Effects of Bacillus subtilis on Rose Growth Promotion and Rhizosphere Microbial Community Changes under Saline–Alkaline Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Test Strain
2.1.2. Soil Composition
2.2. Preparation of Bacterial Agents and Treatment of Seedlings
2.3. Measurement of Physiological Growth Indicators in Roses
2.4. Root Sample Collection
2.5. Root Sample DNA Extraction and High-Throughput Sequencing
2.6. Data Processing and Bioinformatics Analysis
3. Results
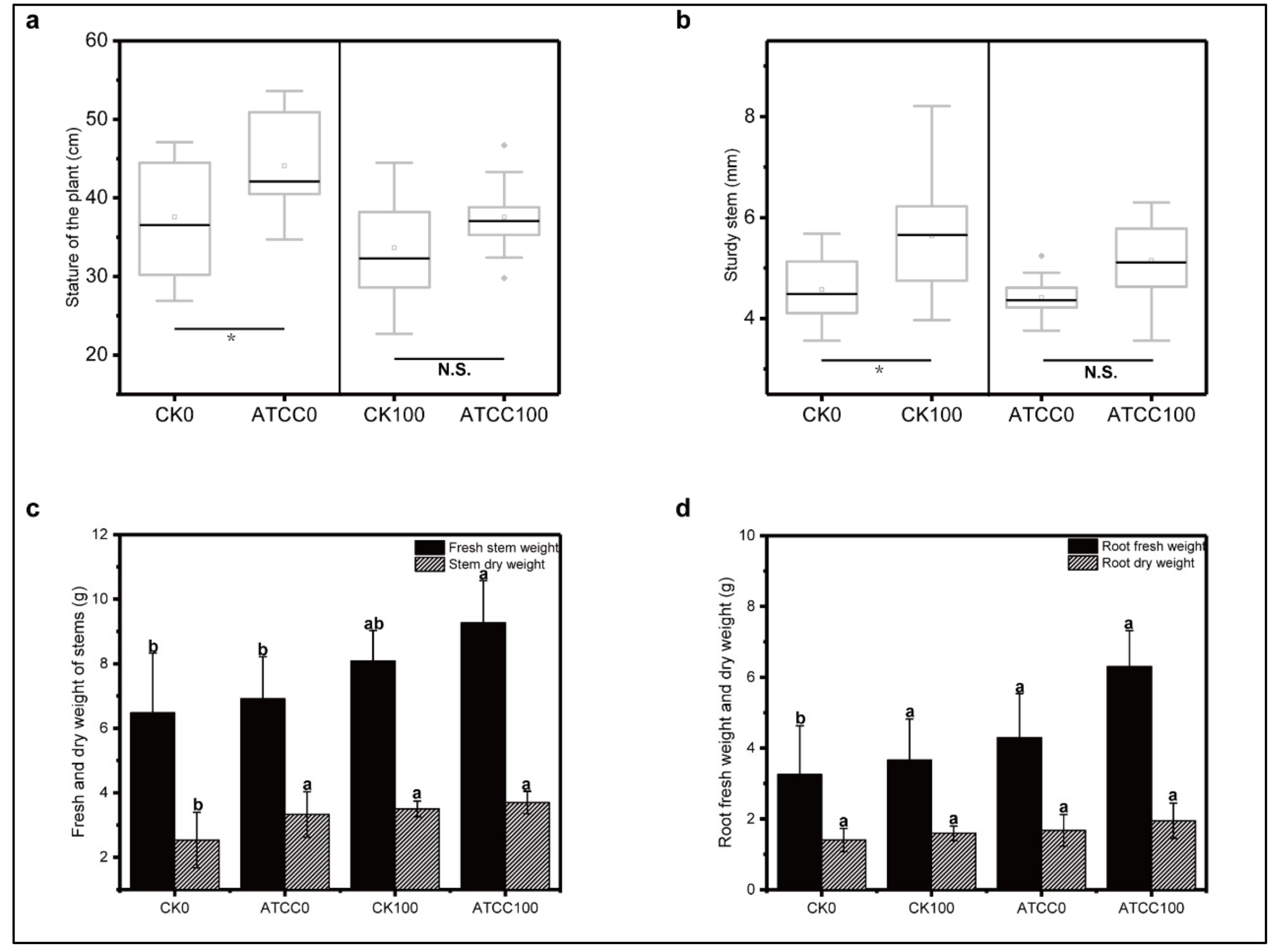
3.1. Growth-Promoting Effects of ATCC6633 Strain on Rose Seedlings under Saline–Alkaline Stress
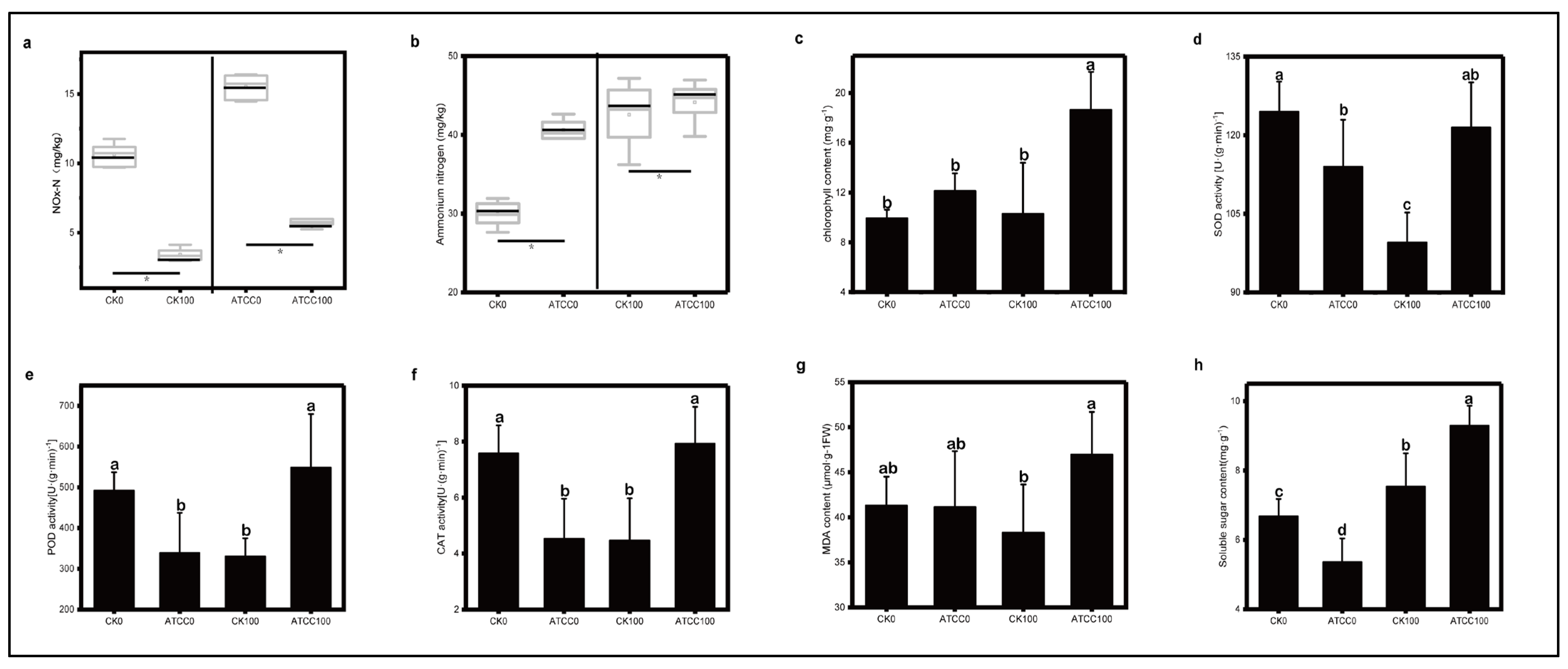
3.2. Effects of ATCC6633 Strain on Soil Nitrogen Content and Photosynthetic Pigments
3.3. Effects of ATCC6633 Strain on Stress Resistance-Related Enzymatic Activities in Roses
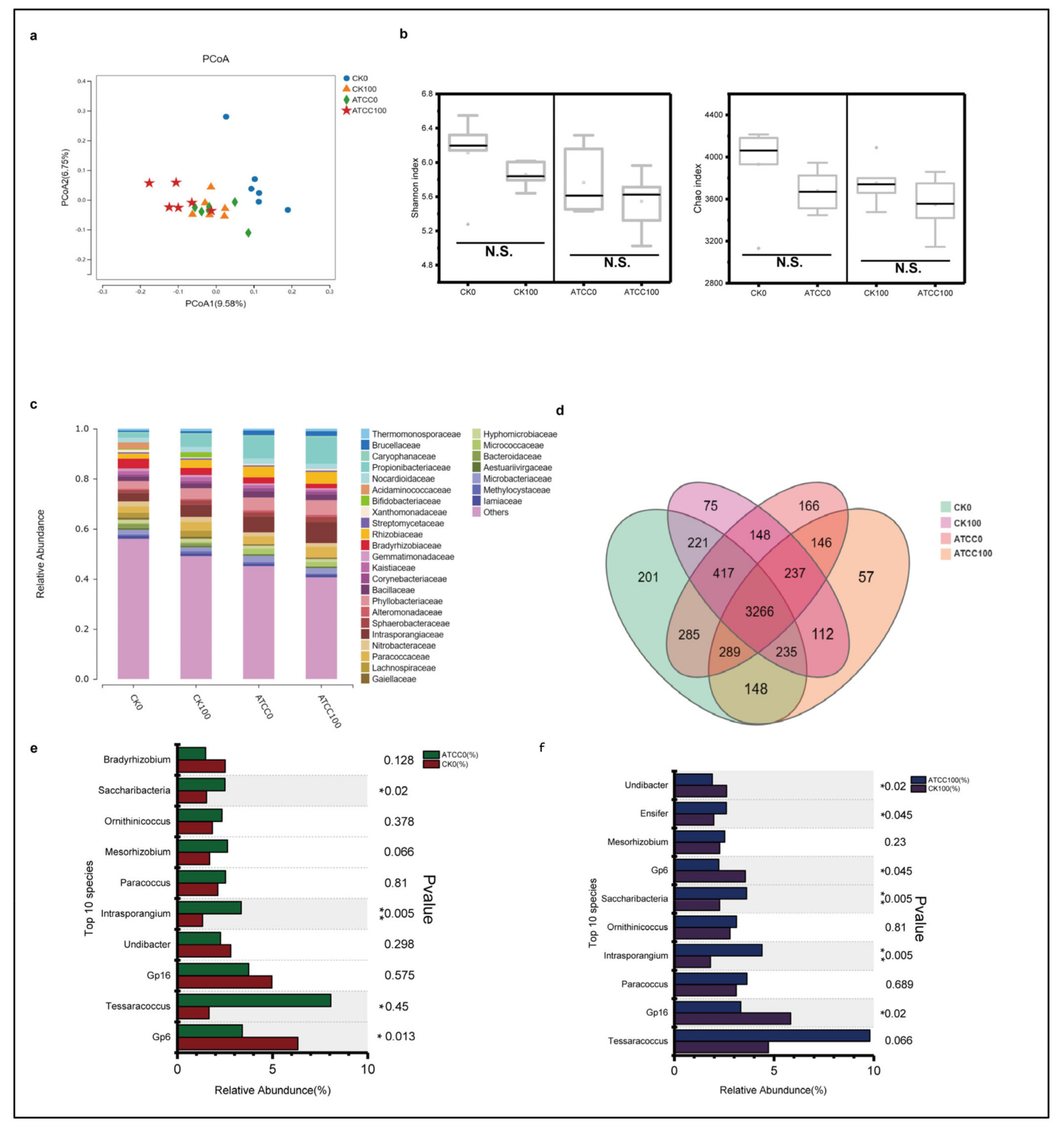
3.4. Characteristics of Root Microbial Community
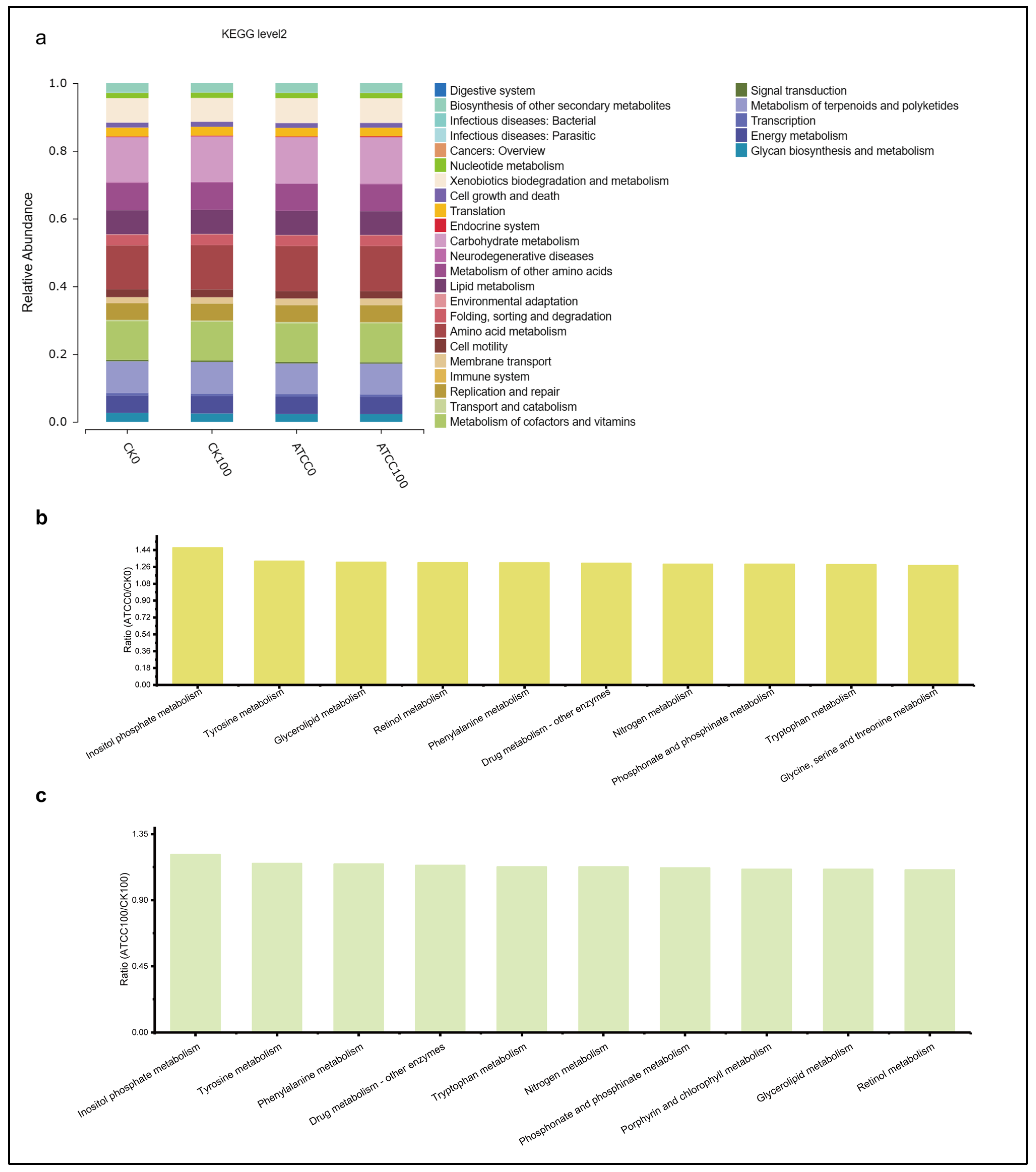
3.5. Differences in the Metabolic Pathways of Root Microbial Communities
4. Discussion
4.1. Impact of ATCC6633 Strain on the Growth Physiology of Rose Seedlings
4.2. Impact of ATCC6633 Strain on Rhizosphere Soil Microbial Communities of Roses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qinglian, M.; Shen, W. Exploration and Analysis of Domestic Saline alkali Land Governance Trends. Hubei Agric. Sci. 2020, 59, 302–306. [Google Scholar] [CrossRef]
- Yao, G.; Mingjian, L.; Xuenan, Z.; Xue, Y.; Zhen, Y. The relationship between the distribution of halophytes in China and the types of saline alkali land. J. Qilu Univ. Technol. 2021, 35, 14–20. [Google Scholar] [CrossRef]
- Ying, Z.; Li, W.; Huili, Z.; Xiaobing, C. Current status and prospects of research on coastal saline alkali land improvement. Chin. Agric. Bull. Sci. Agric. Sin. 2022, 38, 67–74. [Google Scholar]
- Shao, S.; Qi, M.M.; Tao, S.; Lin, J.X.; Wang, Y.N.; Yan, X.F. Physiological and Biochemical Responses of Jerusalem Artichoke Seedlings to Mixed Salt-Alkali Stress Conditions. Not. Bot. Horti Agrobot. Cluj Nap. 2015, 43, 473–478. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Yan, X.; Guo, J. Physiological and transcriptomic analyses of yellow horn (Xanthoceras sorbifolia) provide important insights into salt and saline-alkali stress tolerance. PLoS ONE 2020, 15, e0244365. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tang, J.; Li, Z.; Liu, Y.; Zhou, Z.; Wang, J.; Qu, Y.; Dai, Z. Carbon Mineralization under Different Saline-Alkali Stress Conditions in Paddy Fields of Northeast China. Sustainability 2020, 12, 2921. [Google Scholar] [CrossRef]
- Zhang, L.-h.; Chen, X.-b. Characteristics of ‘salt island’ and ‘fertile island’ for Tamarix chinensis and soil carbon, nitrogen and phosphorus ecological stoichiometry in saline-alkali land. Ying Yong Sheng Tai Xue Bao = J. Appl. Ecol. 2015, 26, 653–658. [Google Scholar]
- Wang, X.-s.; Ren, H.-l.; Wei, Z.-w.; Wang, Y.-w.; Ren, W.-b. Effects of neutral salt and alkali on ion distributions in the roots, shoots, and leaves of two alfalfa cultivars with differing degrees of salt tolerance. J. Integr. Agric. 2017, 16, 1800–1807. [Google Scholar] [CrossRef]
- Wei, Z.; Yujie, F. The physicochemical properties and ecological restoration of saline alkali soil in the Songnen. Acta Pedol. Sin. 2009, 46, 169–172. [Google Scholar]
- Zhang, K.; Li, C.; Li, Z.; Zhang, F.; Zhao, Z.; Tian, C. Characteristics of mineral elements in shoots of three annual halophytes in a saline desert, Northern Xinjiang. J. Arid Land 2013, 5, 244–254. [Google Scholar] [CrossRef]
- Xu, A.; Mu, C.; Li, X.; Lin, J.; Li, Y.; Mu, Y. Salt and alkali stresses effects on contents of organic acids components in wheat seedlings. J. Plant Nutr. 2013, 36, 1056–1064. [Google Scholar] [CrossRef]
- Lu, X.; Ma, L.; Zhang, C.; Yan, H.; Bao, J.; Gong, M.; Wang, W.; Li, S.; Ma, S.; Chen, B. Grapevine (Vitis vinifera) responses to salt stress and alkali stress: Transcriptional and metabolic profiling. BMC Plant Biol. 2022, 22, 528. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.X.; Li, G.T.; Wang, G.H.; Liang, T.Y.; Yan, J.Q.Z.; Li, J.J. Effects of saline-alkali mixed stress on the growth and physiological characteristics of giant juncao (Pennisetum giganteum Z. X. Lin). Appl. Ecol. Environ. Res. 2021, 19, 75–94. [Google Scholar] [CrossRef]
- Li, R.; Shi, F.; Fukuda, K. Interactive effects of salt and alkali stresses on seed germination, germination recovery, and seedling growth of a halophyte Spartina alterniflora (Poaceae). S. Afr. J. Bot. 2010, 76, 380–387. [Google Scholar] [CrossRef]
- Liu, J.; Guo, W.Q.; Shi, D.C. Seed germination, seedling survival, and physiological response of sunflowers under saline and alkaline conditions. Photosynthetica 2010, 48, 278–286. [Google Scholar] [CrossRef]
- Zhao, X.; Yuan, X.; Xing, Y.; Dao, J.; Zhao, D.; Li, Y.; Li, W.; Wang, Z. A meta-analysis on morphological, physiological and biochemical responses of plants with PGPR inoculation under drought stress. Plant Cell Environ. 2023, 46, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Boyce, A.N. Role of Plant Growth Promoting Rhizobacteria in Agricultural Sustainability—A Review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Li, Y.; Tian, S.; Wei, J.; Liao, D.; Feng, M.; Zhang, H.; Zhou, M.; Hang, S. Study on the effects of bacterial residue return on the physicochemical properties, microorganisms, and enzyme activities of vegetable soil. Chin. Agric. Bull. 2020, 36, 98–104. [Google Scholar]
- Wang, Z.; Hu, X.; Solanki, M.K.; Pang, F. A Synthetic Microbial Community of Plant Core Microbiome Can Be a Potential Biocontrol Tool. J. Agric. Food Chem. 2023, 71, 5030–5041. [Google Scholar] [CrossRef]
- Maciag, T.; Koziel, E.; Rusin, P.; Otulak-Koziel, K.; Jafra, S.; Czajkowski, R. Microbial Consortia for Plant Protection against Diseases: More than the Sum of Its Parts. Int. J. Mol. Sci. 2023, 24, 2227. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.-M.; Chang, I.; Noh, D.-H.; Kwon, T.-H.; Muhunthan, B. Improvement of Surface Erosion Resistance of Sand by Microbial Biopolymer Formation. J. Geotech. Geoenviron. Eng. 2018, 144, 06018004. [Google Scholar] [CrossRef]
- Liu, W.; Chen, D.K.; Yin, H.W.; Song, M.; Guo, S.R. Bacillus subtilis for Salt Stress Relief in Vegetable Cultivation. In Proceedings of the International Symposium on Vegetable Safety and Human Health, Beijing, China, 28 February 2010; pp. 237–242. [Google Scholar]
- Xu, Y.J.; Chen, Z.; Li, X.Y.; Tan, J.; Liu, F.; Wu, J.P. Mycorrhizal fungi alter root exudation to cultivate a beneficial microbiome for plant growth. Funct. Ecol. 2023, 37, 664–675. [Google Scholar] [CrossRef]
- Zhou, C.; Li, F.Y.; Xie, Y.; Zhu, L.; Xiao, X.; Ma, Z.Y.; Wang, J.F. Involvement of abscisic acid in microbe-induced saline-alkaline resistance in plants. Plant Signal. Behav. 2017, 12, e1367465. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Li, F.; Zhang, J.; Wei, B.; Jia, Y.; Ynag, H. Optimization of the correction factor for determining soil nitrate nitrogen using UV spectrophotometry. Acta Agric. Boreali-Sin. 2019, 34, 204–212. [Google Scholar]
- Yao, W.; Mou, X.; Wan, S.; Xu, H.; Wang, M.; Zhao, Z. Soil carbon, nitrogen, phosphorus, and sulfur content and their ecological stoichiometric characteristics under different land use methods. Jiangsu Agric. Sci. 2023, 51, 231–239. [Google Scholar] [CrossRef]
- Akbar, A.; Han, B.; Khan, A.H.; Feng, C.; Ullah, A.; Khan, A.S.; He, L.; Yang, X. A transcriptomic study reveals salt stress alleviation in cotton plants upon salt tolerant PGPR inoculation. Environ. Exp. Bot. 2022, 200, 104928. [Google Scholar] [CrossRef]
- Kuramshina, Z.M.; Khairullin, R.M. Improving Salt Stress Tolerance of Triticum aestivum L. with Endophytic Strains of Bacillus subtilis. Russ. J. Plant Physiol. 2023, 70, 53. [Google Scholar] [CrossRef]
- Qi, R.; Lin, W.; Gong, K.; Han, Z.; Ma, H.; Zhang, M.; Zhang, Q.; Gao, Y.; Li, J.; Zhang, X. Bacillus Co-Inoculation Alleviated Salt Stress in Seedlings Cucumber. Agronomy 2021, 11, 966. [Google Scholar] [CrossRef]
- Zhou, M.H.; Butterbach-Bahl, K.; Vereecken, H.; Brüggemann, N. A meta-analysis of soil salinization effects on nitrogen pools, cycles and fluxes in coastal ecosystems. Glob. Chang. Biol. 2017, 23, 1338–1352. [Google Scholar] [CrossRef]
- Zhang, H.; Song, L.; Chen, X.L.; Li, P.C. An Exploration of Seaweed Polysaccharides Stimulating Denitrifying Bacteria for Safer Nitrate Removal. Molecules 2021, 26, 3390. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Yang, Y. How Plants Tolerate Salt Stress. Curr. Issues Mol. Biol. 2023, 45, 5914–5934. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Ma, H.; Jiao, Q.; Ma, C.; Wang, P. Phytohormones: Important Participators in Plant Salt Tolerance. Int. J. Agric. Biol. 2020, 24, 319–332. [Google Scholar]
- Sui, N.; Yang, Z.; Liu, M.; Wang, B. Identification and transcriptomic profiling of genes involved in increasing sugar content during salt stress in sweet sorghum leaves. BMC Genom. 2015, 16, 534. [Google Scholar] [CrossRef]
- Yang, H.-B. Comparative study of osmoticum accumulation in wheat under osmotic and ionic stress. Afr. J. Agric. Res. 2011, 6, 6661–6664. [Google Scholar] [CrossRef]
- Guo, W.-Q.; Zhang, P.-T.; Li, C.-H.; Yin, J.-M.; Han, X.-Y. Recovery of root growth and physiological characters in cotton after salt stress relief. Chil. J. Agric. Res. 2015, 75, 85–91. [Google Scholar] [CrossRef]
- Kwok, A. Interactions between Abscisic Acid and Ethylene in Salt-Stressed Tomato (Solanum lycopersicum L.) Roots. Master’s Thesis, Simon Fraser University, Vancouver, BC, Canada, 2008. [Google Scholar]
- Li, H.; Duijts, K.; Pasini, C.; van Santen, J.E.; Lamers, J.; de Zeeuw, T.; Verstappen, F.; Wang, N.; Zeeman, S.C.; Santelia, D.; et al. Effective root responses to salinity stress include maintained cell expansion and carbon allocation. New Phytol. 2023, 238, 1942–1956. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Zhang, D.; Han, D.; Ren, H.; Wang, Z.; Zhu, Z.; Sun, H.; Wang, L.; Qu, Z.; Lu, W.; et al. Effects of salt stress on soil enzyme activities and rhizosphere microbial structure in salt-tolerant and -sensitive soybean. Sci. Rep. 2023, 13, 17057. [Google Scholar] [CrossRef]
- Neto, A.D.D.; Prisco, J.T.; Enéas, J.; de Abreu, C.E.B.; Gomes, E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. Exp. Bot. 2006, 56, 87–94. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, Y.; Li, X.; Chen, W. Effects of chlorimuron-ethyl on soil microorganisms and enzyme activities under moderate salt stress. Fresenius Environ. Bull. 2018, 27, 2358–2365. [Google Scholar]
- Yang, D.H.; Tang, L.; Cui, Y.; Chen, J.X.; Liu, L.; Guo, C.H. Saline-alkali stress reduces soil bacterial community diversity and soil enzyme activities. Ecotoxicology 2022, 31, 1356–1368. [Google Scholar] [CrossRef] [PubMed]
- Alejandra Hernandez-Ortega, H.; Alarcon, A.; Ferrera-Cerrato, R.; Araceli Zavaleta-Mancera, H.; Antonio Lopez-Delgado, H.; Remedios Mendoza-Lopez, M. Arbuscular mycorrhizal fungi on growth, nutrient status, and total antioxidant activity of Melilotus albus during phytoremediation of a diesel-contaminated substrate. J. Environ. Manag. 2012, 95, S319–S324. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; McCormack, M.L.; Guo, D. Arbuscular mycorrhizal fungal effects on plant competition and community structure. J. Ecol. 2015, 103, 1224–1232. [Google Scholar] [CrossRef]
- Crovadore, J.; Cochard, B.; Chablais, R.; Haenzi, M.; Raffini, F.; Lefort, F. Draft Genome Sequences of Pseudomonas koreensis Strain UASWS1668, Bacillus megaterium Strain UASWS1667, and Paenibacillus sp. Strain UASWS1643, Considered Potential Plant Growth-Promoting Rhizobacteria. Microbiol. Resour. Announc. 2020, 9, e00768-20. [Google Scholar] [CrossRef]
- Kang, S.-M.; Radhakrishnan, R.; You, Y.-H.; Joo, G.-J.; Lee, I.-J.; Lee, K.-E.; Kim, J.-H. Phosphate Solubilizing Bacillus megaterium mj1212 Regulates Endogenous Plant Carbohydrates and Amino Acids Contents to Promote Mustard Plant Growth. Indian J. Microbiol. 2014, 54, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.Q.; Yu, X.A.; Liang, X.J.; Liu, Y.F.; Borriss, R.; Liu, Y.Z. Addition of plant-growth-promoting Bacillus subtilis PTS-394 on tomato rhizosphere has no durable impact on composition of root microbiome. BMC Microbiol. 2017, 17, 131. [Google Scholar] [CrossRef] [PubMed]
- Doronina, N.V.; Trotsenko, Y.A.; Kuznetzov, B.B.; Tourova, T.P. Emended description of Paracoccus kondratievae. Int. J. Syst. Evol. Microbiol. 2002, 52, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.X.; Ge, W.; Liu, X.C.; Zhang, X.M.; Chen, Q.H.; Wu, J.; Li, Z.Y.; Chai, C. Bioremediation of polycyclic aromatic hydrocarbons from an aged contaminated agricultural soil using degrading bacteria and soil amendments. Bioremediat. J. 2022, 26, 305–317. [Google Scholar] [CrossRef]
- Dekkiche, S.; Benguedouar, A.; Sbabou, L.; Taha, K.; Filali-Maltouf, A.; Bena, G. Chickpea (Cicer arietinum) is nodulated by unexpected wide diversity of Mesorhizobium species in Eastern Algeria. Arch. Agron. Soil Sci. 2018, 64, 285–297. [Google Scholar] [CrossRef]
- Rejili, M.; BenAbderrahim, M.A.; Mars, M.; Sherrier, J.D. Novel putative rhizobial species with different symbiovars nodulate Lotus creticus and their differential preference to distinctive soil properties. FEMS Microbiol. Lett. 2020, 367, fnaa084. [Google Scholar] [CrossRef] [PubMed]
- Rejili, M.; Ruiz-Argueso, T.; Mars, M. Novel putative Mesorhizobium and Ensifer genomospecies together with a novel symbiovar psoraleae nodulate legumes of agronomic interest grown in Tunisia. Syst. Appl. Microbiol. 2020, 43, 126067. [Google Scholar] [CrossRef] [PubMed]
- Erturk, Y.; Ercisli, S.; Haznedar, A.; Cakmakci, R. Effects of plant growth promoting rhizobacteria (PGPR) on rooting and root growth of kiwifruit (Actinidia deliciosa) stem cuttings. Biol. Res. 2010, 43, 91–98. [Google Scholar] [CrossRef]
- Saxena, A.K.; Kumar, M.; Chakdar, H.; Anuroopa, N.; Bagyaraj, D.J. Bacillus species in soil as a natural resource for plant health and nutrition. J. Appl. Microbiol. 2020, 128, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Hanke, D.E.; Parmar, P.N.; Caddick, S.E.K.; Green, P.; Brearley, C.A. Synthesis of inositol phosphate ligands of plant hormone-receptor complexes: Pathways of inositol hexakisphosphate turnover. Biochem. J. 2012, 444, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.-M.; Bian, M.-D.; Yang, Z.-M.; Shi, W.-L. Tyrosine phosphorylation mediates starch metabolism in guard cell of Vicia faba. Biologia 2015, 70, 574–580. [Google Scholar] [CrossRef]
- Kang, H.; Jia, C.; Liu, N.; Aboagla, A.A.A.; Chen, W.; Gong, W.; Tang, S.; Hong, Y. Plastid Glycerol-3-phosphate Acyltransferase Enhanced Plant Growth and Prokaryotic Glycerolipid Synthesis in Brassica napus. Int. J. Mol. Sci. 2020, 21, 5325. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Li, J.Q.; Dauk, M.; Huang, Y.; Periappuram, C.; Wei, Y.; Zou, J. Metabolic and Transcriptional Responses of Glycerolipid Pathways to a Perturbation of Glycerol 3-Phosphate Metabolism in Arabidopsis. J. Biol. Chem. 2010, 285, 22955–22963. [Google Scholar] [CrossRef]
- Feduraev, P.; Skrypnik, L.; Riabova, A.; Pungin, A.; Tokupova, E.; Maslennikov, P.; Chupakhina, G. Phenylalanine and Tyrosine as Exogenous Precursors of Wheat (Triticum aestivum L.) Secondary Metabolism through PAL-Associated Pathways. Plants 2020, 9, 476. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, M.; Yu, K.; Liu, H.; Sheng, Q.; Zhang, Y. Effects of Bacillus subtilis on Rose Growth Promotion and Rhizosphere Microbial Community Changes under Saline–Alkaline Stress. Agronomy 2024, 14, 730. https://doi.org/10.3390/agronomy14040730
Zou M, Yu K, Liu H, Sheng Q, Zhang Y. Effects of Bacillus subtilis on Rose Growth Promotion and Rhizosphere Microbial Community Changes under Saline–Alkaline Stress. Agronomy. 2024; 14(4):730. https://doi.org/10.3390/agronomy14040730
Chicago/Turabian StyleZou, Meng, Kai Yu, Hao Liu, Qianqian Sheng, and Yuanlan Zhang. 2024. "Effects of Bacillus subtilis on Rose Growth Promotion and Rhizosphere Microbial Community Changes under Saline–Alkaline Stress" Agronomy 14, no. 4: 730. https://doi.org/10.3390/agronomy14040730
APA StyleZou, M., Yu, K., Liu, H., Sheng, Q., & Zhang, Y. (2024). Effects of Bacillus subtilis on Rose Growth Promotion and Rhizosphere Microbial Community Changes under Saline–Alkaline Stress. Agronomy, 14(4), 730. https://doi.org/10.3390/agronomy14040730




