Abstract
Wheat powdery mildew is a fungal disorder caused by Blumeria graminis f. sp. tritici (Bgt) and is a severe and significant threat to the yield and quality of its host. The most practical and environmentally friendly approach to controlling this disease is through resistance gene identification to develop resistant varieties. Wild germplasm relatives of wheat are a valuable reservoir of genes contributing to resistance against powdery mildew. In our study, we identified the Aegilops tauschii germplasm “KU-2013”, exhibiting seedling resistance to Bgt isolate E09 following hexaploidization. Genetic analysis and chromosomal localization of the powdery mildew resistance gene in doubled haploid (DH) KU-2013 indicated that the disease resistance gene in DHKU-2013 is governed by a dominant gene situated in 5DS, tentatively named PmKu-2013. Following the analysis of PmKu-2013 relative to the genes at the Pm2 locus, it was inferred that PmKu-2013 represented a distinct novel gene separate from Pm2. Using molecular marker analysis, PmKu-2013 was found to be ultimately mapped between the sdau5DS5-3 and sdau5DS6-1 markers, with genetic distances of 0.6 cM and 1.3 cM, respectively. Using markers tightly linked to PmKu-2013, the genotypes of core wheat varieties from various regions were identified, laying the foundation for the transfer and utilization of PmKu-2013 in molecular-assisted selection (MAS) for breeding.
1. Introduction
Wheat (Triticum aestivum L.) is a critical global staple crop worldwide, serving as the primary source of calories for human survival [1]. However, rampant powdery mildew spread, triggered by climate change and shifts in agricultural practices, presents a significant threat to worldwide wheat production and food security [2]. Given the swift evolution of pathogens combined with the emergence of novel virulent strains, numerous specific resistance genes have been rendered ineffective [3,4]. Therefore, it is critical to persistently pursue the identification of novel genes that modulate resistance against powdery mildew.
The wild relatives of wheat contain a rich source of genes that enhance wheat’s potential to withstand biotic and abiotic stresses [5]. The powdery mildew genes, which have been introduced into wheat from wild relatives, mainly consist of Pm2b, Pm4, Pm50, Pm51, Pm53, Pm55, Pm57, Pm62, Pm66, and Pm67 [6]. Currently, the wild wheat relatives primarily include Ae. speltoides, Ae. tauschii, Ae. longissima, Ae. searsii, S. cereale L, D. villosum, Th. intermedium, Th. ponticum, T. monococcum, T. carthlicum, T. dicoccum, T. dicoccoides, T. durum, T. urartu, and T. timopheevi [7]. The transfer and utilization of resistance genes from wild resources is a suitable method of addressing wheat powdery mildew, and the vast and diverse wild germplasm resource pool offers the potential for mining resistance genes against various wheat diseases.
The officially designated powdery mildew genes are situated within 64 chromosomal loci (Pm1–Pm69), with specific equivalences such as Pm8 = Pm17, Pm18 = Pm1c, Pm22 = Pm1e, Pm23 = Pm4c, and Pm31 = Pm21 [8]. Among the reported Pm loci, seven display polyallelic phenomena, including Pm1a-Pm1e, Pm2a-Pm2b-Pm2c, Pm3a-3j, Pm4a-4d, Pm5a-5e, Pm24a-24b, and Pm60-60b [9]. Thirteen Pm genes have undergone successful cloning, including Pm3b, Pm8, Pm17 on chromosome 1AS [10,11,12], Pm1a on chromosome 7AL [13], Pm2 on chromosome 5DS [14], Pm5e on chromosome 7BL [15], and Pm12, Pm21 on the short arm of chromosome 6BS-6SS and 6SL [16,17], Pm40 on chromosome 7BS [18], and Pm41 on chromosome 3BL [19], Pm60/ML1W172/MLWE18 on chromosome 7AL [20,21,22], Pm69 on chromosome 6BL [23], Pm24 on chromosome 1DS [24], WTK4 on chromosome 6DS [25], Pm4 on chromosome 2AL [26], Pm38 on chromosome 7DS [27], and Pm46 on chromosome 4DL [28]. The cloning of these genes will assist in utilizing disease resistance genes throughout breeding and analysis of disease resistance mechanisms. Pm24, WTK4, and Pm4b encode kinases [24,25,26], while Pm38 and Pm46 encode transporter proteins [27,28]. The remaining eight cloned powdery mildew genes encode nucleotide-binding leucine-rich repeat (NLR) proteins [10,11,12,13,14,15,16,17,19,20,21,22,23].
Recently, molecular-assisted selection (MAS) has been widely employed as an important technology in crop breeding, as molecular markers can be used at the seedling stage with high accuracy and low cost. Efficient DNA extraction techniques and detection markers represent critical aspects of implementing MAS. Molecular markers have made significant contributions to the progression of crop breeding [29,30]. Despite the pronounced development of genotyping technologies and platforms [31], molecular markers are favored by breeders due to their unique advantages of high flexibility, low cost, and simple technical operation. The primary goals of this research include: (i) to screen the germplasm resources of Aegilops tauschii for resistance to powdery mildew and perform genetic analysis of resistant genes; (ii) to rapidly locate the chromosomal position of the resistant powdery mildew gene utilizing the BSA method; and (iii) to develop and validate molecular markers that can be effectively employed for MAS.
2. Materials and Methods
2.1. Plant Materials
To characterize powdery mildew resistance traits that will not be masked during hexaploidization, we performed powdery mildew resistance assessments on 68 synthetic hexaploid wheat strains derived from crosses between Aegilops tauschii and Langdon. To assess the genotypes of PmKu-2013 across common wheat germplasm, we chose 112 of the most popular wheat varieties, including 92 originating from wheat-growing regions in China and 20 from outside of China. Additionally, to locate and control the resistance genes against powdery mildew, F1 and 167 F2 individuals were established using powdery-resistant DHKU-2013 and susceptible DH2147 as parents.
2.2. Phenotypic Assessment of Reactions to Powdery Mildew
The powdery mildew identification test was performed in a controlled-environment incubator, maintaining a light cycle of 14 h at 20 °C and 10 h of darkness at 18 °C. DH2147 was planted in each tray to serve as a susceptible control. The response to Bgt inoculation was assessed, and the infection type (IT) was recorded on a scale of 0 to 4 at seven days post-inoculation (dpi). Plants with an IT of 0–2 (exhibiting no disease colonies or spots less than 1 mm in size) were classified as resistant, while those with an IT of 3–4 (exhibiting scattered disease spots greater than 1 mm) were classified as susceptible [32]. The experiment employed the Btg E09 strain of powdery mildew, which was extracted in 1993 and is known to persist in the primary wheat-producing regions of China to the present day [33].
In this experiment, 15 seedlings from each germplasm were utilized as biological duplicates in order to assess 68 synthetic hexaploid wheats and 112 common wheats for their ability to resist powdery mildew. The seedlings were inoculated with the E09 conidia after the first leaf had fully expanded. To ensure consistent inoculation, the fresh strains were dispersed over the plants from above, with the plants being rotated regularly to ensure even coverage. Following inoculation, each plant was placed in a controlled-environment chamber with regulated light and temperature settings. The development of new conidia was carefully monitored and documented for up to a week using predetermined rating standards. Two weeks after inoculation, assessment and documentation were performed. The findings from both assessments were combined in order to determine the overall resistance of the materials to powdery mildew.
After the seedlings of the F2 population reached the two-leaf and one-heart stage, they were fully inoculated with fresh conidia of E09 using the method mentioned above, and in vivo phenotypic identification was performed. The resistance of the F2 population was evaluated after 7 days. Simultaneously, the first leaf was divided into 3 segments (technical replication), with each segment measuring approximately 3–4 cm for in vitro phenotypic identification. The leaves were placed upside up in four-column plates on water agar (1%) with benzimidazole (40 mg/L) to act as a senescence inhibitor.
2.3. BSA and Molecular Markers Analysis
To extract the total genomic DNA from plant tissues, the CTAB method was utilized. Six susceptible pools were developed, selecting ten susceptible individual plants from the F2 population derived from the hybridization of DHKU-2013 and DH2147. The DNA extracted from these six susceptible pools, accompanied by DHKU-2013 and DH2147, was utilized for bulked segregation analysis (BSA).
According to the published genome database of Aegilops tauschii AL8/78 and resequenced data from “KU-2013” and “2147”, InDel molecular markers were constructed for each chromosome of the D genome. A total of 25 marker groups underwent PCR and gel electrophoresis assessments to confirm genuine polymorphism, identifying polymorphic marker distributions on both the long and short arms of each chromosome. In addition, 10 evenly distributed polymorphic marker groups were characterized on the 5DS chromosome.
The PCR reaction components were primarily supplied by Vazyme. Each PCR well contained 5 μL of 2× Taq premix, 0.5 μL of 10 μM/μL forward primer, 0.5 μL of 10 μM/μL reverse primer, 1 μL of template (50–500 ng/μL), and was supplemented with ddH2O to a final volume of 10 μL. The PCR amplification program was established as follows: pre-denaturation was carried out at 94 °C for 3 min, followed by 35 cycles of amplification at 94 °C for 20 s, 55 °C for 20 s, and 72 °C for 20 s. Finally, the PCR product was extended at 72 °C for 5 min and stored at 16 °C. The PCR products were separated using electrophoresis on 8% nondenaturing polyacrylamide gels [34].
2.4. Genetic Analysis and Genetic Mapping
Upon analysis of the phenotypic and genotypic data from the F2 individuals of DHKU-2013 and DH2147, a chi-square (χ2) test was employed to evaluate the deviation of the observed data from the expected segregation ratio.
To construct a genetic map, first we developed multiple sets of markers with polymorphisms between parents for different chromosomal locations of Chr5DS using resequencing data from “2147” and “KU-2013”, as well as published genomic data from AL8/78. Next, the F2 individuals were scanned using all markers developed above to obtain genotypic characterization on each marker. Finally, the phenotypes of powdery mildew resistance and the characterization of genotypes at all markers were entered into the JionMap 4.0 software to generate a genetic linkage map and calculate map distances using the Kosambi function [35]. A LOD score of 3.0 was applied as the linkage threshold. Visualization using MapDraw V2.1 [36].
2.5. Identification of Potential Candidate Genes
When we had localized the target gene within a specific region of the Chr5DS, we conducted preliminary analysis of the genes within this region. Firstly, we utilized the BLAST function of the WheatOmics database [37] to align the flanking marker sequences within the specified region to the “Aegilops tauschii (cv. AL8/78) chromosome”. We obtained the positions of the flanking markers as Chr5D: 45,491,856–45,492,856 and Chr5D: 451,391,561–51,392,561. Subsequently, we employed the JBrowse function of the WheatOmics database to enter the interface from “Aegilops tauschii Aet5 (AL8/78)” [38]. We inputted the chromosome position Chr5D:451,391,561–51,392,561 for gene annotation, selected the “Gene” database for download, and compiled the highly credible genes into Excel. We selectively analyzed genes that were potentially associated with resistance based on previous research on resistant gene types. Similarly, the sequences of the flanking markers in the designated region were compared with the “Chinese Spring RefSeq v2.1 chromosome”, and the Chr5D: 42,320,750–48,039,165 of the extracted annotated gene was obtained. The annotation data was downloaded using the same method as described above for the Chinese Spring gene (RefSeq v2.1) [39] and then structured into a table for the purpose of selecting resistance genes. Finally, we examined the expression patterns of the annotated genes associated with resistance within the localized intervals for the resistant parents DHKU-2013 and DH2147 following inoculation with Bgt isolate E09 at different time points.
RNA was extracted from leaf tissues of DHKU-2013 and DH2147, utilizing NucleoZol reagent (MNG, Düren, Germany). The leaf tissues were collected at 0, 6, 12, 24, 48, and 72 h post-inoculation (hpi) after inoculating with Bgt isolate E09. Subsequently, reverse transcription was performed using approximately 1 μg of RNA using an RT Kit (Takara Standard, Osaka, Japan). The q-PCR assays were performed on an LC480 real-time fluorescent quantitative PCR system using SYBR Premix Ex Taq (Vazyme, Nanjing, China). Each sample was analyzed using three technical replicates, and the expression patterns for each gene were determined as fold changes employing the comparative CT method [40], with actin operating as the normalized internal control. A significant difference analysis was performed using GraphPad Prism 9.0.
2.6. Comparison Pm2 Sequences from KU-2013 and JingY303
To examine the relationship between PmKu-2013 and Pm2, the Pm2 on chromosome 5DS was compared between “KU-2013” and JingY303 in terms of DNA sequence. To validate the differences in the DNA sequences of PmKu-2013 and Pm2, the Pm2 allele was amplified, sequenced, and matched in the susceptible “JingY303” and resistant “KU-2013”.
2.7. Evaluation of the Closely Linked Markers for MAS
We chose 112 elite varieties possessing favorable integrated agronomic traits to assess their resistance to powdery mildew. Thereafter, we examined 112 wheat varieties genotyped with two sets of molecular markers closely associated with PmKu-2013, examined the availability of the linked markers for molecular marker-assisted breeding, and examined the proportion of PmKu-2013 utilized in common varieties.
3. Results
3.1. Identification and Inheritance of Powdery Mildew Resistance in KU-2013
Fourteen of 68 Aegilops tauschii accessions exhibited powdery mildew resistance following inoculation with Bgt E09 at the seedling stage (Table S1). However, only two accessions maintained resistance after hexaploidization. DHKU-2013 exhibited high resistance to IT (1), but DH2147 was highly susceptible (Figure 1). The disease resistance phenotypes of the F1 hybrid of DHKU-2013 and DH2147, encompassing 15 plants, were identical to DHKU-2013 resistance, indicating that the powdery mildew resistance gene in DHKU-2013 was dominant (Figure 1).

Figure 1.
The phenotype of resistant parent DHKu-2013, susceptible parent DH2147, and part of F1 plants were inoculated with powdery mildew Blumeria graminis f. sp. tritici (Bgt) isolate E09. Two-week-old DHKu-2013, DH2147, and F1 plants of their cross were inoculated with Bgt E09. Representative leaves were removed and photographed at 7 days post inoculation (dpi). Bar, 1.5 mm.
The F2 population, composed of 167 individual plants resulting from the cross between DHKU-2013 and DH2147, underwent phenotypic assessment for powdery mildew resistance. Among these, 38 plants were characterized as susceptible, while 129 were resistant. The theoretical segregation ratio of the number of F2 resistant and susceptible plants was found to be 3:1 using a chi-square test, which was aligned with the segregation pattern of mendelian dominant single gene control (χ2 = 0.45, p > 0.05) (Table 1). This finding strongly implies that the resistance to Bgt E09 in DHKU-2013 is modulated by a single dominant gene, named PmKu-2013.

Table 1.
Genetic analysis of powdery mildew resistance gene PmKu-2013 to Bgt E09.
3.2. Chromosomal Localization of PmKu-2013
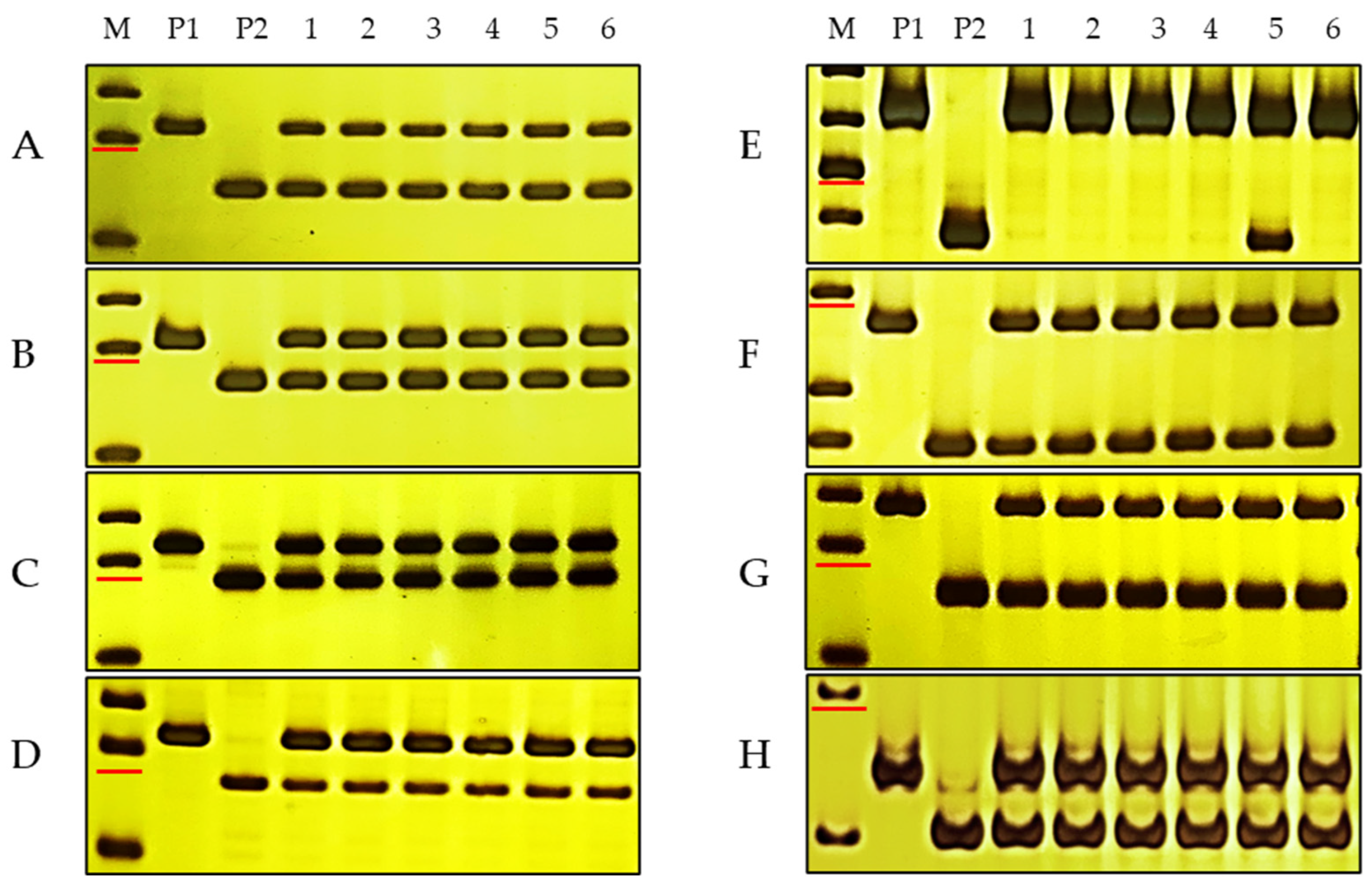
To identify the chromosomal location of PmKu-2013, two preparatory tasks were performed. Firstly, 25 pairs of polymorphic and specific markers were developed between the parental lines on each chromosome arm of the D genome (Table S2). Secondly, six susceptible mixing pools were developed, each consisting of ten susceptible individual plants. PCR analysis using polymorphic markers was performed in the two parent lines, DHKU-2013 and DH2147, as well as the six susceptible pools. The results indicated that specific polymorphic markers on the short arm of chromosome 5 exclusively amplified susceptible genotypes in five susceptible pools, while in one pool, both susceptible and resistant genotypes were found, indicating the potential occurrence of chromosomal recombination in this specific pool. Specific polymorphism primers situated on chromosomes other than 5DS amplified both resistant and susceptible genotypes in all six mixed susceptible pools, similar to 5DL (Figure 2). This evidence indicates a close linkage of the 5DS-specific marker to the powdery mildew resistance locus in DHKU-2013, suggesting that PmKu-2013 is situated on the 5DS of DHKU-2013.
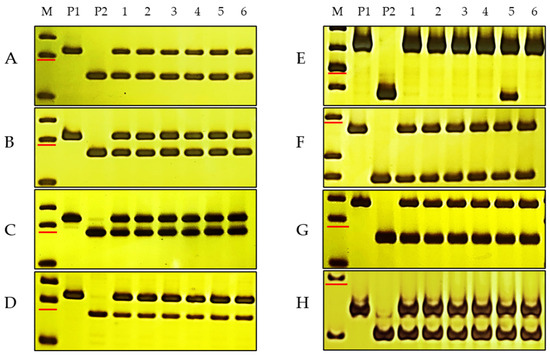
Figure 2.
PCR results of InDel markers on 7 chromosomes in two parents and mixed susceptible pools. M, DNA marker 50 bp DNA Ladder (Dye Plus); P1, DH2147; P2, DHKu-2013; 1–6, 6 mixed susceptible pools. (A–H), molecular markers P18-62-1S1, P18-62-2S3, P18-62-3S5, P18-62-4S1, P18-62-5S3, P18-62-6S4, P18-62-7S6, and P18-62-5L1 for the 7 chromosomes of the D genome. The marker sizes indicated by the red line in a h are 150 bp, 150 bp, 150 bp, 150 bp, 300 bp and 250 bp, 150 bp and 150 bp, respectively.
3.3. Comparison Pm2 between “KU-2013” and “JingY303”
To date, only one powdery mildew resistance gene, Pm2, has been cloned from chromosome 5DS, and the reported alleles at the Pm2 gene locus include Pm2a, Pm2b, Pm2c, Pm48, PmCH1357, PmLX66, and PmND399 [9,14,41,42,43]. After amplifying and sequencing the alleles of Pm2 in resistant “KU-2013” and susceptible “JingY303”, their amino acid sequences were found to be identical, both terminating at amino acid 536 of Pm2 (Figure S2; Table S2). This indicates that the gene controlling powdery mildew resistance in “KU-2013” is not attributed to the Pm2 locus. Additionally, it is speculated that this gene might be prematurely terminated compared to Pm2, causing the loss of its resistance function. Therefore, PmKu-2013 might represent a new gene distinct from Pm2.
3.4. Molecular Mapping of PmKu-2013
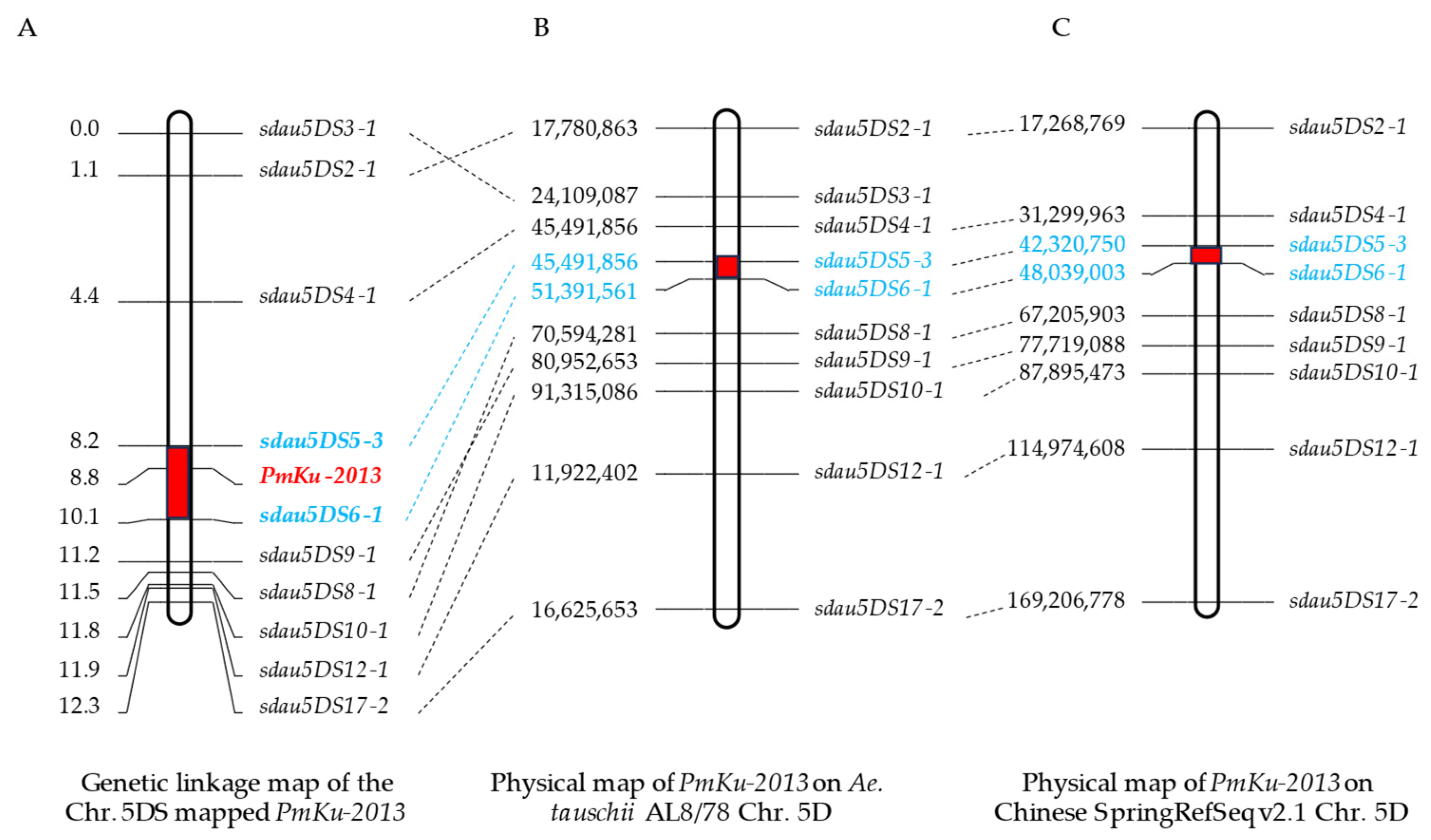
To further situate PmKu-2013, insertions/deletions (InDels) markers were further identified on chromosome 5DS based on resequencing results from “KU-2013” and “2147”. A total of 10 pairs of practical and usable primers were screened (Table 2). Genotyping characterization of 167 individuals from DHKU-2013 and DH2147 was performed using the developed polymorphic markers. The phenotypes of these recombinant plants were combined to genetically map the powdery mildew resistance gene, PmKu-2013 (Figure S1). The results of genetic linkage analysis are shown in Figure 3. Sdau5DS5-3 and sdau5DS6-1 are molecular markers flanking the PmKu-2013 powdery mildew resistance gene with genetic distances of 0.6 cM and 1.3 cM, respectively (Figure 3A). The 10 pairs of linked markers of PmKu-2013 were aligned to the reference genomes of Aegilops tauschii AL8/78 and Triticum aestvium Chinese Spring (CS), respectively. The results revealed a satisfactory collinearity between the mapping interval for PmKu-2013 on DHKU-2013 and the corresponding intervals on AL8/78 and CS. Consequently, PmKu-2013 can be positioned within the physical region of AL8/78’s 5DS from 45.49 to 51.39 Mb, spanning 5.90 Mb, and within the physical region of CS’s 5DS from 42.32 to 48.04 Mb, spanning 5.72 Mb (Figure 3B,C).

Table 2.
Information about InDel markers on 7 chromosomes in the D genome.
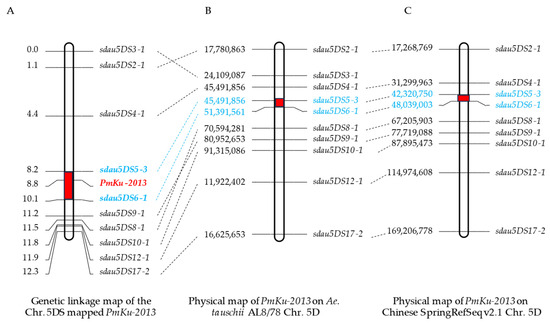
Figure 3.
Mapping of PmKu-2013. (A) Linkage map of PmKu-2013 using the F2 families of DHKu-2013 × DH2147; (B) PmKu-2013 on the Aegilops tauschii (cv. AL8/78) chromosome. The numbers on the left side of (B) represent the physical location of the right marker on the AL8/78; (C) PmKu-2013 on the Chinese Spring RefSeq v2.1. The numbers on the left side of (C) represent the physical location of the right marker on the Chinese Spring. Intervals of PmKu-2013 localization are marked in red in figure. The markers marked in blue font in figures (A–C) are the closest markers on either side of the PmKu-2013.
3.5. Identification of Potential Candidate Genes
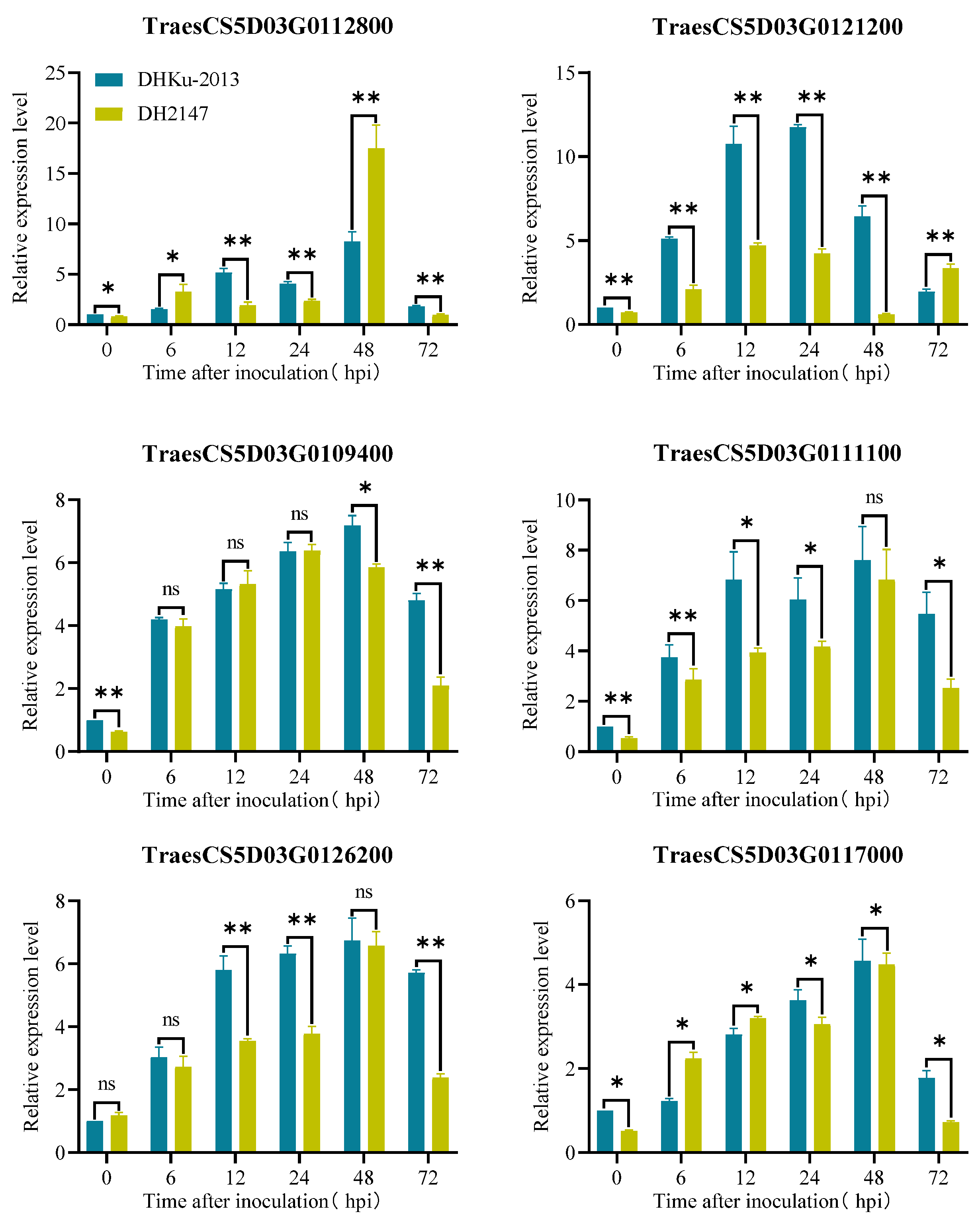
The resistance genes selected from the database of Aegilops tauschii Aet5 (AL8/78) also exist in the Chinese Spring gene (RefSeq v2.1) and will be used as the genes to focus on in the future. There are a total of 77 high-confidence genes within the genetic interval of AL8/78, and they all have corresponding homologous genes on the CS, respectively (Table S3). Scanning the annotation information for 77 high-confidence genes uncovered six high-confidence genes potentially linked to disease resistance, encompassing four genes with R-gene structures, one gene encoding a galacturonic acid-binding cell wall receptor kinase, and one gene encoding a protein related to pathogen attack and keratinization (Table 3). We examined the expression patterns of these genes in the resistant parent DHKU-2013, and the susceptible parent DH2147 following exposure to Bgt isolate E09 at various time points utilizing qRT-PCR. As shown in Figure 4, all genes exhibit an induced expression trend within the parental materials, with varying degrees of induction. For instance, TraesCS5D03G0121200 shows significantly higher induction strength in the resistant parent material relative to the susceptible material. In contrast, TraesCS5D03G0112800, despite fluctuating parental expression levels over the first 48 h, exhibits a significant increase in expression in the susceptible material after 48 h compared to the resistant parent material. Further research is required to identify the candidate gene for PmKu-2013.

Table 3.
Predicted disease resistance-related genes in Chr5D: 45,491,856–51,392,561.
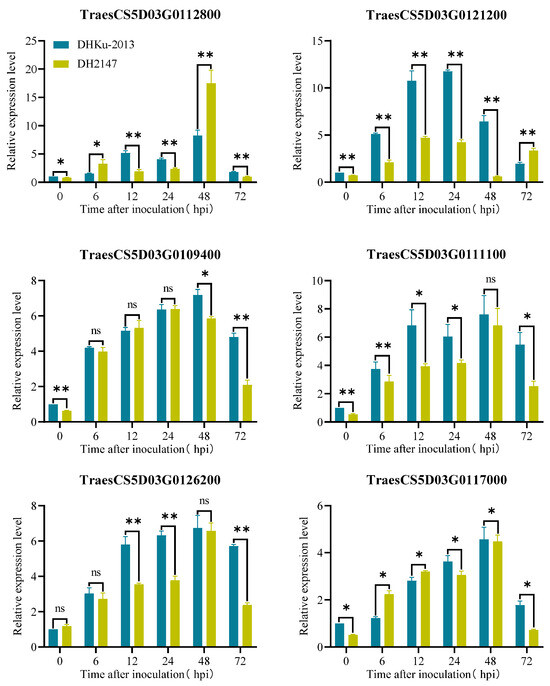
Figure 4.
Expression pattern of potential candidate genes at 0, 6, 12, 24, 48, and 72 h post-inoculation (hpi) after inoculating with Bgt isolate E09. Normalized values of target genes expression relative to Actin were given as mean ± SD from three replicates. Asterisks indicate significant differences (t-tests) between DHKu-2013 and DH2147 at each time point (* p < 0.05, ** p < 0.01, ns: not significant).
3.6. Molecular Markers Closely Linked to PmKu-2013 for MAS
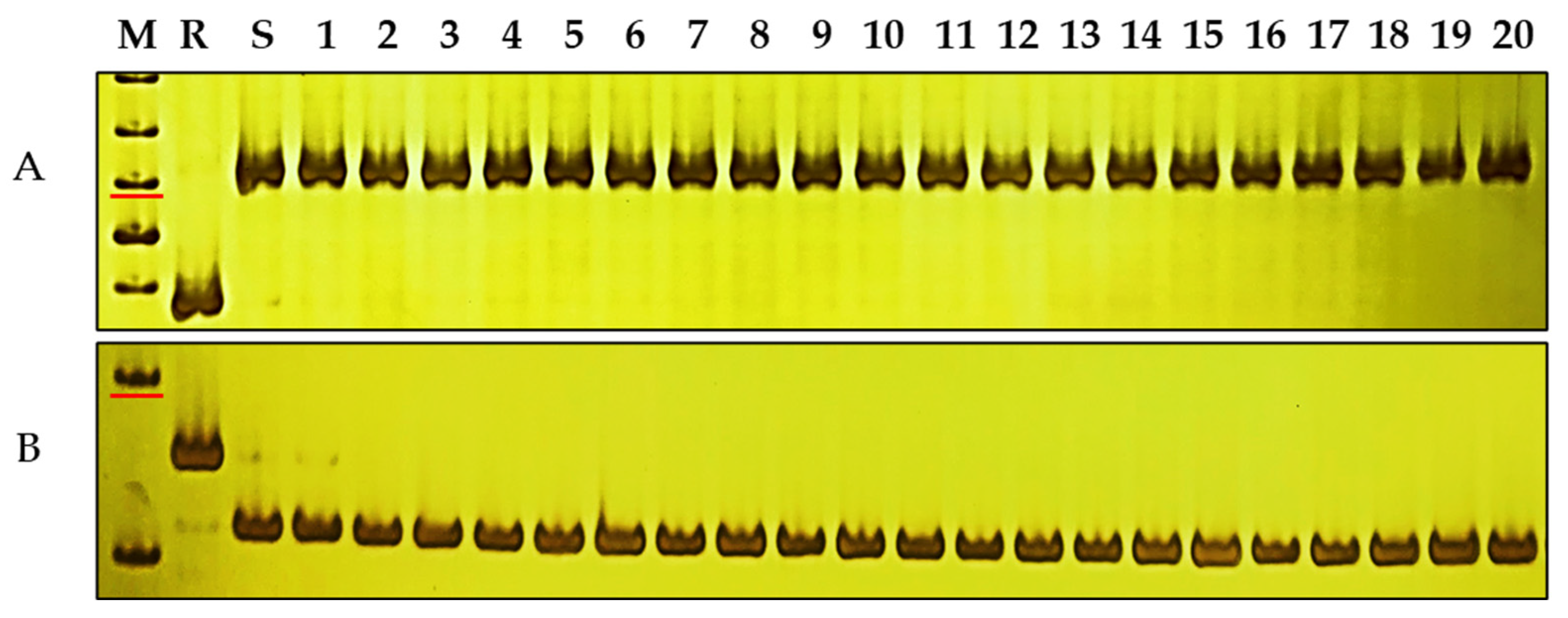
Investigation of powdery mildew resistance in 112 elite wheat varieties with favorable integrated agronomic characteristics demonstrated that 82 were susceptible to E09, while 30 of them exhibited resistance to E09 (Table S4). Two closely related molecular markers, sdau5DS5-3 and sdau5DS8-1, were employed to scan the genotypes of the wheat backbone varieties (Figure 5; Table S4). The findings showed that all germplasm were susceptible genotypes, indicating that PmKu-2013 is not widely employed in breeding and that there is high potential for further utilization, which suggests that both of these two linkage markers are effective and can be valuable in marker-assisted selection (MAS). This indicates that these markers could effectively contribute to the transfer and application of PmKu-2013 in wheat breeding.
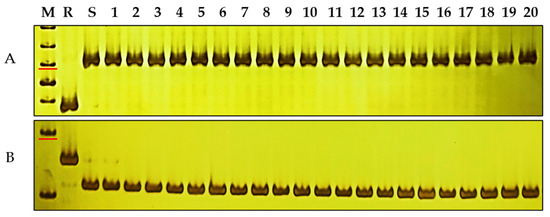
Figure 5.
Amplification patterns of Pmku-2013-linked markers in common wheat. (A) sdau5DS5-3, (B) sdau5DS8-1 in DHKu-2013, DH2147, and 20 wheat varieties susceptible to powdery mildew. M, DNA marker 50 bp DNA Ladder (Dye Plus); lanes R and S, DHKu-2013 and DH2147; and lanes 1–20, Ruihua 618, Zhengmai 168, Yangmai 158, Yannong 33, Huaimai 22, Ningmai 13, Shiluan 02-1, Yumai 108, Hengmai 17207, Fanmai 5, Hengguan 35, Wanke 1838, Lianong 180, Taimai 337, Haoyou 5760, Algarrobo, Lakin, Arduini, Bluesky, Fielder. The marker sizes indicated by the red line are 250 bp.
4. Discussion
The role of Aegilops tauschii in wheat breeding. Research indicates that a previously neglected rare strain, Aegilops tauschii, made an independent contribution during the hybridization process. Genetic diversity was extensive in the AABB genome during the hybridization of hexaploid wheat, while the gene flow in the D genome showed limited diversity [44]. In synthetic hexaploid wheat (SHW), notable improvements in genetic diversity and the acquisition of candidate resistance genes from the Aegilops tauschii germplasm were observed [25]. Furthermore, newly identified genes from Aegilops tauschii for crucial agronomic traits such as resistance to disease and insect pests and yield enhancement demonstrate undeniable potential. Simultaneously, Aegilops tauschii harbors a wealth of allelic diversity that can be harnessed in contemporary wheat breeding initiatives [45]. The discovery of these genes has enhanced the performance of common wheat and paved the way for the identification of a multitude of candidate genes for resistance to disease and insect pests [45]. Breeding programs can be accelerated by developing a genetic map of agronomically important traits to develop durable wheat with sustainable production [46]. Obviously, Aegilops tauschii is attracting growing interest as an ancestral species of wheat, and it plays an indispensable role in wheat breeding.
It is widely acknowledged that the subspecies Ae. tauschii. ssp. strangulata of Aegilops tauschii exerted a pivotal role in the evolution of wheat 8000–10,000 years ago by contributing to the wheat D genome [47]. Ae. tauschii ssp. srangulata is the ancestor of the bread wheat D subgenome [48], and there are more genes in Ae. tauschii ssp. tauschii that are not included in wheat. Therefore, there will be more opportunities to mine novel genes from Ae. tauschii ssp. tauschii. Therefore, it is recommended that priority be given to this subspecies in wheat breeding to improve efficiency.
During the utilization of Aegilops tauschii in the transfer to bread wheat, it is imperative to focus on the suppression of gene expression. In this study, when investigating powdery mildew resistance in crude Aegilops tauschii and wheat synthesized from Aegilops tauschii and Langdon, it was found that many crude Aegilops tauschii showed resistance, whereas resistance was lost after synthesizing hexaploids. The phenomenon of mutual suppression of resistance genes among chromosome groups has been recognized since as early as 1983 [49]. In 2011, it was discovered that the protein product of Pm3 inhibits Pm8 resistance [50]. Similarly, the presence of SuSr-D1 in the cultivated wheat variety “Canthatch” inhibits stem rust resistance [51]. This gene suppression phenomenon has limited the practical application of wild-type resistance genes. Consequently, a viable solution to this phenomenon involves replacing the receptor for hybridization, and there is the option to prioritize resistance genes unaffected by polyploidy for selection. The adept avoidance of this issue is achieved by the discovery of PmKu-2013, making its discovery particularly significant in the field of wheat breeding.
Over the course of three decades, SHWs have been utilized in breeding, leading to the development of hundreds of cultivars through their integration into conventional breeding programs [52]. The SHW methodology, coupled with genomics data obtained from wheat progenitor cells, offers the potential for expedited and efficient creation of new germplasm favored by breeders through marker-assisted breeding, thereby circumventing lengthy periods associated with traditional breeding methods [52]. Hence, the transplantation of PmKu-2013 into wheat can be achieved through molecular-assisted breeding technology, utilizing synthetic hexaploid wheat as an intermediary. Another option is the direct introduction of PmKu-2013 into wheat with the assistance of PmKu-2013. Nevertheless, challenges with hybridization and fruiting may arise during the direct introduction process. Furthermore, PmKu-2013 can be cloned and transferred into wheat via gene editing [53]. Regardless of the chosen approach, further research is essential to investigate the disease resistance of PmKu-2013 in wheat with diverse genetic backgrounds.
5. Conclusions
In conclusion, this found that the dominant control of powdery mildew resistance in Aegilops tauschii “KU-2013” is attributed to PmKu-2013 via BSA and molecular markers. Furthermore, the gene was accurately situated between the markers sdau5DS5-3 and sdau5DS6-1. Only Pm2 has been cloned within this localization in Chr5DS, and PmKu-2013 was found to be a new powdery mildew gene, separate from Pm2, through a comparison of the sequences of Pm2 and the Pm2 allele from “KU-2013”. The molecular markers sdau5DS5-3 and sdau5DS8-1 were effective in MAS when PmKu-2013 was transferred into the promoted wheat varieties. Moreover, genetic scanning of 30 resistant wheat varieties employing linked markers revealed that all shared the same genotype as the susceptible “2147”. This suggests that this gene is likely to have not been widely utilized. Our study holds significance in the exploration and accumulation of powdery mildew-resistant genes, and it contributes to the enhancement of powdery mildew resistance in traditional wheat varieties.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy14040744/s1. Figure S1: Display of PCR results for key markers in genetic maps; Figure S2: Comparison of amino acid sequences of Pm2 and Pm2 allele genes in “Ku-2013” and “JingY303”; Table S1: Identification of powdery mildew in Aegilops tauschii and its synthetic hexaploids; Table S2: Specific marker information for 7 chromosomes in the D genome and primer information for q-PCR; Table S3: Display of predicted results of all genes in Chr5D: 445,489,984–51,389,689 by WheatOmics 1.0; Table S4: Assessment of powdery mildew resistance in 112 wheat cultivars and the potential application of PmKu-2013-linked markers in MAS.
Author Contributions
X.M. designed and supervised this study. W.C. and J.L. arranged the data, analyzed them, performed the experiments, and wrote the manuscript. L.F., D.Q., H.Z., Y.H., M.L. and C.B. collected the plant materials. S.S., X.W., A.L., H.W. and L.K. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2021YFD1200602-5), the National Natural Science Foundation of China (31801352), and the Agricultural Variety Improvement Project of Shandong Province (2023LZGC022).
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Haile, J.K.; N’Diaye, A.; Walkowiak, S.; Nilsen, K.T.; Clarke, J.M.; Kutcher, H.R.; Steiner, B.; Buerstmayr, H.; Pozniak, C.J. Fusarium Head Blight in Durum Wheat: Recent Status, Breeding Directions, and Future Research Prospects. Phytopathology 2019, 109, 1664–1675. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lin, D.; Zhang, Y.; Deng, M.; Chen, Y.; Lv, B.; Li, B.; Lei, Y.; Wang, Y.; Zhao, L.; et al. Genome-edited powdery mildew resistance in wheat without growth penalties. Nature 2022, 602, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Singh, P.K.; Rutkoski, J.; Hodson, D.P.; He, X.; Jørgensen, L.N.; Hovmøller, M.S.; Huerta-Espino, J. Disease Impact on Wheat Yield Potential and Prospects of Genetic Control. Annu. Rev. Phytopathol. 2016, 54, 303–322. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.X.; Xu, X.F.; Ma, X.; Chen, R.Z.; Li, T.Y.; Cao, Y.Y. Virulence structure and its genetic diversity analyses of Blumeria graminis f. sp. tritici isolates in China. BMC Evol. Biol. 2019, 19, 183. [Google Scholar] [CrossRef] [PubMed]
- Cu, L.; Ren, Y.; Murray, T.D.; Yan, W.; Guo, Q.; Niu, Y.; Sun, Y.; Li, H. Development of Perennial Wheat Through Hybridization Between Wheat and Wheatgrasses: A Review. Engineering 2018, 4, 507–513. [Google Scholar] [CrossRef]
- Jia, Z.; Qiu, Y.; Lin, Z.; Wang, K.; Ye, X. Research Progress on Wheat Improvement by Using Desirable Genes from Its Relative Species. Crops 2021, 37, 1–14. [Google Scholar]
- Liu, C.; Han, R.; Wang, X.L.; Gong, W.P.; Cheng, D.G.; Cao, X.Y.; Liu, A.F.; Li, H.S.; Liu, J.J. Research Progress of Wheat Wild Hybridization, Disease Resistance Genes Transfer and Utilization. Sci. Agric. Sin. 2020, 53, 1287–1308. [Google Scholar]
- Jin, Y.; Xiao, L.; Zheng, J.; Su, F.; Yu, Z.; Mu, Y.; Zhang, W.; Li, L.; Han, G.; Ma, P. Genetic Analysis and Molecular Identification of the Powdery Mildew Resistance in 116 Elite Wheat Cultivars/Lines. Plant Dis. 2023, 107, 3801–3809. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Liu, H.; Gu, T.; Xing, L.; Han, G.; Ma, P.; Li, X.; Zhou, Y.; Fan, J.; Li, L.; et al. PM2b, a CC-NBS-LRR protein, interacts with TaWRKY76-D to regulate powdery mildew resistance in common wheat. Front. Plant Sci. 2022, 26, 973065. [Google Scholar] [CrossRef]
- Yahiaoui, N.; Srichumpa, P.; Dudler, R.; Keller, B. Genome analysis at different ploidy levels allows cloning of the powdery mildew resistance gene Pm3b from hexaploid wheat. Plant J. 2004, 37, 528–538. [Google Scholar] [CrossRef]
- Hurni, S.; Brunner, S.; Buchmann, G.; Herren, G.; Jordan, T.; Krukowski, P.; Wicker, T.; Yahiaoui, N.; Mago, R.; Keller, B. Rye Pm8 and wheat Pm3 are orthologous genes and show evolutionary conservation of resistance function against powdery mildew. Plant J. 2013, 76, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Hurni, S.; Ruinelli, M.; Brunner, S.; Sanchez-Martin, J.; Krukowski, P.; Peditto, D.; Buchmann, G.; Zbinden, H.; Keller, B. Evolutionary divergence of the rye Pm17 and Pm8 resistance genes reveals ancient diversity. Plant Mol. Biol. 2018, 98, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, T.; Müller, M.C.; Molnár, I.; Mascher, M.; Holušová, K.; Šimková, H.; Kunz, L.; Zhang, J.; Li, J.; Bhatt, D.; et al. A highly differentiated region of wheat chromosome 7AL encodes a Pm1a immune receptor that recognizes its corresponding AvrPm1a effector from Blumeria graminis. New Phytol. 2021, 229, 2812–2826. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Martín, J.; Steuernagel, B.; Ghosh, S.; Herren, G.; Hurni, S.; Adamski, N.; Vrána, J.; Kubaláková, M.; Krattinger, S.G.; Wicker, T.; et al. Rapid gene isolation in barley and wheat by mutant chromosome sequencing. Genome Biol. 2016, 17, 221. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Guo, G.; Wang, Y.; Hu, T.; Wang, L.; Li, J.; Qiu, D.; Li, Y.; Wu, Q.; Lu, P.; et al. A rare single nucleotide variant in Pm5e confers powdery mildew resistance in common wheat. New Phytol. 2020, 228, 1011–1026. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Liu, C.; Gong, S.; Chen, Z.; Chen, R.; Liu, T.; Liu, R.; Du, H.; Guo, R.; Li, G.; et al. Orthologous genes Pm12 and Pm21 from two wild relatives of wheat show evolutionary conservation but divergent powdery mildew resistance. Plant Commun. 2023, 4, 100472. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Hu, P.; Liu, J.; Witek, K.; Zhou, S.; Xu, J.; Zhou, W.; Gao, L.; Huang, Z.; Zhang, R.; et al. Pm21 from Haynaldia villosa Encodes a CC-NBS-LRR Protein Conferring Powdery Mildew Resistance in Wheat. Mol. Plant 2018, 11, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhong, S.; Chen, C.; Yang, H.; Chen, W.; Tan, F.; Zhang, M.; Chen, W.; Ren, T.; Li, Z.; et al. Identification and Cloning of a CC-NBS-NBS-LRR Gene as a Candidate of Pm40 by Integrated Analysis of Both the Available Transcriptional Data and Published Linkage Mapping. Int. J. Mol. Sci. 2021, 22, 10239. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Fang, T.; Zhang, H.; Xie, C.; Li, H.; Yang, T.; Nevo, E.; Fahima, T.; Sun, Q.; Liu, Z. Molecular identification of a new powdery mildew resistance gene Pm41 on chromosome 3BL derived from wild emmer (Triticum turgidum var. dicoccoides). Theor. Appl. Genet. 2009, 119, 531–539. [Google Scholar] [CrossRef]
- Zou, S.; Wang, H.; Li, Y.; Kong, Z.; Tang, D. The NB-LRR gene Pm60 confers powdery mildew resistance in wheat. New Phytol. 2018, 218, 298–309. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, Y.; Li, B.; Li, J.; Zhang, P.; Xie, J.; Zhang, H.; Guo, G.; Lu, P.; Li, M.; et al. Functional characterization of powdery mildew resistance gene MlIW172, a new Pm60 allele and its allelic variation in wild emmer wheat. J. Genet. Genom. 2022, 49, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhao, F.; Chen, Y.; Zhang, P.; Zhang, H.; Guo, G.; Xie, J.; Dong, L.; Lu, P.; Li, M.; et al. Bulked segregant CGT-Seq-facilitated map-based cloning of a powdery mildew resistance gene originating from wild emmer wheat (Triticum dicoccoides). Plant Biotechnol. J. 2021, 19, 1288–1290. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wei, Z.Z.; Sela, H.; Govta, L.; Klymiuk, V.; Roychowdhury, R.; Chawla, H.S.; Ens, J.; Wiebe, K.; Bocharova, V.; et al. Dissection of a rapidly evolving wheat resistance gene cluster by long-read genome sequencing accelerated the cloning of Pm69. Plant Commun. 2023, 6, 100646. [Google Scholar] [CrossRef]
- Lu, P.; Guo, L.; Wang, Z.; Li, B.; Li, J.; Li, Y.; Qiu, D.; Shi, W.; Yang, L.; Wang, N.; et al. A rare gain of function mutation in a wheat tandem kinase confers resistance to powdery mildew. Nat. Commun. 2020, 11, 680. [Google Scholar] [CrossRef] [PubMed]
- Gaurav, K.; Arora, S.; Silva, P.; Sánchez-Martín, J.; Horsnell, R.; Gao, L.; Brar, G.S.; Widrig, V.; John Raupp, W.; Singh, N.; et al. Population genomic analysis of Aegilops tauschii identifies targets for bread wheat improvement. Nat. Biotechnol. 2022, 40, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Martín, J.; Widrig, V.; Herren, G.; Wicker, T.; Zbinden, H.; Gronnier, J.; Spörri, L.; Praz, C.R.; Heuberger, M.; Kolodziej, M.C.; et al. Wheat Pm4 resistance to powdery mildew is controlled by alternative splice variants encoding chimeric proteins. Nat. Plants 2021, 7, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Krattinger, S.G.; Lagudah, E.S.; Spielmeyer, W.; Singh, R.P.; Huerta-Espino, J.; McFadden, H.; Bossolini, E.; Selter, L.L.; Keller, B. A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science 2009, 323, 1360–1363. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.W.; Herrera-Foessel, S.; Lan, C.; Schnippenkoetter, W.; Ayliffe, M.; Huerta-Espino, J.; Lillemo, M.; Viccars, L.; Milne, R.; Periyannan, S.; et al. A recently evolved hexose transporter variant confers resistance to multiple pathogens in wheat. Nat. Genet. 2015, 47, 1494–1498. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, Z.; Appels, R.; Xia, X. Functional markers in wheat: Current status and future prospects. Theor. Appl. Genet. 2012, 125, 1–10. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, B.; Zhang, J.; Zhang, J.; Li, J. Enhancement of plant variety protection and regulation using molecular marker technology. Acta Agron. Sin. 2022, 48, 1853–1870. [Google Scholar]
- Xiang, M.; Liu, S.; Wang, X.; Zhang, M.; Yan, W.; Wu, J.; Wang, Q.; Li, C.; Zheng, W.; He, Y.; et al. Development of breeder chip for gene detection and molecular-assisted selection by target sequencing in wheat. Mol. Breed. 2023, 43, 13. [Google Scholar] [CrossRef] [PubMed]
- Si, Q.M.; Zhang, X.X.; Duan, X.Y.; Sheng, B.Q.; Zhou, Y.L. On gene analysis and classification of powdery mildew (Erysiphe graminis f. sp. tritici) resistant wheat varieties. Acta Phytopathol. Sin. 1992, 22, 349–355. [Google Scholar]
- Zhou, R.; Zhu, Z.; Kong, X.; Huo, N.; Tian, Q.; Li, P.; Jin, C.; Dong, Y.; Jia, J. Development of wheat near-isogenic lines for powdery mildew resistance. Theor. Appl. Genet. 2005, 110, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.R.; Pena, S.D.; Epplen, J.T. Genetic and population study of a Y-linked tetranucleotide repeat DNA polymorphism with a simple non-isotopic technique. Hum. Genet. 1993, 90, 655–656. [Google Scholar] [CrossRef] [PubMed]
- Kosambi, D.D. The estimation of map distances from recombination values. Ann. Eugen. 1944, 12, 172–175. [Google Scholar] [CrossRef]
- Liu, R.H.; Meng, J.L. MapDraw: A microsoft excel macro for drawing genetic linkage maps based on given genetic linkage data. Yi Chuan 2003, 25, 317–321. [Google Scholar] [PubMed]
- Ma, S.; Wang, M.; Wu, J.; Guo, W.; Chen, Y.; Li, G.; Wang, Y.; Shi, W.; Xia, G.; Fu, D.; et al. WheatOmics: A platform combining multiple omics data to accelerate functional genomics studies in wheat. Mol. Plant 2021, 14, 1965–1968. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, T.; Rodriguez, J.C.; Deal, K.R.; Dubcovsky, J.; McGuire, P.E.; Lux, T.; Spannagl, M.; Mayer, K.F.X.; Baldrich, P.; et al. Aegilops tauschii genome assembly Aet v5.0 features greater sequence contiguity and improved annotation. G3 2021, 11, jkab325. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Wang, L.; Rimbert, H.; Rodriguez, J.C.; Deal, K.R.; De Oliveira, R.; Choulet, F.; Keeble-Gagnère, G.; Tibbits, J.; Rogers, J.; et al. Optical maps refine the bread wheat Triticum aestivum cv. Chinese Spring genome assembly. Plant J. 2021, 107, 303–314. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Xu, H.; Yi, Y.; Ma, P.; Qie, Y.; Fu, X.; Xu, Y.; Zhang, X.; An, D. Molecular tagging of a new broad-spectrum powdery mildew resistance allele Pm2c in Chinese wheat landrace Niaomai. Theor. Appl. Genet. 2015, 128, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jia, H.; Qiao, L.; Fu, B.; Brown-Guedira, G.; Nagarajan, R.; Yan, L. Genetic basis of resistance against powdery mildew in the wheat cultivar “Tabasco”. Mol. Breed. 2023, 43, 56. [Google Scholar] [CrossRef]
- Chen, F.; Jia, H.; Zhang, X.; Qiao, L.; Li, X.; Zheng, J.; Guo, H.; Powers, C.; Yan, L.; Chang, Z. Positional cloning of PmCH1357 reveals the origin and allelic variation of the Pm2 gene for powdery mildew resistance in wheat. Crop J. 2019, 7, 771–783. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, X.; Li, Y.; Xu, J.; Bi, A.; Kang, L.; Xu, D.; Chen, H.; Wang, Y.; Wang, Y.G.; et al. Triticum population sequencing provides insights into wheat adaptation. Nat. Genet. 2020, 52, 1412–1422. [Google Scholar] [CrossRef] [PubMed]
- Delorean, E.; Gao, L.; Lopez, J.F.C.; Open Wild Wheat Consortium; Wulff, B.B.H.; Ibba, M.I.; Poland, J. High molecular weight glutenin gene diversity in Aegilops tauschii demonstrates unique origin of superior wheat quality. Commun. Biol. 2021, 4, 1242. [Google Scholar] [CrossRef]
- Xu, S.; Lyu, Z.; Zhang, N.; Li, M.; Wei, X.; Gao, Y.; Cheng, X.; Ge, W.; Li, X.; Bao, Y.; et al. Genetic mapping of the wheat leaf rust resistance gene Lr19 and development of translocation lines to break its linkage with yellow pigment. Theor. Appl. Genet. 2023, 136, 200. [Google Scholar] [CrossRef] [PubMed]
- Pont, C.; Leroy, T.; Seidel, M.; Tondelli, A.; Duchemin, W.; Armisen, D.; Lang, D.; Bustos-Korts, D.; Goué, N.; Balfourier, F.; et al. Tracing the ancestry of modern bread wheats. Nat. Genet. 2019, 51, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Luo, M.C.; Chen, Z.; You, F.M.; Wei, Y.; Zheng, Y.; Dvorak, J. Aegilops tauschii single nucleotide polymorphisms shed light on the origins of wheat D-genome genetic diversity and pinpoint the geographic origin of hexaploid wheat. New Phytol. 2013, 198, 925–937. [Google Scholar] [CrossRef]
- Kerber, E.R. Suppression of rust resistance in amphiploids of Triticum. In Proceedings of the 6th International Wheat Genetics Symposium, Kyoto, Japan, 28 November–3 December 1983; Sakamoto, S., Ed.; Plant Germ-Plasm Institute, Faculty of Agriculture, Kyoto University: Kyoto, Japan, 1983; pp. 813–817. [Google Scholar]
- McIntosh, R.A.; Zhang, P.; Cowger, C.; Parks, R.; Lagudah, E.S.; Hoxha, S. Rye-derived powdery mildew resistance gene Pm8 in wheat is suppressed by the Pm3 locus. Theor. Appl. Genet. 2011, 123, 359–367. [Google Scholar] [CrossRef]
- Hiebert, C.W.; Moscou, M.J.; Hewitt, T.; Steuernagel, B.; Hernández-Pinzón, I.; Green, P.; Pujol, V.; Zhang, P.; Rouse, M.N.; Jin, Y.; et al. Stem rust resistance in wheat is suppressed by a subunit of the mediator complex. Nat. Commun. 2020, 11, 1123. [Google Scholar] [CrossRef]
- Awan, M.J.A.; Rasheed, A.; Saeed, N.A.; Mansoor, S. Aegilops tauschii presents a genetic roadmap for hexaploid wheat improvement. Trends Genet. 2022, 38, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Hu, J.; Sun, Y.; Li, B.; Zhang, D.; Li, W.; Liu, J.; Li, D.; Gao, C.; Zhang, Y.; et al. Highly efficient heritable genome editing in wheat using an RNA virus and bypassing tissue culture. Mol. Plant 2021, 14, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).