Enhancing Soil Health and Plant Growth through Microbial Fertilizers: Mechanisms, Benefits, and Sustainable Agricultural Practices
Abstract
:1. Introduction
2. Microbial Fertilizers
2.1. Definition of Microbial Fertilizers
2.2. Mechanism of Action of Microbial Fertilizers
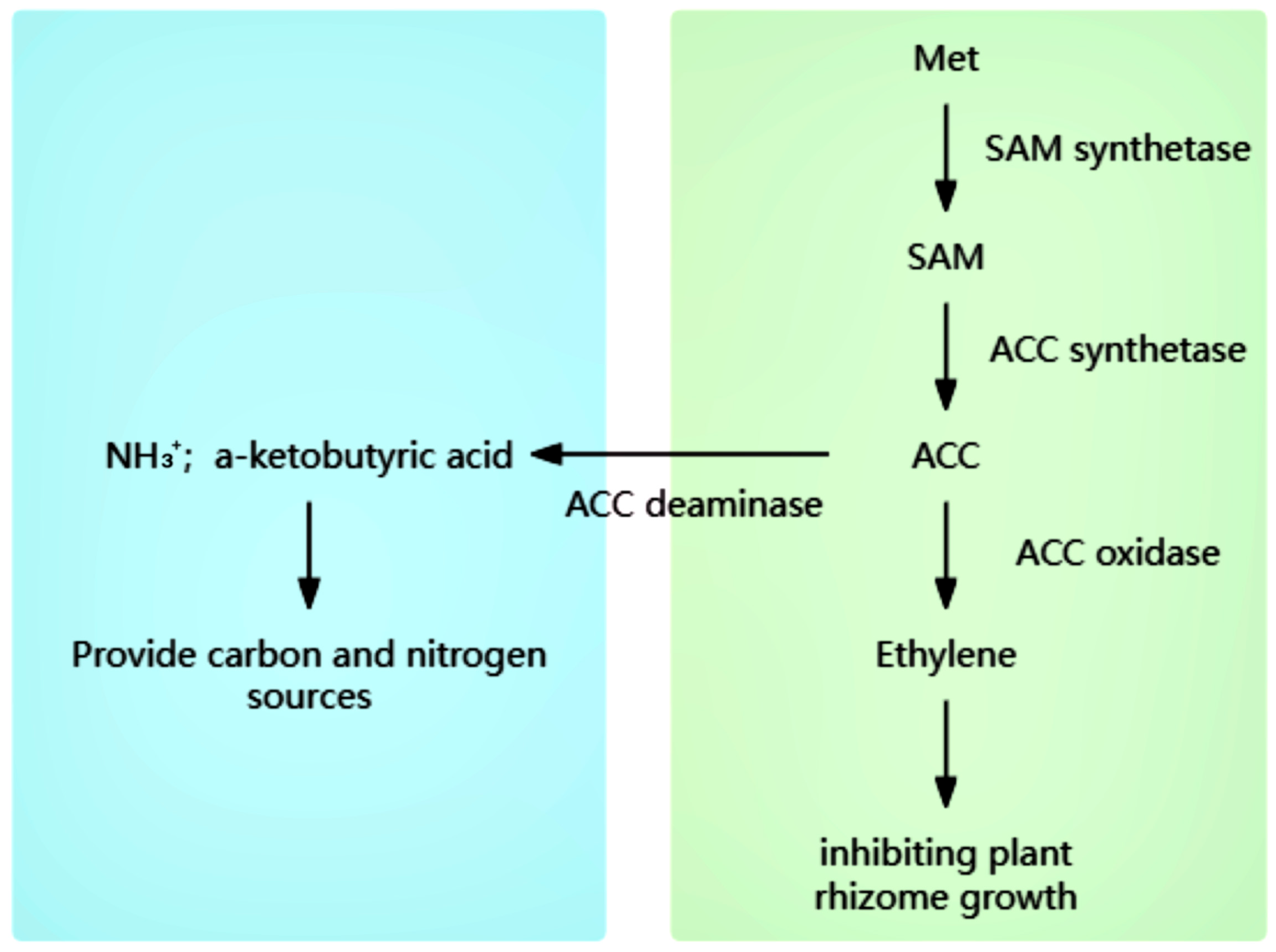
2.2.1. AAC Deamination Enzymatic Probiotics
2.2.2. Produces Plant Hormones and Promotes Plant Growth
2.2.3. Improves Antioxidant Enzyme Activity
2.2.4. Stimulates Plant Enzymes and Signaling Pathways in Plants
2.2.5. Nitrogen Fixation, Potassium Solubilization, Phosphorus Solubilization
3. The Impact of Microbial Fertilizers on the Structure of Soil Microbial Communities
4. The Impact of Microbial Fertilizers on the Structure of Soil Microbial Communities
4.1. Ameliorating Soil Compaction and Promoting Aggregate Formation
4.2. Impact of Soil Microorganisms on Soil pH and Organic Carbon
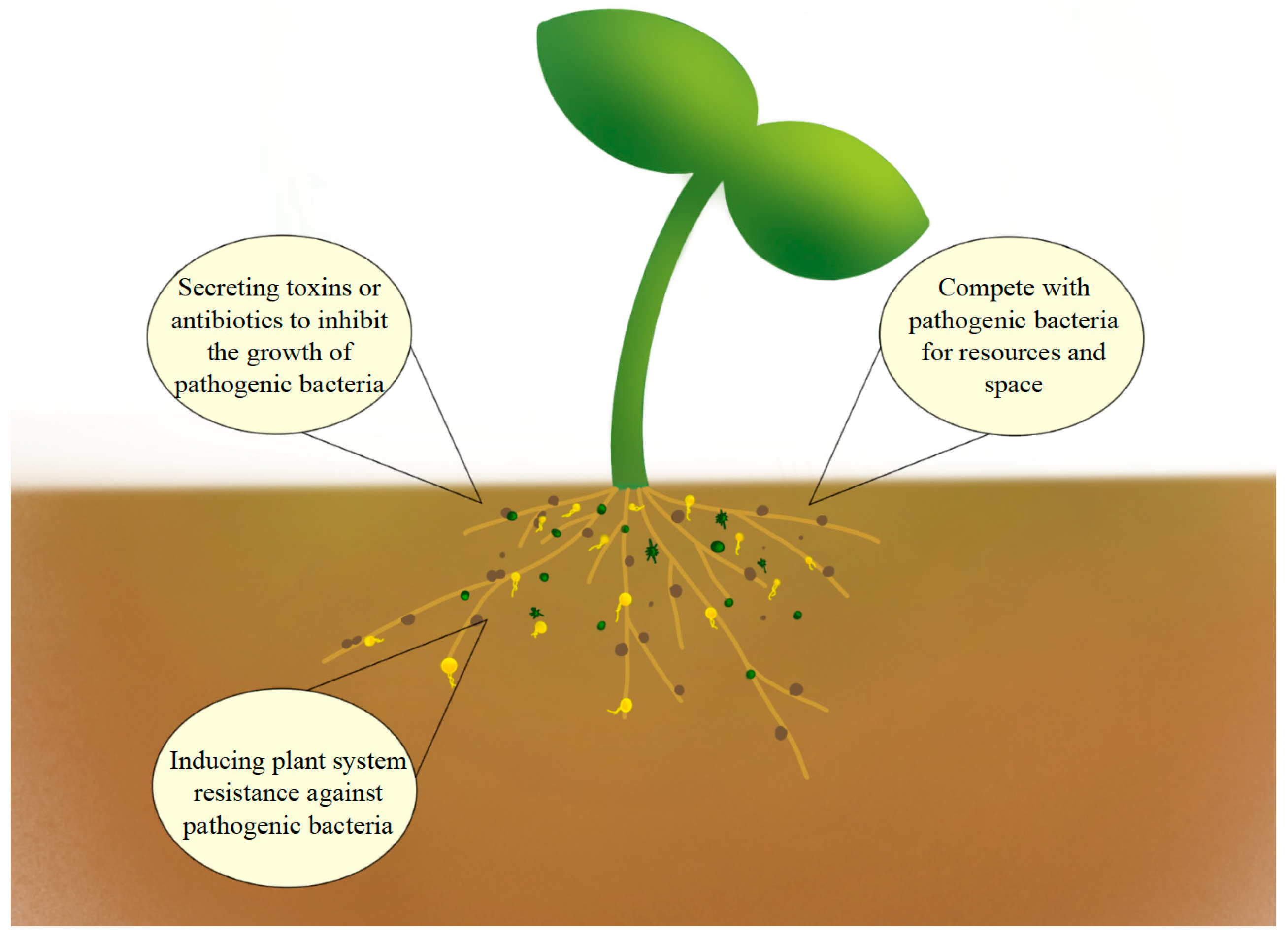
4.3. The Impact of Applying Microbial Fertilizers on Soil Pathogens
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Szilagyi-Zecchin, V.J.; Mógor, Á.F.; Figueiredo, G.G.O. Strategies for Characterization of Agriculturally Important Bacteria. In Microbial Inoculants in Sustainable Agricultural Productivity; Springer: New Delhi, India, 2016; pp. 1–21. [Google Scholar] [CrossRef]
- Liu, H.; Li, S.; Qiang, R.; Lu, E.; Li, C.; Zhang, J.; Gao, Q. Response of Soil Microbial Community Structure to Phosphate Fertilizer Reduction and Combinations of Microbial Fertilizer. Front. Environ. Sci. 2022, 10, 899727. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, J.; Dou, Y.; Chen, M.; Ping, S.; Peng, J.; Lu, W.; Zhang, W.; Yao, Z.; Li, H.; et al. Nitrogen fixation island and rhizosphere competence traits in the genome of root-associated Pseudomonas stutzeri A1501. Proc. Natl. Acad. Sci. USA 2008, 105, 7564–7569. [Google Scholar] [CrossRef]
- Zhongli, C.; Shunpeng, L.; Guoping, F. Isolation of Methyl Parathion-Degrading Strain M6 and Cloning of the Methyl Parathion Hydrolase Gene. Appl. Environ. Microbiol. 2001, 67, 4922–4925. [Google Scholar] [CrossRef]
- Hang, B.-J.; Hong, Q.; Xie, X.-T.; Huang, X.; Wang, C.-H.; He, J.; Li, S.-P. SulE, a Sulfonylurea Herbicide De-Esterification Esterase from Hansschlegelia zhihuaiae S113. Appl. Environ. Microbiol. 2012, 78, 1962–1968. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, J.-G.; Hang, B.-J.; Cai, S.; He, J.; Zhou, S.-G.; Li, S.-P. Cloning of a Novel Arylamidase Gene from Paracoccus sp. Strain FLN-7 That Hydrolyzes Amide Pesticides. Appl. Environ. Microbiol. 2012, 78, 4848–4855. [Google Scholar] [CrossRef]
- Hassen, A.I.; Bopape, F.L.; Sanger, L.K. Sanger Microbial Inoculants as Agents of Growth Promotion and Abiotic Stress Tolerance in Plants. In Microbial Inoculants in Sustainable Agricultural Productivity; Springer: New Delhi, India, 2016; Volume 1, pp. 23–36. [Google Scholar] [CrossRef]
- Hoffman, B.M.; Dean, D.R.; Seefeldt, L.C. Climbing Nitrogenase: Toward a Mechanism of Enzymatic Nitrogen Fixation. Acc. Chem. Res. 2009, 42, 609–619. [Google Scholar] [CrossRef]
- Raman, J.; Kim, J.-S.; Choi, K.R.; Eun, H.; Yang, D.; Ko, Y.-J.; Kim, S.-J. Application of Lactic Acid Bacteria (LAB) in Sustainable Agriculture: Advantages and Limitations. Int. J. Mol. Sci. 2022, 23, 7784. [Google Scholar] [CrossRef]
- Wang, W.; Lu, C. Visualization analysis of big data research based on Citespace. Soft Comput. 2019, 24, 8173–8186. [Google Scholar] [CrossRef]
- Naing, A.H.; Maung, T.T.; Kim, C.K. The ACC deaminase-producing plant growth-promoting bacteria: Influences of bacterial strains and ACC deaminase activities in plant tolerance to abiotic stress. Physiol. Plant. 2021, 173, 1992–2012. [Google Scholar] [CrossRef]
- Singh, R.P.; Ma, Y.; Shadan, A. Perspective of ACC-deaminase producing bacteria in stress agriculture. J. Biotechnol. 2022, 352, 36–46. [Google Scholar] [CrossRef]
- Sarkar, A.; Ghosh, P.K.; Pramanik, K.; Mitra, S.; Soren, T.; Pandey, S.; Mondal, M.H.; Maiti, T.K. A halotolerant Enterobacter sp. displaying ACC deaminase activity promotes rice seedling growth under salt stress. Res. Microbiol. 2018, 169, 20–32. [Google Scholar] [CrossRef]
- Farooq, S.; Danish, S.; Zafar-ul-Hye, M.; Mohsin, F.; Hussain, M. ACC-deaminase producing plant growth promoting rhizobacteria and biochar mitigate adverse effects of drought stress on maize growth. PLoS ONE 2020, 15, e0230615. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Yadav, B.K.; Akhtar, M.S.; Panwar, J. Rhizospheric plantmicrobe interactions: Key factors to soil fertility and plant nutrition. In Plant microbes symbiosis: Applied facets. In Plant Microbes Symbiosis: Applied Facets; Springer: New Delhi, India, 2015; pp. 127–145. [Google Scholar] [CrossRef]
- Bonartsev, A.P.; Zharkova, I.I.; Yakovlev, S.G.; Myshkina, V.L.; Mahina, T.K.; Voinova, V.V.; Zernov, A.L.; Zhuikov, V.A.; Akoulina, E.A.; Ivanova, E.V.; et al. Biosynthesis of poly(3-hydroxybutyrate) copolymers by Azotobacter chroococcum 7B: A precursor feeding strategy. Prep. Biochem. Biotechnol. 2016, 47, 173–184. [Google Scholar] [CrossRef]
- Erturk, Y.; Ercisli, S.; Haznedar, A.; Cakmakci, R. Effects of plant growth promoting rhizobacteria (PGPR) on rooting and root growth of kiwifruit (Actinidia deliciosa) stem cuttings. Biol. Res. 2010, 43, 91–98. [Google Scholar] [CrossRef]
- Khan, M.; Ali, S.; Al Azzawi, T.N.I.; Saqib, S.; Ullah, F.; Ayaz, A.; Zaman, W. The Key Roles of ROS and RNS as a Signaling Molecule in Plant-Microbe Interactions. Antioxidants 2023, 12, 268. [Google Scholar] [CrossRef]
- Sagar, A.; Sayyed, R.Z.; Ramteke, P.W.; Sharma, S.; Marraiki, N.; Elgorban, A.M.; Syed, A. ACC deaminase and antioxidant enzymes producing halophilic Enterobacter sp. PR14 promotes the growth of rice and millets under salinity stress. Physiol. Mol. Biol. Plants 2020, 26, 1847–1854. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, L.; Chen, Y.; Dhanasekaran, S.; Chen, X.; Zhang, X.; Yang, X.; Wu, M.; Song, Y.; Zhang, H. Study on the control effect and physiological mechanism of Wickerhamomyces anomalus on primary postharvest diseases of peach fruit. Int. J. Food Microbiol. 2024, 413, 110575. [Google Scholar] [CrossRef]
- Fang, C.; Fernie, A.R.; Luo, J. Exploring the Diversity of Plant Metabolism. Trends Plant Sci. 2019, 24, 83–98. [Google Scholar] [CrossRef]
- Vukelić, I.D.; Prokić, L.T.; Racić, G.M.; Pešić, M.B.; Bojović, M.M.; Sierka, E.M.; Kalaji, H.M.; Panković, D.M. Effects of Trichoderma harzianum on Photosynthetic Characteristics and Fruit Quality of Tomato Plants. Int. J. Mol. Sci. 2021, 22, 6961. [Google Scholar] [CrossRef]
- Arora, M.; Saxena, P.; Choudhary, D.K.; Abdin, M.Z.; Varma, A. Dual symbiosis between Piriformospora indica and Azotobacter chroococcum enhances the artemisinin content in Artemisia annua L. World J. Microbiol. Biotechnol. 2016, 32, 19. [Google Scholar] [CrossRef]
- Prasanna, R.; Joshi, M.; Rana, A.; Shivay, Y.S.; Nain, L. Influence of co-inoculation of bacteria-cyanobacteria on crop yield and C–N sequestration in soil under rice crop. World J. Microbiol. Biotechnol. 2011, 28, 1223–1235. [Google Scholar] [CrossRef]
- Yang, W.; Gong, T.; Wang, J.; Li, G.; Liu, Y.; Zhen, J.; Ning, M.; Yue, D.; Du, Z.; Chen, G. Effects of Compound Microbial Fertilizer on Soil Characteristics and Yield of Wheat (Triticum aestivum L.). J. Soil Sci. Plant Nutr. 2020, 20, 2740–2748. [Google Scholar] [CrossRef]
- Assainar, S.K.; Abbott, L.K.; Mickan, B.S.; Whiteley, A.S.; Siddique, K.H.M.; Solaiman, Z.M. Response of Wheat to a Multiple Species Microbial Inoculant Compared to Fertilizer Application. Front. Plant Sci. 2018, 9, 416134. [Google Scholar] [CrossRef]
- Tairo, E.V.; Ndakidemi, P.A. Possible benefits of rhizobial inoculation and phosphorus supplementation on nutrition, growth and economic sustainability in grain legumes. Am. J. Res. Commun. 2013, 1, 532–556. [Google Scholar]
- Aasfar, A.; Bargaz, A.; Yaakoubi, K.; Hilali, A.; Bennis, I.; Zeroual, Y.; Meftah Kadmiri, I. Nitrogen Fixing Azotobacter Species as Potential Soil Biological Enhancers for Crop Nutrition and Yield Stability. Front. Microbiol. 2021, 12, 628379. [Google Scholar] [CrossRef]
- Bladergroen, M.R.; Spaink, H.P. Genes and signal molecules involved in the rhizobiaLeguminoseae symbiosis. Curr. Opin. Plant Biol. 1998, 1, 353–359. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The Critical Role of Potassium in Plant Stress Response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Rekha, P.D.; Arun, A.B.; Shen, F.T.; Lai, W.A.; Young, C.C. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl. Soil Ecol. 2006, 34, 33–41. [Google Scholar] [CrossRef]
- Zhang, C.; Kong, F. Isolation and identification of potassium-solubilizing bacteria from tobacco rhizospheric soil and their effect on tobacco plants. Appl. Soil Ecol. 2014, 82, 18–25. [Google Scholar] [CrossRef]
- Etesami, H.; Emami, S.; Alikhani, H.A. Potassium solubilizing bacteria (KSB): Mechanisms, promotion of plant growth, and future prospects—A review. J. Soil Sci. Plant Nutr. 2017, 17, 897–911. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Yang, X.-Z.; Li, Z.; An, X.-H.; Ma, R.-P.; Li, Y.-Q.; Cheng, C.-G. Efficiency of potassium-solubilizing Paenibacillus mucilaginosus for the growth of apple seedling. J. Integr. Agric. 2020, 19, 2458–2469. [Google Scholar] [CrossRef]
- Feng, K.; Lu, H.M.; Sheng, H.J.; Wang, X.L.; Mao, J. Effect of organic ligands on biological availability of inorganic phosphorus in soils. Pedosphere 2004, 14, 85–92. [Google Scholar]
- Wang, W.; Ku, Y.; Xu, G.; Tian, X.; Xie, H.; Yang, X.; Cao, C. Root colonization and growth promotion of soybean, wheat and Chinese cabbage by Bacillus cereus YL6. PLoS ONE 2018, 13, e0200181. [Google Scholar] [CrossRef]
- Marra, L.M.; de Oliveira-Longatti, S.M.; Soares, C.R.F.S.; Olivares, F.L.; Moreira, F.M.d.S. The Amount of Phosphate Solubilization Depends on the Strain, C-Source, Organic Acids and Type of Phosphate. Geomicrobiol. J. 2019, 36, 232–242. [Google Scholar] [CrossRef]
- Habibi, S.; Djedidi, S.; Ohkama-Ohtsu, N.; Sarhadi, W.A.; Kojima, K.; Rallos, R.V.; Ramirez, M.D.A.; Yamaya, H.; Sekimoto, H.; Yokoyama, T. Isolation and Screening of Indigenous Plant Growth-promoting Rhizobacteria from Different Rice Cultivars in Afghanistan Soils. Microbes Environ. 2019, 34, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Hameeda, B.; Harini, G.; Rupela, O.P.; Wani, S.P.; Reddy, G. Growth promotion of maize by phosphate-solubilizing bacteria isolated from composts and macrofauna. Microbiol. Res. 2008, 163, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Finkel, O.M.; Castrillo, G.; Herrera Paredes, S.; Salas González, I.; Dangl, J.L. Understanding and exploiting plant beneficial microbes. Curr. Opin. Plant Biol. 2017, 38, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Pineda, A.; Kaplan, I.; Bezemer, T.M. Steering Soil Microbiomes to Suppress Aboveground Insect Pests. Trends Plant Sci. 2017, 22, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Araujo, R.; Dunlap, C.; Franco, C.M.M. Analogous wheat root rhizosphere microbial successions in field and greenhouse trials in the presence of biocontrol agents Paenibacillus peoriae SP9 and Streptomyces fulvissimus FU14. Mol. Plant Pathol. 2020, 21, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Q.; Adams, C.A.; Sun, Y.; Zhang, S. Effects of microplastics on soil properties: Current knowledge and future perspectives. J. Hazard. Mater. 2022, 424, 127531. [Google Scholar] [CrossRef]
- Frankenberger, W.T.; Dick, W.A. Relationships Between Enzyme Activities and Microbial Growth and Activity Indices in Soil. Soil Sci. Soc. Am. J. 1983, 47, 945–951. [Google Scholar] [CrossRef]
- Liang, J.-P.; Xue, Z.-Q.; Yang, Z.-Y.; Chai, Z.; Niu, J.-P.; Shi, Z.-Y. Effects of microbial organic fertilizers on Astragalus membranaceus growth and rhizosphere microbial community. Ann. Microbiol. 2021, 71, 11. [Google Scholar] [CrossRef]
- Schnurr-Pütz, S.; Bååth, E.; Guggenberger, G.; Drake, H.L.; Kirsten, K. Compaction of forest soil by logging machinery favours occurrence of prokaryotes. FEMS Microbiol. Ecol. 2006, 58, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Briar, S.S.; Fonte, S.J.; Park, I.; Six, J.; Scow, K.; Ferris, H. The distribution of nematodes and soil microbial communities across soil aggregate fractions and farm management systems. Soil Biol. Biochem. 2011, 43, 905–914. [Google Scholar] [CrossRef]
- Bach, E.M.; Hofmockel, K.S. Soil aggregate isolation method affects measures of intra-aggregate extracellular enzyme activity. Soil Biol. Biochem. 2014, 69, 54–62. [Google Scholar] [CrossRef]
- Erktan, A.; Cécillon, L.; Graf, F.; Roumet, C.; Legout, C.; Rey, F. Increase in soil aggregate stability along a Mediterranean successional gradient in severely eroded gully bed ecosystems: Combined effects of soil, root traits and plant community characteristics. Plant Soil 2015, 398, 121–137. [Google Scholar] [CrossRef]
- Valmis, S.; Dimoyiannis, D.; Danalatos, N.G. Assessing interrill erosion rate from soil aggregate instability index, rainfall intensity and slope angle on cultivated soils in central Greece. Soil Tillage Res. 2005, 80, 139–147. [Google Scholar] [CrossRef]
- Forster, S.M. The role of microorganisms in aggregate formation and soil stabilization: Types of aggregation. Arid Soil Res. Rehabil. 1990, 4, 85–98. [Google Scholar] [CrossRef]
- Pokharel, A.K.; Jannoura, R.; Heitkamp, F.; Kleikamp, B.; Wachendorf, C.; Dyckmans, J.; Ludwig, B.; Joergensen, R.G. Development of aggregates after application of maize residues in the presence of mycorrhizal and non-mycorrhizal pea plants. Geoderma 2013, 202–203, 38–44. [Google Scholar] [CrossRef]
- Abiven, S.; Menasseri, S.; Angers, D.A.; Leterme, P. Dynamics of aggregate stability and biological binding agents during decomposition of organic materials. Eur. J. Soil Sci. 2007, 58, 239–247. [Google Scholar] [CrossRef]
- Yang, S.; Du, J.; Xu, G.; Shi, H.; Ni, Q.; Zu, W.; Qin, L. Effects of Microbial Fertilizer and Compound Feritlizer Mixed Application on Yield and Fruit Quality and Flowering and Fruit Setting of Following Year of Apple. Agric. Sci. Technol. 2016, 17, 642. [Google Scholar]
- Yang, J.L.; Zheng, S.J.; He, Y.F.; Matsumoto, H. Aluminium resistance requires resistance to acid stress: A case study with spinach that exudes oxalate rapidly when exposed to Al stress. J. Exp. Bot. 2005, 56, 1197–1203. [Google Scholar] [CrossRef]
- Babourina, O.; Hawkins, B.; Lew, R.R.; Newman, I.; Shabala, S. K+ transport by Arabidopsis root hairs at low pH. Funct. Plant Biol. 2001, 28, 637–643. [Google Scholar] [CrossRef]
- Birgander, J.; Rousk, J.; Olsson, P.A. Comparison of fertility and seasonal effects on grassland microbial communities. Soil Biol. Biochem. 2014, 76, 80–89. [Google Scholar] [CrossRef]
- Li, X.; Kang, X.; Zou, J.; Yin, J.; Wang, Y.; Li, A.; Ma, X. Allochthonous arbuscular mycorrhizal fungi promote Salix viminalis L.–mediated phytoremediation of polycyclic aromatic hydrocarbons characterized by increasing the release of organic acids and enzymes in soils. Ecotoxicol. Environ. Saf. 2023, 249, 114461. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Hayat, R.; Begum, F.; Bohannan, B.J.M.; Inebert, L.; Meyer, K. Variation in Soil Physical, Chemical and Microbial Parameters under Different Land uses in Bagrot Valley, Gilgit, Pakistan Ali. J. Chem. Soc. Pak. 2017, 39, 97–107. [Google Scholar]
- Ali, A.M.; Awad, M.Y.M.; Hegab, S.A.; Gawad, A.M.A.E.; Eissa, M.A. Effect of potassium solubilizing bacteria (Bacillus cereus) on growth and yield of potato. J. Plant Nutr. 2020, 44, 411–420. [Google Scholar] [CrossRef]
- Sabuquillo, P.; Cal, A.D.; Melgarejo, P. Biocontrol of tomato wilt by Penicillium oxalicum formulations in different crop conditions. Biol. Control 2006, 37, 256–265. [Google Scholar] [CrossRef]
- Abhilash, P.C.; Dubey, R.K.; Tripathi, V.; Gupta, V.K.; Singh, H.B. Plant Growth-Promoting Microorganisms for Environmental Sustainability. Trends Biotechnol. 2016, 34, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Jurat-Fuentes, J.L.; Heckel, D.G.; Ferré, J. Mechanisms of Resistance to Insecticidal Proteins from Bacillus thuringiensis. Annu. Rev. Entomol. 2021, 66, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Brescia, F.; Vlassi, A.; Bejarano, A.; Seidl, B.; Marchetti-Deschmann, M.; Schuhmacher, R.; Puopolo, G. Characterisation of the Antibiotic Profile of Lysobacter capsici AZ78, an Effective Biological Control Agent of Plant Pathogenic Microorganisms. Microorganisms 2021, 9, 1320. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.D.; Mendes, S.M.; Parreira, D.F.; Pacheco, R.C.; Marucci, R.C.; Cota, L.V.; Costa, R.V.; Figueiredo, J.E.F. Fungivory: A new and complex ecological function of Doru luteipes (Scudder) (Dermaptera: Forficulidea). Braz. J. Biol. 2021, 82, e238763. [Google Scholar] [CrossRef]
- Nagaraja, H.; Chennappa, G.; Rakesh, S.; Naik, M.K.; Amaresh, Y.S.; Sreenivasa, M.Y. Antifungal activity of Azotobacter nigricans against trichothecene-producing Fusarium species associated with cereals. Food Sci. Biotechnol. 2016, 25, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Koumoutsi, A.; Scholz, R.; Eisenreich, A.; Schneider, K.; Heinemeyer, I.; Morgenstern, B.; Voss, B.; Hess, W.R.; Reva, O.; et al. Comparative analysis of the complete genome sequence of the plant growth-promoting bacterium Bacillus amyloliquefaciens FZB42. Nat. Biotechnol. 2007, 25, 1007–1014. [Google Scholar] [CrossRef]
- Vasanthakumari, M.M.; Shivanna, M.B. Biological control of sorghum anthracnose with rhizosphere and rhizoplane fungal isolates from perennial grasses of the Western Ghats of India. Eur. J. Plant Pathol. 2014, 139, 721–733. [Google Scholar] [CrossRef]
- Stamenković, S.; Beškoski, V.; Karabegović, I.; Lazić, M.; Nikolić, N. Microbial fertilizers: A comprehensive review of current findings and future perspectives. Span. J. Agric. Res. 2018, 16, e09R01. [Google Scholar] [CrossRef]
- Lin, Z.; Pang, S.; Zhang, W.; Mishra, S.; Bhatt, P.; Chen, S. Degradation of Acephate and Its Intermediate Methamidophos: Mechanisms and Biochemical Pathways. Front. Microbiol. 2020, 11, 565022. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Upadhyay, N.; Singh, J.; Singla, S.; Datta, S. Efficient biodegradation of acephate by Pseudomonas pseudoalcaligenes PS-5 in the presence and absence of heavy metal ions [Cu(II) and Fe(III)], and humic acid. 3 Biotech 2017, 7, 262. [Google Scholar] [CrossRef] [PubMed]
- van Wyk, D.A.B.; Adeleke, R.; Rhode, O.H.J.; Bezuidenhout, C.C.; Mienie, C. Ecological guild and enzyme activities of rhizosphere soil microbial communities associated with Bt-maize cultivation under field conditions in North West Province of South Africa. J. Basic Microbiol. 2017, 57, 781–792. [Google Scholar] [CrossRef] [PubMed]



| Microbial Fertilizer Species | Major Microorganisms | Function |
|---|---|---|
| Bacteria fertilizers | Nitrifying bacteria, Azotobacter chroococcum, Azospirillam brasilense, Klebsiella | Convert N2, which cannot be absorbed and utilized by plants, into ammonia or nitrate to promote plant growth |
| Bacillus sp.: Bacillus cereus, Bacillus megaterium, Bacillus mucilaginosus et al. Photosynthetic Bacteria: Rhodopseudomonas capsulate, Rhodopseudomonas sp. et al. Pseudomonas sp.: Klebsiella pneumoniae, Pseudomonas aeruginosa Chromobacterium: Alcaligenes sp. et al. Serratia sp.: Serratia marcescens et al. Thiobacillus: Acidithiobacillus thiooxidans, Thiobacillus thioparus | Insoluble phosphorus is converted into soluble phosphorus, and some microorganisms can also release extracellular enzymes to dissolve minerals and convert them into inorganic phosphorus to promote plant growth. | |
| Bacillus edaphicus, Bacillus mucilaginosus Krassilnikov et al. | Through dissolution and mineralization, the insoluble potassium in the soil is converted into soluble potassium, which destroys the mineral structure of silicate and increases the content of available potassium in the soil. | |
| Lactic acid bacteria | Secretion of acids or antibacterial substances inhibits the growth of pathogenic bacteria in the soil, promotes the growth of plant roots, and improves the absorption of nutrients by plants. | |
| Fungal fertilizers | Mycorrhizal fungi, Ascomycota et al. | It can secrete extracellular enzymes, organic acids, etc., to decompose organic matter in the soil, improve soil structure, and improve soil fertility. |
| Actinomycete fertilizer | Streptomyces jingyangensis, Streptomyces pactum et al. | Secrete antibacterial substances, inhibit the growth of harmful microorganisms, decompose organic matter, reduce soil bulk density, and improve soil physical properties. |
| Common Plant Pathogens | Main Pathogenic Bacteria | Inhibition of Pathogenic Microorganisms | Reference |
|---|---|---|---|
| Rice blast | Magnaporthe oryzae | Fusarium sp., Armillaria novae-zelandiae, Falciphora oryzae | [67] |
| Gray mold | Botrytis cinerea | Bacillus subtilis, Bacillus amyloliquefaciens | [67] |
| Southern corn rust | Puccinia spp. | Doru luteipes | [68] |
| Wheat scab, corn ear rot, Corn stalk rot, etc | Fusarium graminearum | Bacillus cereus, Azotobacter nigricans | [69] |
| Cotton wilt | Fusarium oxysporum | Bacillus amyloliquefaciens | [70] |
| Sorghum anthracnose | Colletotrichum sublineolum | Coccidium, Trichoderma harzianum, Fusarium oxysporum | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Xie, B.; Wan, C.; Song, R.; Zhong, W.; Xin, S.; Song, K. Enhancing Soil Health and Plant Growth through Microbial Fertilizers: Mechanisms, Benefits, and Sustainable Agricultural Practices. Agronomy 2024, 14, 609. https://doi.org/10.3390/agronomy14030609
Wei X, Xie B, Wan C, Song R, Zhong W, Xin S, Song K. Enhancing Soil Health and Plant Growth through Microbial Fertilizers: Mechanisms, Benefits, and Sustainable Agricultural Practices. Agronomy. 2024; 14(3):609. https://doi.org/10.3390/agronomy14030609
Chicago/Turabian StyleWei, Xinpei, Benkang Xie, Chu Wan, Renfeng Song, Wanru Zhong, Shuquan Xin, and Kai Song. 2024. "Enhancing Soil Health and Plant Growth through Microbial Fertilizers: Mechanisms, Benefits, and Sustainable Agricultural Practices" Agronomy 14, no. 3: 609. https://doi.org/10.3390/agronomy14030609
APA StyleWei, X., Xie, B., Wan, C., Song, R., Zhong, W., Xin, S., & Song, K. (2024). Enhancing Soil Health and Plant Growth through Microbial Fertilizers: Mechanisms, Benefits, and Sustainable Agricultural Practices. Agronomy, 14(3), 609. https://doi.org/10.3390/agronomy14030609





