Abstract
Reactive oxygen species (ROS) and salicylic acid (SA) are essential signaling molecules in plant cells that participate in responses to biotic and abiotic stresses. Changes in ROS and SA signals during interactions between pear and the pear scab pathogen Venturia nashicola remain unclear. Herein, we analyzed the roles of ROS in the signal transduction pathway of pear scab resistance using the highly resistant Huangguan and susceptible Xuehua cultivars of pear (Pyrus bretschneideri Rehd). Protoplasts, calluses, and leaves were obtained from 14-year-old pear trees and treated with V. nashicola for different periods. The results showed that ROS rapidly accumulated in protoplasts of both cultivars within a 120-min treatment period, but the fluorescence intensity of ROS differed between cultivars. The H2O2 content in fruit-derived calluses of Huangguan peaked at 48 h post-infection at levels 1.85 times higher than those in Xuehua. Induction of H2O2 by V. nashicola in Huangguan was more intense than in Xuehua over a 96-h treatment period. At 96 h post-infection, the malondialdehyde content in leaves of Huangguan was significantly lower than in Xuehua, while the activities of superoxide dismutase, peroxidase, and catalase, and the relative expression levels of PbMnSOD, PbPOD, and PbCAT genes were higher in Huangguan than Xuehua. V. nashicola infection also caused a continuous increase in the leaf SA content of Huangguan, which was 6.76 times higher than in Xuehua at 96 h post-infection, and V. nashicola exposure upregulated the expression of PbPAL, PbICS, PbPR1, and PbPR5. In summary, both ROS and SA participated in the responses of pear trees to V. nashicola infection and played vital roles in the signal transduction pathway of pear scab resistance.
1. Introduction
Reactive oxygen species (ROS) are the products of some essential physiological activities in plants. ROS with strong oxidizing ability are primarily produced by organelles such as mitochondria and chloroplasts [1,2]. When plants respond to pathogenic invasion, ROS bursts are an early marker in affected cells, and this process can take place within a few minutes of infection. There are two main types of ROS in plants: superoxide anions (O2–) and hydrogen peroxide (H2O2) [3]. O2– can convert into H2O2 spontaneously, and this reaction is accelerated by superoxide dismutase (SOD). H2O2 subsequently acts as a plant signal to participate in the induction of plant resistance responses, and excessive intracellular H2O2 is eventually scavenged by catalase (CAT).
When plants interact incompatibly with pathogens, intracellular ROS levels increase rapidly in the plant, and the H2O2 level regulates the plant hypersensitive response [4]. Under biotic stress, both the scavenging of O2– and enhancement of antioxidant enzyme activity repress the hypersensitive response in the host plant. If the plant antioxidant enzyme system is disrupted, a substantial increase in intracellular ROS occurs, eventually causing cell death [5]. Massive accumulation of intracellular ROS can trigger antioxidant mechanisms in plants. For example, macromolecular antioxidant enzymes, including SOD, peroxidase (POD), and CAT scavenge excessive intracellular ROS, a process which in turn mitigates cell membrane lipid peroxidation damage and cytotoxicity and maintains normal redox homeostasis [6].
During the responses of plants to biotic and abiotic stresses, ROS often act as intracellular signaling molecules and participate in plant immune responses [7]. ROS perform various functions: (i) ROS (e.g., H2O2) exhibit a direct antimicrobial effect; (ii) ROS take part in the regulation of lignin biosynthesis, which in turn enhances the strength of cell walls and hinders the spread of pathogens; (iii) ROS induce systemic acquired resistance in plants, promoting the biosynthesis and accumulation of salicylic acid (SA); (iv) ROS play a role in regulating key transcription factors in signal transduction of disease resistance and activate certain protective enzymes. In addition, there may exist feedback regulation mechanisms between H2O2 and SA. While H2O2 induces the accumulation of SA and results in systemic acquired resistance, SA also increases the level of intracellular H2O2 by inhibiting CAT activity [8,9].
Salicylic acid is a vital signaling molecule involved in plant disease resistance and immune responses. The mechanisms underpinning SA biosynthesis mainly include the phenylalanine ammonia lyase (PAL) pathway and the isochorismate synthase (ICS) pathway. When the enzymatic activity of PAL or ICS is inhibited, the susceptibility of plants to pathogens is enhanced and disease resistance is weakened [10,11]. Moreover, expression of specific genes (e.g., nahG) causes a loss of the biological functions of SA, inhibits the accumulation of SA, and distinctly reduces disease resistance in plants [12]. Under normal growth conditions, SA is mostly stored in a protein-bound form, and only free SA exhibits biological activity [13,14]. In plants, SA-binding proteins (with similar functions to CAT) bind endogenous SA and generate specific signals that trigger the hypersensitive response of plants. Additionally, the normal redox state of cells is altered and NPR1 transcription factors are activated, thereby inducing expression of pathogenicity-related (PR) genes [15].
The pear scab pathogen Venturia nashicola Tanaka et Yamamoto is an obligate fungal parasite that only harms pear trees. Pear scab is among the most serious diseases threatening pear production, and it occurs in the shoots, leaves, and fruit of pear trees [16]. After infection, pear fruits develop dark spots and become deformed, with a bitter taste and a loss of edible value. Because most commercial pear cultivars display a certain level of susceptibility, pear scab causes huge economic losses to fruit growers in years when V. nashicola infection is prevalent. Previous studies have mainly explored the control effects of exogenous hormones or chemical reagents on pear scab or their protective effects on infected plants [17,18]. However, the mechanisms underpinning changes in the intracellular signaling molecules in pear in response to V. nashicola infection have not been reported. It remains unclear whether ROS and SA act as signaling molecules in the response of pear to V. nashicola.
2. Materials and Methods
2.1. Plant Materials and Fungal Pathogen
A highly resistant cultivar (Huangguan) and a susceptible cultivar (Xuehua) of pear (Pyrus bretschneideri Rehd) were used in the study. Xuehua belongs to the traditional Chinese white pear system, while Huangguan is a cross between Xuehua (female parent) and Xinshiji (male parent) [19,20]. Fourteen-year-old pear trees (grafting completed in 2003) of both cultivars were provided by the Fruit Tree Research Institute of Shanxi Agricultural University (Taigu, Shanxi Province, China).
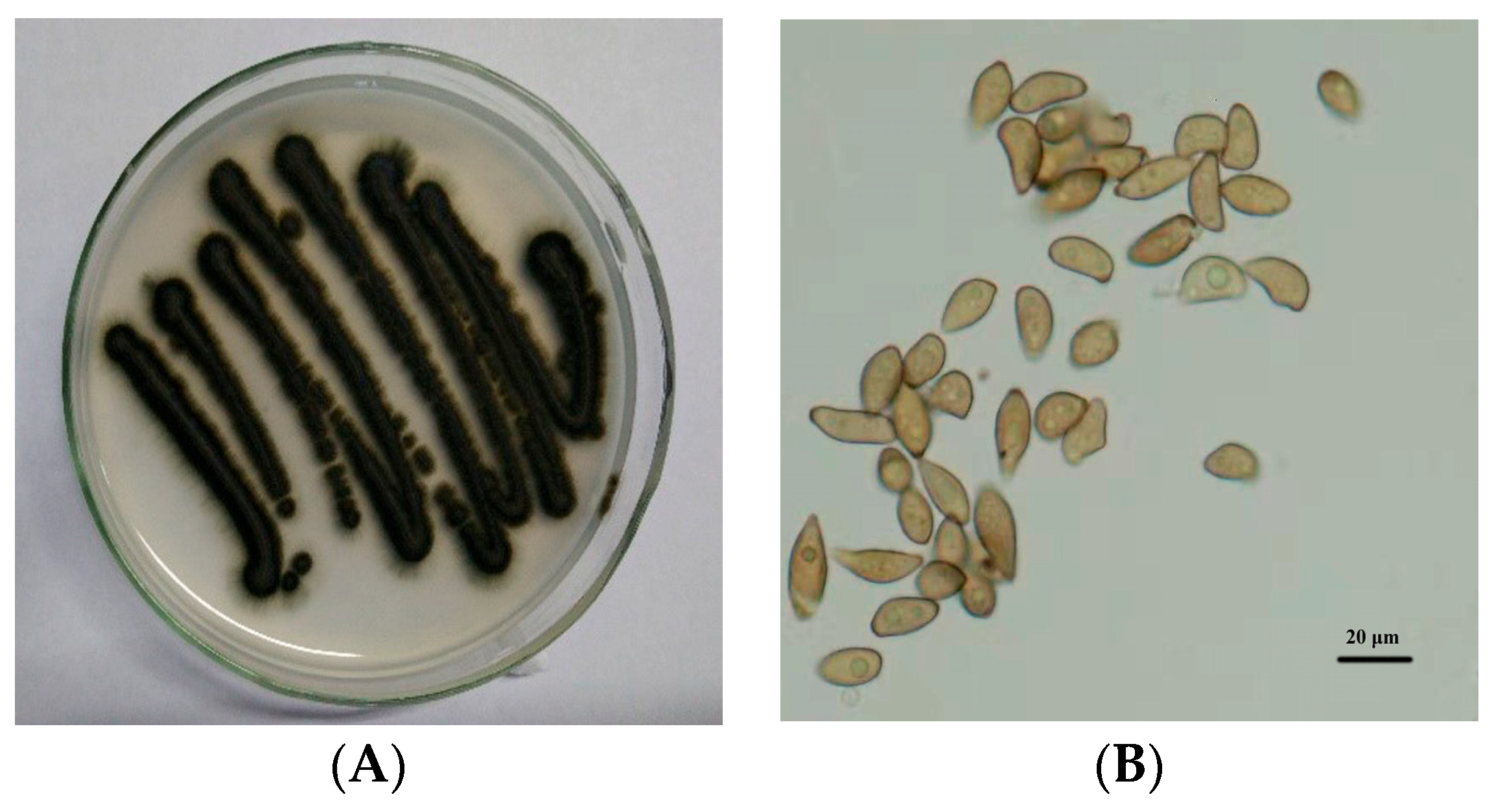
The test strain of V. nashicola was obtained from our laboratory. Leaves with pear scab lesions were collected from a pear orchard in Nan’an Town, Wenshui County, Shanxi Province, China. V. nashicola was isolated and purified from typical diseased leaves, and then inoculated on oat agar plates (Figure 1). The pure culture was preserved at the College of Horticulture, Shanxi Agricultural University.
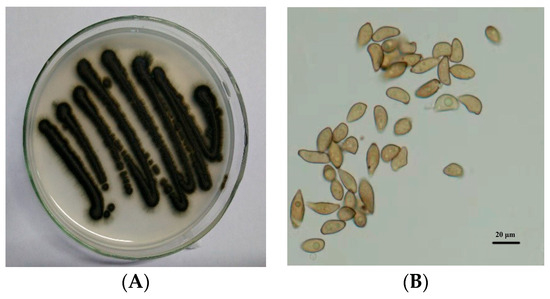
Figure 1.
Colony growth of (A) Venturia nashicola and microscopic morphology of (B) conidia on oat agar medium (25 °C, 10 days).
2.2. Pear Fruit Callus Induction
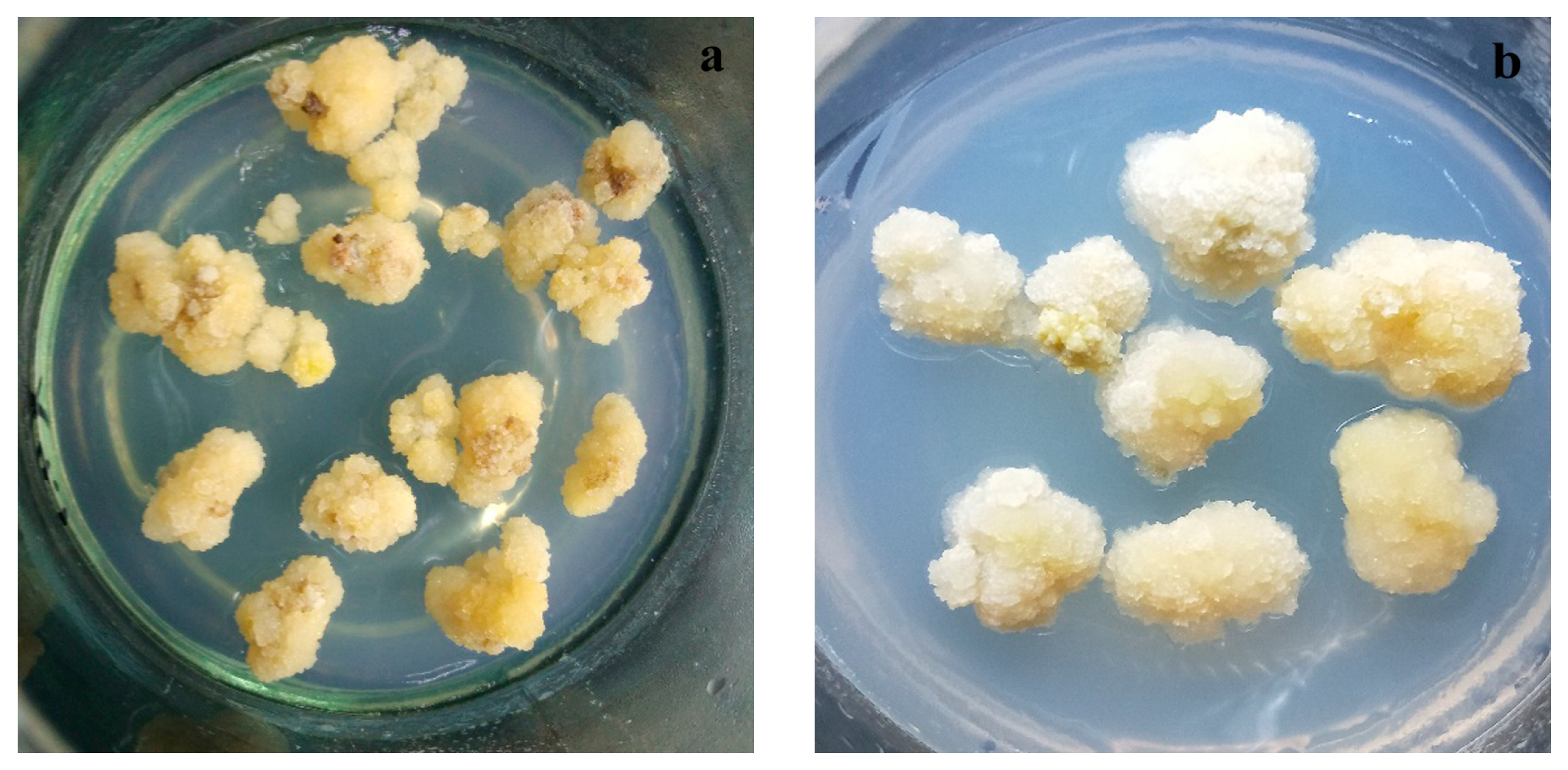
Callus induction of pear fruit was conducted using the method described by Zhao et al. [21]. Mature fruit samples of Huangguan and Xuehua with no evidence of V. nashicola infection were washed with detergent, followed by disinfection with 70% ethanol for 30 s and 0.1% HgCl2 for 20 min. Disinfected fruit samples were then washed in sterile water at least three times. After the fruit was carefully peeled, the pulp was cut into ~1 cm2 slices with a thickness of 1 mm. The slices were placed in Murashige and Skoog medium (6% agar and 3% sucrose, w/v) containing specific hormones for callus induction and subculture. Primary cultures were incubated with a combination of 2,4-dichlorophenoxyacetate (2.0 mg/L) and 6-benzylaminopurine (0.5 mg/L) in darkness at an ambient temperature of ~25 °C and pH 5.8 for ~30 days. Subcultures were incubated with a combination of 2,4-dichlorophenoxyacetate (1.5 mg/L) and 6-benzylaminopurine (1.0 mg/L) under the same conditions for more than 25 days (Figure 2).
Figure 2.
Growth of calluses derived from (a) Huangguan and (b) Xuehua pears.
2.3. Protoplast Isolation and Purification
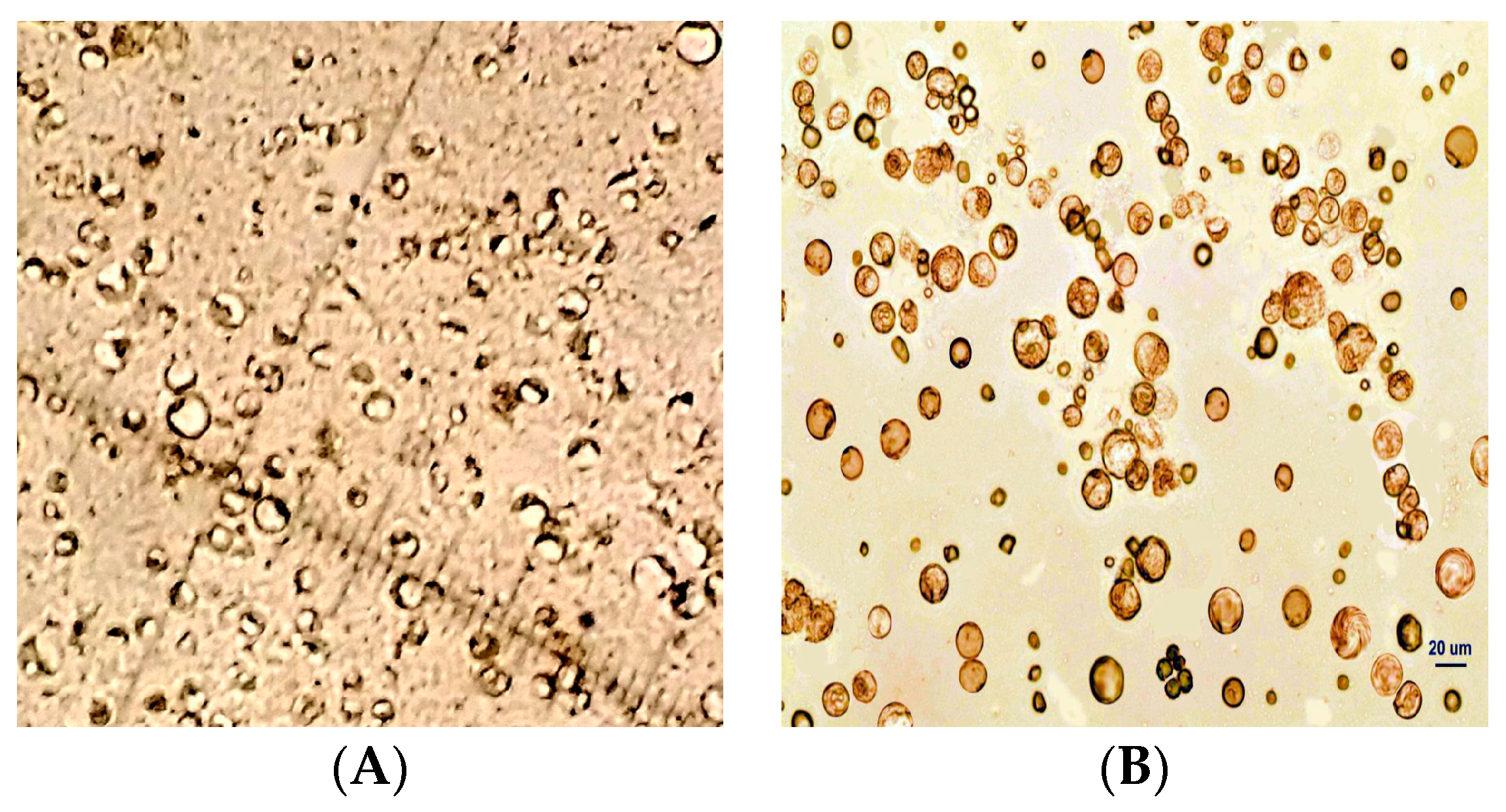
Isolation and purification of protoplasts from fruit-derived calluses followed the method established in our laboratory [22]. Briefly, fruit-derived calluses were cut into pieces using a sterile blade and placed in cell protoplast wash (CPW) medium (0.01% KH2PO4·H2O + 0.13% CaCl2·2H2O) containing 2% cellulose R-10, 0.1% macerozyme R-10, 0.1% pectolase Y-23, 0.4% driselase, and 0.7 mol/L mannitol. Enzymatic hydrolysis was performed in a thermostat rotary shaker at 80 rpm and 28 °C for 10 h. After hydrolysis, the reaction solution was filtered through a 400-mesh nylon mesh. The filtrate was collected and centrifuged at 123× g for 15 min. Protoplasts at the bottom of the tube were cleaned with CPW buffer three times and temporarily stored in CPW buffer containing 0.7 mol/L mannitol. The obtained protoplast mixture was slowly floated on a 1.5 mol/L sucrose solution and centrifuged at 100× g for 3 min. The middle layer of relatively pure protoplasts was carefully aspirated using a pipette for further analysis (Figure 3).
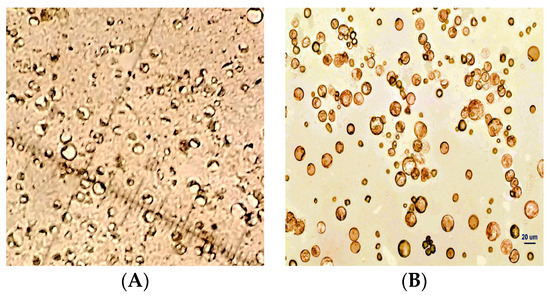
Figure 3.
Protoplasts isolated from pear fruit-derived calluses (A) before and (B) after purification.
2.4. Detection and Quantification of ROS
Purified protoplasts were loaded with the fluorescent probe 2′,7′-dichlorofluorescin diacetate (DCFH-DA, 5 μmol/L; CA1410, Solarbio, Beijing, China) according to the manufacturer’s instructions. Protoplasts were then treated with a conidial suspension of V. nashicola (105–106 conidia/mL) and immediately placed under an inverted fluorescence microscope (IX81; Olympus, Tokyo, Japan) to measure the fluorescence intensity of ROS at 20× magnification. The excitation and emission wavelengths were 488 and 525 nm, respectively. The relative fluorescence intensity of intracellular ROS was measured with five replicates using Image-Pro Plus v6.0 (Media Cybernetics, Rockville, MD, USA).
A conidial suspension of V. nashicola (105 conidia/mL) was prepared in 0.1% sucrose solution and evenly spread on the surface of fruit-derived calluses. Exactly 0.1 g of callus sample that had been infected with V. nashicola (treatments) or not (controls) was weighed into a clean mortar and mixed with 1 mL of 100% acetone. Callus samples were ground thoroughly on ice, and the obtained homogenate was transferred to a 2-mL centrifuge tube. The volume of the homogenate was made up to 1 mL with 100% acetone. The homogenate was centrifuged at 5447× g for 10 min at 4 °C. The supernatant was carefully aspirated using a pipette and kept on ice until analysis. Since H2O2 and Ti(SO4)2 form a yellow titanium peroxide complex with characteristic absorption at 415 nm, a microplate reader (BioTek Synergy, Winooski, VT, USA) was used to measure the absorbance of the reaction solution at 415 nm following the manufacturer’s instructions (BC3595; Solarbio). All measurements were repeated three times.
2.5. Antioxidant Activity Assays
Pear leaves (non-detached) were infected with a conidial suspension of V. nashicola (105 conidia/mL) on both sides, and uninfected leaves served as controls. After 96 h, a 0.5 g sample of infected leaves was weighed into a clean mortar and 2 mL of 10% trichloroacetic acid (TCA) was added. The leaf sample was ground into a homogenate and then transferred into a 10-mL centrifuge tube. The final volume was adjusted to 10 mL using 10% TCA. The homogenate was centrifuged at 1362× g for 10 min at 25 °C. Subsequently, 1 mL of the supernatant was pipetted into a centrifuge tube and mixed with an equal volume of 0.6% thiobarbituric acid prepared with 10% TCA. The mixture was incubated in a boiling water bath for 15 min and then quickly cooled on ice. The malondialdehyde (MDA) content in fruit-derived calluses of both pear cultivars was determined using an ultraviolet-visible spectrophotometer (Shimadzu, Kyoto, Japan). Determination and calculation were performed using five replicates according to the method of Chen et al. [23].
Antioxidant enzyme activity assays were conducted according to the method of Wang et al. [24]. Briefly, 0.5 g of pear leaves that had been infected with V. nashicola (treatments) or not (controls) was weighed into a pre-cooled mortar. The sample was fully ground with 5 mL of phosphate-buffered saline (pH 7.8), and the resulting homogenate was transferred into a 10-mL centrifuge tube. After refrigerated centrifugation (>15 min, 8510× g), the supernatant was collected and kept on ice or in a refrigerator at 4 °C before use. SOD, POD, and CAT activities in supernatants were measured with three replicates each using an ultraviolet-visible spectrophotometer (Shimadzu) at wavelengths of 560, 470, and 240 nm, respectively.
2.6. SA Analysis
Extraction of SA from pear leaves was conducted at 96 h post-infection following the method of Gao and Zhang [25]. The concentration of SA in sample extracts was analyzed using high-performance liquid chromatography (U3000; Thermo Fisher, Waltham, MA, USA) equipped with an Agilent TC-C18 column (250 × 4.6 mm, 5 μm; Santa Clara, CA, USA). The chromatographic conditions were as follows: column temperature, 30 °C; detection wavelength, 280 nm; flow rate of the autosampler, 1 mL/min; injection volume, 20 μL. The mobile phase consisted of methanol and 65% acetonitrile (50:50; v/v). The 65% acetonitrile was prepared using chromatography-grade acetonitrile and purified water.
2.7. Gene Expression Analysis
Total RNA was isolated from callus and leaf samples using CTAB-LiCl according to the method of Jaakola et al. [26].The RNA extracts were treated with DNase I using the following: 5 µg of RNA, 1.2 µL of 10× reaction buffer with MgCl2, 2 µL of DNase I, and 0.5 µL of Ribolock RNAase inhibitor. The reaction system was made up to a final volume of 12 µL with DEPC-treated water and incubated in a water bath at 37 °C for 30 min, followed by 60 °C for 10 min to inactivate DNase I. DNase-treated RNA was reverse-transcribed into complementary DNA using a PrimeScript RT kit (TaKaRa, Otsu, Shiga, Japan). Specific gene primers used for reverse transcription PCR (RT-PCR) were designed using Primer Premier v5.0 (Premier Biosoft International, San Francisco, CA, USA) based on the mRNA sequences of pear-related genes retrieved from the NCBI database https://www.ncbi.nlm.nih.gov/ (accessed on 5 February 2019). Intron-spanning primers were synthesized by BGI (Beijing, China), and primer sequences are listed in Table 1.

Table 1.
Primers used for reverse transcription PCR of target genes in pear.
PCR samples contained 1 μL cDNA, 10 μL qPCR Mix (TaKaRa), and 0.25 μL each of forward and reverse primers, with nuclease-free water to a final volume of 20 µL. The reaction conditions were as follows: 94 °C for 5 min; 35 cycles at 94 °C for 30 s, 55 °C for 30 s, 72 °C for 30 s; 72 °C for 5 min; 4 °C until removal. PCR products were semi-quantitatively analyzed by 1% agarose gel electrophoresis, and the results were examined using Quantity One v4.6.2 software (Bio-Rad, Hercules, CA, USA) to obtain the grayscale values of DNA bands. The ratio between the grayscale values of each target gene and the internal reference gene (Actin) was calculated as the relative expression level of the target gene.
2.8. Statistical Analysis
Data are expressed as mean ± standard deviation. The significance of differences between mean values (p < 0.05) was analyzed by t-test using SAS v9.0 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. ROS Levels in Pear Protoplasts
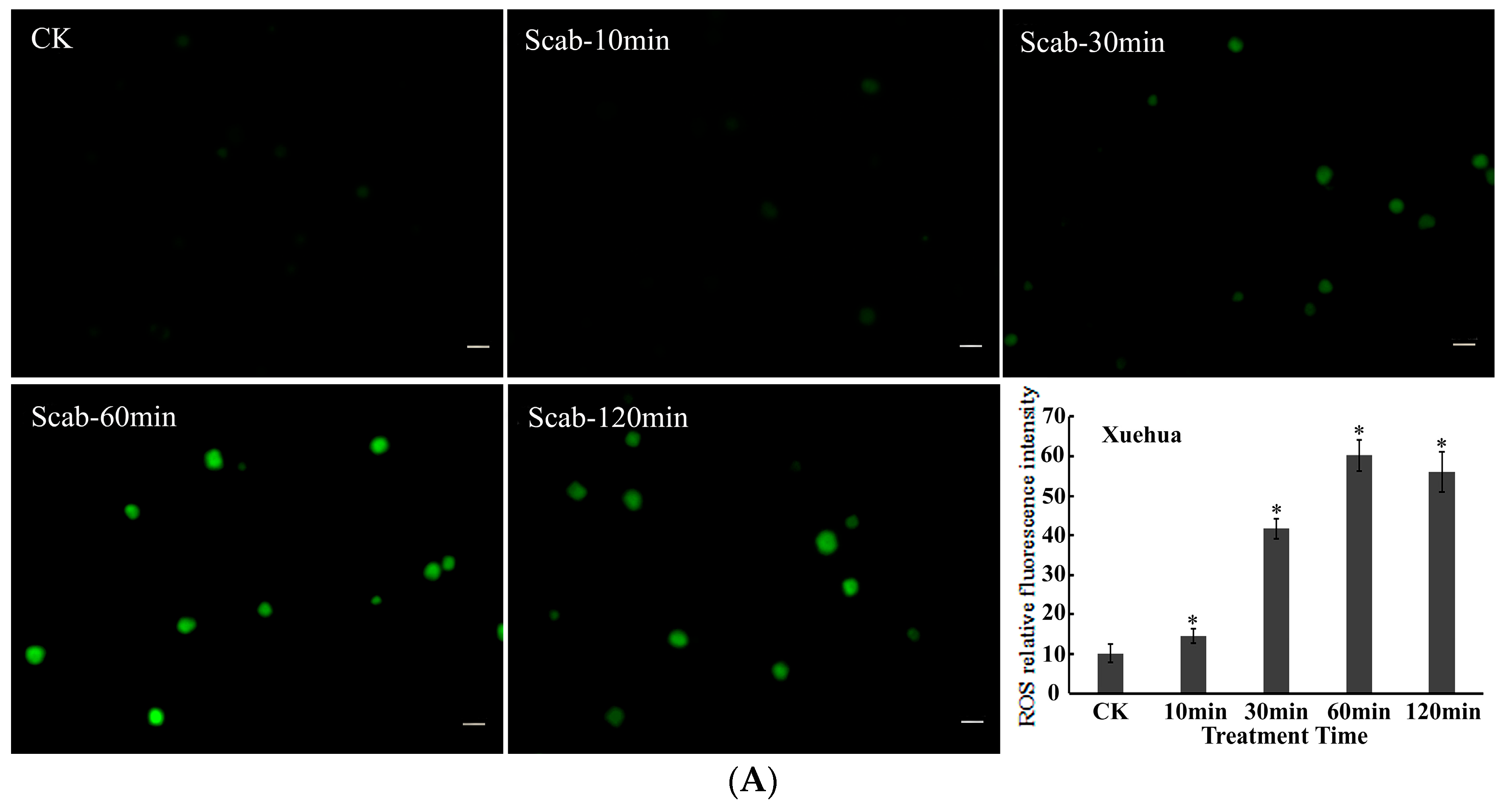
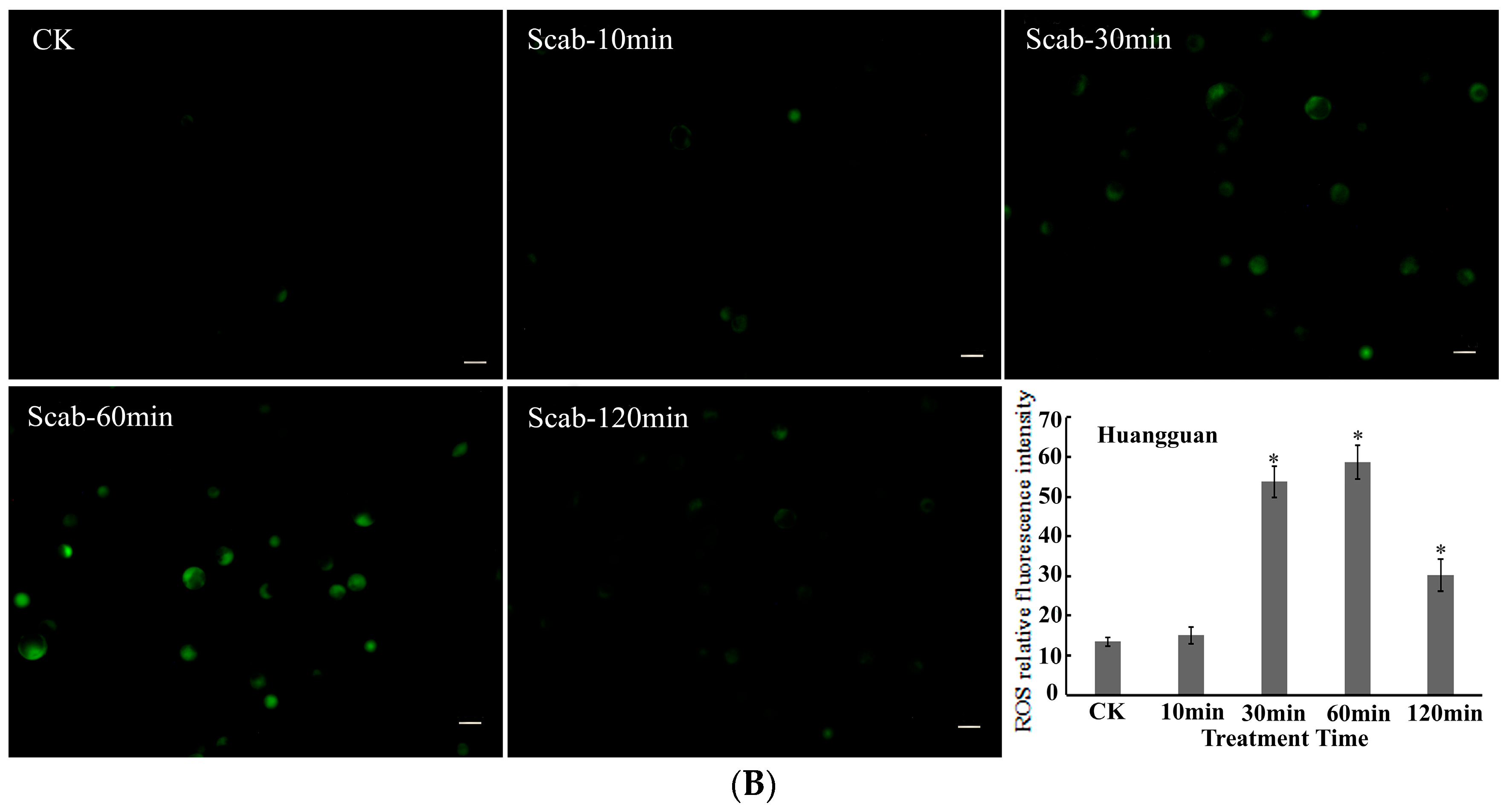
Dynamic changes in ROS levels in pear protoplasts induced by V. nashicola were analyzed using the DCFH-DA fluorescent probe. It was found that the fluorescence intensity of intracellular ROS gradually increased in the susceptible pear cultivar Xuehua over the 120 min treatment with V. nashicola. Induction of ROS production was observed within 10 min, and the highest fluorescence intensity occurred at 60 min, which was ~5.91 times higher than that of the control. The fluorescence intensity was then maintained at a relatively high level from 60 to 120 min (Figure 4A).
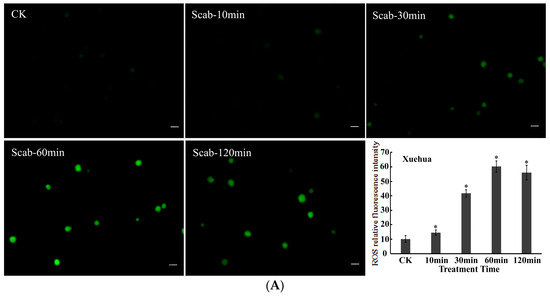
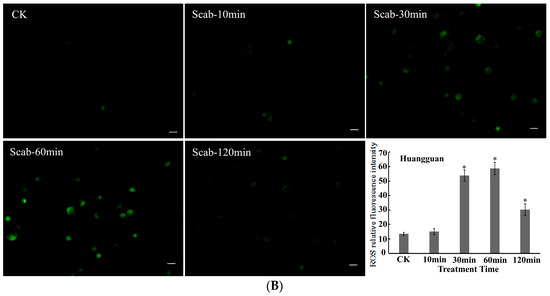
Figure 4.
V. nashicola-induced changes in the fluorescence intensity of reactive oxygen species (ROS) in (A) Xuehua and (B) Huangguan pear protoplasts. Fluorescence microphotographs were taken at 20× magnification and show dynamic changes in the fluorescence intensity of ROS at 0 min (Ctrl), 10 min (Scab-10 min), 30 min (Scab-30 min), 60 min (Scab-60 min), and 120 min (Scab-120 min) following V. nashicola infection (bars = 20 μm). Histograms show the relative fluorescence intensity of intracellular ROS at different timepoints analyzed using Image-Pro Plus v6.0 software.Asterisks denote significant differences from control (* p < 0.05).
Compared with Xuehua, there were differences in the changes in intracellular ROS levels for the resistant pear cultivar Huangguan after treatment with V. nashicola. Specifically, intracellular ROS seemed to be in an unstimulated state and remained at the initial low level within the first 10 min. The fluorescence intensity of ROS increased substantially from 10 to 30 min, then reached the maximum level at 60 min (p < 0.05). However, this state did not last long, and the ROS fluorescence intensity of Huangguan decreased markedly at 120 min, which was significantly lower than that of Xuehua at the same timepoint (p < 0.05; Figure 4B).
3.2. H2O2 Content in Pear Calluses
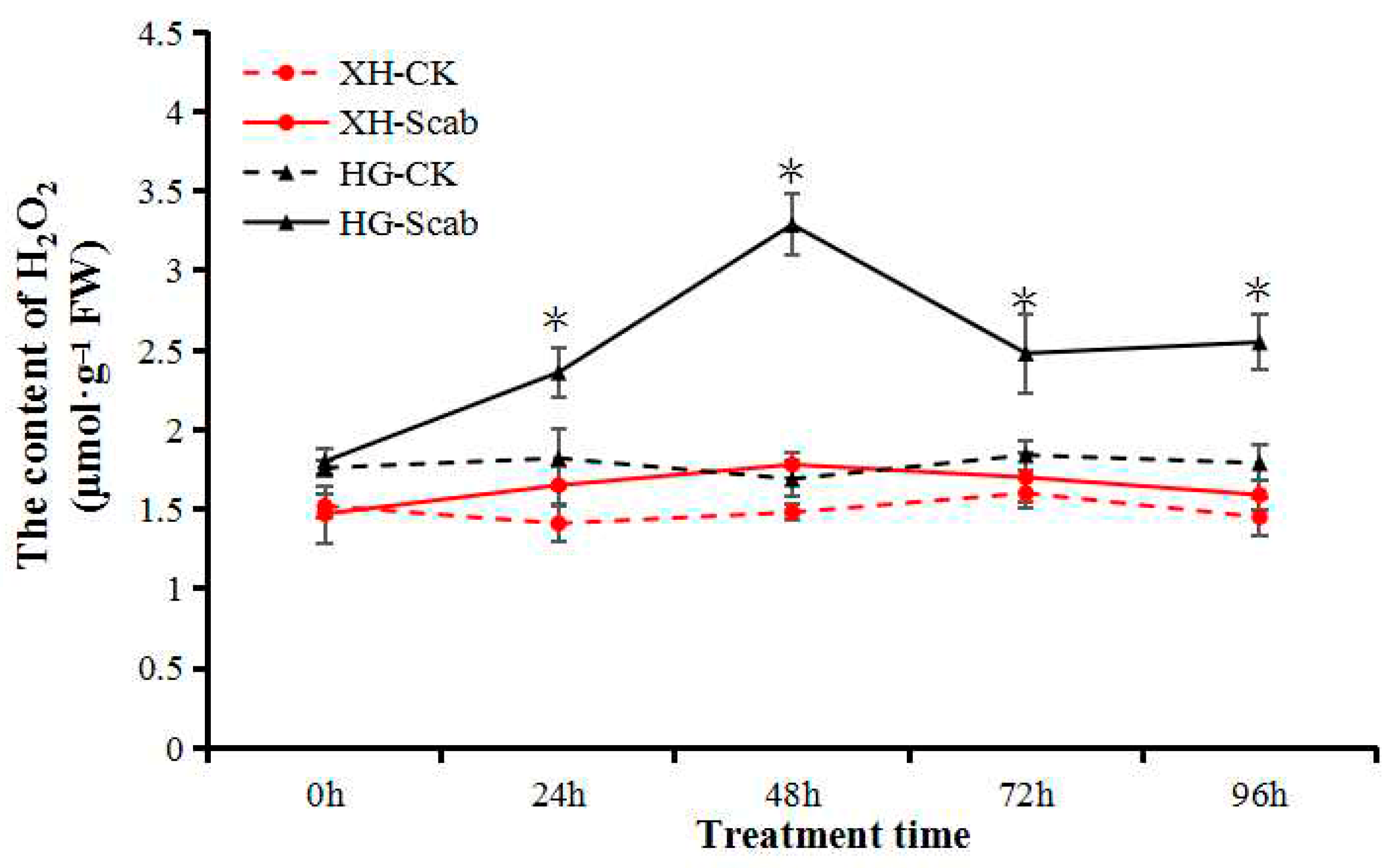
The H2O2 content in fruit-derived calluses of both pear cultivars was measured following infection by V. nashicola. There were no significant differences in the H2O2 content of uninfected controls between cultivars during a 96-h treatment period, and the initial H2O2 content of Huangguan was slightly higher than that of Xuehua. The H2O2 content of Xuehua was essentially unaffected by the presence of V. nashicola, and only a slight but not significant increase occurred at 48 h (Figure 5).
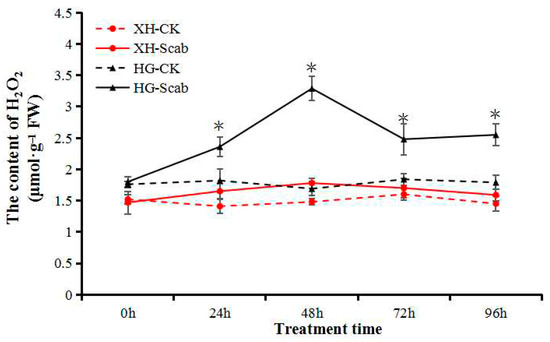
Figure 5.
V. nashicola-induced changes in H2O2 content in Xuehua and Huangguan fruit calluses. XH, Xuehua; HG, Huangguan; CK, uninfected control; Scab, infected by V. nashicola. * p < 0.05.
In Huangguan, the H2O2 content was significantly higher than that of the control at 24 h, and the highest level was reached at 48 h post-infection, which was 1.85 times higher than that of Xuehua. Despite slight decreases at 72 and 96 h, the H2O2 content was maintained at a relatively high level and was still significantly higher than that of the control (Figure 5). The results indicate that H2O2 participated in the response of the resistant pear cultivar Huangguan to V. nashicola.
3.3. Antioxidant Activity in Pear Leaves
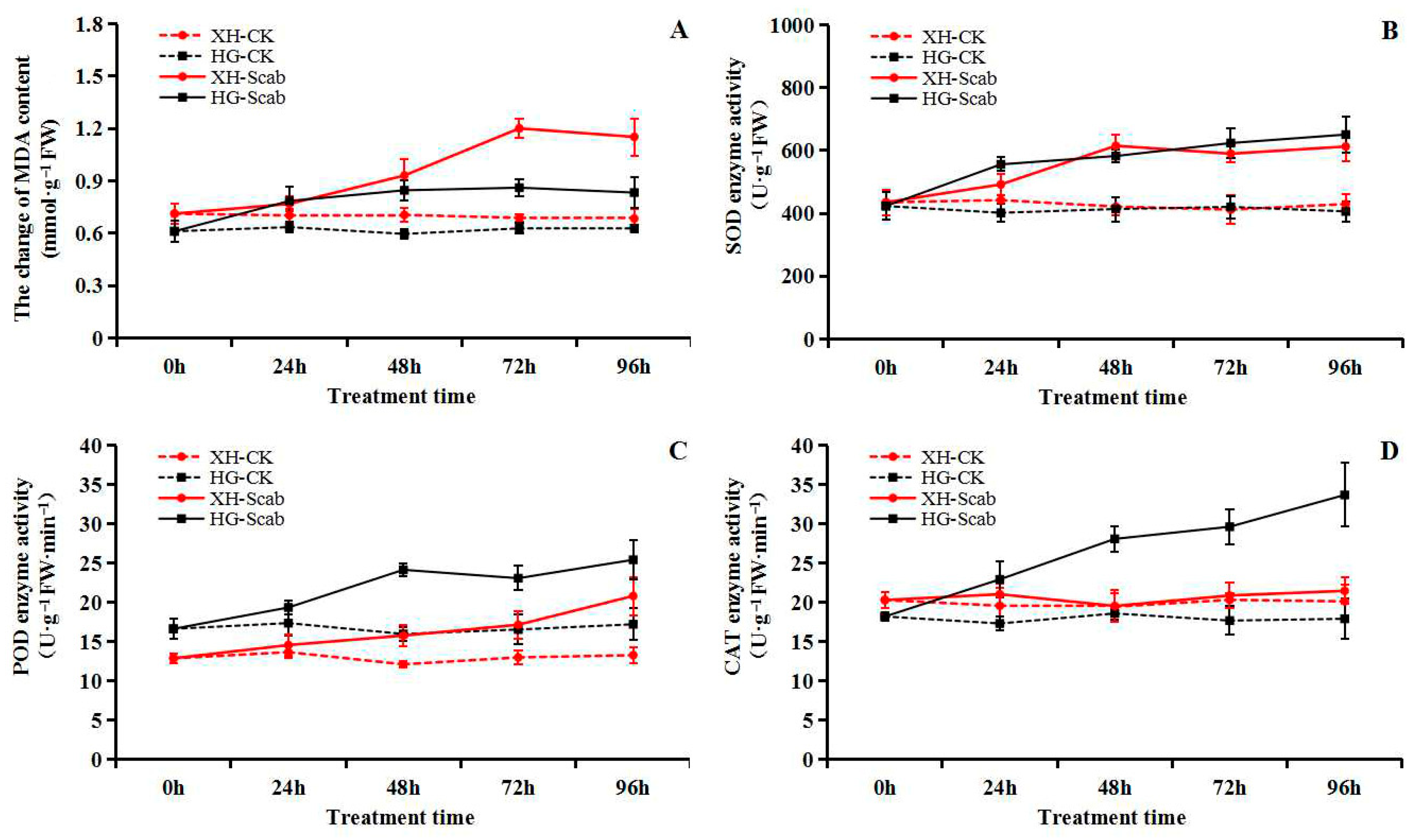
Within 96 h of V. Nashicola treatment, the MDA content of the two pear varieties showed a trend of first rising and then falling, and reached its peak at 72 h, when the MDA content of the Xuehua pear and Huangguan pear was 1.20 and 0.86 mmol·g−1, 1.69 times and 1.41 times higher than before infection, respectively (Figure 6A). In addition, V. Nashicola treatment activated the antioxidant oxidase system of pear, and induced the continuous increase of SOD, POD and CAT activities. After 96 h of treatment, the activities of the three enzymes reached a high level, and the SOD activities of Xuehua pear and Huangguan pear were 612.83 and 650.81 U·g−1, POD activity were 20.80 and 25.41 U·g−1·min−1, and CAT activity were 21.45 and 33.67 U·g−1·min−1, respectively. During the treatment period, the degree of antioxidant enzymes induced by pathogens was more significant, and the activity of the three enzymes were higher than that of the Xuehua pear (Figure 6B–D).
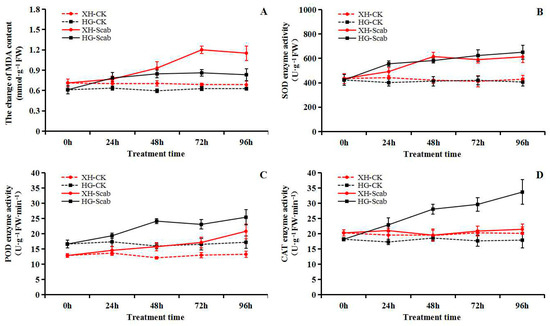
Figure 6.
Dynamic changes of malondialdehyde (MDA) content and antioxidant enzyme activity (superoxide dismutase, SOD; peroxidase, POD; catalase, CAT) in pear infected by V. nashicola (within 96 h). (A) MDA content; (B) SOD enzyme activity; (C) POD enzyme activity; (D) CAT enzyme activity.
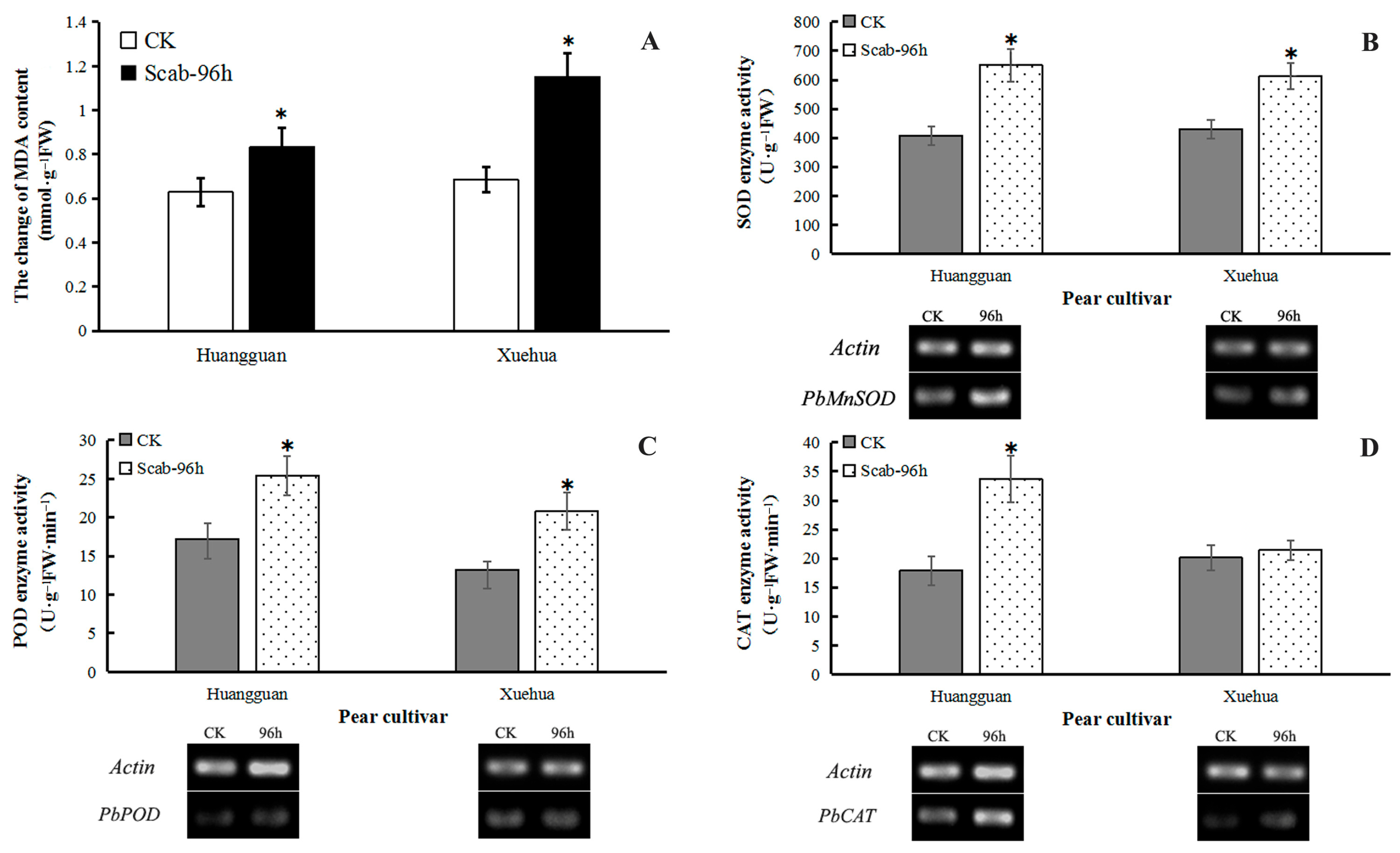
The MDA content in the leaves of both Huangguan and Xuehua was increased significantly at 96 h after infection by V. nashicola. Induction of MDA production was more distinct in the susceptible cultivar Xuehua, with the MDA content reaching ~141% of the control. This result showed that during V. nashicola infection of pear leaves, the membrane structure of leaf cells was affected, and the degree of damage was significantly higher in Xuehua than in Huangguan (Figure 7A).
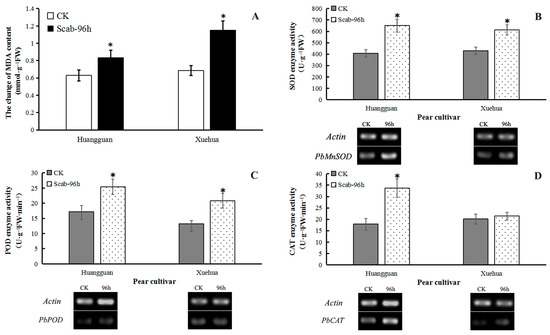
Figure 7.
Effects of V. nashicola infection on MDA content and antioxidant enzyme activity in two different pear cultivars. CK, uninfected control; Scab-96 h, fruit-derived calluses at 96 h post-infection with V. nashicola. (A) MDA content; (B) SOD enzyme activity and PbMnSOD gene expression; (C) POD enzyme activity and PbPOD gene expression; (D) CAT enzyme activity and PbCAT gene expression. * p < 0.05.
V. nashicola infection also induced a significant increase in the enzyme activities of SOD and POD and the expression levels of PbMnSOD and PbPOD genes in the leaves of both cultivars at 96 h. In Xuehua, SOD activity was increased by 42.68% and the relative expression level of PbMnSOD was 2.65 times higher than that of the control. In Huangguan, SOD activity was increased by 60.22% and the relative expression level of PbMnSOD was 1.89 times higher than that of the control (Figure 7B). Similarly, POD activity was increased by 56.98% and 47.82% in Xuehua and Huangguan, respectively, while the relative expression level of POD was 1.08 and 1.32 times higher than that of the control, respectively (Figure 7C).
Furthermore, V. nashicola infection enhanced CAT activity in the leaves of both cultivars. This increase was significant in Huangguan (88.21%) but not in Xuehua (6.67%), compared with the control. The relative expression level of PbCAT was 2.74 and 1.60 times higher than that of the control, respectively (Figure 7D).
3.4. SA Content and SA Biosynthesis Gene Expression in Pear Leaves
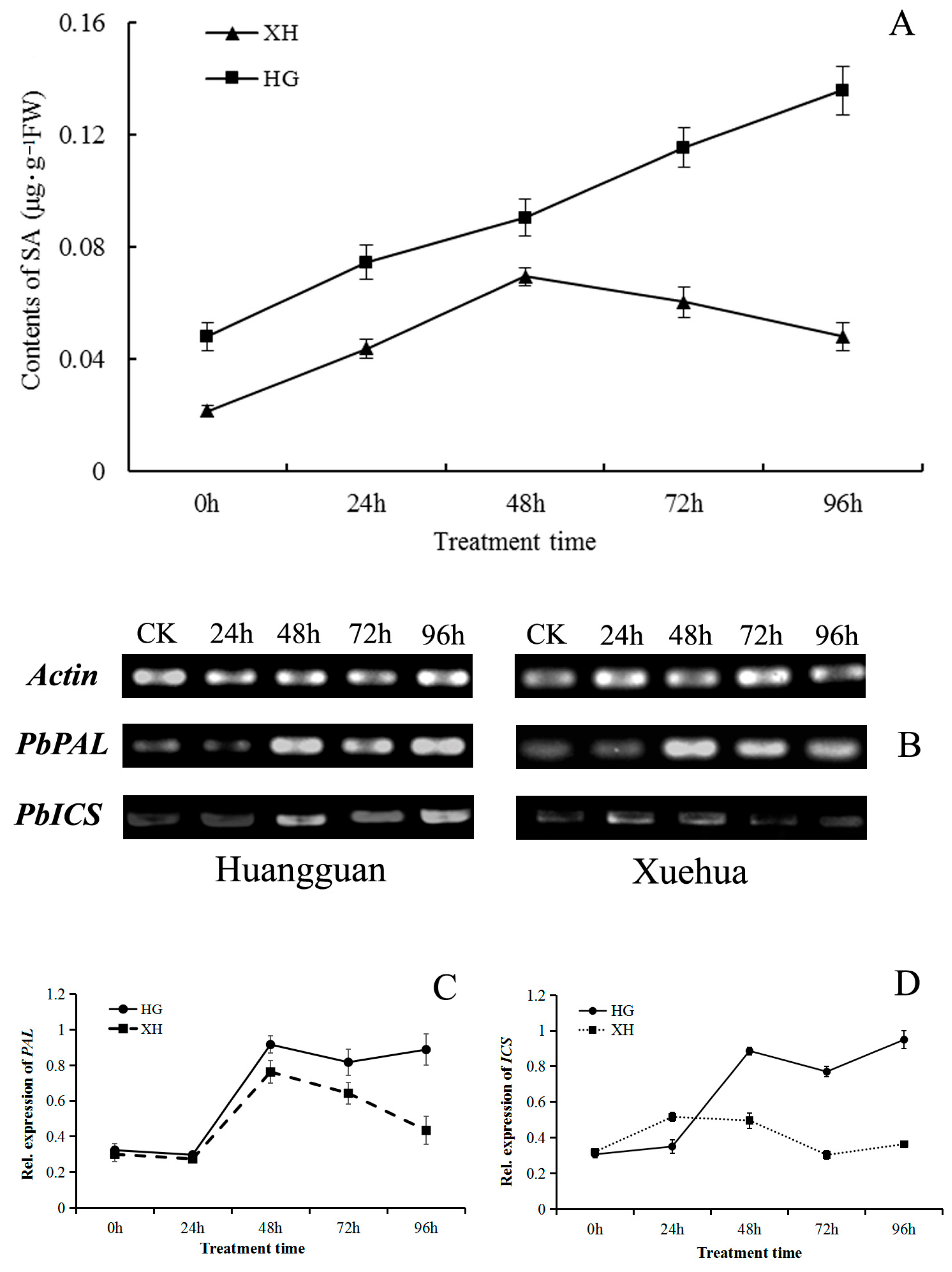
Irrespective of V. nashicola infection, the free SA content in Huangguan leaves was consistently higher than that in Xuehua leaves (Figure 8A). The leaf SA content increased continuously over time in Huangguan within 96 h post-infection. Although there was also a significant increase in SA content in Xuehua within 48 h post-infection, the SA content was not always maintained at a high level; a remarkable decrease in SA occurred in Xuehua between 48–96 h, and the SA content basically returned to the initial level at 96 h, which only accounted for 14.80% of the free SA content in Huangguan.
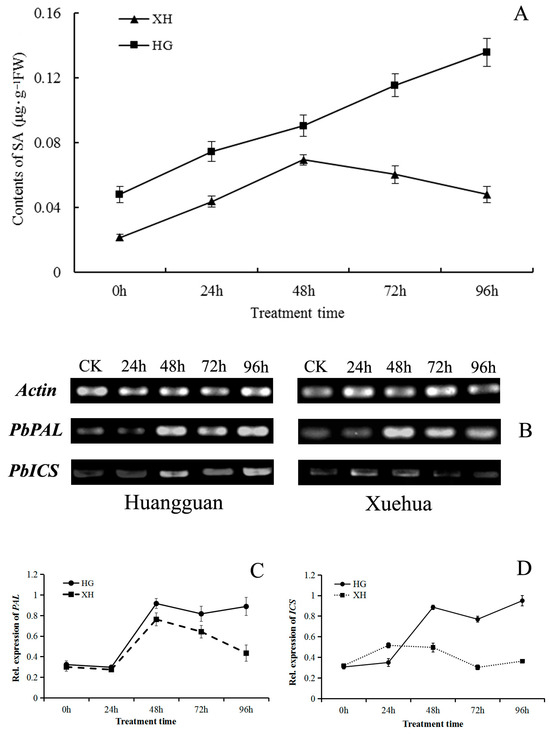
Figure 8.
Effects of V. nashicola infection on salicylic acid (SA) biosynthesis in pear leaves. (A) Free SA content in Huangguan (HG) and Xuehua (XH); (B) expression of SA biosynthesis genes PbPAL and PbICS detected by semi-quantitative PCR; (C,D) relative expression changes of PbPAL and PbICS genes analyzed using Quantity One software; 0 h and CK, uninfected controls; 24 h–96 h, timepoints post-infection by V. nashicola.
The relative expression levels of two important SA biosynthesis genes (PbPAL and PbICS) in pear leaves were analyzed. Both genes were induced by V. nashicola in both pear cultivars. Compared with controls, expression levels of the two genes varied with time (Figure 8B). The expression patterns of PbPAL were basically consistent in the two cultivars within 72 h of infection; gene expression levels first decreased then increased rapidly, then decreased again. Expression of PbPAL was maintained at a relatively high level in Huangguan at 96 h post-infection, but the corresponding gene expression level in Xuehua was relatively low, equivalent to only 48.82% of that of Huangguan (Figure 8C).
Expression of the PbICS gene was continuously upregulated over time in Huangguan within 48 h post-infection (Figure 8D). Despite a temporal decrease at 72 h, expression of PbICS continued to increase and reached its highest level at 96 h post-infection. In Xuehua, induction of PbICS by V. nashicola was observed at a relatively low level. The relative expression level of PbICS in Xuehua was consistently lower than that of Huangguan, except at 24 h post-infection, when the PbICS expression level of Xuehua was 1.48 times higher than that of Huangguan. The relative expression level of PbICS in Xuehua was only 38.21% of that of Huangguan at 96 h of post-infection.
3.5. Pathogenesis-Related Gene Expression in Pear Leaves
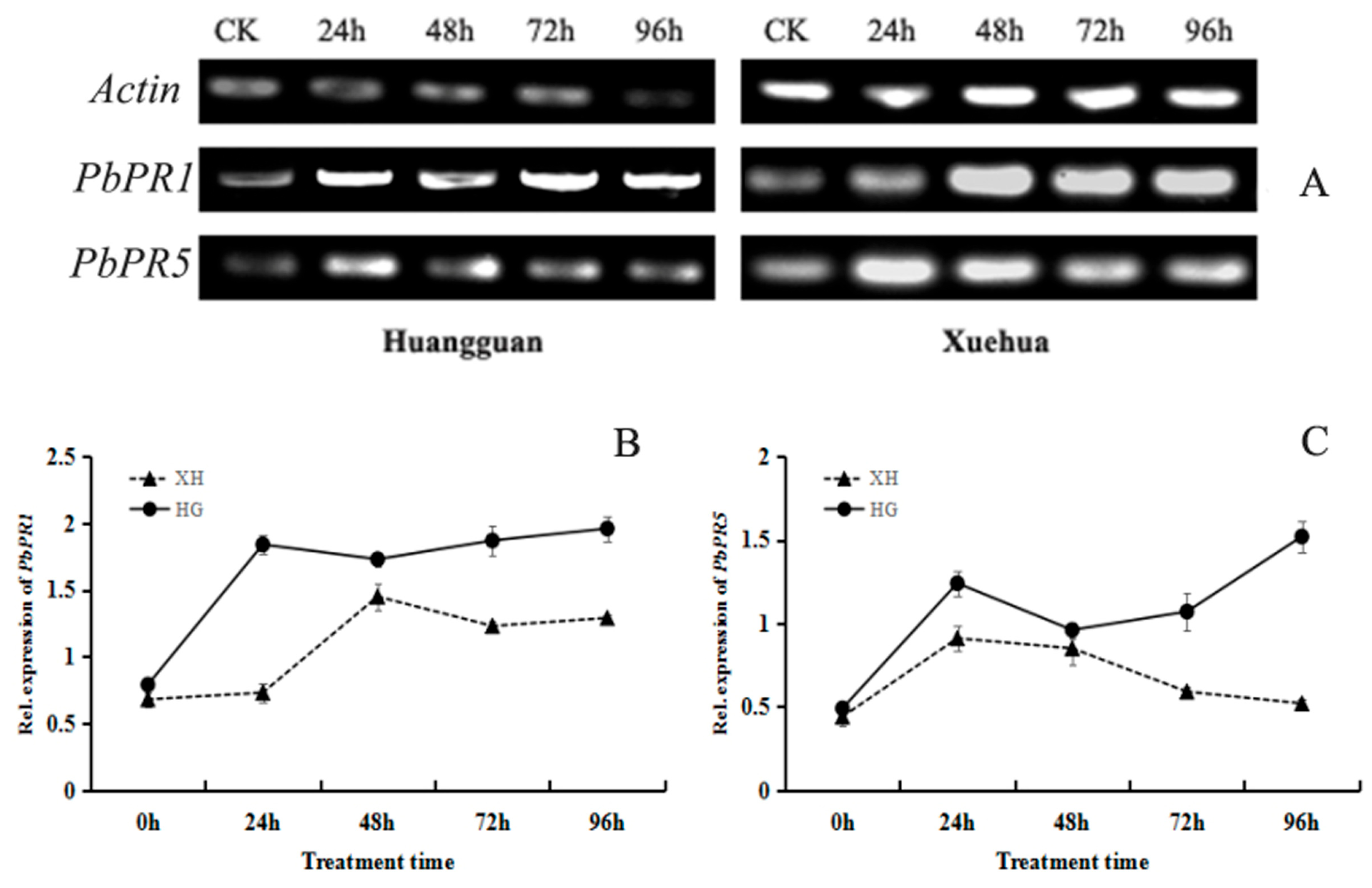
Semi-quantitative PCR analysis revealed that V. nashicola exposure induced specific changes in the expression levels of PbPR1 and PbPR5 genes in the leaves of both pear cultivars (Figure 9A). When the resistant cultivar Huangguan was infected by V. nashicola, the expression level of PbPR1 rapidly increased and reached a peak at 24 h; afterwards, gene expression was maintained at a relatively stable level from 48 to 96 h. When the susceptible cultivar Xuehua was infected, expression of the PbPR1 gene was also significantly upregulated at 48 h, but expression then decreased slowly and remained at a relatively low level from 72 to 96 h (Figure 9B). PbPR1 was consistently expressed at a higher level in Huangguan than in Xuehua at the same timepoint within the 96-h treatment period.
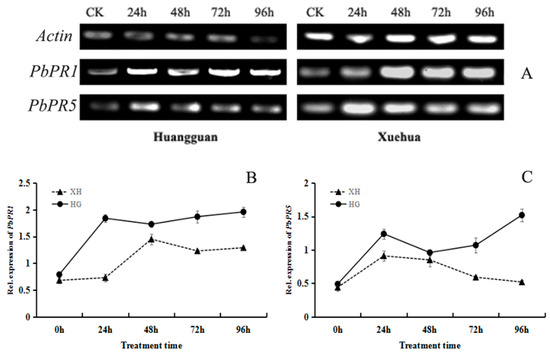
Figure 9.
Effects of V. nashicola infection on expression of pathogenicity-related genes in pear leaves. (A) Expression of PbPR1 and PbPR5 detected by semi-quantitative PCR; (B,C) relative expression changes of PbPR1 and PbPR5 genes analyzed using Quantity One software. CK, uninfected control; 24 h–96 h, timepoints post-infection by V. nashicola. HG, Huangguan’; XH, Xuehua.
In Huangguan, V. nashicola exposure upregulated the expression of PbPR5 in two stages; there was a rapid increase within 24 h post-infection, and then a steady increase over the 48–96 h post-infection. However, in Xuehua, PbPR5 was expressed at relatively high levels only within 48 h post-infection, and relative expression levels were then lower at other timepoints and showed no significant differences (Figure 9C). Overall, when exposed to V. nashicola, the expression levels of PR genes in pear leaves were higher in the resistant cultivar Huangguan than in the susceptible cultivar Xuehua.
4. Discussion
In response to long-term exposure to biotic and abiotic stresses (e.g., drought, salinity, and pathogens), plants have evolved a complex signaling network to ensure normal growth and development [27]. ROS are signaling molecules involved in plant responses to environmental changes or pathogenic invasion [28]. In the present study, we found that V. nashicola infection induced the generation of intracellular ROS fluorescence within a short time and resulted in the continuous accumulation of ROS over a certain period in two pear cultivars with contrasting resistance to pear scab. In addition, levels of H2O2, the most stable signaling molecule among ROS, was differentially abundant in the two pear cultivars exposed to V. nashicola. These results suggest that ROS, including H2O2, participate in the signal transduction of plant resistance to pear scab.
Our previous study showed that V. nashicola infection caused the influx of extracellular Ca2+ in pear; intracellular Ca2+ concentration increased, while the corresponding calcium sensors (e.g., CaMs and CDPKs) were also activated [22]. When plants are exposed to biotic stress, ROS accumulate in apoplasts through apoplast NADPH oxidase and cell wall POD. This ROS accumulation activates cell membrane Ca2+ channels and causes Ca2+ influx, leading to an increase in intracellular Ca2+ and ROS concentrations. Moreover, H2O2 enters the cell through aquaporin on the cell membrane [29,30,31,32]. Furthermore, plant–pathogen interactions drive intracellular organelles (e.g., chloroplasts, mitochondria, and peroxisomes) to produce ROS signals and trigger resistance responses [33]. All these mechanisms may contribute to the changes in intracellular ROS fluorescence in both pear cultivars following V. nashicola infection. The differential abundance of ROS between the susceptible and resistant cultivars may be due to differences in the activity of NADPH oxidase and the stress level experienced by organelles. However, the specific mechanisms require further investigation.
When ROS accumulate excessively in plants, the normal redox homeostasis of cells is disrupted, causing nucleic acid damage, metalloenzyme inactivation, and phospholipid peroxidation, and in severe cases, the cell membrane system is destroyed, leading to cell death [5]. In the present study, we found that following V. nashicola infection, a much larger increase in the MDA content occurred in the susceptible cultivar Xuehua than in the resistant cultivar Huangguan. MDA is one of the major products of membrane lipid peroxidation [34]. The V. nashicola-induced increase in MDA indicates that damage to the cell membrane system of pear leaves was more serious in Xuehua than in Huangguan.
Plants have evolved multiple antioxidant mechanisms to scavenge excessive ROS, which in turn minimizes or prevents the oxidative damage and poisoning of plant cells [35,36]. By assaying antioxidant enzyme activities, we found that V. nashicola infection induced an increase in SOD, POD, and CAT activities in pear leaves, and the induction effects were generally higher in the resistant cultivar Huangguan than in the susceptible cultivar Xuehua. The relatively weak ROS scavenging ability of the antioxidant system in Xuehua may be one of the reasons why this cultivar is more prone to stress and exhibits greater susceptibility to pear scab. In addition, SOD can transform O2– radicals into H2O2, while CAT is mainly responsible for the removal of H2O2 in peroxisomes. Therefore, the higher activity of antioxidant enzymes in Huangguan may be responsible for the later decrease in the fluorescence intensity of intracellular ROS following V. nashicola infection.
SA is a small endogenous hormone synthesized in chloroplasts, which plays an essential role in plant PAMP-triggered immunity, effector-triggered immunity, and systemic acquired resistance [37]. There is evidence that SA participates in and regulates various physiological processes, such as immune responses, plant senescence, and abiotic stress responses [38]. SA positively regulates plant resistance to biotrophic pathogens and negatively regulates plant resistance to necrotrophic pathogens [39,40]. The results of the present study showed that due to the induction effect of V. nashicola, there was a continuous increase in the free SA content of pear leaves in the resistant cultivar Huangguan, whereas the SA content was increased only at specific timepoints post-infection in the susceptible pear cultivar Xuehua. In addition, the leaf SA content of Huangguan was considerably higher than that of Xuehua. These results indicate that the SA signal transduction pathway plays a vital role in plant-pathogen interactions and enhancing pear scab resistance.
The biosynthesis of SA is essential for enhancing the resistance of plants to pathogens. Yang et al. [41] found that an enhancement of the SA biosynthesis pathway in Arabidopsis increases resistance to Pseudomonas syringae, whereas a mutant in which the SA biosynthesis pathway was blocked displayed considerably reduced resistance to this pathogen. In the present study, we analyzed the expression of two genes encoding PAL and ICS, key enzymes in the SA biosynthesis pathway. It was found that although V. nashicola affected the expression levels of both PbPAL and PbICS genes in both pear cultivars, overall, gene expression in the resistant cultivar Huangguan was induced by V. nashicola to a higher degree, and the resulting gene expression levels were higher than those of the susceptible cultivar Xueli. Su et al. [42] also observed an increase in the expression levels of PbPAL and PbICS genes and free SA content in Scutellaria baicalensis seedlings exposed to stress, and the relative expression levels of PbPAL and PbICS were positively correlated with free SA content. Therefore, like SA, PAL and ICS can be indirectly used as indicators of plant disease resistance. Furthermore, our results suggest that the biosynthesis of PAL, ICS, and SA is closely associated with resistance to pear scab.
In this study, V. nashicola infection induced an increase in intracellular ROS (H2O2) levels and facilitated the accumulation of SA in pear leaves. Both ROS and H2O2 levels first increased rapidly and then decreased considerably in the resistant cultivar Huangguan, whereas this pattern was not observed in the susceptible cultivar Xuehua. This seems to be a specific signal produced by Huangguan pear cells infected by V. nashicola, which plays a role in regulating SA biosynthesis and inducing high expression levels of PR genes. However, the potential interaction mechanisms between ROS and SA need to be further explored.
Ca2+-dependent ROS can increase the endogenous SA content in plants and simultaneously induce PR gene expression [43]. H2O2 enhances caspase enzyme activity, contributing to programmed cell death and SA accumulation [44]. In addition, H2O2 is a signaling molecule that activates PAL in plant cells, inducing PR protein expression and inducing systemic acquired resistance [45]. PR proteins are key components in the SA signaling pathway, highly conserved in the plant kingdom, and widely involved in complex defense responses in plant-pathogen interactions [46,47]. In the present study, expression of both PbPR1 and PbPR5 genes was upregulated in pear leaves exposed to V. nashicola, and higher expression levels were observed in the resistant cultivar Huangguan compared with the susceptible cultivar Xuehua. This result indicates that SA may positively regulate PbPR1 and PbPR5 expression by mediating the expression of NPR1 transcription factors, while PR gene expression levels are positively correlated with plant resistance to pear scab.
As a common fungal disease in Asian pear production, scab is still controlled by sterol demethylation inhibitor (DMI) fungicides [48,49]. However, with the widespread use of fungicides, fungal resistance is increasing, and the problem of drug residues is particularly prominent. Under the condition of not affecting plant growth and human health, the development and application of plant disease resistance inducers with new mechanisms to activate the plant’s own disease resistance signal pathway, and strengthen the comprehensive prevention and control of orchard diseases to reduce the disease pressure will become an important control strategy for pear trees against scab in the future [50]. Previous studies have shown that H2O2 signals actively participate in plant regulation of drought, cold damage, high salt and other stresses [51]. The results of this study suggested that there might be some potential interaction mechanism between ROS (H2O2) and SA accumulation (plant immunity). Therefore, exogenous application of H2O2 might activate the SA-mediated resistance signal network, thereby improving the resistance of pear trees to V. nashicola. The selection of the optimal application period and the optimal reagent concentration will be the key points of future research.
5. Conclusions
In summary, this study measured ROS fluorescence intensity, H2O2 content, antioxidant activity, SA biosynthesis, and PR gene expression in two cultivars with contrasting resistance to pear scab following V. nashicola infection. V. nashicola infection induced transient changes in intracellular ROS (H2O2) in both resistant Huangguan and susceptible Xuehua pear cultivars, but the changes in ROS signal differed between cultivars. With the accumulation of ROS, lipid peroxidation of cell membranes was aggravated, and the antioxidant enzyme system was activated. Compared with Xuehua, stress was lower in Huangguan in which antioxidant enzyme activities and related gene expression levels were generally higher. In addition, V. nashicola induced the biosynthesis of endogenous SA and expression of PR genes (PbPR1 and PbPR5), and the induction effects were greater in Huangguan than in Xuehua. The results indicate that transient ROS bursts and continuous SA accumulation in plant cells are essential physiological processes that facilitate resistance to V. nashicola infection in pear. This study unravels the mechanisms underpinning the responses of pear to V. nashicola and highlight the roles of ROS and SA in the signal transduction pathway of pear scab resistance. The results supplied new insights into the roles of ROS and SA in the signal transduction pathways of pear scab resistance. In addition, the results of this study will provide some new ideas for the research and development of pear disease resistance regulators and the improvement of pear tree anti-scab control strategy.
Author Contributions
Literature search, X.Z. and Y.S.; conceptualization, Y.L. and L.L.; methodology, Y.L., P.Z., X.Z. and Y.S.; data collection, X.Z. and P.Z.; data analysis, Y.L., P.Z. and X.Z.; data interpretation, Y.L. and P.Z.; writing, Y.L. and L.L.; visualization, Y.S.; supervision, Y.S. and L.L.; funding acquisition, Y.S. and L.L.; All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Central Government Guides Local Funds for Science and Technology Development of China (grant No. YDZJSX2022A042).
Data Availability Statement
All the research data is contained within the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mansoor, S.; Ali, A.; Kour, N.; Bornhorstet, J.; AlHarbi, K.; Rinklebe, J.; Moneim, D.A.E.; Ahmad, P.; Chung, Y.S. Heavy metal induced oxidative stress mitigation and ROS scavenging in plants. Plants 2023, 12, 3003. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Jóźwiak, W.; Politycka, B. Effect of selenium on alleviating oxidative stress caused by a water deficit in cucumber roots. Plants 2019, 8, 217. [Google Scholar] [CrossRef] [PubMed]
- Niwas, R.; Gupta, R.N.; Rani, N.H.K. Systemic resistance: Plant responses to interaction with fungal bio-control agents. J. Eco-Friendly Agric. 2022, 17, 363–368. [Google Scholar] [CrossRef]
- Yang, R.; Wang, J.; Cai, Z.; Shen, Y.; Gan, Z.; Duan, B.; Yuan, J.; Huang, T.; Zhang, W.; Du, H.; et al. Transcriptome profiling to elucidate mechanisms of the enhancement of the resistance to Botryosphaeria dothidea by nitric oxide in postharvest kiwifruit during storage. LWT-Food Sci. Technol. 2022, 159, 113187. [Google Scholar] [CrossRef]
- Trillo-Hernández, E.A.; Sierra, A.D.; Tiznado-Hernández, M.E. Role of reactive oxygen species in the initiation of plant retrograde signaling. Phyton-Int. J. Exp. Bot. 2022, 91, 905–913. [Google Scholar] [CrossRef]
- Sandalio, L.M.; Rodriguez-Serrano, M.; Romero-Puertas, M.C.; Rio, L.A. Imaging of reactive oxygen species and nitric oxide in vivo in plant tissues. Methods Enzymol. 2008, 440, 397–409. [Google Scholar] [CrossRef]
- Saleem, M.; Fariduddin, Q.; Castroverde, C.D.M. Salicylic acid: A key regulator of redox signalling and plant immunity. Plant Physiol. Biochem. 2021, 168, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Chakraborty, N.; Acharya, K. Unraveling the role of nitric oxide in regulation of defense responses in chilli against Alternaria leaf spot disease. Physiol. Mol. Plant Pathol. 2021, 114, 101621. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, G.; Yang, S.; Zhang, J.; Deng, Y.; Qi, J.; Wu, J.; Fu, D.; Wang, W.; Hao, Q. Overexpression of isochorismate synthase enhances drought tolerance in barley. J. Plant Physiol. 2021, 260, 153404. [Google Scholar] [CrossRef]
- Huang, X.; Bai, X.; Xie, Z.; Fahad, S.; Gbokie, T., Jr.; Lu, Y.; Guo, T.; Li, J.; Zhang, Z.; Wu, W.; et al. De novo transcriptome assembly of Coffea liberica reveals phylogeny and expression atlas of phenylalanine ammonia-lyase genes in Coffea species. Ind. Crops Prod. 2023, 192, 116029. [Google Scholar] [CrossRef]
- Kachroo, P.; Yoshioka, K.; Shah, J.; Dooner, H.K.; Klessig, D.F. Resistance to turnip crinkle virus in Arabidopsis is regulated by two host genes and is salicylic acid dependent but NPR1, ethylene, and jasmonate independent. Plant Cell 2000, 12, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Prakash, V.; Singh, V.P.; Tripathi, D.K.; Sharma, S.; Corpas, F.J. Nitric oxide (NO) and salicylic acid (SA): A framework for their relationship in plant development under abiotic stress. Plant Biol. 2021, 23, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Himeno, N.; Saburi, W.; Wakuta, S.; Takeda, R.; Matsui, H. Identification of rice β-glucosidase with high hydrolytic activity towards salicylic acid β-D-glucoside. Biosci. Biotechnol. Biochem. 2013, 77, 934–939. [Google Scholar] [CrossRef]
- Ding, L.N.; Li, Y.T.; Wu, Y.Z.; Li, T.; Geng, R.; Cao, J.; Zhang, W.; Tan, X.L. Plant disease resistance-related signaling pathways: Recent progress and future prospects. Int. J. Mol. Sci. 2022, 23, 16200. [Google Scholar] [CrossRef] [PubMed]
- Li, B.H.; Zhao, M.Q. Studies on parasitic position and pathogenicity of Venturia nashicola. Acta Phytopathol. Sin. 1999, 4, 345–348. [Google Scholar] [CrossRef]
- Jing, S.Y.; Liu, Y.; Liu, H.F.; Li, L.L. Methyl jasmonate regulates protective enzyme activities to improve resistance to Venturia nashicola in pear (Pyrus bretschneideri Rehd.). Eur. J. Plant Pathol. 2020, 158, 789–797. [Google Scholar] [CrossRef]
- Nakao, S.; Watanabe, H.; Yano, T.; Yamaoka, Y.; Ishii, H. Control efficacy of the systemic acquired resistance (SAR) inducer acibenzolar-S-methyl against Venturia nashicola in Japanese pear orchards. J. Gen. Plant Pathol. 2021, 87, 307–315. [Google Scholar] [CrossRef]
- Sun, Y.H.; Wang, Y.T.; Li, Y.; Jia, E.J. A medium maturity, scab resistant new pear variety-Huang guan. China Fruits 1997, 1, 6. [Google Scholar] [CrossRef]
- Dong, X.G.; Tian, L.M.; Cao, Y.F. Evaluation of resistance to scab in pear germplasms. J. Plant Genet. Resour. 2012, 13, 571–576. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, J.S.; Wang, Y.G.; Feng, W.X.; Xu, W.X. Effects of infection of epiphyte caused fruit rot on defense mechanism in pear fruit callus. Acta Bot. Boreal-Occident. Sin. 2010, 30, 2219–2224. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.B.; Li, J.H.; Li, L.L. Responses of intracellular Ca2+ and its sensors to Venturia nashicola infection in pear (Pyrus bretschneideri) with differing resistance. Sci. Hortic. 2019, 243, 552–558. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Q.; Fu, M. Postharvest Low Temperature Conditioning Reduces Peel Browning and Improves Fruit Quality in Storage and Subsequent Shelf Life of Huangguan Pear. Food Nutr. Sci. 2015, 6, 1351–1361. [Google Scholar] [CrossRef]
- Wang, Y.J.; Yang, J.S.; Yuan, C.P.; Liu, J.G.; Li, D.H.; Dong, S.T. Characteristics of senescence and antioxidant enzyme activities in leaves at different plant parts of summer maize with the super-high yielding potential after anthesis. Acta Agron. Sin. 2013, 39, 2183–2191. [Google Scholar] [CrossRef]
- Gao, L.J.; Zhang, Y.X. Effects of salicylic acid on the expression of SOD, PPO isozymes and NPR1 in Pear. Atca Hortic. Sin. 2012, 40, 41–48. [Google Scholar] [CrossRef]
- Jaakola, L.; Pirttilä, A.M.; Halonen, M.; Hohtola, A. Isolation of high quality RNA from bilberry (Vaccinium myrtillus L.) fruit. Mol. Biotechnol. 2001, 19, 201–203. [Google Scholar] [CrossRef]
- Lim, S.D.; Kim, S.; Gilroy, S.; Cushman, J.C.; Choi, W. Quantitative ROS bioreporters: A robust toolkit for studying biological roles of ROS in response to abiotic and biotic stresses. Physiol. Plant. 2019, 165, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Waszczak, C.; Carmody, M.; Kangasjarviet, J. Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef] [PubMed]
- Bowler, C.; Fluhr, R. The role of calcium and activated oxygens as signals for controlling cross-tolerance. Trends Plant Sci. 2000, 5, 241–246. [Google Scholar] [CrossRef]
- Mansoor, S.; Wani, O.A.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive oxygen species in plants: From source to sink. Antioxidants 2022, 11, 225. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Jin, L.; Peng, R. Crosstalk between Ca2+ and other regulators assists plants in responding to abiotic stress. Plants 2022, 11, 1351. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Chi, Y.; Jiang, Z.H.; Xu, Y.; Xie, L.; Huang, F.; Wan, D.; Ni, J.; Yuan, F.; Wu, X.; et al. Hydrogen peroxide sensor HPCA1 is an LRR receptor kinase in Arabidopsis. Nature 2020, 578, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Camejo, D.; Guzmán-Cedeño, A.; Moreno, A. Reactive oxygen species, essential molecules, during plant-pathogen interactions. Plant Physiol. Biochem. 2016, 103, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Ma, M.; Ge, G.; Suleman, M.; Li, H. The role of cyanide-resistant respiration in Solanum tuberosum L. against high light stress. Plant Biol. 2020, 22, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Shah, A.; Karthik, K.; Rathinam, M.; Rai, V.; Chaudhary, N.; Sreevathsa, R. Reactive oxygen species in plants: An invincible fulcrum for biotic stress mitigation. Appl. Microbiol. Biot. 2022, 106, 5945–5955. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.K.; Jayalakshmi, K.; Tilgam, J.; Gupta, A.; Nagaraju, Y.; Kumar, A.; Hamid, S.; Singh, H.V.; Minkina, T.; Rajput, V.D.; et al. ROS generated from biotic stress: Effects on plants and alleviation by endophytic microbes. Front. Plant Sci. 2022, 13, 1042936. [Google Scholar] [CrossRef] [PubMed]
- Miyaji, N.; Shimizu, M.; Takasaki-Yasuda, T.; Dennis, E.S.; Fujimoto, R. The transcriptional response to salicylic acid plays a role in Fusarium yellows resistance in Brassica rapa L. Plant Cell Rep. 2021, 40, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.P.; Dong, X.N. Perception of the plant immune signal salicylic acid. Curr. Opin. Plant Biol. 2014, 20, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Niu, L.; Wang, C.; Wei, L.; Pan, Y.; Liao, W. Hydrogen peroxide is involved in salicylic acid-induced adventitious rooting in cucumber under cadmium stress. J. Plant Biol. 2022, 65, 43–52. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Y.; Zhang, Z.; Wei, J. Hydrogen peroxide promotes programmed cell death and salicylic acid accumulation during the induced production of sesquiterpenes in cultured cell suspensions of Aquilaria sinensis. Funct. Plant Biol. 2015, 42, 337–346. [Google Scholar] [CrossRef]
- Yang, L.; Li, B.; Zheng, X.Y.; Li, J.; Yang, M.; Dong, X.N.; He, G.M.; An, C.C.; Deng, X.Y. Salicylic acid biosynthesis is enhanced and contributes to increased biotrophic pathogen resistance in Arabidopsis hybrids. Nat. Commun. 2015, 6, 7309. [Google Scholar] [CrossRef]
- Su, H.; Song, S.; Yan, X.; Fang, L.; Zeng, B.; Zhu, Y. Endogenous salicylic acid shows different correlation with baicalin and baicalein in the medicinal plant Scutellaria baicalensis Georgi subjected to stress and exogenous salicylic acid. PLoS ONE 2018, 13, e0192114. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Wang, C.; Lu, W.; Jing, B.J.; Hua, X. Proline induces calcium-mediated oxidative burst and salicylic acid signaling. Amino Acids 2011, 40, 1473–1484. [Google Scholar] [CrossRef]
- Takumi, K.; Nonaka, G. Bacterial cysteine-inducible cysteine resistance systems. J. Bacteriol. 2016, 198, 1384–1392. [Google Scholar] [CrossRef]
- Nguyen, H.P.; Chakravarthy, S.; Velasquez, A.C.; Mclane, H.L.; Martin, G.B. Methods to study PAMP-triggered immunity using tomato and Nicotiana benthamiana. Mol. Plant Microbe 2010, 23, 991–1104. [Google Scholar] [CrossRef]
- Verk, M.V.; Pappaioannou, D.; Neeleman, L.; Bol, J.F.; Linthorst, H.J.M. A novel WRKY transcription factor is required for induction of PR-1a gene expression by salicylic acid and bacterial elicitors. Plant Physiol. 2008, 146, 1983–1995. [Google Scholar] [CrossRef]
- Islam, M.T.; Han, M.G.; Ziemann, M.; Hussain, H.I.; Cahill, D. Phaeophyceaean (brown algal) extracts activate plant defense systems in Arabidopsis thaliana challenged With Phytophthora cinnamomi. Front. Plant Sci. 2020, 11, 852. [Google Scholar] [CrossRef]
- Ishii, H.; Cools, H.J.; Nishimura, K.; Borghi, L.; Kikuhara, K.; Yamaoka, Y. DMI-fungicide resistance in Venturia nashicola, the causal agent of Asian pear scab—How reliable are mycelial growth tests in culture? Microorganisms 2021, 9, 1377. [Google Scholar] [CrossRef]
- Vielba-Fernández, A.; Polonio, A.; Ruiz-Jiménez, L.; Vicente, A.; Pérez-García, A.; Fernández-Ortuño, D. Fungicide resistance in powdery mildew fungi. Microorganisms 2020, 8, 1431. [Google Scholar] [CrossRef]
- Corkley, I.; Fraaije, B.; Hawkins, N. Fungicide resistance management: Maximizing the effective life of plant protection products. Plant Pathol. 2022, 71, 150–169. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, S.; Jiang, H.; Zhang, X.; Gao, H.; Yang, P.; Hu, T. Melatonin-induced cold and drought tolerance is regulated by brassinosteroids and hydrogen peroxide signaling in perennial ryegrass. Environ. Exp. Bot. 2022, 196, 104815. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).