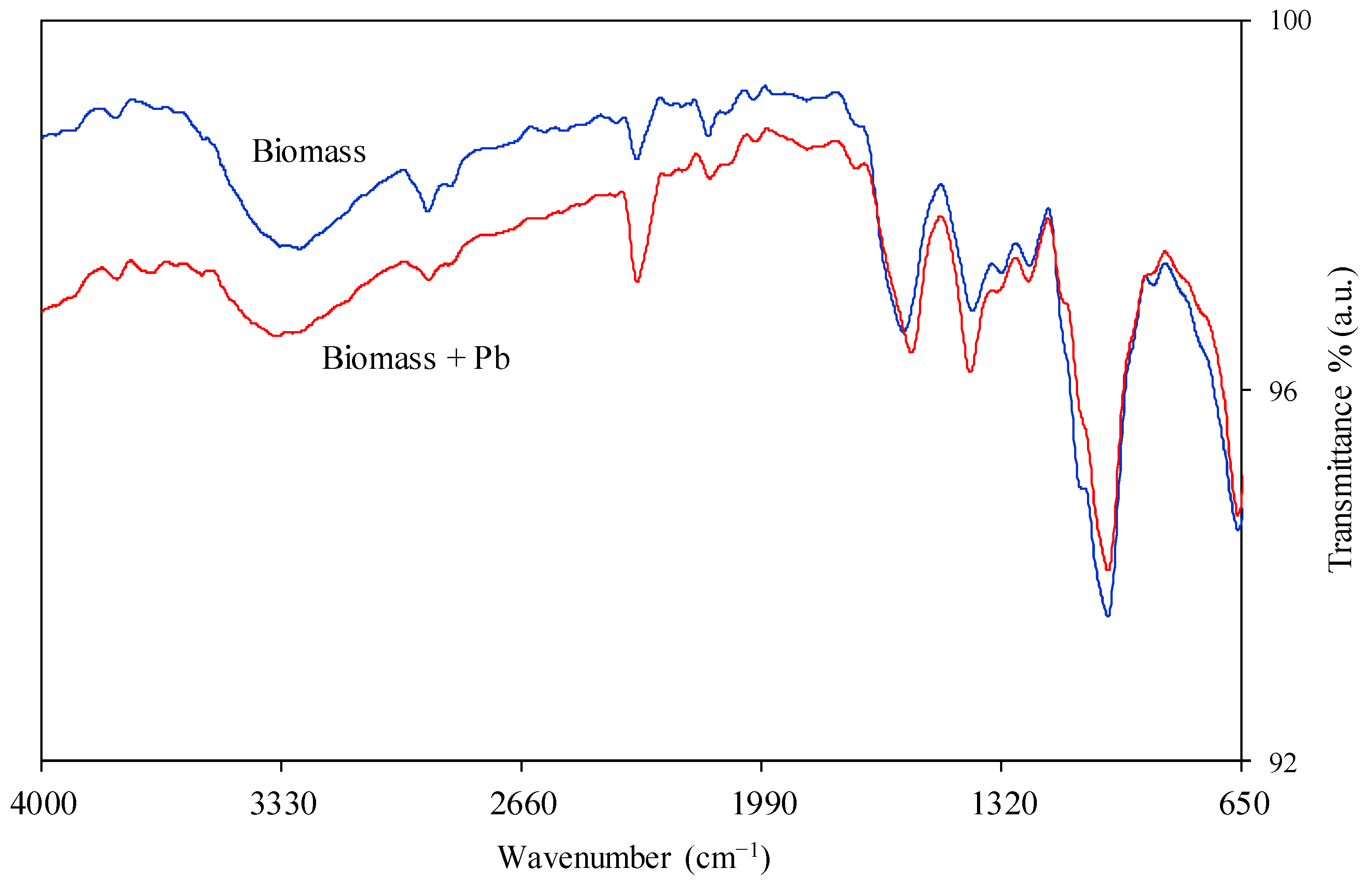
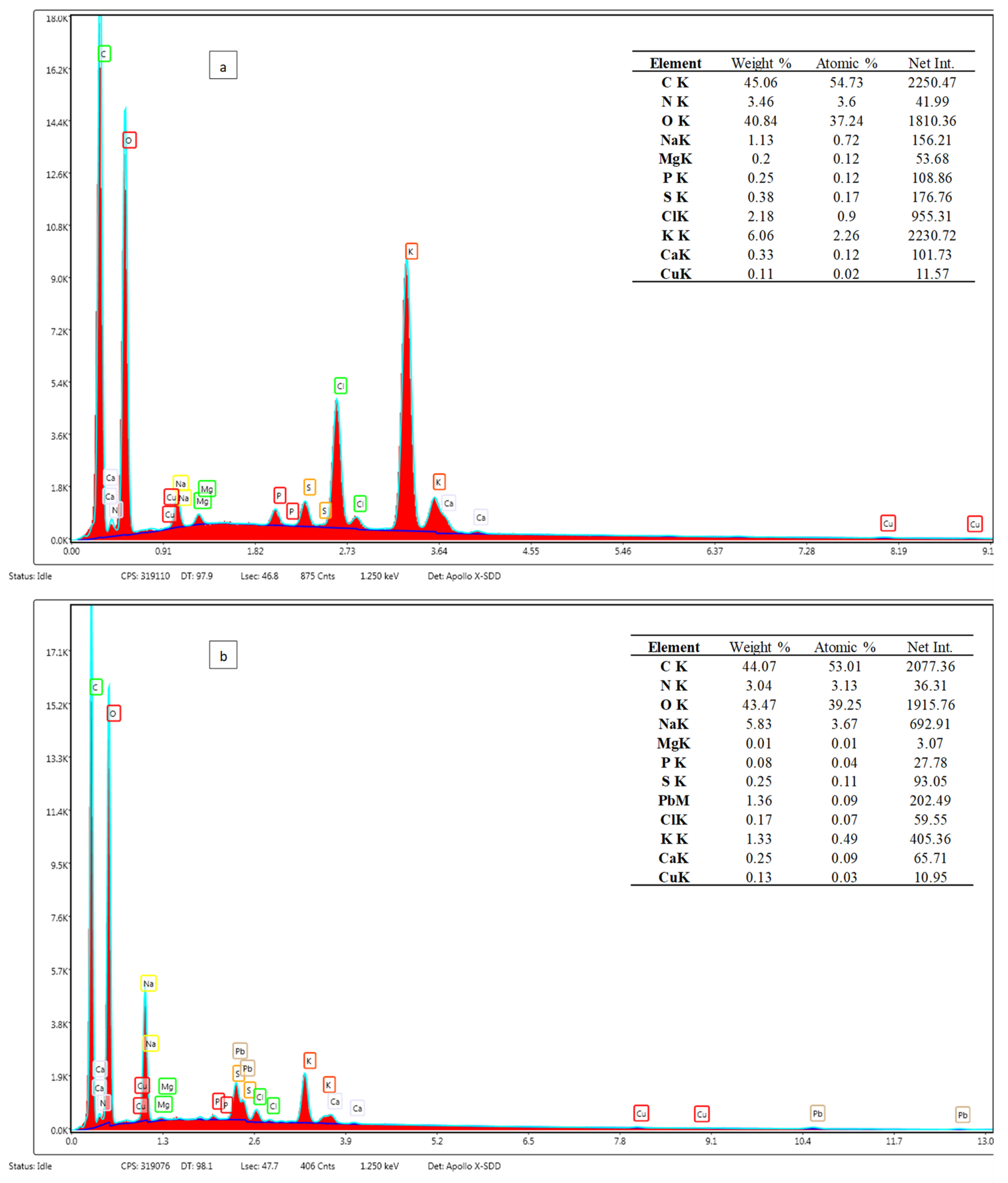
3.2. Sorbent Characterisation
3.3. Metal Removal Experiments
3.3.1. Effect of Ratio Mass of Biosorbent/Volume of Pb(II) Solution
3.3.2. Effect of pH
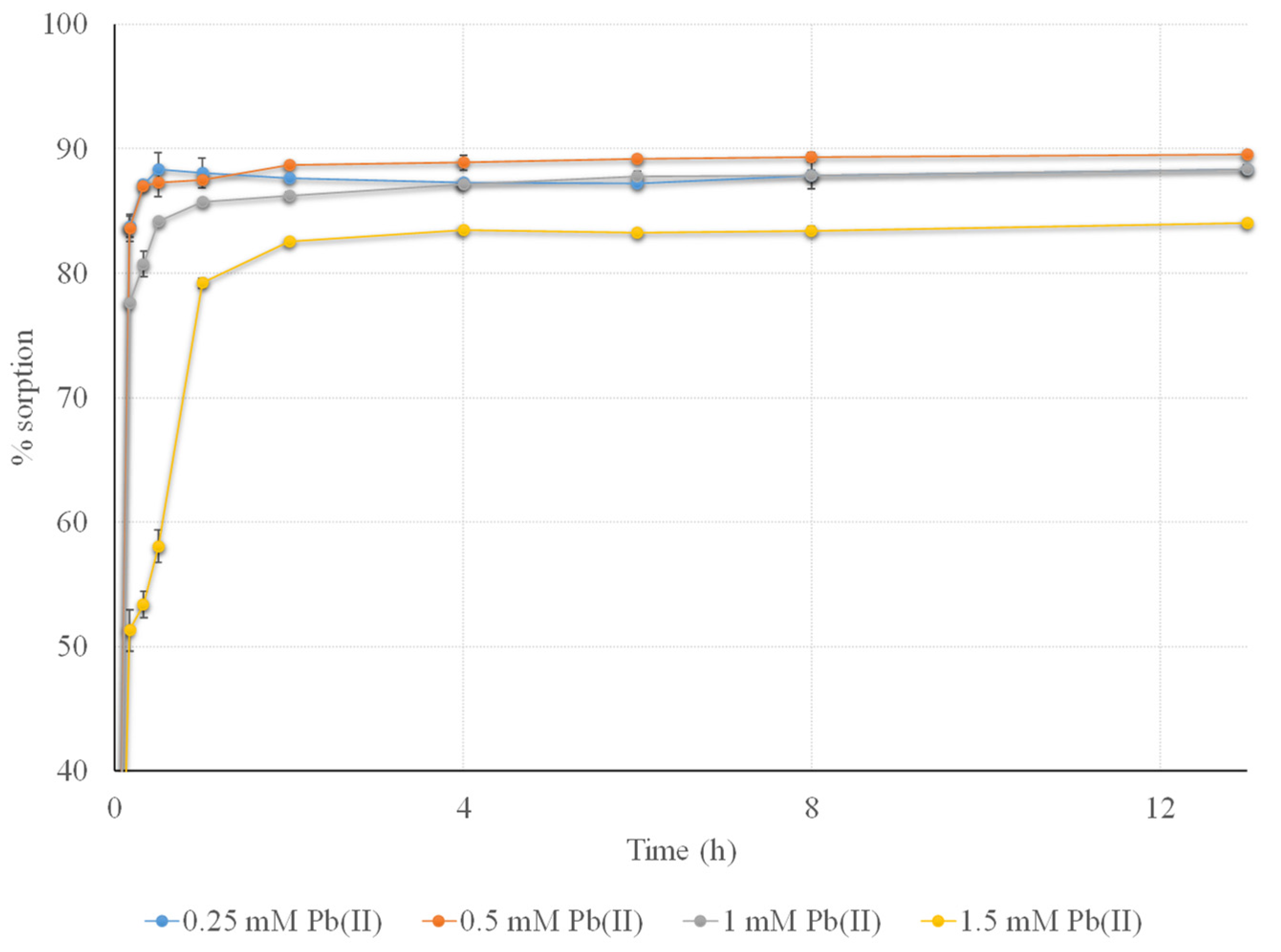
3.3.3. Effect of Sorption Time and Initial Concentration of Pb(II) on the Removal
3.4. Mechanisms of the Pb(II) Sorption
3.4.1. Sorption Isotherm
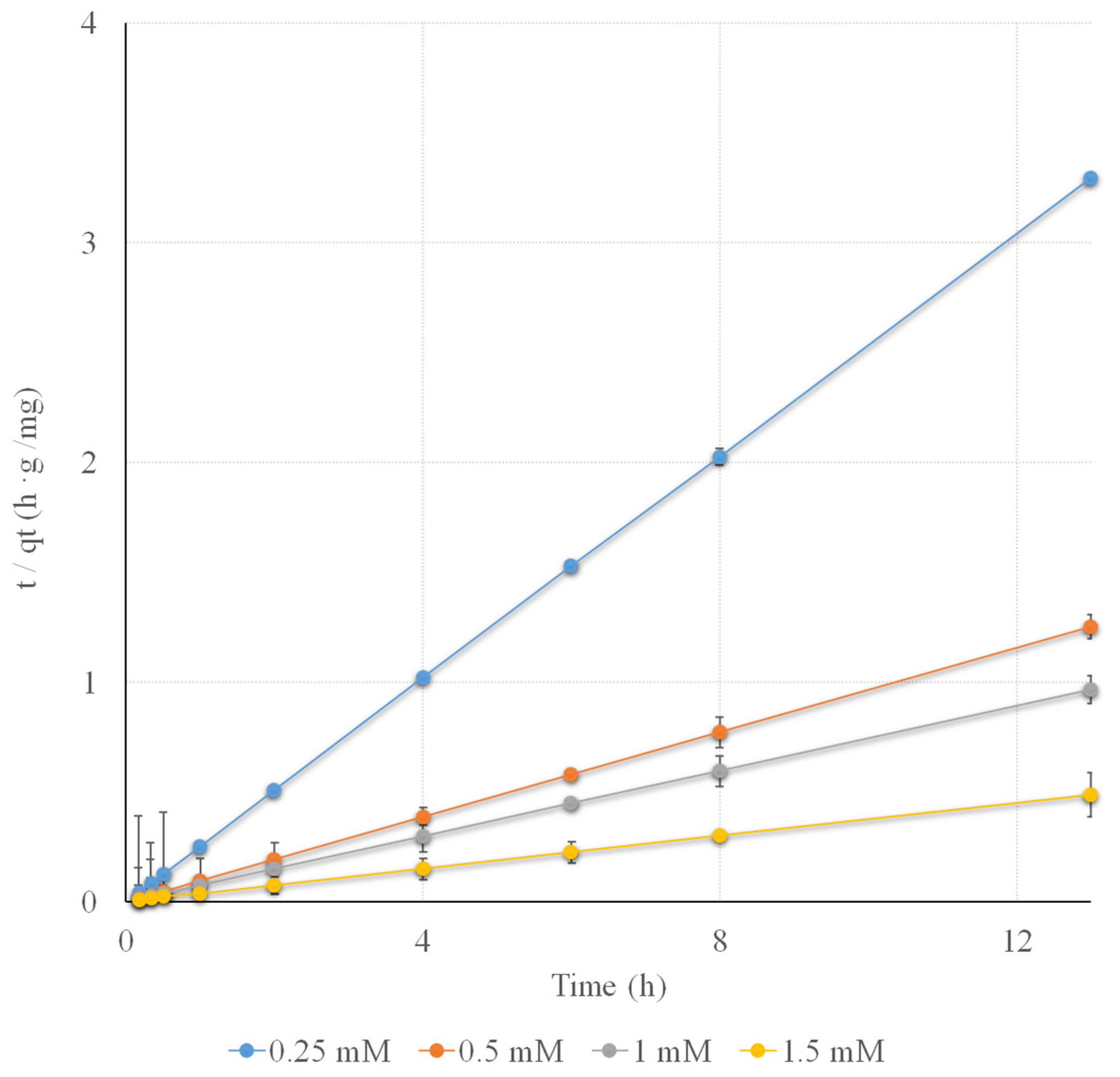
3.4.2. Sorption Kinetics
3.4.3. Sorption Thermodynamic Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beni, A.A.; Esmaeili, A. Biosorption, an efficient method for removing heavy metals from industrial effluents: A review. Environ. Technol. Innov. 2020, 17, 100503. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, K.; Sinha, M.; Baral, E.R.; Koteswararao, R.; Dhyani, A.; Cho, M.H.; Cho, S. Bio-sorbents, industrially important chemicals and novel materials from citrus processing waste as a sustainable and renewable bioresource: A review. J. Adv. Res. 2020, 23, 61–82. [Google Scholar] [CrossRef] [PubMed]
- Mangwandi, C.; Kurniawan, T.A.; Albadarin, A.B. Comparative biosorption of chromium (VI) using chemically modified date pits (CM-DP) and olivestone (CM-OS): Kinetics, isotherms and influence of co-existing ions. Chem. Eng. Res. Des. 2020, 156, 251–262. [Google Scholar] [CrossRef]
- Itankar, N.; Patil, Y. Employing waste to manage waste: Utilizing waste biomaterials for the elimination of hazardous contaminant [Cr(VI)] from aqueous matrices. J. Contam. Hydrol. 2021, 239, 103775. [Google Scholar] [CrossRef] [PubMed]
- Debnath, B.; Haldar, D.; Purkait, M.K. Environmental remediation by tea waste and its derivative products: A review on present status and technological advancements. Chemosphere 2022, 300, 134480. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wei, W.; Chen, H.; Ni, B.-J. Recent advances in waste-derived functional materials for wastewater remediation. Eco-Environ. Health 2022, 1, 86–104. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, A.; Kubaczynski, A.; Szewczuk-Karpisz, K. Assessment of agricultural waste biochars for remediation of degraded water-soil environment: Dissolved organic carbon release and immobilization of impurities in one- or two-adsorbate systems. Waste Manag. 2023, 155, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Mikulewicz, M. Green analytical methods of metals determination in biosorption studies. Trends in Anal. Chem. 2019, 116, 264–265. [Google Scholar] [CrossRef]
- WHO 2021. Fact Sheets for Lead Poisoning. Available online: https://www.who.int/news-room/fact-sheets/detail/lead-poisoning-and-health (accessed on 21 November 2023).
- Raj, K.; Das, A.P. Lead pollution: Impact on environment and human health and approach for a sustainable solution. Environ. Chem. Ecotoxicol. 2023, 5, 79–85. [Google Scholar] [CrossRef]
- Dongre, R.S. Lead: Toxicological Profile, Pollution Aspects and Remedial Solutions. In Lead Chemistry; Chooto, P., Ed.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Debnath, B.; Saha, I.; Mukherjee, T.; Mitra, S.; Das, A.; Das, A. Chapter 12—Sorbents from waste materials: A circular economic approach. In Sorbents Materials for Controlling Environmental Pollution; Núñez-Delgado, A., Ed.; Elsevier: Lugo, Spain, 2021; pp. 285–322. ISBN 9780128200421. [Google Scholar] [CrossRef]
- Vasić, V.; Kukić, D.; Šćiban, M.; Đurišić-Mladenović, N.; Velić, N.; Pajin, B.; Crespo, J.; Farre, M.; Šereš, Z. Lignocellulose-based biosorbents for the removal of contaminants of emerging concern (CECs) from water: A Review. Water 2023, 15, 1853. [Google Scholar] [CrossRef]
- Basu, M.; Guha, A.K.; Ray, L. Adsorption of lead on cucumber peel. J. Clean. Prod. 2017, 151, 603–615. [Google Scholar] [CrossRef]
- Parlayici, S.; Pehlivan, E. Comparative study of Cr(VI) removal by bio-waste adsorbents: Equilibrium, kinetics, and thermodynamic. J. Anal. Sci. Technol. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Kabir, M.M.; Mouna, S.S.P.; Akter, S.; Khandaker, S.; Didar-ul-Alam, M.; Bahadur, N.M.; Mohinuzzaman, M.; Islam, M.A.; Shenashen, M.A. Tea waste based natural adsorbent for toxic pollutant removal from waste samples. J. Mol. Liq. 2021, 322, 115012. [Google Scholar] [CrossRef]
- Nie, L.; Chang, P.; Liang, S.; Hu, K.; Hua, D.; Lio, S.; Sun, J.; Sun, M.; Wang, T.; Okoro, O.V.; et al. Polyphenol rich green tea waste hydrogel for removal of copper and chromium ions from aqueous solution. Clean. Eng. Technol. 2021, 4, 100167. [Google Scholar] [CrossRef]
- Matei, E.; Râpa, M.; Predescu, A.M.; Turcanu, A.A.; Vidu, R.; Predescu, C.; Bobirica, C.; Bobirica, L.; Orbeci, C. Valorization of agri-food wastes as sustainable eco-materials for wastewater treatment: Current state and new perspectives. Materials 2021, 14, 4581. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.J.; Li, J.Y.; Yuan, J.H.; Xu, R.K. Adsorption of Cu(II) by biochars generated from three crop straws. Chem. Eng. J. 2011, 172, 828–834. [Google Scholar] [CrossRef]
- Feizi, M.; Jalali, M. Removal of heavy metals from aqueous solutions using sunflower, potato, canola and walnut shell residues. J. Taiwan Inst. Chem. Eng. 2015, 54, 125–136. [Google Scholar] [CrossRef]
- Bartczak, P.; Norman, M.; Klapiszewski, L.; Karwanska, N.; Kawalec, M.; Baczynska, M.; Wysokowski, M.; Zdarta, J.; Ciesielczyk, F.; Jesionowski, T. Removal of nickel(II) and lead(II) ions from aqueous solution using peat as a low-cost adsorbent: A kinetic and equilibrium study. Arab. J. Chem. 2018, 11, 1209–1222. [Google Scholar] [CrossRef]
- Alslaibi, T.M.; Ismail, A.; Mohd, A.A.; Foul, A.A. Heavy metals removal from wastewater using agricultural wastes as adsorbents: A review. Int. J. Chem. Environ. Eng. 2014, 5, 7–10. [Google Scholar]
- Zevallos Torres, L.A.; Lorenci Woiciechowski, A.; de Andrade Tanobe, V.O.; Karp, S.G.; Guimarães Lorenci, L.C.; Faulds, C.; Soccol, C.R. Lignin as a potential source of high-added value compounds: A review. J. Clean. Product. 2020, 263, 121499. [Google Scholar] [CrossRef]
- Philippini, R.R.; Martiniano, S.E.; Ingle, A.P.; Franco Marcelino, P.R.; Silva, G.M.; Barbosa, F.G.; dos Santos, J.C.; da Silva, S.S. Agroindustrial byproducts for the generation of biobased products: Alternatives for sustainable biorefineries. Front. Energy Res. 2020, 8, 1–23. [Google Scholar] [CrossRef]
- Langston, F.; Redha, A.A.; Nash, G.R.; Bows, J.R.; Torquati, L.; Gidley, M.J.; Cozzolino, D. Qualitative analysis of broccoli (Brassica oleracea var. italica) glucosinolates: Investigating the use of mid-infrared spectroscopy combined with hemometrics. J. Food Compos. Anal. 2023, 123, 105532. [Google Scholar] [CrossRef]
- Latté, K.P.; Appel, K.E.; Lampen, A. Health benefits and possible risks of broccoli—An overview. Food Chem. Toxicol. 2011, 49, 3287–3309. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, L.; Ser, S.L.; Cumming, J.R.; Ku, K.M. Comparative Phytonutrient Analysis of Broccoli By-Products: The Potentials for Broccoli By-Product Utilization. Molecules 2018, 23, 900. [Google Scholar] [CrossRef] [PubMed]
- Petkowicz, C.L.O.; Williams, P.A. Pectins from food waste: Characterization and functional properties of a pectin extracted from broccoli stalk. Food Hydrocolloids 2020, 107, 105930. [Google Scholar] [CrossRef]
- Landin-Sandoval, V.J.; Mendoza-Castillo, D.I.; Seliem, M.K.; Mobarak, M.; Villanueva-Mejia, F.; Bonilla-Petriciolet, A.; Navarro-Santos, P.; Reynel-Ávila, H.E. Physicochemical analysis of multilayer adsorption mechanism of anionic dyes on lignocellulosic biomasses via statistical physics and density functional theory. J. Mol. Liq. 2021, 322, 114511. [Google Scholar] [CrossRef]
- Fooddata Central for Broccoli. FDC ID: 168510 NDB Number:11742. US Department of Agriculture, USDA. 2019. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/168510/nutrients (accessed on 15 February 2024).
- Domínguez-Perles, R.; Martínez-Ballesta, M.C.; Carvajal, M.; García-Viguera, C.; Moreno, D.A. Broccoli-derived by-products—A promising source of bioactive ingredients. J. Food Sci. 2010, 75, C383–C392. [Google Scholar] [CrossRef]
- Prade, T.; Muneer, F.; Berndtsson, E.; Nynäs, A.L.; Svensson, S.E.; Newson, W.R.; Johansson, E. Protein fractionation of broccoli (Brassica oleracea, var. Italica) and kale (Brassica oleracea, var. Sabellica) residual leaves—A pre-feasibility assessment and evaluation of fraction phenol and fibre content. Food Bioprod. Process. 2021, 130, 229–243. [Google Scholar] [CrossRef]
- Campas-Baypoli, O.N.; Sanchez-Machado, D.I.; Bueno-Solano, C.; Nuñez-Gastelum, J.A.; Reyes-Moreno, C.; Lopez-Cervantes, J. Biochemical composition and physicochemical properties of broccoli flours. Int. J. Food Sci. Nutr. 2009, 60, 163–173. [Google Scholar] [CrossRef]
- Salim, N.S.; Garièpy, Y.; Raghavan, V. Effects of operating factors on osmotic dehydration of broccoli stalk slices. Cogent Food Agric. 2016, 2, 1134025. [Google Scholar] [CrossRef]
- Quintero-Herrera, S.; García-León, A.M.; Botello-Álvarez, J.E.; Estrada-Baltazar, A.; Abel-Seabra, J.E.; Padilla-Rivera, A.; Rivas-García, P. The use of broccoli agro-industrial waste in dairy cattle diet for environmental mitigation. Clean. Environ. Syst. 2021, 2, 100035. [Google Scholar] [CrossRef]
- Peixiu, L.; Meixuan, Z.; Mei, Y.; Xiaoying, L.; Wenqiang, L.; Min, Y.; Yupeng, L. Study on the nutrient components and physicochemical properties of broccoli flour. Sci. Technol. Food Ind. 2022, 11, 326–333. [Google Scholar] [CrossRef]
- Gavilanes-Terán, I.; Jara-Samaniego, J.; Idrovo-Novillo, J.; Bustamante, M.A.; Moral, R.; Paredes, C. Windrow composting as horticultural waste management strategy—A case study in Ecuador. Waste Manag. 2016, 48, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.H.; Zuo, A.Y.; Wang, D.G.; Pan, H.Y.; Zheng, W.B.; Qian, Z.C.; Zou, X.T. Effects of broccoli stems and leaves meal on production performance and egg quality of laying hens. Anim. Feed Sci. Technol. 2011, 170, 117–121. [Google Scholar] [CrossRef]
- Landing-Sandoval, V.J.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A.; Aguayo-Villarreal, I.A.; Reynel-Avila, H.E.; Gonzalez-Ponce, H.A. Valorization of agri-food industry wastes to prepare adsorbents for heavy metal removal from water. J. Environ. Chem. Eng. 2020, 8, 104067. [Google Scholar] [CrossRef]
- Pholosi, A.; Ofomaja, A.E.; Naidoo, E.B. Effect of chemical extractants on the biosorptive properties of pine cone powder: Influence on lead(II) removal mechanism. J. Saudi Chem. Soc. 2013, 17, 77–86. [Google Scholar] [CrossRef]
- Horwitz, W. (Ed.) AOAC-ASTM Method: Cation exchange capacity (63). In AOAC: Official Methods of Analysis, 13th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1980; 29p. [Google Scholar]
- do Nascimento, J.M.; Diniz de Oliveira, J.; Gomez Ferreira Leite, S. Chemical characterization of biomass flour of the babassu coconut mesocarp (Orbignya speciosa) during biosorption process of copper ions. Environ. Technol. Innov. 2019, 16, 100440. [Google Scholar] [CrossRef]
- Boehm, H.P. Some aspects of the surface chemistry of carbon black and other carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, X.; Shi, L.; Li, J.; Li, S.; Lü, J.; Li, Y. Efficient removal of lead from solution by celery-derived biochars rich in alkaline minerals. Bioresour. Technol. 2017, 235, 185–192. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Kabiri-Samani, M. Effective removal of Ni(II) from aqueous solutions by modification of nano particles of clinoptilolite with dimethylglyoxime. J. Hazard. Mater. 2013, 260, 339–349. [Google Scholar] [CrossRef]
- Ofomaja, A.E.; Naidoo, E.B.; Modise, S.J. Surface modification of pine cone powder and its application for removal of Cu(II) from wastewater. Desal. Water Treat. 2012, 19, 275–285. [Google Scholar] [CrossRef]
- Landi, E.; Medri, V.; Papa, E.; Dedecek, J.; Klein, P.; Benito, P.; Vaccari, A. Alkali-bonded ceramics with hierarchical tailored porosity. Appl. Clay Sci. 2013, 73, 56–64. [Google Scholar] [CrossRef]
- Khelaifia, F.Z.; Hazourli, S.; Nouacer, S.; Rahima, H.; Ziati, M. Valorization of raw biomaterial waste-date stones-for Cr(VI) adsorption in aqueous solution: Thermodynamics, kinetics and regeneration studies. Int. Biodeterior. Biodegrad. 2016, 114, 76–86. [Google Scholar] [CrossRef]
- Turco, A.; Pennetta, A.; Caroli, A.; Mazzotta, E.; Monteduro, A.G.; Primiceri, E.; de Benedetto, G.; Malitesta, C. Easy fabrication of mussel inspired coated foam and its optimization for the facile removal of copper from aqueous solutions. J. Colloid Interface Sci. 2019, 552, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zang, G.-L.; Zhao, Q. Removal of copper ions by functionalized biochar based on a multicomponent Ugi reaction. RSC Adv. 2021, 11, 25880–25891. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Equeenuddin, S.M. Adsorption of hexavalent chromium using natural goethite: Isotherm thermodynamic and kinetic study. J. Geol. Soc. India 2019, 93, 285–292. [Google Scholar] [CrossRef]
- Saha, B.; Orvig, C. Biosorbents for hexavalent chromium elimination from industrial and municipal effluents. Coord. Chem. Rev. 2010, 254, 2959–2972. [Google Scholar] [CrossRef]
- Önal, Y. Kinetics of adsorption of dyes from aqueous solution using activated carbon prepared from waste apricot. J. Hazard. Mater. 2006, 137, 1719–1728. [Google Scholar] [CrossRef]
- Cheung, W.; Porter, J.F.; Mckay, G. Sorption kinetic analysis for the removal of cadmium ions from efuents using bone char. Water. Res. 2001, 35, 605–612. [Google Scholar] [CrossRef]
- Foroutan, R.; Esmaeili, H.; Fard, M.K. Equilibrium and kinetic studies of Pb(II) biosorption from aqueous solution using shrimp peel. Int. Res. J. Appl. Basic Sci. 2015, 9, 1954–1965. [Google Scholar]
- Milmile, S.N.; Pande, J.V.; Karmakar, S.; Bansiwal, A.; Chakrabarti, T.; Biniwale, R.B. Equilibrium isotherm and kinetic modeling of the adsorption of nitrates by anion exchange Indion NSSR resin. Desalination 2011, 276, 38–44. [Google Scholar] [CrossRef]
- Bazargan-Lari, R.; Zafarani, H.R.; Bahrololoom, M.E.; Nemati, A. Removal of Cu(II) ions from aqueous solutions by low-cost natural hydroxyapatite/chitosan composite: Equilibrium, kinetic and thermodynamic studies. J. Taiwan Inst. Chem. Eng. 2014, 45, 1642–1648. [Google Scholar] [CrossRef]
- Ibrahim, W.M.; Hassan, A.F.; Azab, Y.A. Biosorption of toxic heavy metals from aqueous solution by Ulva lactuca activated carbon. Egypt. J. Basic. Appl. Sci. 2016, 3, 241–249. [Google Scholar] [CrossRef]
- Hu, F.; Ragauskas, A. Suppression of pseudo-lignin formation under dilute acid pretreatment conditions. RSC Adv. 2014, 4, 4317–4323. [Google Scholar] [CrossRef]
- Tran, H.A.; van de Steene, L.; Dung, D. Pyrolysis and char oxidation of biomass and coal blends: Kinetic study using Thermogravimetric Analysis. In IOP Conference Series: Earth and Environmental Science; IOP Publishing Ltd: Bristol, UK, 2018; Volume 159, p. 012035. Available online: https://iopscience.iop.org/article/10.1088/1755-1315/159/1/012035 (accessed on 15 February 2024).
- Hackbarth, F.V.; Girardi, F.; de Souza, S.M.A.G.U.; de Souza, A.A.U.; Boaventura, R.A.R.; Vilar, V.J.P. Marine macroalgae Pelvetia canaliculata (Phaeophyceae) as a natural cation exchanger for cadmium and lead ions separation in aqueous solutions. Chem. Eng. J. 2014, 242, 294–305. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, J.; Liu, H.; Peng, Q.; Zhou, H.; Zhang, X. Adsorption characteristics and mechanisms of Pb2+ and Cd2+ by a new agricultural waste–Caragana korshinskii biomass derived biochar. Environ. Sci. Pollut. Res. 2021, 28, 13800–13818. [Google Scholar] [CrossRef] [PubMed]
- Revelou, P.K.; Kokotou, M.G.; Pappas, C.S.; Constantinou-Kokotou, V. Direct determination of total isothiocyanate content in broccoli using attenuated total reflectance infrared Fourier transform spectroscopy. J. Food Compos. Anal. 2017, 61, 47–51. [Google Scholar] [CrossRef]
- Redha, A.A.; Torquati, L.; Langston, F.; Nash, G.R.; Gidley, M.J.; Cozzolino, D. Determination of glucosinolates and isothiocyanates in glucosinolate-rich vegetables and oilseeds using infrared spectroscopy: A systematic review. Crit. Rev. Food Sci. Nutr. 2023, 10, 1–17. [Google Scholar] [CrossRef]
- Kostic, M.; Radovic, M.; Mitrovic, J.; Antonijevic, M.; Bojic, D.; Petrovic, M.; Bojic, A. Using xanthated Lagenaria vulgaris shell biosorbent for removal of Pb(II) ions from wastewater. J. Iran chem. Soc. 2014, 11, 565–578. [Google Scholar] [CrossRef]
- Anu Rana, N.; Kumari, N.; Tyagi, M.; Jagadevan, S. Leaf-extract mediated zero-valent iron for oxidation of Arsenic (III): Preparation, characterization and kinetics. Chem. Eng. J. 2018, 347, 91–100. [Google Scholar] [CrossRef]
- Farinella, N.V.; Matos, G.D.; Arruda, M.A.Z. Grape bagasse as a potential biosorbent of metals in effluent treatments. Bioresour. Technol. 2007, 98, 1940–1946. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Martin, A.; Reynolds-Marzal, D.; Martin, A.; Velazquez, R.; Poblaciones, M.J. Combined Foliar Zinc and Nitrogen Application in Broccoli (Brassica oleracea var. italica L.): Effects on Growth, Nutrient Bioaccumulation, and Bioactive Compounds. Agronomy 2021, 11, 548. [Google Scholar] [CrossRef]
- Sgriccia, N.; Hawley, M.C.; Misra, M. Characterization of natural fiber surfaces and natural fiber composites. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1632–1637. [Google Scholar] [CrossRef]
- Wang, S.; Shi, Z.; Jin, Y.; Zaini, I.N.; Li, Y.; Tang, C.; Mu, W.; Wen, Y.; Jiang, J.; Jönsson, P.G.; et al. A machine learning model to predict the pyrolytic kinetics of different types of feedstocks. Energy Convers. Manag. 2022, 260, 115613. [Google Scholar] [CrossRef]
- Stavridou, E.; Kristensen, H.L.; Krumbein, A.; Schreiner, M.; Thorup-Kristensen, K. Effect of differential N and S competition in Inter- and sole cropping of Brassica species and lettuce on glucosinolate concentration. J. Agric. Food Chem. 2012, 60, 6268–6278. [Google Scholar] [CrossRef] [PubMed]
- Idris, M.O.; Usman, A.O.; Musa, Q.; Suleiman, A.I.; Emmanuel, J.L.; Sambo, P. Biomass-Derived Activated Carbon: A viable material for remediation of Pb2+ and 2, 4-Dichlorophenol (2, 4 DCP) through Adsorption. J. Adv. Res. Appl. Sci. Eng. Technol. 2021, 25, 37–45. [Google Scholar] [CrossRef]
- Daifullah, A.A.M.; Girgis, B.S.; Gad, H.M.H. Utilization of agro-residues (rice husk) in small waste water treatment plans. Mater. Let. 2003, 57, 1723–1731. [Google Scholar] [CrossRef]
- Hopkins, D.; Hawboldt, K. Biochar for the removal of metals from solution: A review of lignocellulosic and novel marine feedstocks. J. Environ. Chem. Eng. 2020, 8, 103975. [Google Scholar] [CrossRef]
- Liu, Y.; Li, W.; Sun, X.; Li, S.; Wang, C.; Zhang, R. Adsorption of lead ions by green waste compost and its mechanism. J. Soils Sediments 2023, 23, 299–311. [Google Scholar] [CrossRef]
- Abdallah, M.M.; Ahmad, M.N.; Walker, G.; Leahy, J.J.; Kwapinski, W. Batch and continuous systems for Zn, Cu, and Pb metal ions adsorption on spent mushroom compost biochar. Ind. Eng. Chem. Res. 2019, 58, 7296–7307. [Google Scholar] [CrossRef]
- Lavado-Meza, C.; de la Cruz-Cerrón, L.; Lavado-Puente, C.; Angeles-Suazo, J.; Dávalos-Prado, J.Z. Efficient Lead Pb(II) removal with chemically modified nostoc commune biomass. Molecules 2023, 28, 268. [Google Scholar] [CrossRef] [PubMed]
- Kouassi, N.L.B.; N’goran, K.P.D.A.; Blonde, L.D.; Diabate, D.; Albert, T. Simultaneous removal of Copper and Lead from industrial effluents using corn cob activated carbon. Chem. Afr. 2023, 6, 733–745. [Google Scholar] [CrossRef]
- Qin, H.; Hu, T.; Zhai, Y.; Lu, N.; Aliyeva, J. The improved methods of heavy metals removal by biosorbents: A review. Environ. Pollut. 2020, 258, 113777. [Google Scholar] [CrossRef]
- Bernal-Jácome, L.A.; Olvera-Izaguirre, L.; Gallegos García, M.; Delgado-Delgado, R.; Espinosa Rodríguez, M.A. Adsorption of Lead (II) from aqueous solution using adsorbents obtained from nanche stone (Byrsonima Crassifolia). J. Mex. Chem. Soc. 2020, 64, 301–315. [Google Scholar] [CrossRef]
- Mani, R.S. Hard Soft Acid Base Theory (Hsab Theory) of Ralph Pearson. Int. J. Sci. Res. 2018, 7, 29–31. [Google Scholar]
- Kowalik, M.; Masternak, J.; Brzeski, J.; Daszkiewicz, M.; Barszcz, B. Effect of a lone electron pair and tetrel interactions on the structure of Pb(II) CPs constructed from pyrimidine carboxylates and auxiliary inorganic ions. Polyhedron 2022, 219, 115818. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, L.; Chu, G. Comparison of the Sorption of Cu(II) and Pb(II) by Bleached and Activated Biochars: Insight into Complexation and Cation–π Interaction. Agronomy 2023, 13, 1282. [Google Scholar] [CrossRef]
- Wu, J.; Wang, T.; Zhang, Y.; Pan, W.-P. The distribution of Pb(II)/Cd(II) adsorption mechanisms on biochars from aqueous solution: Considering the increased oxygen functional groups by HCl treatment. Bioresour. Technol. 2019, 291, 121859. [Google Scholar] [CrossRef]
- Olu-Owolabi, B.I.; Alabi, A.H.; Unuabonah, E.I.; Diagboya, P.N.; Böhm, L.; Düring, R.-A. Calcined biomass-modified bentonite clay for removal of aqueous metal ions. J. Environ. Chem. Eng. 2016, 4, 1376–1382. [Google Scholar] [CrossRef]
- Cruz-Lopes, L.P.; Macena, M.; Esteves, B.; Guiné, R.P.F. Ideal pH for the adsorption of metal ions Cr6+, Ni2+, Pb2+ in aqueous solution with different adsorbent materials. Open Agric. 2021, 6, 115–123. [Google Scholar] [CrossRef]
- Azouaou, N.; Mokaddem, H.; Allalou, O.; Boudechiche, N.; Sadaoui, Z. Synergistic effect of cafeterias and domestic wastes for the removal of lead from aqueous solution. Reac. Kinet. Mech. Cat. 2022, 135, 403–424. [Google Scholar] [CrossRef]
- Boudrahem, F.; Aissani-Benissad, F.; Audonnet, F.; Vial, C. Kinetics and equilibrium adsorption of lead (II) ions on olive residues: Effects of chemical activation. Alger. J. Environ. Sci. Technol. 2021, 7, 1715–1738. [Google Scholar]
- Šoštarić, T.D.; Petrović, M.S.; Pastor, F.T.; Lončarević, D.R.; Petrović, J.T.; Milojković, J.V.; Stojanović, M.D. Study of heavy metals biosorption on native and alkali-treated apricot shells and its application in wastewater treatment. J. Mol. Liq. 2018, 259, 340–349. [Google Scholar] [CrossRef]
- Mahyoob, W.; Alakayleh, Z.; Hajar, H.A.A.; Al-Mawla, L.; Altwaiq, A.M.; Al-Remawi, M.; Al-Akayleh, F. A novel co-processed olive tree leaves biomass for lead adsorption from contaminated water. J. Contam. Hydrol. 2022, 248, 104025. [Google Scholar] [CrossRef] [PubMed]
- Afroze, S.; Sen, T.K. A Review on Heavy Metal Ions and Dye Adsorption from Water by Agricultural Solid Waste Adsorbents. Water Air Soil Pollut. 2018, 229, 225. [Google Scholar] [CrossRef]
- Wijitkosum, S. Biochar derived from agricultural wastes and wood residues for sustainable agricultural and environmental applications. Int. Soil Water Conserv. Res. 2022, 10, 335–341. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, W.; Shen, G.; Ji, G.; Zhang, Y.; Gao, C.; Han, L. Carbonization and ball milling on the enhancement of Pb(II) adsorption by wheat straw: Competitive effects of ion exchange and precipitation. Bioresour. Technol. 2019, 273, 70–76. [Google Scholar] [CrossRef]
- Devi, B.; Goswami, M.; Rabha, S.; Kalita, S.; Sarma, H.P.; Devi, A. Efficacious Sorption Capacities for Pb(II) from Contaminated Water: A Comparative Study Using Biowaste and Its Activated Carbon as Potential Adsorbents. ACS Omega 2023, 8, 15141–15151. [Google Scholar] [CrossRef]
- Raji, Z.; Karim, A.; Karam, A.; Khalloufi, S. Adsorption of Heavy Metals: Mechanisms, Kinetics, and Applications of Various Adsorbents in Wastewater Remediation—A Review. Waste 2023, 1, 775–805. [Google Scholar] [CrossRef]
- Ramos-Ramírez, E.; Gutiérrez Ortega, N.L.; Contreras Soto, C.A.; Olguín Gutiérrez, M.T. Adsorption isotherm studies of chromium (VI) from aqueous solutions using sol–gel hydrotalcite-like compounds. J. Hazard. Mater. 2009, 172, 1527–1531. [Google Scholar] [CrossRef]
- Yang, W.; Lu, C.; Liang, B.; Yin, C.; Lei, G.; Wang, B.; Zhou, X.; Zhen, J.; Quan, S.; Jing, Y. Removal of Pb(II) from Aqueous Solution and Adsorption Kinetics of Corn Stalk Biochar. Separations 2023, 10, 438. [Google Scholar] [CrossRef]
- Escudero, C.; Poch, J.; Villaescusa, I. Modelling of breakthrough curves of single and binary mixtures of Cu(II), Cd(II), Ni(II) and Pb(II) sorption onto grape stalks waste. Chem. Eng. J. 2013, 217, 129–138. [Google Scholar] [CrossRef]
- Kanu, S.A.; Moyo, M.; Zvinowanda, C.M.; Okonkwo, J.O. Biosorption of Pb(II) from aqueous solution using Rooibos shoot powder (RSP). Desalin. Water Treat. 2015, 57, 5614–5622. [Google Scholar] [CrossRef]
- Li, W.; Zhang, L.B.; Peng, J.H.; Li, N.; Zhang, S.M.; Guo, S.H. Tobacco stems as a low-cost adsorbent for the removal of Pb(II) from wastewater: Equilibrium and kinetic studies. Ind. Crop. Prod. 2008, 28, 294–302. [Google Scholar] [CrossRef]
- Temkin, M.I.; Pyzhev, V. Kinetics of ammonia synthesis on promoted iron catalyst. Acta Phys. Chim. USSR 1940, 12, 327–356. [Google Scholar]
- Rangabhashiyam, S.; Anu, N.; Giri Nandagopal, M.S.; Selvaraju, N. Relevance of isotherm models in biosorption of pollutants by agricultural byproducts. J. Environ. Chem. Eng. 2014, 2, 398–414. [Google Scholar] [CrossRef]
- Zhang, J.; Li, T.; Li, X.; Liu, Y.; Li, N.; Wang, Y.; Li, X. A key role of inner-cation-π interaction in adsorption of Pb(II) on carbon nanotubes: Experimental and DFT studies. J. Hazard. Mater. 2021, 412, 125187. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, X.; Luo, W. Metal removal with two biochars made from municipal organic waste: Adsorptive characterization and surface complexation modeling. Toxicol. Environ. Chem. 2015, 97, 1–30. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, Y.; Jin, F.; McMillan, O.; Al-Tabbaa, A. Qualitative and quantitative characterisation of adsorption mechanisms of lead on four biochars. Sci. Total Environ. 2017, 609, 1401–1410. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Villaescusa, I. The Chemistry Behind the Use of Agricultural Biomass as Sorbent for Toxic Metal Ions: pH Influence, Binding Groups, and Complexation Equilibria. In Biomass: Detection, Production and Usage; InTech: London, UK, 2011. [Google Scholar] [CrossRef]
- Zhang, Z.; Gu, P.; Zhang, M.; Yan, S.; Dong, L.; Zhang, G. Synthesis of a robust layered metal sulfide for rapid and effective removal of Sr2+ from aqueous solutions. Chem. Eng. J. 2019, 372, 1205–1215. [Google Scholar] [CrossRef]
- Khani, M.H.; Keshtkar, A.R.; Ghannadi, M.; Pahlavanzadeh, H. Equilibrium, kinetic and thermodynamic study of the biosorption of uranium onto Cystoseria indica algae. J. Hazard. Mater. 2008, 150, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Horsfall, M.; Spiff, A. Effects of Temperature on the Sorption of Pb2+ and Cd2+ from Aqueous Solution by Caladium bicolor (Wild Cocoyam) Biomass. Electron. J. Biotechnol. 2005, 8, 162–168. [Google Scholar] [CrossRef]

| Parameter | Average Value | SD |
|---|---|---|
| Iodine adsorption capacity (mg I2 g−1 biomass) | 619.7 | 33.3 |
| Cation exchange capacity, CEC (meq H+ exchange 100 g−1 biomass = cmol kg−1 biomass) | 30.7 | 0.01 |
| Functional groups (mmol g−1) | ||
| Basic | 1.085 | 0.021 |
| Acid | 1.257 | 0.012 |
| Carboxylic group | 1.216 | 0.012 |
| Lactonic group | 0.006 | 0.013 |
| Phenolic group | 0.035 | 0.013 |
| pH of biomass | 6.88 | 0.02 |
| pHpzc | 6.25 | 0.02 |
| Conductivity (µS cm−1) | 1566 | 22 |
| Bulk density, BD (g mL−1) | 0.52 | 0.01 |
| Apparent density, AD (g mL−1) | 0.65 | 0.03 |
| Porosity (%) | 20.6 | 3.2 |
| Specific surface area, SSA (m2 g−1) | 15.3 | 2.7 |
| Isotherm Models | Isotherm Constants and Determination Coefficients a | |
|---|---|---|
| Parameter | Values at 23 °C | |
| Langmuir | KL (L mg−1//L mmol−1) | 0.013//2.78 |
| qmax (mg g−1//mmol g−1) | 64.9//0.31 | |
| RL | 0.19–0.62 | |
| R2 | 0.873 | |
| MSE | 0.023 | |
| Freundlich | KF (mg g−1//mmol g−1) | 1.11//0.005 |
| 1/n | 0.82 | |
| R2 | 0.969 | |
| MSE | 0.005 | |
| Dubinin–Radushkevich | B (mol2 J−2) | 7.28 × 10−9 |
| qmax (mg g−1//mmol g−1) | 587//2.83 | |
| E (kJ mol−1) | 8.29 | |
| R2 | 0.981 | |
| MSE | 0.018 | |
| Temkin | B | 9.93 |
| KT (L mg−1//L mmol−1) | 0.24//50.4 | |
| bT (J mol−1) | 248 | |
| R2 | 0.980 | |
| MSE | 2.69 | |
| Kinetic Models | Kinetic Parameters and Determination Coefficients b | ||||
|---|---|---|---|---|---|
| Parameter a | 0.25 mmol L−1 | 0.5 mmol L−1 | 1 mmol L−1 | 1.5 mmol L−1 | |
| Pseudo-first-order | k1 (min−1) | 3.78 × 10−4 | 7.73 × 10−3 | 0.01 | 0.03 |
| qe (mg g−1//mmol g−1) | 0.02//1.14 × 10−4 | 0.30//1.43 × 10−3 | 1.11//5.35 × 10−3 | 12.2//0.06 | |
| qe exp (mg g−1//mmol g−1) | 3.95//0.02 | 10.3//0.05 | 13.4//0.07 | 27.9//0.14 | |
| R2 | 0.008 | 0.989 | 0.942 | 0.968 | |
| MSE | 0.05 | 1.43 × 10−3 | 0.03 | 0.05 | |
| Pseudo-second-order | k2 (g mg−1 min−1//mmol−1 min−1) | 0.85//1.76 × 102 | 0.09 | 0.04//7.96 | 4.22 × 10−3//0.87 |
| qe (mg g−1//mmol g−1) | 3.95//0.02 | 10.4//0.05 | 13.5//0.07 | 27.0//0.13 | |
| qe exp (mg g−1//mmol g−1) | 3.95//0.02 | 10.3//0.05 | 13.4//0.07 | 27.9//0.14 | |
| h (mg g−1min−1//mmol g−1 min−1) | 13.2//0.06 | 10.1//0.05 | 6.95//0.03 | 3.08//0.02 | |
| R2 | 0.999 | 0.999 | 0.999 | 0.999 | |
| MSE | 0.03 | 1.86 × 10−3 | 2.11 × 10−3 | 0.03 | |
| Elovich | a (mg g−1 min−1) | non fitted | 2.86 × 1048 | 1.26 × 1012 | 0.57 |
| b (g mg−1) | 11.6 | 2.57 | 0.32 | ||
| R2 | 0.985 | 0.876 | 0.768 | ||
| MSE | 2.10 × 10−4 | 0.05 | 5.55 | ||
| Ritchie’s second order | k2_R (min−1) | 1.00 | 0.88 | 1.19 | 3.55 |
| R2 | 0.056 | 0.965 | 0.755 | 0.751 | |
| MSE | 23.3 × 103 | 1.85 × 102 | 1.05 × 104 | 5.66 × 104 | |
| First order reversible | k1 (min−1) | 0.02 | 0.03 | 0.02 | 0.03 |
| k−1 (min−1) | 3.34 × 10−3 | 3.20 × 10−3 | 2.73 × 10−3 | 6.71 × 10−3 | |
| R2 | R2 < 0 | R2 < 0 | R2 < 0 | 0.947 | |
| MSE | 13.3 | 6.83 | 3.78 | 0.15 | |
| Intraparticle diffusion | Kd (mg g−1 min−1/2) | 9.0 × 10−5 | 0.02 | 0.07 | 0.50 |
| C (mg g−1) | 3.94 | 10.0 | 12.1 | 17.7 | |
| R2 | 0.0007 | 0.9417 | 0.712 | 0.582 | |
| MSE | 6.74 × 10−4 | 8.19 × 10−4 | 0.11 | 10.0 | |
| Temperature (°C/K) | ΔG° (kJ mol−1) | ΔH° (kJ mol−1) | ΔS° (J mol−1 K−1) | S* | Ea (kJ mol−1) |
|---|---|---|---|---|---|
| 18/291 | −4.17 | 8.98 | 45.20 | 0.006 | 7.66 |
| 23/296 | −4.40 | ||||
| 30/303 | −4.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granado-Castro, M.D.; Galindo-Riaño, M.D.; Gestoso-Rojas, J.; Sánchez-Ponce, L.; Casanueva-Marenco, M.J.; Díaz-de-Alba, M. Ecofriendly Application of Calabrese Broccoli Stalk Waste as a Biosorbent for the Removal of Pb(II) Ions from Aqueous Media. Agronomy 2024, 14, 554. https://doi.org/10.3390/agronomy14030554
Granado-Castro MD, Galindo-Riaño MD, Gestoso-Rojas J, Sánchez-Ponce L, Casanueva-Marenco MJ, Díaz-de-Alba M. Ecofriendly Application of Calabrese Broccoli Stalk Waste as a Biosorbent for the Removal of Pb(II) Ions from Aqueous Media. Agronomy. 2024; 14(3):554. https://doi.org/10.3390/agronomy14030554
Chicago/Turabian StyleGranado-Castro, María Dolores, María Dolores Galindo-Riaño, Jesús Gestoso-Rojas, Lorena Sánchez-Ponce, María José Casanueva-Marenco, and Margarita Díaz-de-Alba. 2024. "Ecofriendly Application of Calabrese Broccoli Stalk Waste as a Biosorbent for the Removal of Pb(II) Ions from Aqueous Media" Agronomy 14, no. 3: 554. https://doi.org/10.3390/agronomy14030554
APA StyleGranado-Castro, M. D., Galindo-Riaño, M. D., Gestoso-Rojas, J., Sánchez-Ponce, L., Casanueva-Marenco, M. J., & Díaz-de-Alba, M. (2024). Ecofriendly Application of Calabrese Broccoli Stalk Waste as a Biosorbent for the Removal of Pb(II) Ions from Aqueous Media. Agronomy, 14(3), 554. https://doi.org/10.3390/agronomy14030554










