Contact Electrification of Liquid Droplets Impacting Living Plant Leaves
Abstract
:1. Introduction
2. Materials and Methods
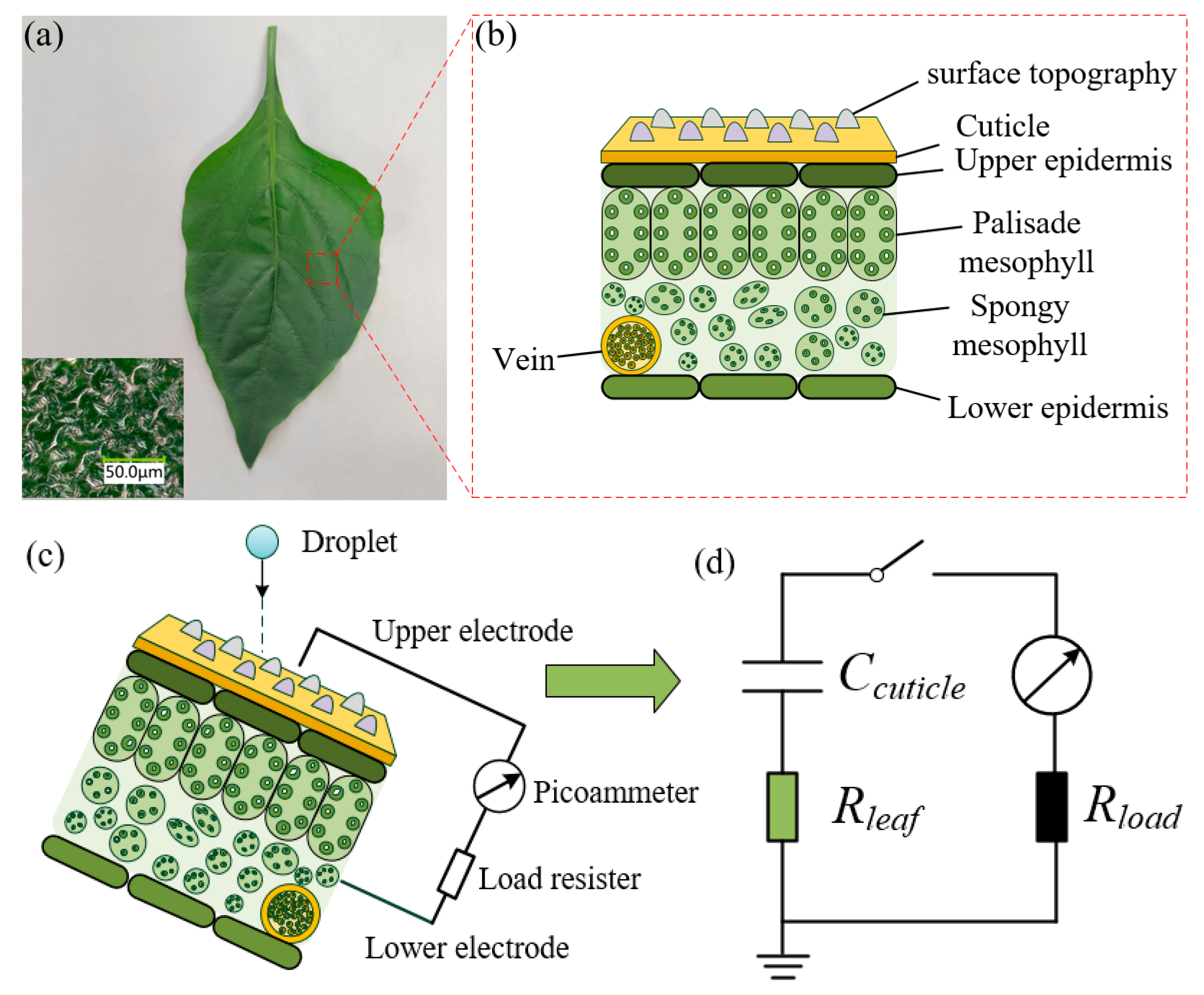
2.1. Plant Species and Preparation
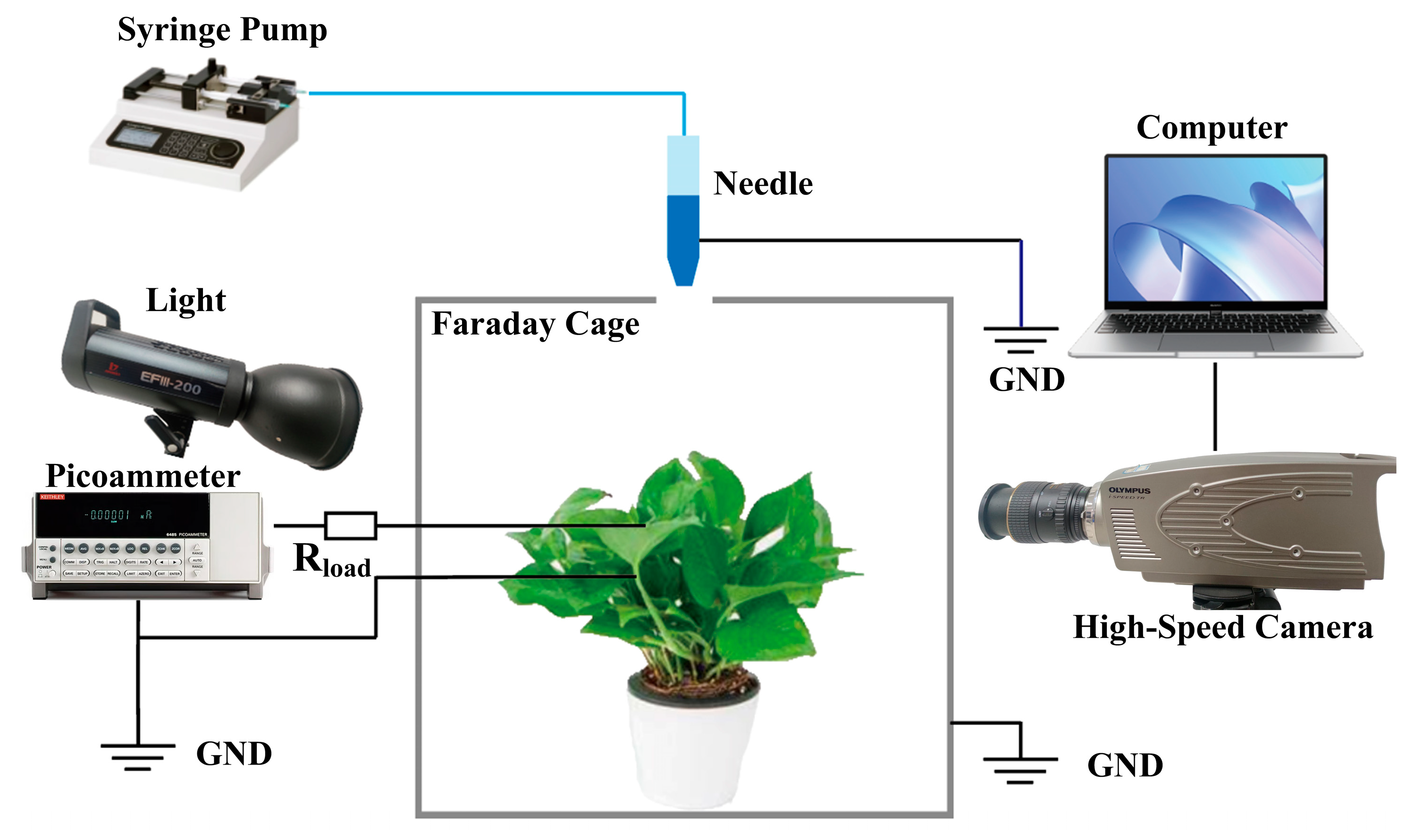
2.2. Dedicated Measurement Set-Up
2.3. Contact Angle and Surface Topography Measurements
2.4. Measurement of Droplet Velocity and Diameter
2.5. Data and Statistical Analysis
3. Results
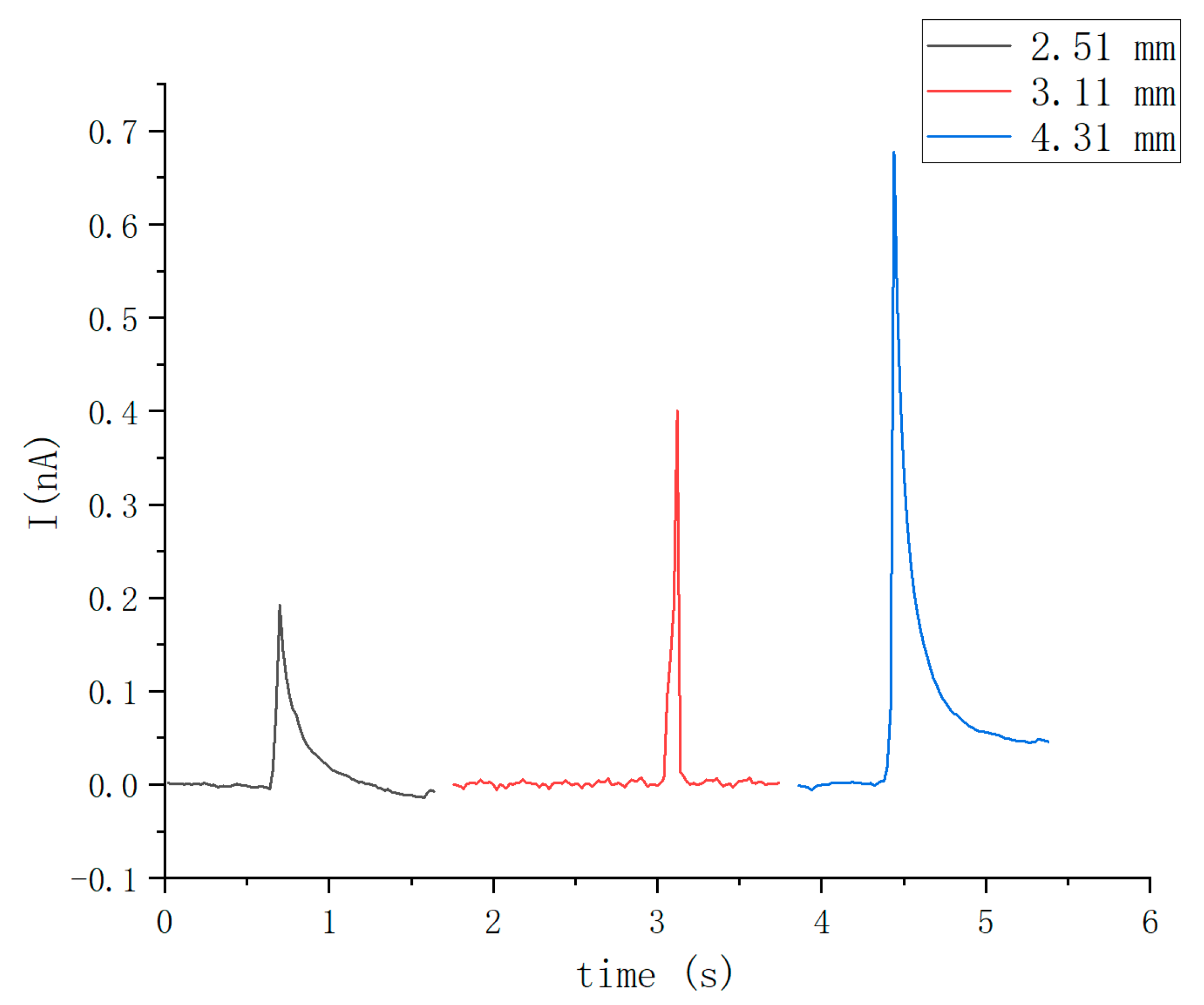
3.1. Electric Signals Generated by Droplet–Leaf Interaction
3.2. Influence of Droplet Diameter on the Electric Signals
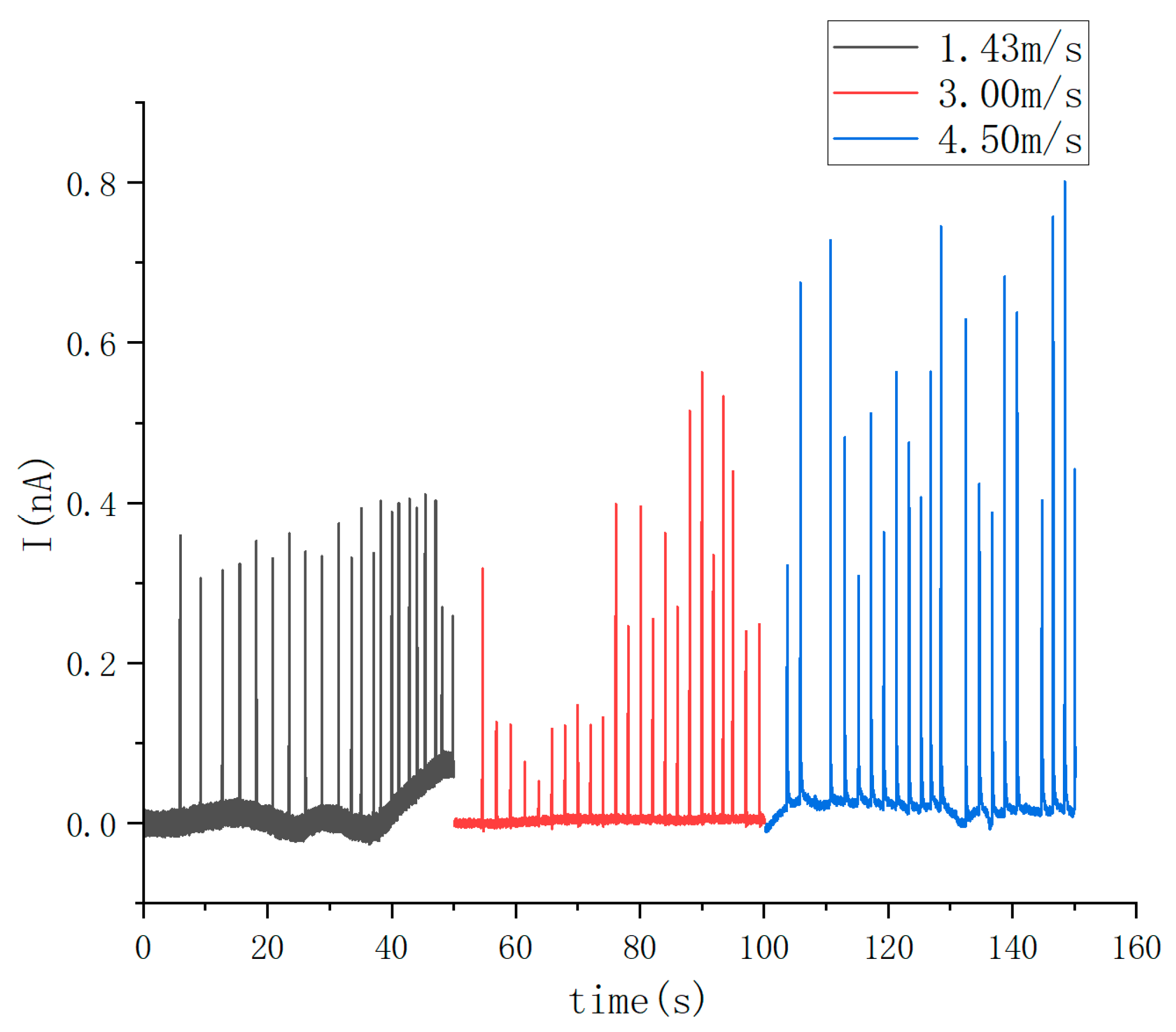
3.3. Influence of Droplet Velocity on the Electric Signals
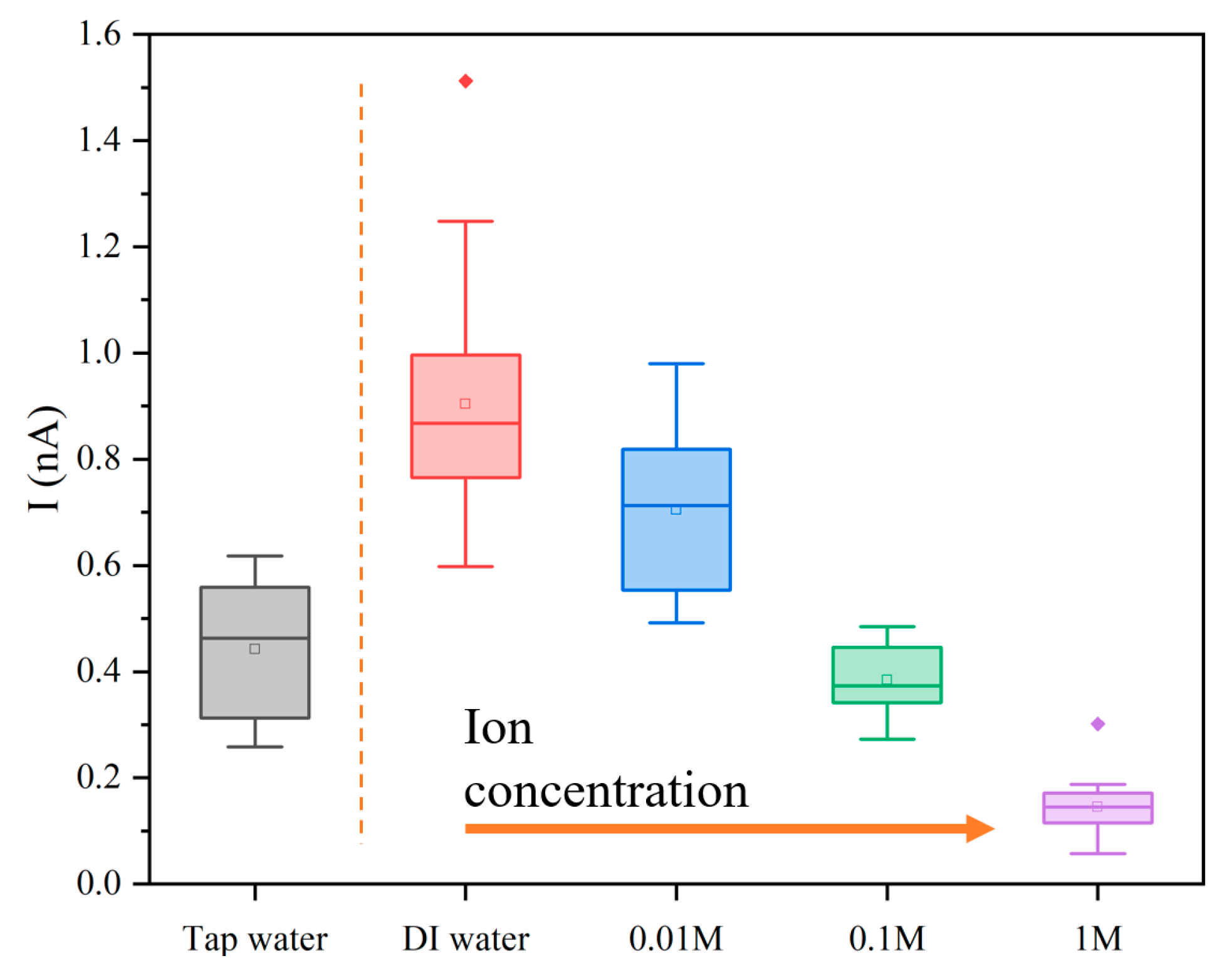
3.4. Influence of Ion Concentration on the Electric Signals
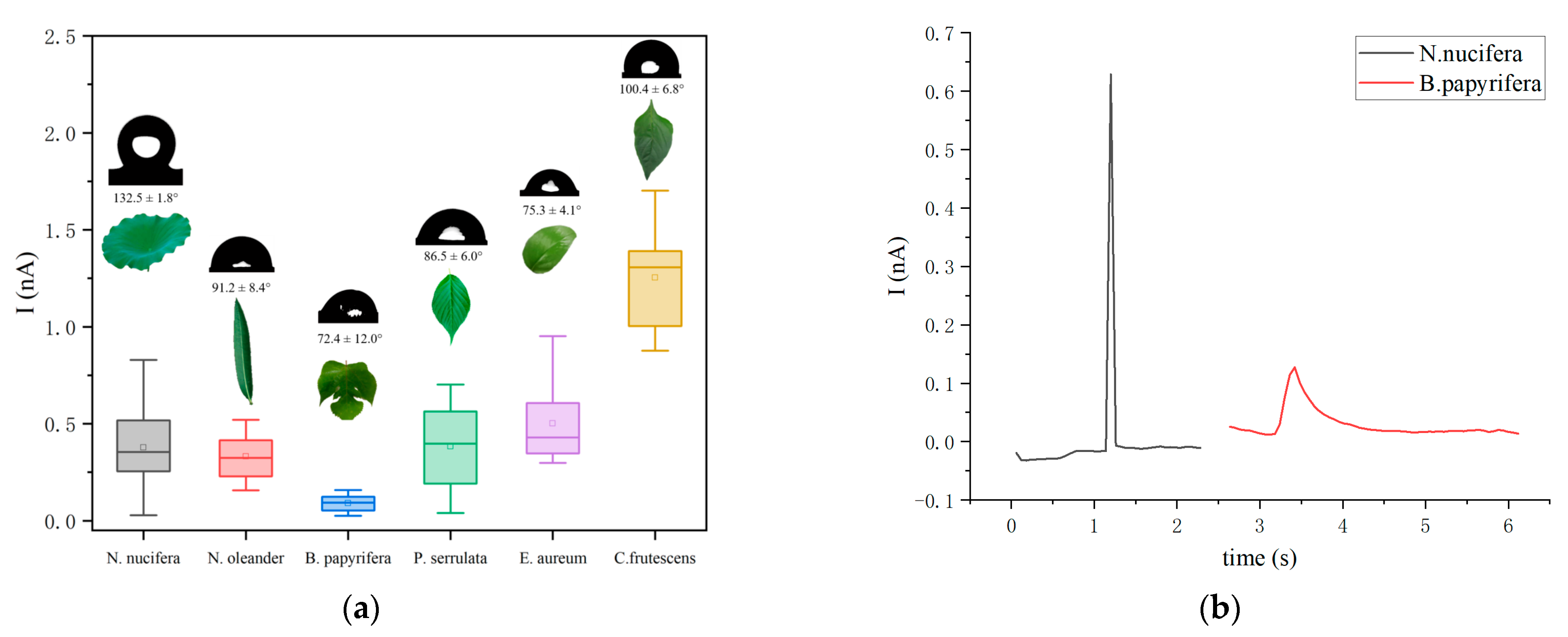
3.5. Comparison with Different Plant Species Surfaces
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gilet, T.; Bourouiba, L. Fluid fragmentation shapes rain-induced foliar disease transmission. J. R. Soc. Interface 2015, 12, 20141092. [Google Scholar] [CrossRef] [PubMed]
- Roth-Nebelsick, A.; Konrad, W.; Ebner, M.; Miranda, T.; Thielen, S.; Nebelsick, J.H. When rain collides with plants-patterns and forces of drop impact and how leaves respond to them. J. Exp. Bot. 2022, 73, 1155–1175. [Google Scholar] [CrossRef]
- Patel, M.K.; Sahoo, H.K.; Nayak, M.K.; Ghanshyam, C. Plausibility of variable coverage high range spraying: Experimental studies of an externally air-assisted electrostatic nozzle. Comput. Electron. Agric. 2016, 127, 641–651. [Google Scholar] [CrossRef]
- White, D.; Gurung, S.; Zhao, D.; Tabler, T.; McDaniel, C.; Styles, D.; McKenzie, S.; Farnell, Y.; Farnell, M. Foam or spray application of agricultural chemicals to clean and disinfect layer cages. J. Appl. Poult. Res. 2018, 27, 416–423. [Google Scholar] [CrossRef]
- Ranjan, R.; Das, A.K. A review on surface protective coating using cold spray cladding technique. Mater. Today Proc. 2022, 56, 768–773. [Google Scholar] [CrossRef]
- Deng, Q.; Wang, H.; Xie, Z.; Zhou, X.; Tian, Y.; Zhang, Q.; Zhu, X.; Chen, R.; Liao, Q. Behaviors of the water droplet impacting on subcooled superhydrophobic surfaces in the electrostatic field. Chem. Eng. Sci. 2023, 266, 118282. [Google Scholar] [CrossRef]
- Lin, J.; Ma, J.; Liu, K.; Huang, X.; Xiao, L.; Ahmed, S.; Dong, X.; Qiu, B. Development and test of an autonomous air-assisted sprayer based on single hanging track for solar greenhouse. Crop Prot. 2021, 142, 105502. [Google Scholar] [CrossRef]
- Lin, S.; Zhao, B.; Zou, S.; Guo, J.; Wei, Z.; Chen, L. Impact of viscous droplets on different wettable surfaces: Impact phenomena, the maximum spreading factor, spreading time and post-impact oscillation. J. Colloid Interface Sci. 2018, 516, 86–97. [Google Scholar] [CrossRef]
- Lauderbaugh, L.K.; Ginebra-Solanellas, R.M.; Holder, C.D.; Webb, R. A biomechanical model of leaf inclination angle oscillations after raindrop impact. Environ. Exp. Bot. 2021, 190, 104586. [Google Scholar] [CrossRef]
- Ma, J.; Liu, K.; Dong, X.; Huang, X.; Ahmad, F.; Qiu, B. Force and motion behaviour of crop leaves during spraying. Biosyst. Eng. 2023, 235, 83–99. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, Y.; Li, Y.; Chen, X.; Shen, H. A Review: Contact Electrification on Special Interfaces. Front. Mater. 2022, 9, 909746. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Z.; Xu, G.; Xu, J.; Wang, Z.; Zi, Y. Fully biodegradable water droplet energy harvester based on leaves of living plants. ACS Appl. Mater. Interfaces 2020, 12, 56060–56067. [Google Scholar] [CrossRef]
- Armiento, S.; Filippeschi, C.; Meder, F.; Mazzolai, B. Liquid-solid contact electrification when water droplets hit living plant leaves. Commun. Mater. 2022, 3, 79. [Google Scholar] [CrossRef]
- Nan, Y.; Shao, J.; Willatzen, M.; Wang, Z.L. Understanding Contact Electrification at Water/Polymer Interface. Research 2022, 2022, 9861463. [Google Scholar] [CrossRef]
- Zheng, H.; Wu, H.; Yi, Z.; Song, Y.; Xu, W.; Yan, X.; Zhou, X.; Wang, S.; Wang, Z. Remote-Controlled Droplet Chains-Based Electricity Generators. Adv. Energy Mater. 2023, 13, 2203825. [Google Scholar] [CrossRef]
- Lin, S.; Zheng, M.; Wang, Z.L. Detecting the Liquid–Solid Contact Electrification Charges in a Liquid Environment. J. Phys. Chem. C 2021, 125, 14098–14104. [Google Scholar] [CrossRef]
- Lin, S.; Xu, L.; Chi Wang, A.; Wang, Z.L. Quantifying electron-transfer in liquid-solid contact electrification and the formation of electric double-layer. Nat. Commun. 2020, 11, 399. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Chen, X.; Wang, Z.L. Contact Electrification at the Liquid-Solid Interface. Chem. Rev. 2022, 122, 5209–5232. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Lin, S.; Wang, Z.L. Effect of Surface Pre-Charging and Electric Field on the Contact Electrification between Liquid and Solid. J. Phys. Chem. C 2022, 126, 8897–8905. [Google Scholar] [CrossRef]
- Tang, Z.; Lin, S.; Wang, Z.L. Quantifying Contact-Electrification Induced Charge Transfer on a Liquid Droplet after Contacting with a Liquid or Solid. Adv. Mater. 2021, 33, e2102886. [Google Scholar] [CrossRef]
- Sun, M.; Lu, Q.; Wang, Z.L.; Huang, B. Understanding contact electrification at liquid-solid interfaces from surface electronic structure. Nat. Commun. 2021, 12, 1752. [Google Scholar] [CrossRef] [PubMed]
- Nauruzbayeva, J.; Sun, Z.; Gallo, A., Jr.; Ibrahim, M.; Santamarina, J.C.; Mishra, H. Electrification at water-hydrophobe interfaces. Nat. Commun. 2020, 11, 5285. [Google Scholar] [CrossRef] [PubMed]
- Mats, L.; Bramwell, A.; Dupont, J.; Liu, G.; Oleschuk, R. Electrowetting on superhydrophobic natural (Colocasia) and synthetic surfaces based upon fluorinated silica nanoparticles. Microelectron. Eng. 2015, 148, 91–97. [Google Scholar] [CrossRef]
- Li, X.; Tian, H.; Shao, J.; Ding, Y.; Chen, X.; Wang, L.; Lu, B. Decreasing the Saturated Contact Angle in Electrowetting-on-Dielectrics by Controlling the Charge Trapping at Liquid-Solid Interfaces. Adv. Funct. Mater. 2016, 26, 2994–3002. [Google Scholar] [CrossRef]
- Law, S.E. Agricultural electrostatic spray application: A review of significant research and development during the 20th century. J. Electrost. 2001, 51, 25–42. [Google Scholar]
- Maski, D.; Durairaj, D. Effects of electrode voltage, liquid flow rate, and liquid properties on spray chargeability of an air-assisted electrostatic-induction spray-charging system. J. Electrost. 2010, 68, 152–158. [Google Scholar] [CrossRef]
- Salcedo, R.; Llop, J.; Campos, J.; Costas, M.; Gallart, M.; Ortega, P.; Gil, E. Evaluation of leaf deposit quality between electrostatic and conventional multi-row sprayers in a trellised vineyard. Crop Prot. 2020, 127, 104964. [Google Scholar] [CrossRef]
- Patel, M.K.; Praveen, B.; Sahoo, H.K.; Patel, B.; Kumar, A.; Singh, M.; Nayak, M.K.; Rajan, P. An advance air-induced air-assisted electrostatic nozzle with enhanced performance. Comput. Electron. Agric. 2017, 135, 280–288. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, H.; Ozkan, H.E.; Thistle, H.W. Evaporation rate and development of wetted area of water droplets with and without surfactant at different locations on waxy leaf surfaces. Biosyst. Eng. 2010, 106, 58–67. [Google Scholar] [CrossRef]
- Armiento, S.; Meder, F.; Mazzolai, B. Device for Simultaneous Wind and Raindrop Energy Harvesting Operating on the Surface of Plant Leaves. IEEE Robot. Autom. Lett. 2023, 8, 2269–2276. [Google Scholar] [CrossRef]
- Yoo, D.; Kim, S.J.; Joung, Y.; Jang, S.; Choi, D.; Kim, D.S. Lotus leaf-inspired droplet-based electricity generator with low-adhesive superhydrophobicity for a wide operational droplet volume range and boosted electricity output. Nano Energy 2022, 99, 107361. [Google Scholar] [CrossRef]
- Yan, Z.; Xu, D.; Lin, Z.; Wang, P.; Cao, B.; Ren, H.; Song, F.; Wan, C.; Wang, L.; Zhou, J. Highly stretchable van der Waals thin films for adaptable and breathable electronic membranes. Science 2022, 375, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Jie, Y.; Jia, X.; Zou, J.; Chen, Y.; Wang, N.; Wang, Z.L.; Cao, X. Natural Leaf Made Triboelectric Nanogenerator for Harvesting Environmental Mechanical Energy. Adv. Energy Mater. 2018, 8, 1703133. [Google Scholar] [CrossRef]
- Meder, F.; Must, I.; Sadeghi, A.; Mondini, A.; Filippeschi, C.; Beccai, L.; Mattoli, V.; Pingue, P.; Mazzolai, B. Energy Conversion at the Cuticle of Living Plants. Adv. Funct. Mater. 2018, 28, 1806689. [Google Scholar] [CrossRef]
- Xu, W.; Zheng, H.; Liu, Y.; Zhou, X.; Zhang, C.; Song, Y.; Deng, X.; Leung, M.; Yang, Z.; Xu, R.X.; et al. A droplet-based electricity generator with high instantaneous power density. Nature 2020, 578, 392–396. [Google Scholar] [CrossRef]
- Wu, H.; Mendel, N.; van Der Ham, S.; Shui, L.; Zhou, G.; Mugele, F. Charge Trapping-Based Electricity Generator (CTEG): An ultrarobust and high efficiency nanogenerator for energy harvesting from water droplets. Adv. Mater. 2020, 32, 2001699. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Mendel, N.; van den Ende, D.; Zhou, G.; Mugele, F. Energy harvesting from drops impacting onto charged surfaces. Phys. Rev. Lett. 2020, 125, 078301. [Google Scholar] [CrossRef]
- Zhang, N.; Gu, H.; Lu, K.; Ye, S.; Xu, W.; Zheng, H.; Song, Y.; Liu, C.; Jiao, J.; Wang, Z. A universal single electrode droplet-based electricity generator (SE-DEG) for water kinetic energy harvesting. Nano Energy 2021, 82, 105735. [Google Scholar] [CrossRef]
- Gennes, P.-G.; Brochard-Wyart, F.; Quéré, D. Capillarity and Wetting Phenomena: Drops, Bubbles, Pearls, Waves; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Vasandani, P.; Mao, Z.-H.; Jia, W.; Sun, M. Relationship between triboelectric charge and contact force for two triboelectric layers. J. Electrost. 2017, 90, 147–152. [Google Scholar] [CrossRef]
- Cao, S.; Zhang, H.; Cui, X.; Yuan, Z.; Ding, J.; Sang, S. Fully-Enclosed Metal Electrode-Free Triboelectric Nanogenerator for Scavenging Vibrational Energy and Alternatively Powering Personal Electronics. Adv. Eng. Mater. 2019, 21, 1800823. [Google Scholar] [CrossRef]
- Kim, D.W.; Kim, S.W.; Jeong, U. Lipids: Source of static electricity of regenerative natural substances and nondestructive energy harvesting. Adv. Mater. 2018, 30, 1804949. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, W.; Gao, Z.; Dong, X.; Chen, J.; Qiu, B. Contact Electrification of Liquid Droplets Impacting Living Plant Leaves. Agronomy 2024, 14, 573. https://doi.org/10.3390/agronomy14030573
Hu W, Gao Z, Dong X, Chen J, Qiu B. Contact Electrification of Liquid Droplets Impacting Living Plant Leaves. Agronomy. 2024; 14(3):573. https://doi.org/10.3390/agronomy14030573
Chicago/Turabian StyleHu, Wei, Zhouming Gao, Xiaoya Dong, Jian Chen, and Baijing Qiu. 2024. "Contact Electrification of Liquid Droplets Impacting Living Plant Leaves" Agronomy 14, no. 3: 573. https://doi.org/10.3390/agronomy14030573





