Polyphenol Profile, Antioxidant Activity and Yield of Cynara cardunculus altilis in Response to Nitrogen Fertilisation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site, Climate, and Soil Characteristics
2.2. Plant Material, Experimental Design, and Management Practices
2.3. Harvesting of Raw Material and Post-Harvest Treatment
2.4. Chemicals and Standards
2.5. Sample Preparation
2.6. Determination of Total Polyphenols and Flavonoids
- A—absorbance of the test solution,
- K—conversion factor for quercetin acid K = 3.5087,
- M—raw material weight.
2.7. HPLC Analysis
2.8. Radical Scavenging Activity Assay
2.8.1. 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulphonic Acid (ABTS) Assay
2.8.2. Ferric Reducing Antioxidant Power (FRAP) Assay
2.8.3. 2,2-diphenyl-1-picrylhydrazyl (DPPH) Assay
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effect of N Rate and Form on Leaf Biomass Yields
3.2. Effect of N Rate and Form on Polyphenol Profile
3.3. Effect of N Rate and Form on Radical Scavenging Activity
3.4. Effect of Growing Season on Biomass Yield, Polyphenol Profile and Radical Scavenging Activity
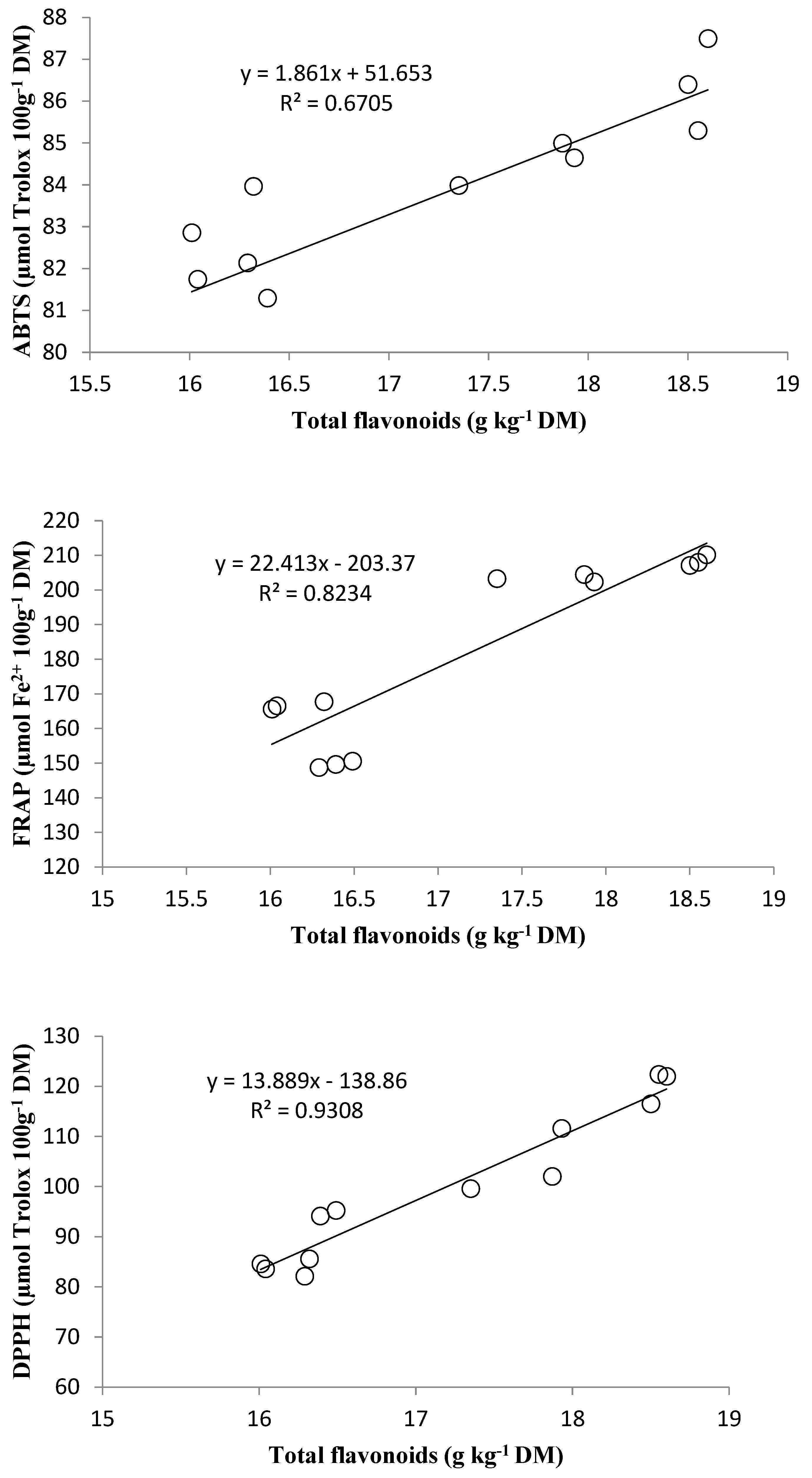
3.5. Correlation Analysis of Parameters under Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Portis, E.; Scaglione, D.; Acquadro, A.; Mauromicale, G.; Mauro, R.; Knapp, S.; Lanteri, S. Genetic mapping and identification of QTL for earliness in the globe artichoke/cultivated cardoon complex. BMC Res. Notes 2012, 5, 252. [Google Scholar] [CrossRef] [PubMed]
- Pandino, G.; Mauromicale, G. Globe artichoke and cardoon forms between traditional and modern uses. Acta Hort. 2020, 1284, 1–18. [Google Scholar] [CrossRef]
- Sałata, A.; Lombardo, S.; Pandino, G.; Mauromicale, G.; Buczkowska, H.; Nurzyńska-Wierdak, R. Biomass yield and polyphenol compounds profile in globe artichoke as affected by irrigation frequency and drying temperature. Ind. Crops Prod. 2022, 176, 114375. [Google Scholar] [CrossRef]
- Sałata, A.; Sękara, A.; Pandino, G.; Mauromicale, G.; Lombardo, S. Living mulch as sustainable tool to improve leaf biomass and phytochemical yield of Cynara cardunculus var. altilis. Agronomy 2023, 13, 1274. [Google Scholar] [CrossRef]
- Borgognone, D.; Cardarelli, M.; Rea, E.; Lucini, L.; Colla, G. Salinity source-induced changes in yield, mineral composition, phenolic acids and flavonoids in leaves of artichoke and cardoon grown in floating system. J. Sci. Food Agric. 2014, 94, 1231–1237. [Google Scholar] [CrossRef]
- Kollia, E.; Markaki, P.; Zoumpoulakis, P.; Proestos, C. Antioxidant activity of Cynara scolymus L. and Cynara cardunculus L. extracts obtained by different extraction techniques. Nat. Prod. Res. 2017, 31, 1163–1167. [Google Scholar] [CrossRef]
- Pandino, G.; Bonomo, A.; Scavo, A.; Mauromicale, G.; Lombardo, S. Caffeoylquinic acids and flavones profile in Cynara cardunculus L. seedlings under controlled conditions as affected by light and water-supply treatments. Sci. Hortic. 2022, 302, 111180. [Google Scholar] [CrossRef]
- Del Bò, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic review on polyphenol intake and health outcomes: Is there sufficient evidence to define a health-promoting polyphenol-rich dietary pattern? Nutrients 2019, 11, 1355. [Google Scholar] [CrossRef]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, 14264. [Google Scholar] [CrossRef]
- Rossoni, G.; Grande, S.; Galli, C.; Visioli, F. Wild artichoke prevents the age associated loss of vasomotor function. J. Agric. Food Chem. 2005, 53, 10291–10296. [Google Scholar] [CrossRef]
- Gebhardt, R.; Fausel, M. Antioxidant and hepatoprotective effects of artichoke extracts and constituents in cultured rat hepatocytes. Toxicol Vitr. 1997, 11, 669–672. [Google Scholar] [CrossRef]
- Speroni, E.; Cervellati, R.; Covoni, P.; Guizzardi, S.; Renzulli, C.; Guerra, M. Efficacy of different Cynara scolymus preparation of liver complaints. J. Ethnopharmacol. 2003, 86, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Kraft, K. Artichoke leaf extract-recent findings reflecting effects on lipid metabolism, liver, and gastrointestinal tracts. Phytomedicine 1997, 4, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Bundy, R.; Walker, A.F.; Middleton, R.W.; Wallis, C.; Simpson, H.C.R. Artichoke leaf extract (Cynara scolymus) reduced plasma cholesterol in otherwise healthy hypercholesterolemic adults: A randomized, double blind placebo controlled trial. Phytomedicine 2008, 15, 668–675. [Google Scholar] [CrossRef]
- Gebhardt, R. Choleretic and anticholestatic activities of flavonoids of artichoke (Cynara cardunculus L. subsp. scolymus (L.) Hayek). Acta Hort. 2005, 681, 429–436. [Google Scholar] [CrossRef]
- De Falco, B.; Incerti, G.; Amato, M.; Lanzotti, V. Artichoke: Botanical, agronomical, phytochemical, and pharmacological overview. Phytochem. Rev. 2015, 14, 993–1018. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Mauromicale, G.; Williamson, G. Characterization of phenolic acids and flavonoids in leaves, stems, bracts and edible parts of globe artichokes. Acta Hort. 2012, 942, 413–417. [Google Scholar] [CrossRef]
- Lombardo, S.; Scavo, A.; Pandino, G.; Cantone, M.; Mauromicale, G. Improvement in the cynaropicrin, caffeoylquinic acid and flavonoid content of globe artichokes with gibberellic acid Treatment. Plants 2022, 11, 1845. [Google Scholar] [CrossRef] [PubMed]
- Pandino, G.; Meneghini, M.; Tavazza, R.; Lombardo, S.; Mauromicale, G. Phytochemicals accumulation and antioxidant activity in callus and suspension cultures of Cynara scolymus L. Plant Cell Tissue Organ Cult. 2017, 128, 223–230. [Google Scholar] [CrossRef]
- Ghoneim, I.M. Effect of biofertilizer types under varying nitrogen levels on vegetative growth, head yield and quality of globe artichoke (Cynara scolymus L.). J. Agric. Environ. Sci. Alex. Univ. Egypt 2005, 4, 1–23. [Google Scholar]
- Elia, A.; Conversa, G. Mineral nutrition aspects in artichoke growing. Acta Hortic. 2007, 730, 239–249. [Google Scholar] [CrossRef]
- Shinohara, T.; Shinsuke, A.; Kil, Y.S.; Leskovar, D.I. Irrigation and nitrogen management of artichoke: Yield, head quality, and phenolic content. HortScience 2011, 46, 377–386. [Google Scholar] [CrossRef]
- Saleh, S.A.; Zaki, M.F.; Tantawy, A.S.; Salama, Y.A.M. Response of artichoke productivity to different proportions of nitrogen and potassium fertilizers. Int. J. Chem. Technol. Res. 2016, 9, 25–33. [Google Scholar]
- Lombardo, S.; Pandino, G.; Mauromicale, G. The nutraceutical response of two globe artichoke cultivars to contrasting NPK fertilizer regimes. Food Res. Int. 2015, 76, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.; Restuccia, C.; Muratore, G.; Barbagallo, R.N.; Licciardello, F.; Pandino, G.; Mauromicale, G. Effect of nitrogen fertilization on the overall quality of minimally processed globe artichoke heads. J. Sci. Food Agric. 2017, 97, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.; Pandino, G.; Mauromicale, G. Minerals profile of two globe artichoke cultivars as affected by NPK fertilizer regimes. Food Res. Int. 2017, 100, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Baier, C.; Eich, J.; Grün, M.; Wagenbreth, D.; Zimermann, R. Artichoke leaves used for herbal drug production: Influence of nitrogen fertilization on yield and on pharmaceutical quality. Acta Hort. 2005, 681, 545–554. [Google Scholar] [CrossRef]
- Matthes, C.; Honermeier, B. Cultivation of the artichoke as a medicinal plant under temperate climate condition in Germany. Acta Hort. 2007, 630, 483–489. [Google Scholar] [CrossRef]
- Montesano, V.; Negro, D.; Sonnante, G.; Laghetti, G.; Urbano, M. Polyphenolic compound variation in globe artichoke cultivars as affected by fertilization and biostimulants application. Plants 2022, 11, 2067. [Google Scholar] [CrossRef]
- Sabir, M.; Hanafi, M.M.; Malik, M.T.; Aziz, T.; Zia-ur-Rehman, M.; Ahmad, R.A.; Hakeem, K.R.; Shahid, M. Differential effect of nitrogen forms on physiological parameters and micronutrient concentration in maize (Zea mays L.). Aust. J. Crop Sci. 2013, 7, 1836–1842. [Google Scholar]
- Sun, Y.D.; Lou, W.R.; Liu, H.C. Effects of different nitrogen forms on the nutritional quality and physiological characteristics of Chinese chive seedlings. Plant. Soil Environ. 2014, 60, 216–220. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources, 2014. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; update 2015; World Soil Resources Reports No.106; FAO: Rome, Italy, 2014; p. 192. [Google Scholar]
- Sałata, A.; Gortat, M.; Buczkowska, H. Leaf petioles blanching influence on the yield and chemical composition of cardoon (Cynara cardunculus L.). Acta Sci. Pol. Hortorum Cultus 2017, 16, 41–56. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 97, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Pharmacopoeia Poland; Polish Pharmaceutical Society: Warszawa, Poland, 2002; Volume 6, pp. 880–881.
- IUPAC. Nomenclature of cyclitols. Biochem. J. 1976, 153, 23–31. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Gouveia, S.C.; Castilho, P.C. Antioxidant potential of Artemisia argentea L’Hér alcoholic extract and its relation with the phenolic composition. Food Res. Int. 2011, 44, 1620–1631. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, S.M.; Chun, J.; Lee, H.B.; Lee, J. Influence of heat treatment on the antioxidant activities and polyphenolic compounds of Shiitake (Lentinus edodes) mushroom. Food Chem. 2006, 99, 381–387. [Google Scholar] [CrossRef]
- Negro, D.; Montesano, V.; Sonnante, G.; Rubino, P.; De Lisi, A.; Sarli, G. Fertilization strategies on cultivars of globe artichoke: Effects on yield and quality performance. J. Plant Nutr. 2016, 39, 279–287. [Google Scholar] [CrossRef]
- Radušienė, J.; Marksa, M.; Ivanauskas, L.; Jakštas, V.; Çalişkan, Ö.; Kurt, D.; Odabaş, M.S.; Çirak, C. Effect of nitrogen on herb production, secondary metabolites and antioxidant activities of Hypericum pruinatum under nitrogen application. Ind. Crops Prod. 2019, 139, 111519. [Google Scholar] [CrossRef]
- Stefanelli, D.; Goodwin, I.; Jones, R. Minimal nitrogen and water use in horticulture: Effects on quality and content of selected nutrients. Food Res. Int. 2010, 43, 1833–1843. [Google Scholar] [CrossRef]
- Munene, R.; Changamu, E.; Korir, N.; Joseph, G.O. Effects of different nitrogen forms on growth, phenolics, flavonoids and antioxidant activity in amaranth species. Trop. Plant Res. 2017, 4, 81–89. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.M.; Lu, Y.T.; Qiu, Q.L.; Fan, D.M.; Wang, X.C.; Zheng, X.Q. Influence of different nitrogen sources on carbon and nitrogen metabolism and gene expression in tea plants (Camellia sinensis L.). Plant Physiol. Biochem. 2021, 167, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Yang, H.; Wei, Z.; Yang, H.; Fan, S.; Wu, W.; Lyu, L.; Li, W. Effects of different nitrogen forms on blackberry fruit quality. Foods 2023, 12, 2318. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.; Zangerl, A.; DeLucia, E.; Berenbaum, M. The carbon–nutrient balance hypothesis: Its rise and fall. Ecol. Lett. 2001, 4, 86–95. [Google Scholar] [CrossRef]
| Growing Season | Month | Average/Sum | ||||||
|---|---|---|---|---|---|---|---|---|
| April | May | June | July | August | September | October | ||
| Average Temperature (°C) | ||||||||
| 2018 | 13.4 | 17.1 | 18.8 | 20.7 | 20.7 | 15.5 | 10.0 | 16.6 |
| 2019 | 9.5 | 13.4 | 21.5 | 19.4 | 20.3 | 14.5 | 11.0 | 15.6 |
| 1955–2012 | 7.4 | 13.0 | 16.2 | 17.8 | 17.1 | 12.6 | 12.4 | 13.7 |
| Total Rainfall (mm) | ||||||||
| 2018 | 40 | 56 | 65 | 124 | 72 | 68 | 36 | 461 |
| 2019 | 49 | 93 | 37 | 38 | 102 | 52 | 29 | 400 |
| 1955–2012 | 39 | 58 | 66 | 84 | 69 | 54 | 58 | 428 |
| Growing Season | Month | Temperature (°C) | Insolation (Sum of Hours) | |
|---|---|---|---|---|
| Average Maximum | Average Minimum | |||
| 2018 | April | 15.1 | 13.3 | 216 |
| May | 18.8 | 14.4 | 245 | |
| June | 20.4 | 16.5 | 206 | |
| July | 22.6 | 19.4 | 169 | |
| August | 23.2 | 18.1 | 214 | |
| September | 20.3 | 17.7 | 165 | |
| October | 19.7 | 16.8 | 134 | |
| Average/Total | 20.0 | 16.6 | 209/1349 | |
| 2019 | April | 14.1 | 10.3 | 183 |
| May | 18.0 | 13.4 | 128 | |
| June | 22.2 | 15.5 | 253 | |
| July | 23.1 | 18.4 | 198 | |
| August | 24.2 | 19.1 | 205 | |
| September | 18.3 | 16.6 | 140 | |
| October | 17.7 | 15.8 | 138 | |
| Average/Total | 19.6 | 15.5 | 178/1245 | |
| Characteristics Soil | ||||||||
|---|---|---|---|---|---|---|---|---|
| Silt (%) | Loam (%) | Sand (%) | pH in H2O | Na (mg L−1) | Cl (mg L−1) | Salinity (g L−1 NaCl) | Organic Matter %C | C/N |
| 69 | 10 | 21 | 7.36 | 57.1 | 6.13 | 0.29 | 1.42 | 11.3 |
| Macro and Microelement Content (mg L−1) | ||||||||
| N-NO3 | P | K | Ca | Mg | Zn | Mn | Cu | Fe |
| 10.6 | 204 | 132 | 1293 | 106 | 8.72 | 12.80 | 5.24 | 42.8 |
| Treatment | Source of Variation | Leaf Air-Dried Biomass (g plant−1) | Yield of Leaf Air-Dried Biomass (t ha−1) |
|---|---|---|---|
| N rate (N) kg ha−1 | 0 | 89.50 b ± 0.16 | 4.27 b ± 0.05 |
| 60 | 165.17 a ± 0.22 | 6.40 a ± 0.06 | |
| 120 | 160.83 a ± 0.25 | 6.07 a ± 0.19 | |
| 180 | 162.50 a ± 0.28 | 6.22 a ± 0.20 | |
| p-value | <0.001 | <0.001 | |
| N form (F) | NO3 | 143.25 b ± 0.18 | 5.91 a ± 0.18 |
| NH4 | 139.25 b ± 0.27 | 5.26 b ± 0.16 | |
| Urea | 151.00 a ± 0.23 | 6.05 a ± 0.22 | |
| p-value | 0.001 | 0.001 | |
| Growing season (S) | 2018 | 149.00 a ± 0.24 | 6.30 a ± 0.43 |
| 2019 | 140.25 a ± 0.19 | 5.18 b ± 0.38 | |
| p-value | 0.340 | 0.009 | |
| N × F | p-value | 0.284 | 0.065 |
| N × S | p-value | 0.034 | 0.008 |
| F × S | p-value | 0.206 | 0.065 |
| N × F × S | p-value | 0.002 | 0.162 |
| Treatment | Source of Variation | Total Flavonoids (g Quercitin kg−1 DM) | Total Polyphenols (g Gallic Acid kg−1 DM) |
|---|---|---|---|
| N rate (N) kg ha−1 | 0 | 17.93 b ± 0.15 | 33.05 a ± 1.33 |
| 60 | 16.01 c ± 0.25 | 32.52 a ± 3.14 | |
| 120 | 18.50 a ± 0.18 | 33.28 a ± 1.36 | |
| 180 | 16.39 c ± 0.27 | 28.12 b ± 0.96 | |
| p-value | 0.008 | <0.001 | |
| N form (F) | NO3 | 17.33 a ± 0.25 | 33.58 a ± 1.01 |
| NH4 | 17.14 a ± 0.18 | 30.60 a ± 1.05 | |
| Urea | 17.15 a ± 0.29 | 31.05 a ± 0.95 | |
| p-value | 0.430 | 0.345 | |
| Growing season (S) | 2018 | 17.83 a ± 0.25 | 33.23 a ± 1.98 |
| 2019 | 16.59 b ± 0.27 | 30.26 b ± 1.05 | |
| p-value | <0.001 | 0.001 | |
| N × F | p-value | 0.065 | 0.060 |
| N × S | p-value | 0.322 | 0.716 |
| F × S | p-value | 0.868 | 0.145 |
| N × F × S | p-value | 0.003 | <0.001 |
| Main Factor | Source of Variation | Chlorogenic Acid | Caffeic Acid | Cynarin |
|---|---|---|---|---|
| N rate (N) kg ha−1 | 0 | 542.26 b ± 0.32 | 71.65 a ± 0.09 | 63.35 b ± 0.02 |
| 60 | 449.57 c ± 0.28 | 75.77 a ± 0.05 | 82.33 a ± 0.05 | |
| 120 | 692.80 a ± 0.45 | 67.77 b ± 0.03 | 82.10 a ± 0.06 | |
| 180 | 511.43 b ± 0.30 | 52.29 c ± 0.02 | 58.60 c ± 0.18 | |
| p-value | <0.001 | <0.001 | <0.001 | |
| N form (F) | NO3 | 572.67 a ± 0.48 | 61.91 b ± 0.02 | 65.11 b ± 0.04 |
| NH4 | 515.94 a ± 0.51 | 61.54 b ± 0.05 | 73.75 a ± 0.08 | |
| Urea | 558.43 a ± 0.45 | 77.17 a ± 0.09 | 75.93 a ± 0.12 | |
| p-value | 0.170 | 0.034 | 0.001 | |
| Growing season (S) | 2018 | 650.11 a ± 0.65 | 67.77 a ± 0.05 | 76.43 a ± 0.07 |
| 2019 | 447.92 b ± 0.24 | 65.97 a ± 0.02 | 66.76 b ± 0.09 | |
| p-value | 0.006 | 0.054 | <0.001 | |
| N × F | p-value | 0.378 | 0.115 | 0.474 |
| N × S | p-value | 0.007 | 0.698 | 0.326 |
| F × S | p-value | 0.268 | 0.898 | 0.075 |
| N × F × S | p-value | 0.567 | <0.001 | <0.001 |
| Main Factor | Source of Variation | Luteolin | Luteolin-7-O-Glucoside | Luteolin-7-O-Rutinoside |
|---|---|---|---|---|
| N rate (N) kg ha−1 | 0 | 40.76 a ± 0.09 | 13.86 a ± 0.01 | 113.43 a ± 0.35 |
| 60 | 37.23 b ± 0.02 | 12.66 b ± 0.05 | 115.15 a ± 0.35 | |
| 120 | 40.90 a ± 0.05 | 13.91 a ± 0.01 | 115.30 a ± 0.36 | |
| 180 | 35.12 b ± 0.11 | 11.94 b ± 0.05 | 115.20 a ± 0.41 | |
| p-value | <0.001 | 0.005 | 0.105 | |
| N form (F) | NO3 | 37.38 b ± 0.06 | 12.71 b ± 0.05 | 113.43 b ± 0.45 |
| NH4 | 39.15 a ± 0.02 | 13.31 a ± 0.01 | 116.15 a ± 0.34 | |
| Urea | 38.98 a ± 0.09 | 13.25 a ± 0.03 | 114.73 b ± 0.38 | |
| p-value | 0.001 | 0.013 | <0.001 | |
| Growing season (S) | 2018 | 42.15 a ± 0.07 | 14.33 a ± 0.04 | 116.52 a ± 0.15 |
| 2019 | 34.86 b ± 0.13 | 11.85 b ± 0.01 | 113.02 b ± 0.16 | |
| p-value | <0.001 | <0.001 | <0.001 | |
| N × F | p-value | 0.205 | 0.216 | 0.208 |
| N × S | p-value | 0.367 | 0.766 | 0.056 |
| F × S | p-value | 0.407 | 0.366 | 0.344 |
| N × F× S | p-value | 0.676 | <0.001 | <0.001 |
| Main Factor | Source of Variation | ABTS Assay (µmol Trolox 100 g−1 DM) | FRAP Assay (µmol Fe2+ 100 g−1 DM | DPPH Assay (µmol Trolox 100 g−1 DM) |
|---|---|---|---|---|
| N rate (N) kg ha−1 | 0 | 86.65 a ± 0.64 | 203.36 b ± 1.90 | 101.60 b ± 0.80 |
| 60 | 84.86 a ± 0.46 | 166.61 c ± 0.94 | 84.59 c ± 0.63 | |
| 120 | 84.40 a ± 0.40 | 207.09 a ± 1.47 | 106.51 a ± 0.79 | |
| 180 | 70.30 b ± 0.39 | 148.65d ± 0.83 | 74.15d ± 0.57 | |
| p-value | 0.041 | 0.023 | 0.001 | |
| N form (F) | NO3 | 82.50 a ± 0.42 | 187.33 a ± 0.94 | 96.33 a ± 0.54 |
| NH4 | 75.03 b ± 0.36 | 167.40 b ± 0.75 | 82.59 b ± 0.79 | |
| Urea | 87.13 a ± 0.65 | 189.56 a ± 0.89 | 96.21 a ± 0.49 | |
| p-value | <0.001 | 0.040 | <0.001 | |
| Growing season (S) | 2018 | 88.04 a ± 0.69 | 191.41 a ± 1.24 | 101.1 a ± 0.25 |
| 2019 | 75.05 b ± 0.45 | 171.45 b ± 0.83 | 82.31 b ± 0.19 | |
| p-value | <0.001 | 0.005 | <0.001 | |
| N × F | p-value | 0.910 | 0.343 | 0.056 |
| N × S | p-value | 0.407 | 0.517 | 0.096 |
| F × S | p-value | 0.055 | 0.645 | 0.065 |
| N × F × S | p-value | 0.003 | <0.001 | <0.001 |
| Nitrogen Rate (kg ha−1) | Parameter | TF | TP | Lut | L7G | L7R | Ch | Caf |
|---|---|---|---|---|---|---|---|---|
| 0 | TF | 1 | ||||||
| TP | 0.77 | 1 | ||||||
| Lut | 0.75 | 0.97 | 1 | |||||
| L7G | 0.70 | 0.91 | 0.58 | 1 | ||||
| L7R | 0.81 | 0.82 | 0.74 | 0.69 | 1 | |||
| Ch | 0.93 | 0.73 | ns | ns | ns | 1 | ||
| Caf | 0.86 | 0.84 | ns | ns | ns | 0.73 | 1 | |
| Cyn | 0.88 | 0.80 | ns | ns | ns | 0.89 | 0.74 | |
| 60 | TF | 1 | ||||||
| TP | 0.56 | 1 | ||||||
| Lut | 0.73 | 0.70 | 1 | |||||
| L7G | 0.69 | 0.64 | ns | 1 | ||||
| L7R | 0.79 | 0.89 | ns | 0.41 | 1 | |||
| Ch | 0.99 | 0.78 | ns | 0.58 | ns | 1 | ||
| Caf | 0.98 | 0.75 | ns | ns | ns | 0.58 | 1 | |
| Cyn | 0.86 | 0.74 | ns | ns | ns | 0.70 | 0.75 | |
| 120 | TF | 1 | ||||||
| TP | 0.87 | 1 | ||||||
| Lut | 0.80 | 0.79 | 1 | |||||
| L7G | 0.88 | 0.84 | ns | 1 | ||||
| L7R | 0.91 | 0.63 | ns | 0.67 | 1 | |||
| Ch | 0.86 | 0.84 | ns | ns | ns | 1 | ||
| Caf | 0.97 | 0.92 | ns | ns | ns | 0.67 | 1 | |
| Cyn | 0.85 | 0.91 | ns | ns | ns | 0.70 | 0.79 | |
| 180 | TF | 1 | ||||||
| TP | 0.33 | 1 | ||||||
| Lut | 0.46 | 0.45 | 1 | |||||
| L7G | 0.34 | 0.32 | ns | 1 | ||||
| L7R | 0.48 | 0.33 | ns | 0.34 | 1 | |||
| Ch | 0.48 | 0.31 | ns | ns | ns | 1 | ||
| Caf | 0.37 | 0.33 | ns | ns | ns | 0.32 | 1 | |
| Cyn | 0.42 | 0.33 | ns | ns | ns | 0.30 | 0.30 |
| Nitrogen Form | Parameter | TF | TP | Lut | L7G | L7R | Ch | Caf |
|---|---|---|---|---|---|---|---|---|
| NO3 | TF | 1 | ||||||
| TP | 0.60 | 1 | ||||||
| Lut | 0.56 | 0.98 | 1 | |||||
| L7G | ns | 0.67 | ns | 1 | ||||
| L7R | ns | 0.83 | ns | 0.34 | 1 | |||
| Ch | 0.97 | 0.51 | ns | ns | ns | 1 | ||
| Caf | 0.93 | 0.45 | ns | ns | ns | 0.67 | 1 | |
| Cyn | 0.96 | 0.59 | ns | ns | ns | ns | 0.61 | |
| NH4 | TF | 1 | ||||||
| TP | 0.41 | 1 | ||||||
| Lut | 0.47 | 0.95 | 1 | |||||
| L7G | ns | 0.88 | ns | 1 | ||||
| L7R | ns | 0.74 | ns | ns | 1 | |||
| Ch | 0.89 | 0.74 | ns | ns | ns | 1 | ||
| Caf | 0.98 | 0.66 | ns | ns | 0.44 | 0.64 | 1 | |
| Cyn | 0.93 | 0.75 | ns | 0.35 | 0.32 | 0.65 | ns | |
| Urea | TF | 1 | ||||||
| TP | 0.33 | 1 | ||||||
| Lut | 0.48 | 0.48 | 1 | |||||
| L7G | ns | 0.42 | ns | 1 | ||||
| L7R | ns | 0.36 | 0.66 | 0.73 | 1 | |||
| Ch | 0.61 | ns | ns | ns | ns | 1 | ||
| Caf | 0.78 | ns | ns | ns | ns | ns | 1 | |
| Cyn | 0.77 | 0.33 | ns | ns | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sałata, A.; Nurzyńska-Wierdak, R.; Lombardo, S.; Pandino, G.; Mauromicale, G.; Ibáñez-Asensio, S.; Moreno-Ramón, H.; Kalisz, A. Polyphenol Profile, Antioxidant Activity and Yield of Cynara cardunculus altilis in Response to Nitrogen Fertilisation. Agronomy 2024, 14, 739. https://doi.org/10.3390/agronomy14040739
Sałata A, Nurzyńska-Wierdak R, Lombardo S, Pandino G, Mauromicale G, Ibáñez-Asensio S, Moreno-Ramón H, Kalisz A. Polyphenol Profile, Antioxidant Activity and Yield of Cynara cardunculus altilis in Response to Nitrogen Fertilisation. Agronomy. 2024; 14(4):739. https://doi.org/10.3390/agronomy14040739
Chicago/Turabian StyleSałata, Andrzej, Renata Nurzyńska-Wierdak, Sara Lombardo, Gaetano Pandino, Giovanni Mauromicale, Sara Ibáñez-Asensio, Héctor Moreno-Ramón, and Andrzej Kalisz. 2024. "Polyphenol Profile, Antioxidant Activity and Yield of Cynara cardunculus altilis in Response to Nitrogen Fertilisation" Agronomy 14, no. 4: 739. https://doi.org/10.3390/agronomy14040739







