Temporal Synchronization of Nitrogen and Sulfur Fertilization: Impacts on Nutrient Uptake, Use Efficiency, Productivity, and Relationships with Other Micronutrients in Soybean
Abstract
1. Introduction
2. Materials and Methods
2.1. Location Description, Experimental Design, and Crop Husbandry
2.2. Chemical Analysis of Plant and Soil Samples
| GYF | : Signifies the grain yield with N or S application. |
| GYC | : Represents the grain yield for the control group. |
| F | : Denotes the amount of applied fertilizers, whether N or S. |
| GNUF | : Stands for the grain nutrient uptake with N or S application. |
| GNUC | : Indicates the grain nutrient uptake for the control group. |
2.3. Statistical Analysis
3. Results
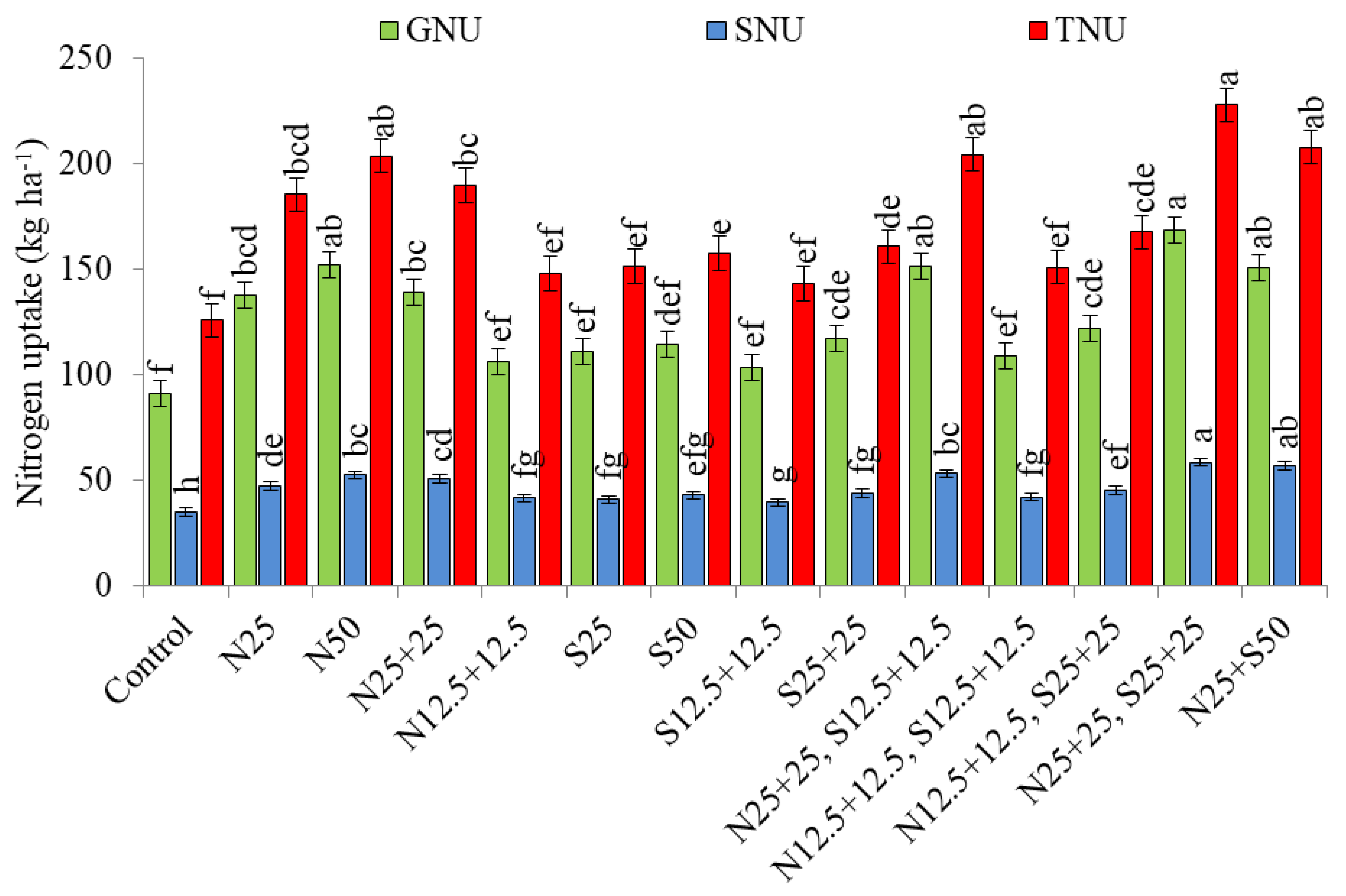
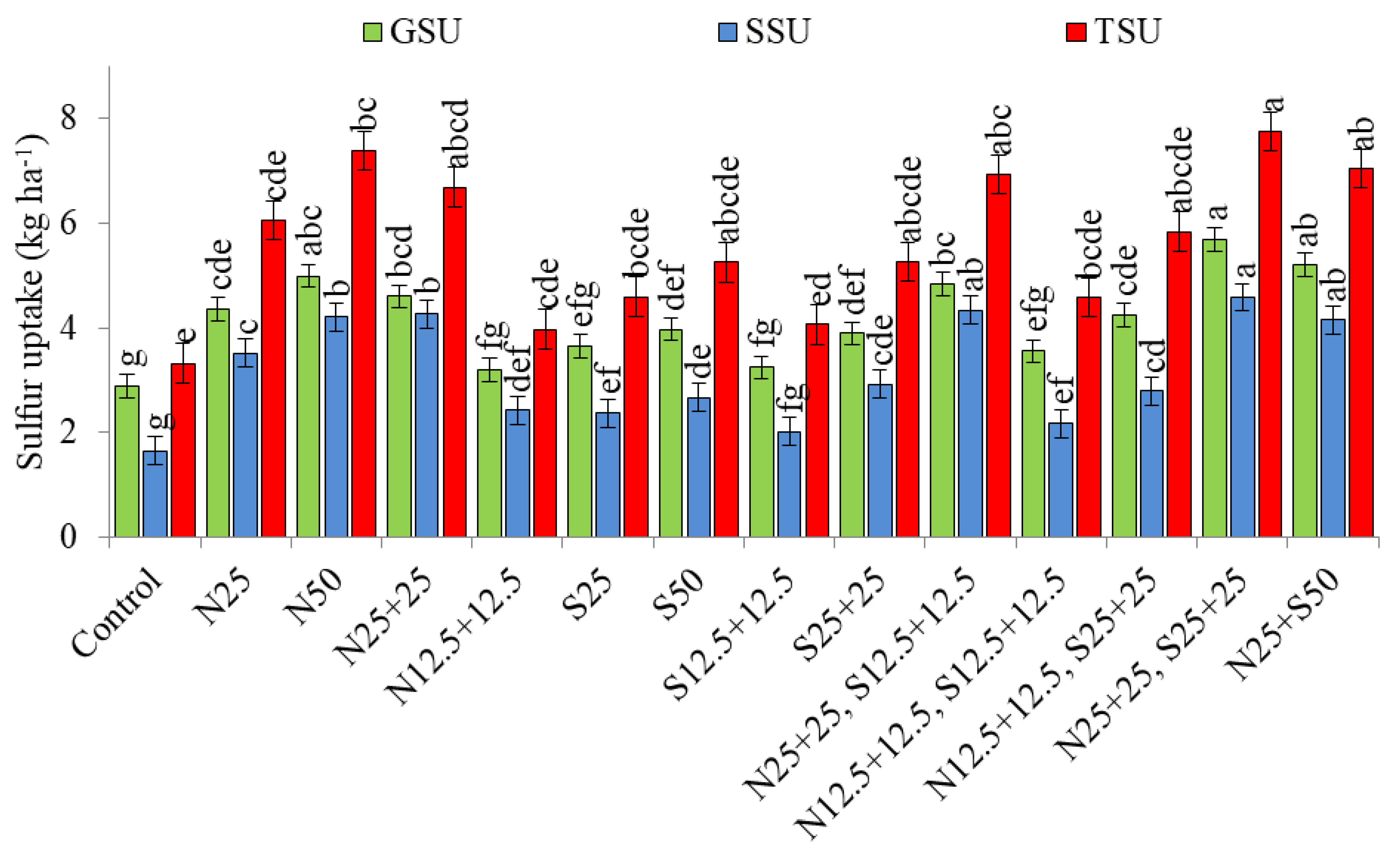
3.1. Nutrient Uptake (kg ha−1)
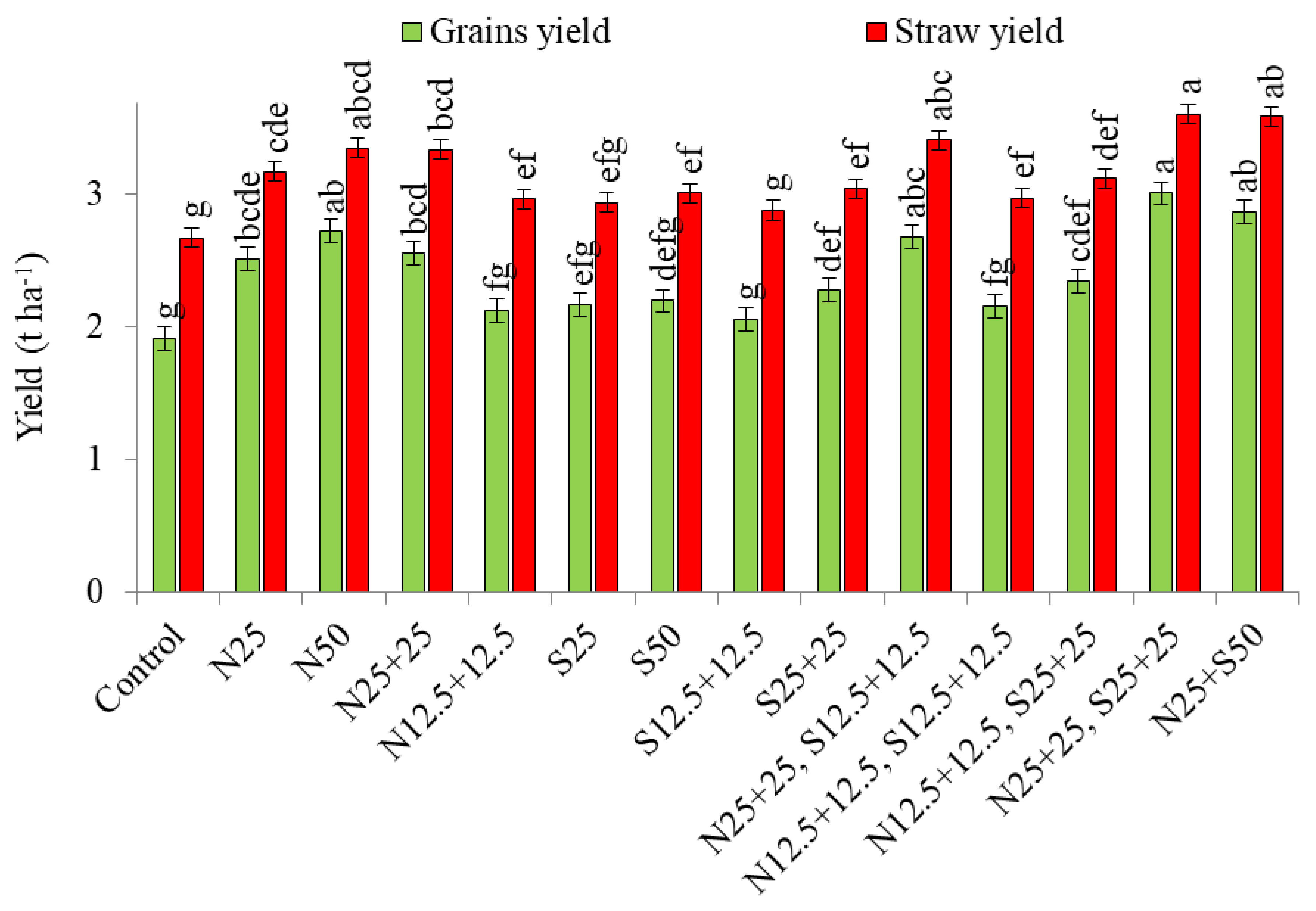
3.2. Seed and Straw Yield (mg ha−1)
3.3. Zn and Fe Content of Seeds and Soil (mg kg−1)
3.4. Nutrient Use Efficiency
3.5. Pearson Correlation and Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wilson, R.F. Soybean: Market Driven Research Needs. In Genetics and Genomics of Soybean; Springer: New York, NY, USA, 2008; pp. 3–15. [Google Scholar]
- Mahmoud, A.A.; Natarajan, S.S.; Bennett, J.O.; Mawhinney, T.P.; Wiebold, W.J.; Krishnan, H.B. Effect of Six Decades of Selective Breeding on Soybean Protein Composition and Quality: A Biochemical and Molecular Analysis. J. Agric. Food Chem. 2006, 54, 3916–3922. [Google Scholar] [CrossRef]
- Khalili, A.; Dhar, S.; Mohammadi, M.; Rasooli, M. Effect of nitrogen on yield and yield attributes of soybean in Kunar, Afghanistan. E-Planet 2021, 19, 46–49. [Google Scholar]
- Khalili, A.; Ramesh, A.; Sharma, M.P. Effect of nitrogen and sulfur applications on growth, chlorophyll content and yield of soybean (Glycine max L.) Merrill. E-Planet 2023, 21, 29–34. [Google Scholar]
- Luo, C.; Branlard, G.; Griffin, W.B.; McNeil, D.L. The effect of nitrogen and sulphur fertilisation and their interaction with genotype on wheat glutenins and quality parameters. J. Cereal Sci. 2000, 31, 185–194. [Google Scholar] [CrossRef]
- Yu, Z.; Juhasz, A.; Islam, S.; Diepeveen, D.; Zhang, J.; Wang, P.; Ma, W. Impact of mid-season sulphur deficiency on wheat nitrogen metabolism and biosynthesis of grain protein. Sci. Rep. 2018, 8, 2499. [Google Scholar] [CrossRef]
- Bellaloui, N.; Bruns, H.A.; Abbas, H.K.; Mengistu, A.; Fisher, D.K.; Reddy, K.N. Agricultural practices altered soybean seed protein, oil, fatty acids, sugars, and minerals in the Midsouth USA. Front. Plant Sci. 2015, 6, 1–14. [Google Scholar] [CrossRef]
- Sinclair, T.R.; de Wit, C.T. Analysis of the Carbon and Nitrogen Limitations to Soybean Yield. Agron. J. 1976, 68, 319–324. [Google Scholar] [CrossRef]
- Giller, K.E.; Cadisch, G. Future Benefits from Biological Nitrogen Fixation: An Ecological Approach to Agriculture. In Management of Biological Nitrogen Fixation for the Development of More Productive and Sustainable Agricultural Systems, Extended versions of papers presented at the Symposium on Biological Nitrogen Fixation for Sustainable Agriculture at the 15th Congress of Soil Science, Acapulco, Mexico, 1994; Springer: Berlin/Heidelberg, Germany, 1995; pp. 255–277. [Google Scholar]
- Cassman, K.G.; Dobermann, A.; Walters, D.T. Agroecosystems, nitrogen-use efficiency, and nitrogen management. Ambio. A J. Hum. Environ. 2002, 31, 132–140. [Google Scholar] [CrossRef]
- Salvagiotti, F.; Castellarín, J.M.; Miralles, D.J.; Pedrol, H.M. Sulfur fertilization improves nitrogen use efficiency in wheat by increasing nitrogen uptake. Field Crop. Res. 2009, 113, 170–177. [Google Scholar] [CrossRef]
- Timsina, J.; Connor, D. Productivity and management of rice–wheat cropping systems: Issues and challenges. Field Crop. Res. 2001, 69, 93–132. [Google Scholar] [CrossRef]
- Salvagiotti, F.; Cassman, K.; Specht, J.; Walters, D.; Weiss, A.; Dobermann, A. Nitrogen uptake, fixation and response to fertilizer N in soybeans: A review. Field Crop. Res. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- Scheerer, U.; Haensch, R.; Mendel, R.R.; Kopriva, S.; Rennenberg, H.; Herschbach, C. Sulphur flux through the sulphate assimilation pathway is differently controlled by adenosine 5′-phosphosulphate reductase under stress and in transgenic poplar plants overexpressing γ-ECS, SO, or APR. J. Exp. Bot. 2009, 61, 609–622. [Google Scholar] [CrossRef]
- Ingenbleek, Y.; Kimura, H. Nutritional essentiality of sulfur in health and disease. Nutr. Rev. 2013, 71, 413–432. [Google Scholar] [CrossRef]
- Salvagiotti, F.; Miralles, D.J. Radiation interception, biomass production and grain yield as affected by the interaction of nitrogen and sulfur fertilization in wheat. Eur. J. Agron. 2008, 28, 282–290. [Google Scholar] [CrossRef]
- Arata, A.F.; Lerner, S.E.; Tranquilli, G.E.; Arrigoni, A.C.; Rondanini, D.P. Nitrogen× sulfur interaction on fertiliser-use efficiency in bread wheat genotypes from the Argentine Pampas. Crop Pasture Sci. 2017, 68, 202–212. [Google Scholar] [CrossRef]
- Gaspar, A.P.; Laboski, C.A.; Naeve, S.L.; Conley, S.P. Dry Matter and Nitrogen Uptake, Partitioning, and Removal across a Wide Range of Soybean Seed Yield Levels. Crop. Sci. 2017, 57, 2170–2182. [Google Scholar] [CrossRef]
- Bender, R.R.; Haegele, J.W.; Below, F.E. Nutrient Uptake, Partitioning, and Remobilization in Modern Soybean Varieties. Agron. J. 2015, 107, 563–573. [Google Scholar] [CrossRef]
- Erenoglu, E.B.; Kutman, U.B.; Ceylan, Y.; Yildiz, B.; Cakmak, I. Improved nitrogen nutrition enhances root uptake, root-to-shoot translocation and remobilization of zinc (65Zn) in wheat. New Phytol. 2011, 189, 438–448. [Google Scholar] [CrossRef]
- Kutman, U.B.; Kutman, B.Y.; Ceylan, Y.; Ova, E.A.; Cakmak, I. Contributions of root uptake and remobilization to grain zinc accumulation in wheat depending on post-anthesis zinc availability and nitrogen nutrition. Plant Soil. 2012, 361, 177–187. [Google Scholar] [CrossRef]
- Khalili, A.; Qayyum, A.; Khan, S.U.; Ullah, I.; Khalofah, A. Role of Temporal Zn Fertilization along with Zn Solubilizing Bacteria in Enhancing Zinc Content, Uptake, and Zinc Use Efficiency in Wheat Genotypes and Its Implications for Agronomic Biofortification. Agronomy 2023, 13, 2677. [Google Scholar] [CrossRef]
- Persson, D.P.; de Bang, T.C.; Pedas, P.R.; Kutman, U.B.; Cakmak, I.; Andersen, B.; Finnie, C.; Schjoerring, J.K.; Husted, S. Molecular speciation and tissue compartmentation of zinc in durum wheat grains with contrasting nutritional status. New Phytol. 2016, 211, 1255–1265. [Google Scholar] [CrossRef]
- Sohrabi, Y.; Habibi, A.; Mohammadi, K.; Sohrabi, M.; Heidari, G.; Khalesro, S.; Khalvandi, M. Effect of nitrogen (N) fertilizer foliar-applied iron (Fe) fertilizer at various reproductive stages on yield yield component chemical composition of soybean (Glycine max L. Merr.) seed. Afr. J. Biotechnol. 2012, 11, 9599–9605. [Google Scholar]
- Kumar, S.; Wani, J.A.; Lone, B.A.; Fayaz, A.; Singh, P.; Qayoom, S.; Dar, Z.A.; Ahmed, N. Effect of Phosphorus and Sulphur on Nutrient and Amino Acids Content of Soybean (Glycine max L. Merrill) under ‘Alfisols’. J. Exp. Agric. Int. 2017, 16, 1–7. [Google Scholar]
- Sharma, A.; Sharma, S. Effect of nitrogen and sulphur nutrition on yield parameters and protein composition in soybean [Glycine max (L.) Merrill]. J. Appl. Nat. Sci. 2014, 6, 402–408. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Miller, J.D. The measurement of civic scientific literacy. Public Underst. Sci. 1998, 7, 203–223. [Google Scholar] [CrossRef]
- Burkitbayev, M.; Bachilova, N.; Kurmanbayeva, M.; Tolenova, K.; Yerezhepova, N.; Zhumagul, M.; Mamurova, A.; Turysbek, B.; Demeu, G. Effect of sulfur-containing agrochemicals on growth, yield, and protein content of soybeans (Glycine max (L.) Merr.). Saudi. J. Biol. Sci. 2021, 28, 891–900. [Google Scholar] [CrossRef]
- Głowacka, A.; Jariene, E.; Flis-Olszewska, E.; Kiełtyka-Dadasiewicz, A. The Effect of nitrogen and sulphur application on soybean productivity traits in temperate climates conditions. Agronomy 2023, 13, 780. [Google Scholar] [CrossRef]
- Schweiger, P.; Hofer, M.; Hartl, W.; Wanek, W.; Vollmann, J. N2 fixation by organically grown soybean in Central Europe: Method of quantification and agronomic effects. Eur. J. Agron. 2012, 41, 11–17. [Google Scholar] [CrossRef]
- Jarecki, W.; Bobrecka-Jamro, D. Effect of fertilization with nitrogen and seed inoculation with nitragina on seed quality of soya bean (Glycine max (L.) Merrill). Acta Sci. Polonorum. Agric. 2015, 14, 51–59. [Google Scholar]
- Lorenc-Kozik, A.M.; Pisulewska, E. Wplyw zroznicowanego nawozenia azotem i mikroelementami na plonowanie wybranych odmian soi. Rośliny Oleiste-Oilseed Crops 2003, 24, 131–142. [Google Scholar]
- Bobrecka-Jamro, D.; Jarecki, W.; Buczek, J. Response of soya bean to different nitrogen fertilization levels. J. Elem. 2018, 23, 559–568. [Google Scholar]
- Lošák, T.; Ševčík, M.; Plchová, R.; Von Bennewitz, E.; Hlušek, J.; Elbl, J.; Buňka, F.; Polášek, Z.; Antonkiewicz, J.; Varga, L. Nitrogen and sulphur fertilisation affecting soybean seed spermidine content. J. Elem. 2018, 23, 581–588. [Google Scholar]
- Namvar, A.; Sharifi, R.S. Phenological and morphological response of chickpea (Cicer arietinum L.) to symbiotic and mineral nitrogen fertilization. Zemdirbysté-Agric. 2011, 98, 121–130. [Google Scholar]
- Gai, Z.; Zhang, J.; Li, C. Effects of starter nitrogen fertilizer on soybean root activity, leaf photosynthesis and grain yield. PLoS ONE 2017, 12, e0174841. [Google Scholar] [CrossRef]
- Van Kessel, C.; Hartley, C. Agricultural management of grain legumes: Has it led to an increase in nitrogen fixation? Field Crops Res. 2000, 65, 165–181. [Google Scholar] [CrossRef]
- Khalili, A.; Dhar, S.; Rasrat, N.A.; Faiz, M.A.; Dass, A.; Varghese, E. Effect of nitrogen management on yield and economics of maize (Zea mays L.) in Kandahar region of Afghanistan. Ann. Agric. Res. 2016, 37, 236–342. [Google Scholar]
- Sharma, O.P.; Tiwari, S.C.; Raghuwanshi, R.K. Effect of doses and sources of sulphur on nodulation, yield, oil and protein content of soybean and soil properties. Soybean Res. 2004, 2, 35–40. [Google Scholar]
- Shi, R.; Zhang, Y.; Chen, X.; Sun, Q.; Zhang, F.; Römheld, V.; Zou, C. Influence of long-term nitrogen fertilization on micronutrient density in grain of winter wheat (Triticum aestivum L.). J. Cereal Sci. 2010, 51, 165–170. [Google Scholar] [CrossRef]
- Cakmak, I.; Kutman, U.B. Agronomic biofortification of cereals with zinc: A review. Eur. J. Soil Sci. 2018, 69, 172–180. [Google Scholar] [CrossRef]
- Cakmak, I.; Kalayci, M.; Kaya, Y.; Torun, A.A.; Aydin, N.; Wang, Y.; Arisoy, Z.; Erdem, H.A.M.İ.D.E.; Yazici, A.; Gokmen, O. Biofortification and localization of zinc in wheat grain. J. Agric. Food Chem. 2010, 58, 9092–9102. [Google Scholar] [CrossRef]
- Kutman, U.B.; Yildiz, B.; Cakmak, I. Improved nitrogen status enhances zinc and iron concentrations both in the whole grain and the endosperm fraction of wheat. J. Cereal Sci. 2011, 53, 118–125. [Google Scholar] [CrossRef]
- Barunawati, N.; Giehl, R.F.H.; Bauer, B.; Wirén, N.V. The influence of inorganic nitrogen fertilizer forms on micronutrient retranslocation and accumulation in grains of winter wheat. Front. Plant Sci. 2013, 4, 320. [Google Scholar] [CrossRef] [PubMed]
- Kutman, U.B.; Yildiz, B.; Cakmak, I. Effect of nitrogen on uptake, remobilization and partitioning of zinc and iron throughout the development of durum wheat. Plant Soil 2011, 342, 149–164. [Google Scholar] [CrossRef]
- Aciksoz, B.S.; Yazicici, A.; Ozturk, L.; Cakmak, I. Biofortification of wheat with iron through soil and foliar application of nitrogen and iron fertilizers. Plant Soil 2011, 349, 215–225. [Google Scholar] [CrossRef]
- Khalili, A.; Dhar, S.; Dass, A.; Ahmad Faiz, M.; Varghese, E. Agronomic indices of nitrogen use efficiency and maize yield response to various rates, time of application and their interaction effect in Kandahar region of Afghanistan. Agronomic indices of nitrogen use efficiency and maize yield response. Ann. Agric. Res. 2018, 39, 347–353. [Google Scholar]



| Treatment Coding | Treatment Details | |
|---|---|---|
| N | S | |
| N0S0 | 0 kg ha−1 nitrogen | 0 kg ha−1 sulfur |
| N(25) | Basal dose at a rate of 25 kg ha−1 nitrogen | - |
| N(50) | Basal dose at a rate of 50 kg ha−1 nitrogen | - |
| N(25+25) | Basal dose at a rate of 25 kg ha−1 nitrogen + Split application of 25 kg ha−1 nitrogen at the R2 stage | - |
| N(12.5+12.5) | Basal dose at a rate of 12.5 kg ha−1 nitrogen + Split application of 12.5 kg ha−1 nitrogen at the R2 stage | - |
| S(25) | - | Basal dose at a rate of 25 kg ha−1 sulfur |
| S(50) | - | Basal dose at a rate of 50 kg ha−1 sulfur |
| S(12.5+12.5) | - | Basal dose at a rate of 12.5 kg ha−1 sulfur + Split application of 12.5 kg ha−1 sulfur at the R2 stage |
| S(25+25) | - | Basal dose at a rate of 25 kg ha−1 sulfur + Split application of 25 kg ha−1 sulfur at the R2 stage |
| N(25+25), S(12.5+12.5) | Basal dose at a rate of 25 kg ha−1 nitrogen + Split application of 25 kg ha−1 nitrogen at the R2 stage | basal dose at a rate of 12.5 kg ha−1 sulfur + Split application of 12.5 kg ha−1 sulfur at the R2 stage |
| N(12.5+12.5), S(12.5+12.5) | Basal dose at a rate of 12.5 kg ha−1 nitrogen + Split application of 12.5 kg ha−1 nitrogen at the R2 stage | Basal dose at a rate of 12.5 kg ha−1 sulfur + Split application of 12.5 kg ha−1 sulfur at the R2 stage |
| N(12.5+12.5), S(25+25) | Basal dose at a rate of 12.5 kg ha−1 nitrogen + Split application of 12.5 kg ha−1 nitrogen at the R2 stage | Basal dose at a rate of 25 kg ha−1 sulfur + Split application of 25 kg ha−1 sulfur at the R2 stage |
| N(25+25), S(25+25) | Basal dose at a rate of 25 kg ha−1 nitrogen + Split application of 25 kg ha−1 nitrogen at the R2 stage | Basal dose at a rate of 25 kg ha−1 sulfur + Split application of 25 kg ha−1 sulfur at the R2 stage |
| N(25), S(50) | Basal dose at a rate of 25 kg ha−1 nitrogen | Basal dose at a rate of 50 kg ha−1 sulfur |
| Treatment | Soil Zn Content (mg kg−1) | Soil Fe Content (mg kg−1) | Soil Zn Content (mg kg−1) | Soil Fe Content (mg kg−1) | Seed Zn Content (mg kg−1) | Seed Fe Content (mg kg−1) |
|---|---|---|---|---|---|---|
| R2 | R5 | |||||
| Control | 0.68 ± 0.04 g | 3.58 ± 0.04 g | 0.76 ± 0.02 g | 3.80 ± 0.02 i | 34.8 ± 1.7 h | 78.9 ± 2.4 f |
| N(25) | 0.79 ± 0.03 cd | 3.80 ± 0.05 c | 0.84 ± 0.03 c | 4.09 ± 0.03 d | 42.5 ± 1.3 cd | 112.1 ± 3.9 c |
| N(50) | 0.85 ± 0.02 b | 3.91 ± 0.04 b | 0.88 ± 0.02 b | 4.14 ± 0.06 cd | 46.3 ± 1.5 b | 113.9 ± 5.6 c |
| N(25+25) | 0.81 ± 0.02 c | 3.76 ± 0.03 cd | 0.89 ± 0.06 b | 4.19 ± 0.03 bc | 46.4 ± 1.2 b | 125.3 ± 7.1 b |
| N(12.5+12.5) | 0.72 ± 0.02 f | 3.69 ± 0.04 ef | 0.77 ± 0.02 efg | 3.91 ± 0.03 fgh | 36.4 ± 1.0 fgh | 81.0 ± 2.9 ef |
| S(25) | 0.73 ± 0.04 ef | 3.71 ± 0.06 de | 0.74 ± 0.02 g | 3.88 ± 0.05 gh | 37.1 ± 1.2 fg | 102.4 ± 2.5 d |
| S(50) | 0.76 ± 0.01 de | 3.73 ± 0.02 de | 0.79 ± 0.01 cdef | 3.94 ± 0.04 efg | 39.7 ± 1.3 e | 104.8 ± 1.4 d |
| S(12.5+12.5) | 0.71 ± 0.02 fg | 3.64 ± 0.02 f | 0.75 ± 0.04 g | 3.88 ± 0.03 ef | 35.3 ± 0.6 gh | 86.8 ± 3.4 e |
| S(25+25) | 0.76 ± 0.02 de | 3.79 ± 0.04 c | 0.79 ± 0.03 def | 3.95 ± 0.04 ab | 40.8 ± 1.3 de | 100.5 ± 4.7 d |
| N(25+25), S(12.5+12.5) | 0.88 ± 0.04 ab | 3.87 ± 0.05 b | 0.91 ± 0.03 ab | 4.21 ± 0.07 ab | 46.6 ± 0.7 b | 126.9 ± 2.7 ab |
| N(12.5+12.5), S(12.5+12.5) | 0.78 ± 0.05 cd | 3.73 ± 0.03 de | 0.81 ± 0.03 cde | 3.91 ± 0.04 fgh | 36.8 ± 0.9 fg | 84.08 ± 7.7 ef |
| N(12.5+12.5), S(25+25) | 0.77 ± 0.03 d | 3.79 ± 0.04 c | 0.82 ± 0.05 cd | 3.98 ± 0.03 e | 37.7 ± 0.8 f | 88.2 ± 6.0 e |
| N(25+25), S(25+25) | 0.90 ± 0.05 a | 3.99 ± 0.07 a | 0.94 ± 0.04 a | 4.27 ± 0.04 a | 49.1 ± 0.9 a | 133.2 ± 2.8 a |
| N(25), S(50) | 0.87 ± 0.03 b | 3.91 ± 0.05 b | 0.91 ± 0.04 ab | 4.16 ± 0.05 ab | 43.6 ± 1.51 c | 115.2 ± 2.0 c |
| LSD (p = 0.05) | 0.04 | 0.06 | 0.04 | 0.06 | 1.9 | 7.2 |
| Treatment | N Agronomic Efficiency kg kg−1 | N Physiological Efficiency kg kg−1 | N Recovery Efficiency % | S Agronomic Efficiency kg kg−1 | S Physiological Efficiency kg kg−1 | S Recovery Efficiency |
|---|---|---|---|---|---|---|
| N(25) | 23.96 | 10.07 | 238 | - | - | - |
| N(50) | 16.12 | 10.36 | 156 | - | - | - |
| N(25+25) | 16.12 | 10.00 | 129 | - | - | - |
| N(12.5+12.5) | 8.36 | 9.41 | 89 | - | - | - |
| S(25) | - | - | - | 10.00 | 196.85 | 5.1 |
| S(50) | - | - | - | 5.62 | 145.60 | 3.9 |
| S(12.5+12.5) | - | - | - | 5.52 | 186.49 | 3.0 |
| S(25+25) | - | - | - | 7.20 | 185.57 | 3.9 |
| N(25+25), S(12.5+12.5) | 15.32 | 9.77 | 157 | 30.64 | 210.44 | 14.4 |
| N(12.5+12.5), S(12.5+12.5) | 9.52 | 9.48 | 101 | 9.52 | 146.91 | 5.0 |
| N(12.5+12.5), S(25+25) | 17.16 | 10.29 | 167 | 8.58 | 170.24 | 5.0 |
| N(25+25), S(25+25) | 21.86 | 10.73 | 204 | 21.86 | 246.73 | 8.9 |
| N(25), S(50) | 38.28 | 11.68 | 333 | 19.14 | 257.26 | 7.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalili, A.; Khalofah, A.; Ramesh, A.; Sharma, M.P. Temporal Synchronization of Nitrogen and Sulfur Fertilization: Impacts on Nutrient Uptake, Use Efficiency, Productivity, and Relationships with Other Micronutrients in Soybean. Agronomy 2024, 14, 570. https://doi.org/10.3390/agronomy14030570
Khalili A, Khalofah A, Ramesh A, Sharma MP. Temporal Synchronization of Nitrogen and Sulfur Fertilization: Impacts on Nutrient Uptake, Use Efficiency, Productivity, and Relationships with Other Micronutrients in Soybean. Agronomy. 2024; 14(3):570. https://doi.org/10.3390/agronomy14030570
Chicago/Turabian StyleKhalili, Azizullah, Ahlam Khalofah, Aketi Ramesh, and Mahaveer P. Sharma. 2024. "Temporal Synchronization of Nitrogen and Sulfur Fertilization: Impacts on Nutrient Uptake, Use Efficiency, Productivity, and Relationships with Other Micronutrients in Soybean" Agronomy 14, no. 3: 570. https://doi.org/10.3390/agronomy14030570
APA StyleKhalili, A., Khalofah, A., Ramesh, A., & Sharma, M. P. (2024). Temporal Synchronization of Nitrogen and Sulfur Fertilization: Impacts on Nutrient Uptake, Use Efficiency, Productivity, and Relationships with Other Micronutrients in Soybean. Agronomy, 14(3), 570. https://doi.org/10.3390/agronomy14030570







