Resistance to Site-Specific Succinate Dehydrogenase Inhibitor Fungicides Is Pervasive in Populations of Black and Yellow Sigatoka Pathogens in Banana Plantations from Southeastern Brazil
Abstract
:1. Introduction
2. Materials and Methods
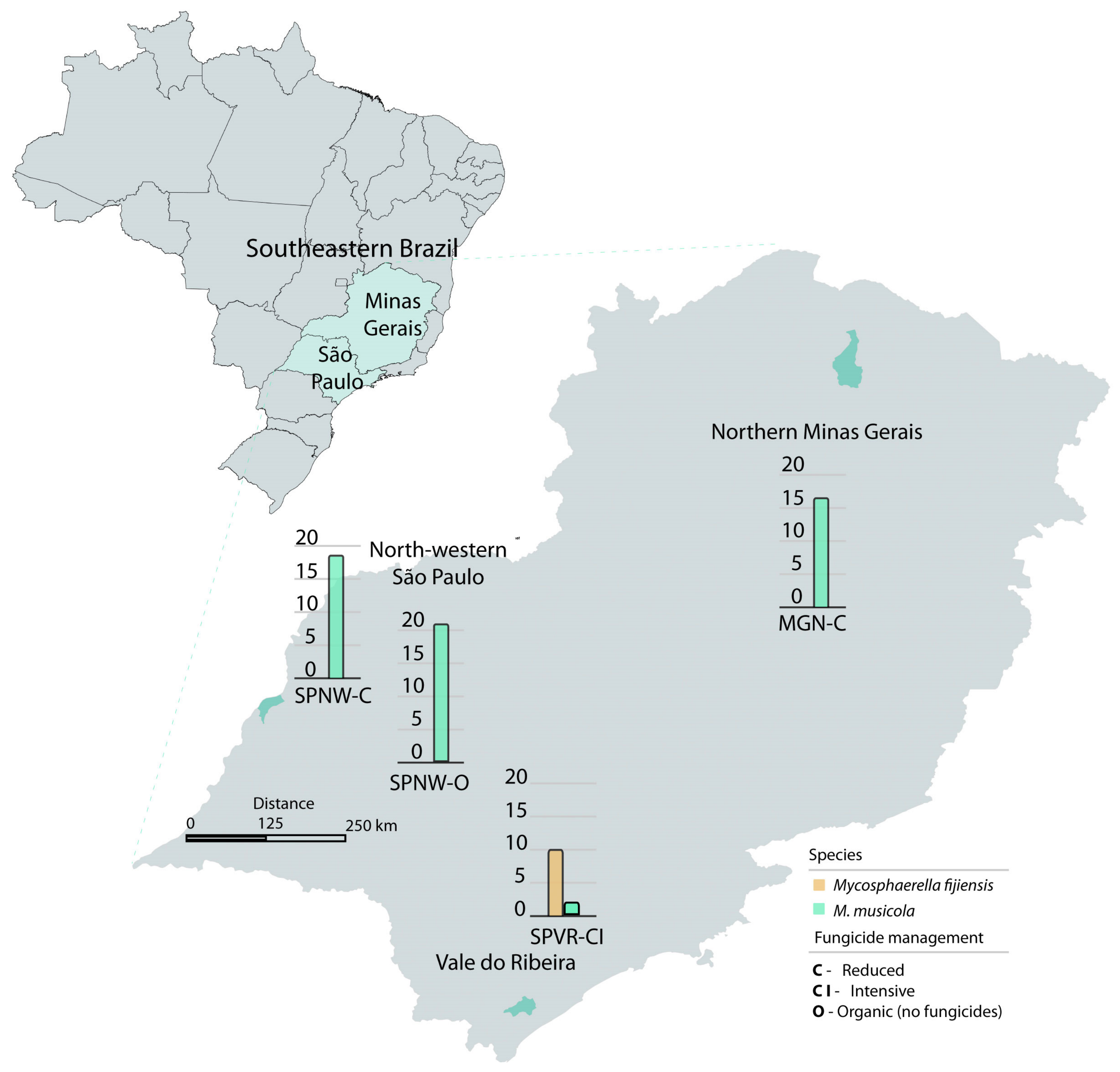
2.1. Sampling Area
2.2. Pathogen Strain Isolation, Identification, and Storage
2.3. Fungicide Sensitivity Evaluation
SDHI Fungicide Sensitivity Testing
- RRMycosphaerella = relative reduction of resazurin due to the metabolic activity;
- T = absorbance reading at 569 nm;
- T0 = reading at time zero, immediately after adding RZ to the microplate wells;
- T24 = reading time 24 h after adding RZ.
2.4. Determination of Mutations in the SdhB, SdhC, SdhC2, and SdhD Genes
2.5. Sensitivity to Efflux Pump Substrates
2.6. Statistical Analysis
3. Results
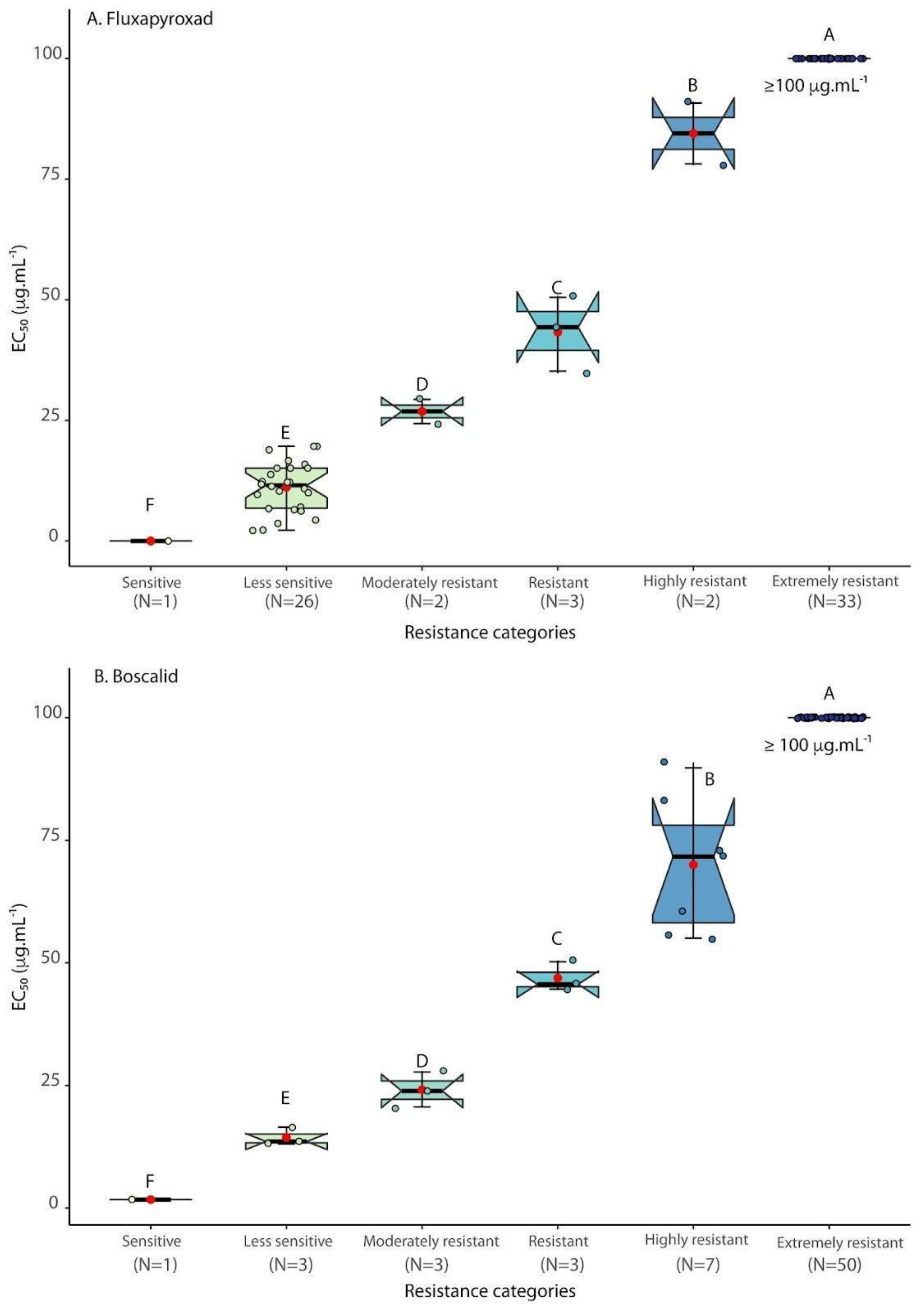
3.1. SDHI Fungicides Sensitivity Testing
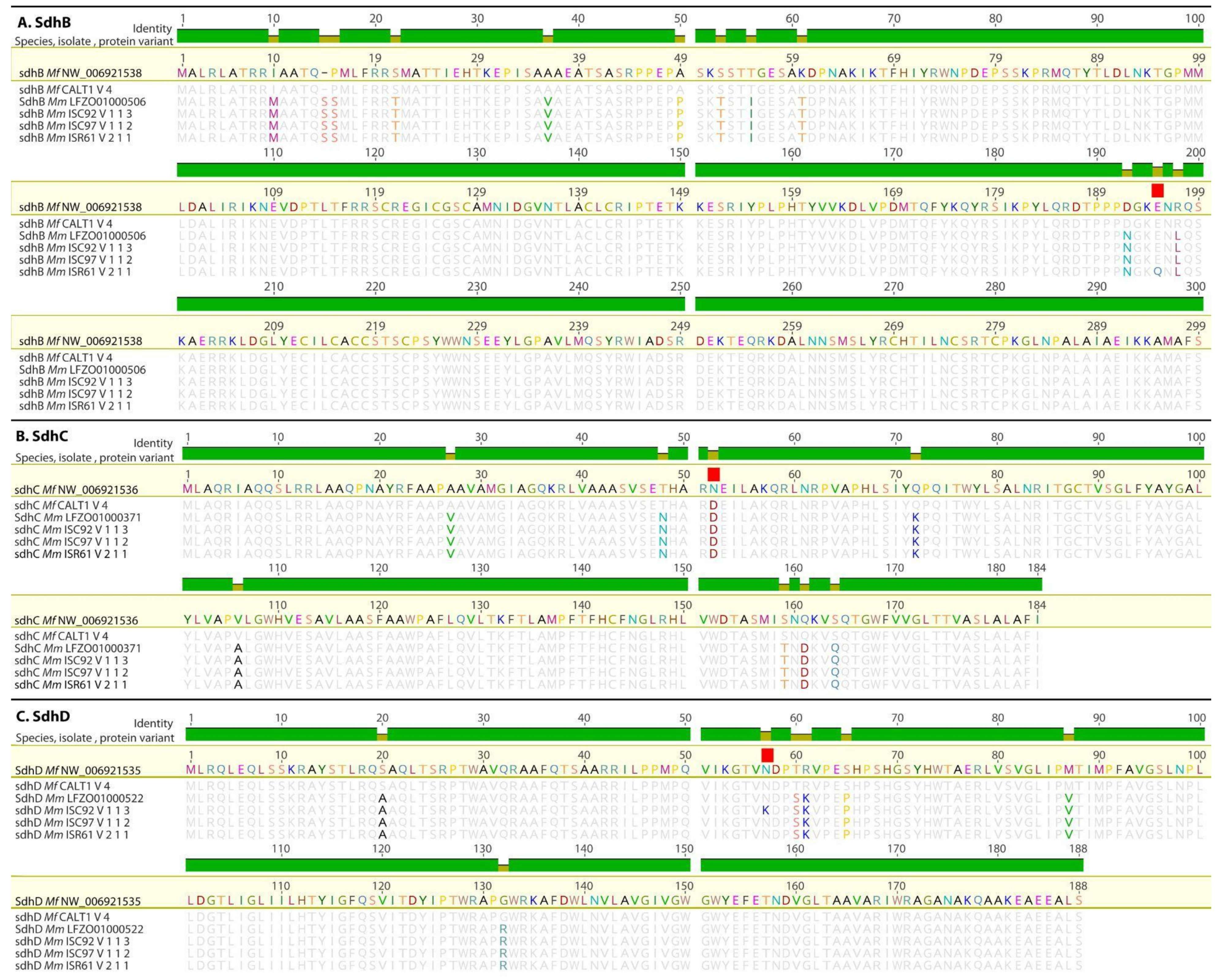
3.2. Detection of Mutations in the Sdh Genes
3.2.1. Mutations in the SdhB, SdhC, and SdhD Genes from Mf and SDHI Sensitivity
3.2.2. Mutations in the SdhB, SdhC, and SdhD Genes from Mm
3.2.3. Examination of an Additional SdhC Paralog from Mf and Mm
3.3. Efflux Pump Inhibition Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friesen, T.L. Combating the Sigatoka Disease Complex on Banana. PLoS Genet. 2016, 12, e1006234. [Google Scholar] [CrossRef] [PubMed]
- Brito, F.S.D.; Fraaije, B.; Miller, R.N.G. Sigatoka Disease Complex of Banana in Brazil: Management Practices and Future Directions. Outlooks Pest Manag. 2015, 26, 78–81. [Google Scholar] [CrossRef]
- Brito, F.S.D.; Santos, J.R.P.; Azevedo, V.C.R.; Peixouto, Y.S.; de Oliveira, S.A.; Ferreira, C.F.; Haddad, F.; Amorim, E.P.; Fraaije, B.; Miller, R.N.G. Genetic Diversity and Azole Fungicide Sensitivity in Pseudocercospora musae Field Populations in Brazil. Front. Microbiol. 2020, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.J. Dominância Da Sigatoka-Negra Em Bananais Do Vale Do Ribeira. Fitopatol. Bras. 2005, 30, 193. [Google Scholar]
- Rocha, F.S.; Catão, H.C.R.M.; Muniz, M.F.S. Aspectos Diagnósticos Entre Mycosphaerella Spp. Da Bananeira, Distribuição e Manejo No Brasil. Enciclopédia Biosf. 2012, 8, 64–84. [Google Scholar]
- Nomura, E.S.; Moraes, W.d.S.; Kobori, R.T.; Penteado, L.A.d.C. Doenças. In Cultivo da Bananeira; Manual Técnico; Coordenadoria de Assistência Técnica Integral (CATI): Campinas, Brazil, 2020; pp. 107–134. ISBN CDD.634.722. [Google Scholar]
- Oliveira, T.Y.K.; Silva, T.C.; Moreira, S.I.; Christiano, F.S.; Gasparoto, M.C.G.; Fraaije, B.A.; Ceresini, P.C. Evidence of Resistance to QoI Fungicides in Contemporary Populations of Mycosphaerella Fijiensis, M. Musicola and M. Thailandica from Banana Plantations in Southeastern Brazil. Agronomy 2022, 12, 2952. [Google Scholar] [CrossRef]
- Malimpensa, J.R. Caracterização da Resistência a Fungicidas em Populações de Fungos Associados a Lesões de Sigatokas em Bananais do Vale do Ribeira (SP). Masters’ Thesis, Instituto Biológico, São Paulo, Brazil, 2018. [Google Scholar]
- Gomes, L.; Douhan, G.; Bibiano, L.; Maffia, L.; Mizubuti, E. Mycosphaerella musicola Identified as the Only Pathogen of the Sigatoka Disease Complex Present in Minas Gerais State, Brazil. Plant Dis. 2013, 97, 1537–1543. [Google Scholar] [CrossRef]
- Gomes, L.I.S.; Douhan, G.W.; Lehner, M.S.; Bibiano, L.B.J.; Mizubuti, E.S.G. Yellow Sigatoka Epidemics Caused by a Panmictic Population of Mycosphaerella musicola in Brazil. Plant Pathol. 2018, 67, 295–302. [Google Scholar] [CrossRef]
- Gasparotto, L.; Pereira, J.C.R.; Pereira, M.C.N.; Costa, M.M. Controle Químico da Sigatoka Negra da Bananeira. I–Trifloxistrobin, Propiconazole e Difenoconazole; Comunicado Técnico; Embrapa: Manaus, Brazil, 2000; p. 1. [Google Scholar]
- Ramos, J.B.; Bragança, C.A.D.; Rocha, L.S.; Oliveira, A.d.S.; Cordeiro, Z.J.M.; Haddad, F. First Report of Black Sigatoka of Banana Caused by Mycosphaerella Fijiensis in Bahia, Brazil. Plant Dis. 2018, 102, 2035. [Google Scholar] [CrossRef]
- Arzanlou, M.; Abeln, E.C.A.; Kema, G.H.J.; Waalwijk, C.; Carlier, J.; Vries, I.d.; Guzmán, M.; Crous, P.W. Molecular Diagnostics for the Sigatoka Disease Complex of Banana. Phytopathology 2007, 97, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Moraes, W.d.S.; Nomura, E.S. Monitoramento Da Sigatoka e Controle Químico. In Cultivo da Bananeira; Manual Técnico; Coordenadoria de Assistência Técnica Integral (CATI): Campinas, Brazil, 2020; pp. 135–144. ISBN CDD.634.722. [Google Scholar]
- Ghini, R.; Hamada, E.; Gonçalves, R.R.V.; Gasparotto, L.; Pereira, J.C.R. Análise de Risco Das Mudanças Climáticas Globais Sobre a Sigatoka-Negra Da Bananeira No Brasil. Fitopatol. Bras. 2007, 32, 197–204. [Google Scholar] [CrossRef]
- Bendini, H.d.N. Processamento Digital de Imagens Para Inferência de Risco de Doença Fúngica Da Bananicultura. Masters’ Thesis, Federal University of São Carlos (UFScar), São Carlos, Brazil, 2012. [Google Scholar]
- Bendini, H.d.N.; Moraes, W.d.S.; Silva, S.H.M.G.d.; Tezuka, E.S.; Cruvinel, P.E. Análise de Risco Da Ocorrência de Sigatoka-Negra Baseada Em Modelos Polinomiais: Um Estudo de Caso. Trop. Plant Pathol. 2013, 38, 35–43. [Google Scholar] [CrossRef]
- Cordeiro, Z.J.M. Doenças. In A Cultura da Banana: Aspectos Técnicos, Sócio Ecnonômicos e Agroinstriais; Embrapa SPI, CNPMF: Cruz das Almas, Brazil, 1997; pp. 353–408. ISBN 85-7383-001-8. [Google Scholar]
- Rocha, H.S. Epidemiology of Yellow Sigatoka, Phenols Quantification in Banana Varieties and Phylogenetic Analysis of Mycosphaerella musicola Isolates Using Microsatellites. Ph.D. Thesis, Universidade Federal de Lavras, Lavras, Brazil, 2008. [Google Scholar]
- Beltrán-García, M.J.; Prado, F.M.; Oliveira, M.S.; Ortiz-Mendoza, D.; Scalfo, A., Jr.; Medeiros, M.H.G.; White, J.F.; Mascio, P.D. Singlet Molecular Oxygen Generation by Light-Activated DHN-Melanin of the Fungal Pathogen Mycosphaerella Fijiensis in Black Sigatoka Disease of Bananas. PLoS ONE 2014, 9, e91616. [Google Scholar] [CrossRef]
- Burt, P.J.A. Windborne Dispersal of Sigatoka Leaf Spot Pathogens. Grana 1994, 33, 108–111. [Google Scholar] [CrossRef]
- Manzo-Sánchez, G.; Orozco-Santos, M.; Islas-Flores, I.; Martínez-Bolaños, L.; Guzmán-González, S.; Leopardi-Verde, C.L.; Canto-Canché, B. Genetic Variability of Pseudocercospora Fijiensis, the Black Sigatoka Pathogen of Banana (Musa Spp.) in Mexico. Plant Pathol. 2019, 68, 513–522. [Google Scholar] [CrossRef]
- Mendoza, M.J.; Ardales, E. Population Structure of the Banana Black Sigatoka Pathogen [Pseudocercospora Fijiensis (M. Morelet) Deighton] in Luzon, Philippines. Philipp. Agric. Sci. 2019, 102, 211–219. [Google Scholar]
- Cañas-Gutiérrez, G.P.; Angarita-Velásquez, M.J.; Restrepo-Flórez, J.M.; Rodríguez, P.; Moreno, C.X.; Arango, R. Analysis of the CYP51 Gene and Encoded Protein in Propiconazole-Resistant Isolates of Mycosphaerella Fijiensis. Pest Manag. Sci. 2009, 65, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Trujillo, C.; Chong, P.; Stergiopoulos, I.; Cordovez, V.; Guzman, M.; De Wit, P.J.G.M.; Meijer, H.J.G.; Scalliet, G.; Sierotzki, H.; Lilia Peralta, E.; et al. A New Mechanism for Reduced Sensitivity to Demethylation-Inhibitor Fungicides in the Fungal Banana Black Sigatoka Pathogen Pseudocercospora Fijiensis. Mol. Plant Pathol. 2018, 19, 1491–1503. [Google Scholar] [CrossRef] [PubMed]
- Grice, K.; Vawdrey, L.; Stammler, G.; Koch, A.; Wilson, D.; Matthews, N. Yellow Sigatoka (Mycosphaerella musicola) Populations Develop Resistance to QoI Fungicides in Australia. In Modern Fungicides and Antifungal Compounds; Dehne, H.W., Deising, H.B., Fraaije, B., Gisi, U., Hermann, D., Mehl, A., Oerke, E.C., Russell, P.E., Stammler, G., Kuck, K.H., et al., Eds.; Deutsche Phytomedizinische Gesellschaft: Braunschweig, Germany, 2013; Volume 7, ISBN 978-3-941261-13-6. [Google Scholar]
- Churchill, A.C.L. Mycosphaerella Fijiensis, the Black Leaf Streak Pathogen of Banana: Progress towards Understanding Pathogen Biology and Detection, Disease Development, and the Challenges of Control. Mol. Plant Pathol. 2011, 12, 307–328. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide Emergence of Resistance to Antifungal Drugs Challenges Human Health and Food Security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, N.J.; Fraaije, B.A. Fitness Penalties in the Evolution of Fungicide Resistance. Annu. Rev. Phytopathol. 2018, 56, 339–360. [Google Scholar] [CrossRef]
- Lucas, J.A.; Hawkins, N.J.; Fraaije, B.A. The Evolution of Fungicide Resistance. Adv. Appl. Microbiol. 2015, 90, 29–92. [Google Scholar] [CrossRef]
- Mair, W.; Lopez-Ruiz, F.; Stammler, G.; Clark, W.; Burnett, F.; Hollomon, D.; Ishii, H.; Thind, T.S.; Brown, J.K.; Fraaije, B.; et al. Proposal for a Unified Nomenclature for Target-Site Mutations Associated with Resistance to Fungicides. Pest Manag. Sci. 2016, 72, 1449–1459. [Google Scholar] [CrossRef]
- McDonald, B.A.; Linde, C. Pathogen Population Genetics, Evolutionary Potential, and Durable Resistance. Annu. Rev. Phytopathol. 2002, 40, 349–379. [Google Scholar] [CrossRef]
- Sang, H.; Lee, H.B. Molecular Mechanisms of Succinate Dehydrogenase Inhibitor Resistance in Phytopathogenic Fungi. Res. Plant Dis. 2020, 26, 1–7. [Google Scholar] [CrossRef]
- Fraaije, B.; Atkins, S.; Diez, P.; Hawkins, N. Evolution and Spread of SDHI-Resistant Alleles within Field Populations of Zymoseptoria tritici in the UK. In Proceedings of the Plant Health 2019, Cleveland, OH, USA, 3–7 August 2019; APS the American Phytopathological Society: Cleveland, OH, USA, 2019. [Google Scholar]
- Klappach, K.; Stammler, G. Resistance of Plant Pathogens to Succinate Dehydrogenase Inhibitor (SDHI) Fungicides (FRAC Code 7). In Fungicide Resistance in North America; Mycology; Klappach, K., Stammler, G., Eds.; APS the American Phytopathological Society: Saint Paul, Brazil, 2019; pp. 85–95. ISBN 978-0-89054-622-2. [Google Scholar]
- Sierotzki, H.; Scalliet, G. A Review of Current Knowledge of Resistance Aspects for the Next-Generation Succinate Dehydrogenase Inhibitor Fungicides. Phytopathology 2013, 103, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Brent, K.J.; Hollomon, D.W. Fungicide Resistance in Crop Pathogens: How Can It Be Managed? 2nd ed.; FRAC Monograph; Fungicide Resistance Action Comittee: Brussels, Belgium, 2007; ISBN 90-72398-07-6. [Google Scholar]
- Ōmura, S.; Shiomi, K. Discovery, Chemistry, and Chemical Biology of Microbial Products. Pure Appl. Chem. 2007, 79, 581–591. [Google Scholar] [CrossRef]
- Amiri, A.; Zuniga, A.I.; Peres, N.A. Mutations in the Membrane-Anchored SdhC Subunit Affect Fitness and Sensitivity to Succinate Dehydrogenase Inhibitors in Botrytis Cinerea Populations from Multiple Hosts. Phytopathology 2020, 110, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Ayer, K.M.; Villani, S.M.; Choi, M.-W.; Cox, K.D. Characterization of the VisdhC and VisdhD Genes in Venturia inaequalis, and Sensitivity to Fluxapyroxad, Pydiflumetofen, Inpyrfluxam, and Benzovindiflupyr. Plant Dis. 2019, 103, 1092–1100. [Google Scholar] [CrossRef]
- De Miccolis Angelini, R.M.; Masiello, M.; Rotolo, C.; Pollastro, S.; Faretra, F. Molecular Characterisation and Detection of Resistance to Succinate Dehydrogenase Inhibitor Fungicides in Botryotinia Fuckeliana (Botrytis cinerea). Pest Manag. Sci. 2014, 70, 1884–1893. [Google Scholar] [CrossRef] [PubMed]
- Förster, H.; Luo, Y.; Hou, L.; Adaskaveg, J.E. Mutations in Sdh Gene Subunits Confer Different Cross-Resistance Patterns to SDHI Fungicides in Alternaria alternata Causing Alternaria Leaf Spot of Almond in California. Plant Dis. 2022, 106, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Cui, K.; Song, Y.; Li, T.; Liu, N.; Mu, W.; Liu, F. Activity of the Novel Succinate Dehydrogenase Inhibitor Fungicide Pydiflumetofen Against SDHI-Sensitive and SDHI-Resistant Isolates of Botrytis cinerea and Efficacy Against Gray Mold. Plant Dis. 2020, 104, 2168–2173. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.A.; Stammler, G.; May De Mio, L.L. Multiple Resistance to DMI, QoI and SDHI Fungicides in Field Isolates of Phakopsora pachyrhizi. Crop Prot. 2021, 145, 105618. [Google Scholar] [CrossRef]
- Peng, J.; Sang, H.; Proffer, T.J.; Gleason, J.; Outwater, C.A.; Jung, G.; Sundin, G.W. A Method for the Examination of SDHI Fungicide Resistance Mechanisms in Phytopathogenic Fungi Using a Heterologous Expression System in Sclerotinia sclerotiorum. Phytopathology 2021, 111, 819–830. [Google Scholar] [CrossRef]
- Prasad, R.; Rawal, M.K. Efflux Pump Proteins in Antifungal Resistance. Front. Pharmacol. 2014, 5, 202. [Google Scholar] [CrossRef]
- Rehfus, A.; Strobel, D.; Bryson, R.; Stammler, G. Mutations in Sdh Genes in Field Isolates of Zymoseptoria tritici and Impact on the Sensitivity to Various Succinate Dehydrogenase Inhibitors. Plant Pathol. 2018, 67, 175–180. [Google Scholar] [CrossRef]
- Veloukas, T.; Kalogeropoulou, P.; Markoglou, A.N.; Karaoglanidis, G.S. Fitness and Competitive Ability of Botrytis cinerea Field Isolates with Dual Resistance to SDHI and QoI Fungicides, Associated with Several sdhB and the Cytb G143A Mutations. Phytopathology 2014, 104, 347–356. [Google Scholar] [CrossRef]
- Yamashita, M.; Fraaije, B. Non-target Site SDHI Resistance Is Present as Standing Genetic Variation in Field Populations of Zymoseptoria tritici. Pest Manag. Sci. 2018, 74, 672–681. [Google Scholar] [CrossRef]
- Steinhauer, D.; Salat, M.; Frey, R.; Mosbach, A.; Luksch, T.; Balmer, D.; Hansen, R.; Widdison, S.; Logan, G.; Dietrich, R.A.; et al. A Dispensable Paralog of Succinate Dehydrogenase Subunit C Mediates Standing Resistance towards a Subclass of SDHI Fungicides in Zymoseptoria tritici. PLoS Pathog. 2019, 15, e1007780. [Google Scholar] [CrossRef]
- FRAC—Fungicide Resistance Action Committee. Recommendations for SDHI Fungicides. Available online: https://www.frac.info/frac-teams/working-groups/sdhi-fungicides/recommendations-for-sdhi (accessed on 22 February 2023).
- FRAC—Fungicide Resistance Action Committee. Recommendations for Bananas. Available online: https://www.frac.info/frac-teams/working-groups/banana-group/recommendations-for-bananas (accessed on 22 February 2023).
- MAPA—Ministério da Agricultura Pecuária e Abastecimento—Brazil Agrofit—Sistemas de Agrotóxicos Fitossanitários, Coordenação Geral de Agrotóxicos e Afins. Available online: http://agrofit.agricultura.gov.br/agrofit_cons/principal_agrofit_cons. (accessed on 5 February 2023).
- Rangel, A.; Penteado, L.A.C.; Tonet, R.M. Cultura Da Banana, 2nd ed.; Boletim Técnico; Coordenadoria de Assistência Técnica Integral (CATI): Campinas, Brazil, 2002.
- Silva, T.C.; Moreira, S.I.; Assis, F.G.; Vicentini, S.N.C.; Silva, A.G.; Oliveira, T.Y.K.; Christiano, F.S.; Custódio, A.A.P.; Leite, R.P.; Gasparoto, M.C.G.; et al. An Accurate, Affordable, and Precise Resazurin-Based Digital Imaging Colorimetric Assay for the Assessment of Fungicide Sensitivity Status of Fungal Populations. Agronomy 2023, 13, 343. [Google Scholar] [CrossRef]
- Ma, B.; Uddin, W.; Olaya, G. Baseline and Non-Baseline Sensitivity of Magnaporthe Oryzae Isolates from Perennial Ryegrass to Azoxystrobin in the Northeastern United States. Can. J. Plant Pathol. 2009, 31, 57–64. [Google Scholar] [CrossRef]
- Vargas, M.H. ED50plus v.1.0. Available online: https://archive.org/details/ed50v10_zip (accessed on 2 March 2022).
- R Development Core Team R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 2 March 2022).
- Oliveira, T.Y.K. de Evidence of Resistance to Strobilurin Fungicides in Contemporary Populations of Mycosphaerella fijiensis and M. musicola from Banana Plantations in Southeastern Brazil. Masters’ Thesis, São Paulo State University (UNESP), Ilha Solteira, Brazil, 2022. [Google Scholar]
- Perlin, M.H.; Andrews, J.; San Toh, S. Essential Letters in the Fungal Alphabet: ABC and MFS Transporters and Their Roles in Survival and Pathogenicity. Adv. Genet. 2014, 85, 201–253. [Google Scholar] [PubMed]
- Prates, R.A.; Kato, I.T.; Ribeiro, M.S.; Tegos, G.P.; Hamblin, M.R. Influence of Multidrug Efflux Systems on Methylene Blue-Mediated Photodynamic Inactivation of Candida Albicans. J. Antimicrob. Chemother. 2011, 66, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gupta, V.K.; Pathania, R. Efflux Pump Inhibitors for Bacterial Pathogens: From Bench to Bedside. Indian J. Med. Res. 2019, 149, 129. [Google Scholar] [PubMed]
- Tegos, G.P.; Masago, K.; Aziz, F.; Higginbotham, A.; Stermitz, F.R.; Hamblin, M.R. Inhibitors of Bacterial Multidrug Efflux Pumps Potentiate Antimicrobial Photoinactivation. Antimicrob. Agents Chemother. 2008, 52, 3202–3209. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.; Ma, J.; Rothnie, A.; Biggin, P.C.; Kerr, I.D. Towards Understanding Promiscuity in Multidrug Efflux Pumps. Trends Biochem. Sci. 2014, 39, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.G. (São Paulo State University—UNESP, São Paulo, Brazil). Personal communication, 2023. [Google Scholar]
- Stammler, G.; Glättli, A.; Koch, A.; Schlehuber, S. Mutations in the Target Protein Conferring Resistance to SDHI Fungicides. In Proceedings of the Modern Fungicides and Antifungal Compounds VI. 16th International Reinhardsbrunn Symposium, Friedrichroda, Germany, 25–29 April 2010. [Google Scholar]
- Hu, M.-J.; Fernández-Ortuño, D.; Schnabel, G. Monitoring Resistance to SDHI Fungicides in Botrytis cinerea from Strawberry Fields. Plant Dis. 2016, 100, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, S.N.; Casado, P.S.; de Carvalho, G.; Moreira, S.I.; Dorigan, A.F.; Silva, T.C.; Silva, A.G.; Custódio, A.A.; Gomes, A.C.S.; Nunes Maciel, J.L. Monitoring of Brazilian Wheat Blast Field Populations Reveals Resistance to QoI, DMI, and SDHI Fungicides. Plant Pathol. 2022, 71, 304–321. [Google Scholar] [CrossRef]
- Sierotzki, H.; Parisi, S.; Steinfeld, U.; Tenzer, I.; Poirey, S.; Gisi, U. Mode of Resistance to Respiration Inhibitors at the Cytochrome Bc1 Enzyme Complex of Mycosphaerella Fijiensis Field Isolates. Pest Manag. Sci. 2000, 56, 833–841. [Google Scholar] [CrossRef]
- Avenot, H.; Sellam, A.; Michailides, T. Characterization of Mutations in the Membrane-Anchored Subunits AaSDHC and AaSDHD of Succinate Dehydrogenase from Alternaria alternata Isolates Conferring Field Resistance to the Fungicide Boscalid. Plant Pathol. 2009, 58, 1134–1143. [Google Scholar] [CrossRef]
- Avenot, H.F.; Sellam, A.; Karaoglanidis, G.; Michailides, T.J. Characterization of Mutations in the Iron-Sulphur Subunit of Succinate Dehydrogenase Correlating with Boscalid Resistance in Alternaria alternata from California Pistachio. Phytopathology 2008, 98, 736–742. [Google Scholar] [CrossRef]
- de Miccolis Angelini, R.M.; Habib, W.; Rotolo, C.; Pollastro, S.; Faretra, F. Selection, Characterization and Genetic Analysis of Laboratory Mutants of Botryotinia fuckeliana (Botrytis cinerea) Resistant to the Fungicide Boscalid. Eur. J. Plant Pathol. 2010, 128, 185–199. [Google Scholar] [CrossRef]
- Miyamoto, T.; Ishii, H.; Seko, T.; Kobori, S.; Tomita, Y. Occurrence of Corynespora cassiicola Isolates Resistant to Boscalid on Cucumber in Ibaraki Prefecture, Japan. Plant Pathol. 2009, 58, 1144–1151. [Google Scholar] [CrossRef]
- Miyamoto, T.; Ishii, H.; Stammler, G.; Koch, A.; Ogawara, T.; Tomita, Y.; Fountaine, J.M.; Ushio, S.; Seko, T.; Kobori, S. Distribution and Molecular Characterization of Corynespora cassiicola Isolates Resistant to Boscalid. Plant Pathol. 2010, 59, 873–881. [Google Scholar] [CrossRef]
- Scalliet, G.; Bowler, J.; Luksch, T.; Kirchhofer-Allan, L.; Steinhauer, D.; Ward, K.; Niklaus, M.; Verras, A.; Csukai, M.; Daina, A.; et al. Mutagenesis and Functional Studies with Succinate Dehydrogenase Inhibitors in the Wheat Pathogen Mycosphaerella graminicola. PLoS ONE 2012, 7, e35429. [Google Scholar] [CrossRef]
- Fernández-Ortuño, D.; Torés, J.A.; de Vicente, A.; Pérez-García, A. Mechanisms of Resistance to QoI Fungicides in Phytopathogenic Fungi. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2008, 11, 1–9. [Google Scholar]
- Omrane, S.; Sghyer, H.; Audéon, C.; Lanen, C.; Duplaix, C.; Walker, A.; Fillinger, S. Fungicide Efflux and the MgMFS 1 Transporter Contribute to the Multidrug Resistance Phenotype in Zymoseptoria tritici Field Isolates. Environ. Microbiol. 2015, 17, 2805–2823. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, M.; Leroch, M.; Mosbach, A.; Walker, A.-S.; Fillinger, S.; Mernke, D.; Schoonbeek, H.-J.; Pradier, J.-M.; Leroux, P.; De Waard, M.A. Fungicide-Driven Evolution and Molecular Basis of Multidrug Resistance in Field Populations of the Grey Mould Fungus Botrytis cinerea. PLoS Pathog. 2009, 5, e1000696. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, S.N.C.; Moreira, S.I.; da Silva, A.G.; de Oliveira, T.Y.K.; Silva, T.C.; Assis Junior, F.G.; Krug, L.D.; de Paiva Custódio, A.A.; Leite Júnior, R.P.; Teodoro, P.E.; et al. Efflux Pumps and Multidrug-Resistance in Pyricularia oryzae Triticum Lineage. Agronomy 2022, 12, 2068. [Google Scholar] [CrossRef]
- Vicentini, S.N.C.; Krug, L.D.; Ceresini, P.C.; Custódio, A.A.P.; Leite, R.P., Jr.; West, J.S.; Fraaije, B.A. Can Fungal Aerobiology Tools Help Tracking Fungicide Resistance Alleles and Block the Spread of Fungicide Resistance in the Agroecosystem? In Proceedings of the VI EpidemioBrasil: Brazilian Workshop of Plant Disease Epidemiology; Canale, M.C., Nesi, C.N., Bergamim Filho, A., Del Ponte, E., Amorim, L., May De Mio, L.L., Laranjeira, F., Eds.; Epagri: Chapecó, Brazil, 2022; pp. 25–26. ISBN 978-65-998614-0-6. [Google Scholar]
- Vicentini, S.N.C.; Hawkins, N.J.; King, K.M.; Moreira, S.I.; de Paiva Custódio, A.A.; Leite Júnior, R.P.; Portalanza, D.; Garcés-Fiallos, F.R.; Krug, L.D.; West, J.S.; et al. Aerobiology of the Wheat Blast Pathogen: Inoculum Monitoring and Detection of Fungicide Resistance Alleles. Agronomy 2023, 13, 1238. [Google Scholar] [CrossRef]




| Target | Primers | Sequence (5′-3′) | Amplicon Size (bp) | Annealing Temperature (°C) |
|---|---|---|---|---|
| MfSdhB | SdhB_Mf_F42 | TTCTGCTTCACCACGTCTCC | 1164 | 55.0 |
| SdhB_Mf_R1205 | TGTGAGTCTGCCTATCATGA | |||
| MfSdhC | SdhC_Mf_F84 | TGTTTGTCTACACCAGCACTG | 849 | 58.0 |
| SdhC_Mf_R932 | AAGCCAAAGTGAGTTGCCCA | |||
| MfSdhC2 | Sdh_altC_Mf_F35 | TCGAAGTGATGCAGGATAAGAATC | 341 | 58.4 |
| Sdh_altC_Mf_R341 | AGTGAGCCGAAGATATGCAAG | |||
| MfSdhD | SdhD_Mf_F92 | TCTGTCTTCCCACCTCTCAC | 996 | 58.5 |
| SdhD_Mf_R1087 | GCCACGGGATTGAGCTGTTG | |||
| MmSdhB | SdhB_Mm_F103 | CCTCCCCTCTGCTCATTACG | 996 | 59.5 |
| SdhB_Mm_R1098 | CACCACCCCACCACATACC | |||
| MmSdhC | SdhC_Mm_F100 | TGTCTGTCTACACCAGCACTG | 946 | 58.0 |
| SdhC_Mm_R845 | CAACCTGCAAACCAAGACCC | |||
| MmSdhC2 | Sdh_altC_Mm_620F | CACAATCATTCATAACTCGGCG | 845 | 58.1 |
| Sdh_altC_Mm_1464R | TCGGAAAGTAGACATCGACAAC | |||
| MmSdhD | SdhD_Mm_F153 | CCCACCCTGATCTTTTGCAT | 899 | 58.0 |
| SdhD_Mm_R1051 | CCATACCACAAAGCGAGCCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, T.C.; Moreira, S.I.; de Souza, D.M.; Christiano, F.S., Jr.; Gasparoto, M.C.G.; Fraaije, B.A.; Goldman, G.H.; Ceresini, P.C. Resistance to Site-Specific Succinate Dehydrogenase Inhibitor Fungicides Is Pervasive in Populations of Black and Yellow Sigatoka Pathogens in Banana Plantations from Southeastern Brazil. Agronomy 2024, 14, 666. https://doi.org/10.3390/agronomy14040666
Silva TC, Moreira SI, de Souza DM, Christiano FS Jr., Gasparoto MCG, Fraaije BA, Goldman GH, Ceresini PC. Resistance to Site-Specific Succinate Dehydrogenase Inhibitor Fungicides Is Pervasive in Populations of Black and Yellow Sigatoka Pathogens in Banana Plantations from Southeastern Brazil. Agronomy. 2024; 14(4):666. https://doi.org/10.3390/agronomy14040666
Chicago/Turabian StyleSilva, Tatiane C., Silvino I. Moreira, Daniel M. de Souza, Felix S. Christiano, Jr., Maria C. G. Gasparoto, Bart A. Fraaije, Gustavo H. Goldman, and Paulo C. Ceresini. 2024. "Resistance to Site-Specific Succinate Dehydrogenase Inhibitor Fungicides Is Pervasive in Populations of Black and Yellow Sigatoka Pathogens in Banana Plantations from Southeastern Brazil" Agronomy 14, no. 4: 666. https://doi.org/10.3390/agronomy14040666





