Effect of Mild Organic Substitution on Soil Quality and Microbial Community
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Experiment Conditions
2.2. Design of the Experiment
- No N fertilizer (CK)
- Chemical N fertilizer (MF)
- 20% organic manure replacing chemical fertilizer (20% OF)
- 40% organic manure replacing chemical fertilizer (40% OF)
2.3. Sampling and Analysis
2.3.1. Sample Collection
2.3.2. Soil Chemical Property
2.3.3. Soil Enzyme
2.3.4. DNA Extraction and High-Throughput Sequencing Analysis
2.3.5. Data and Statistical Analyses
3. Results
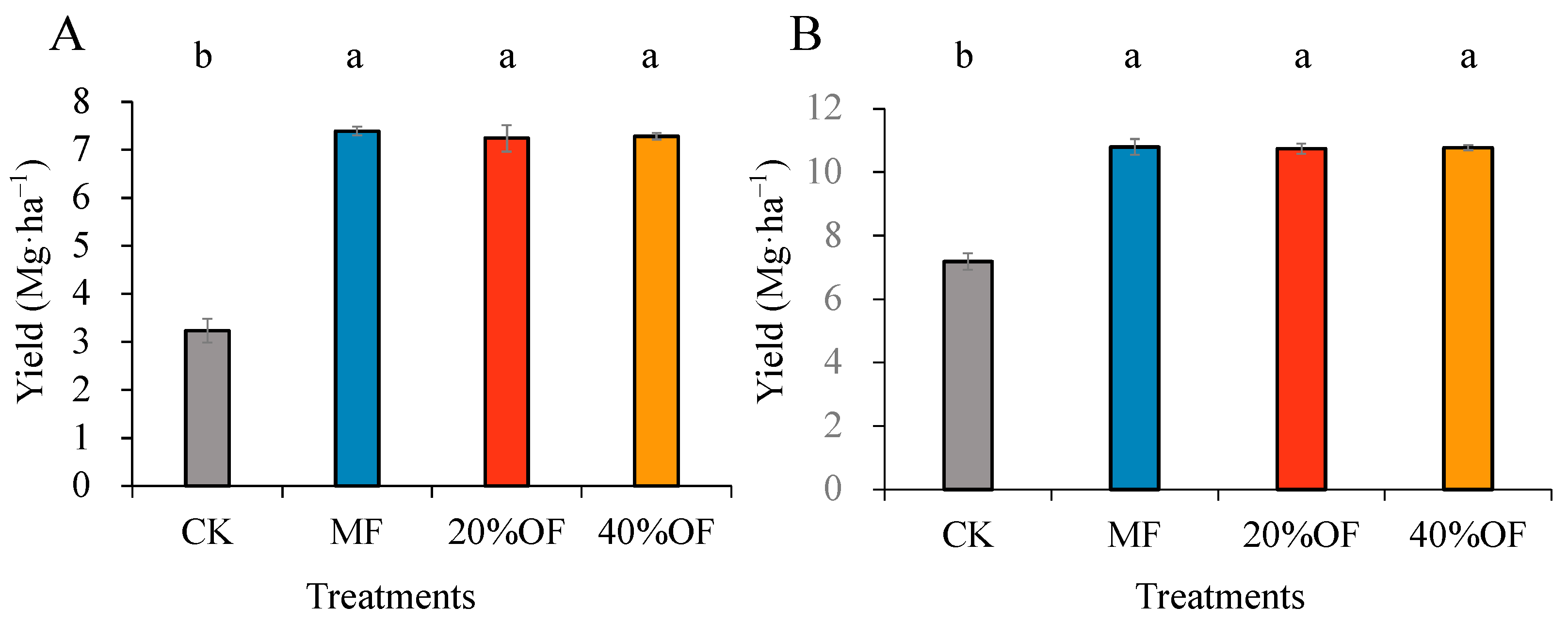
3.1. Crop Yield
3.2. Soil Chemical Properties
3.3. Soil Enzyme Activity
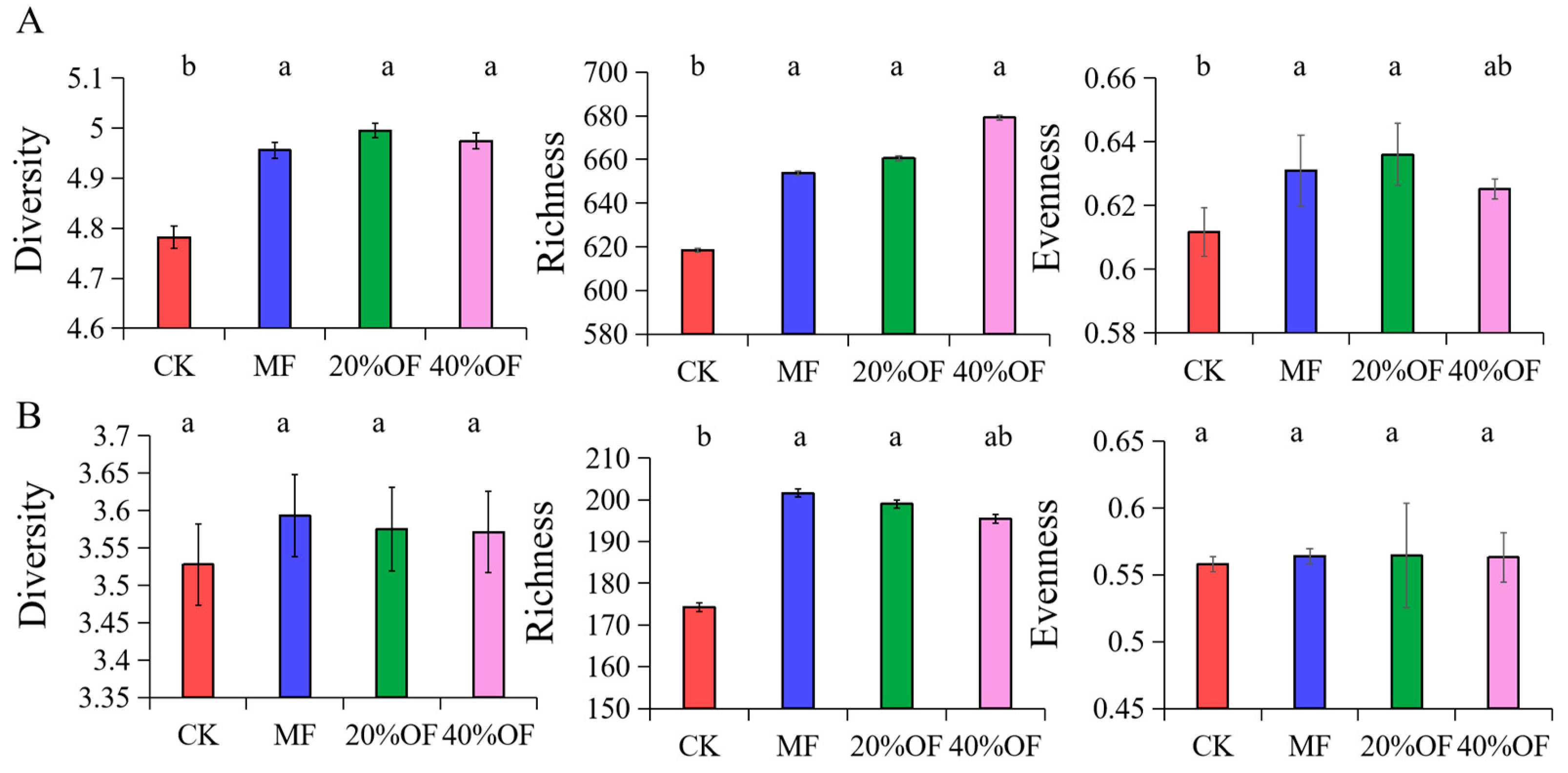
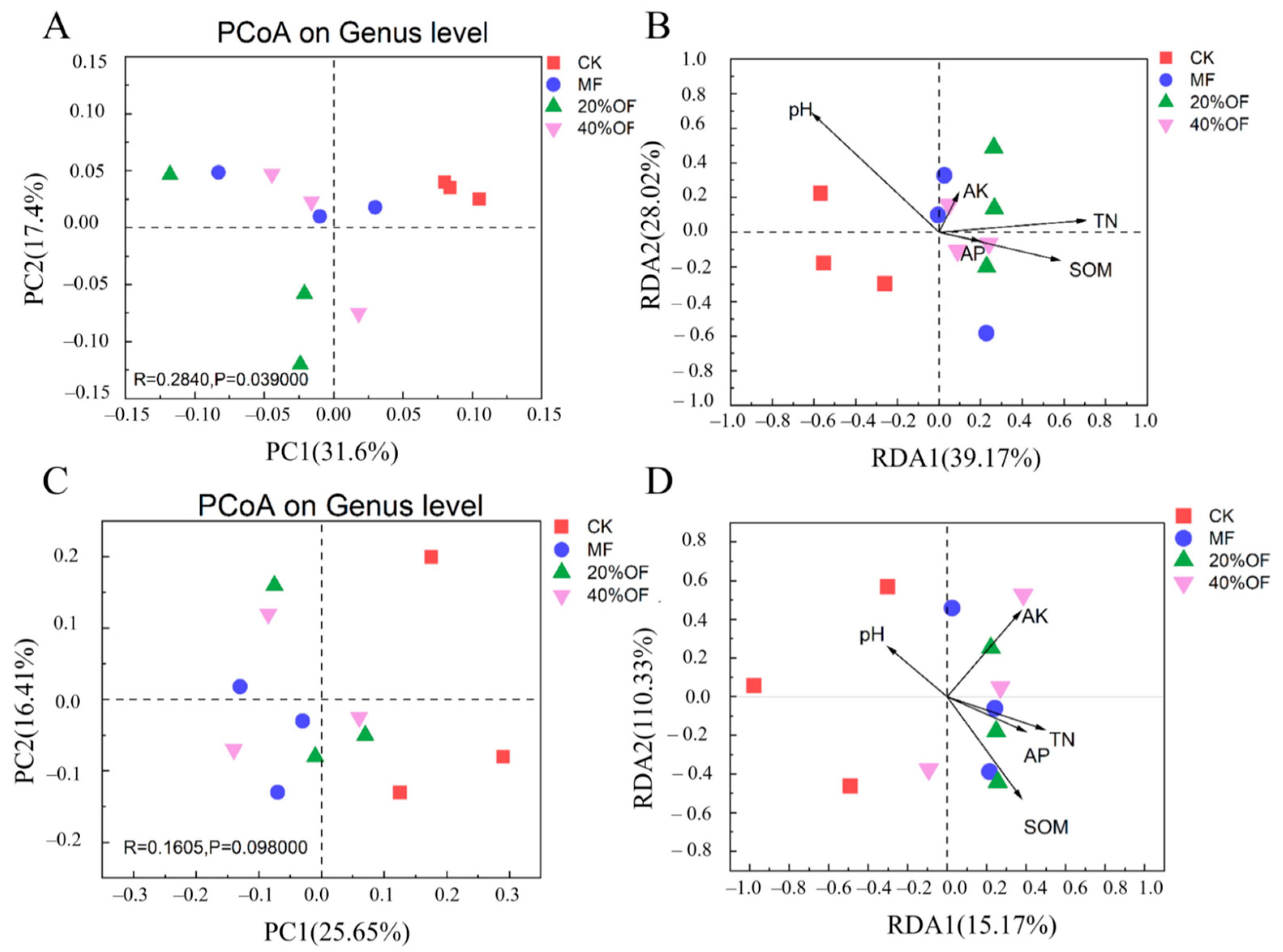
3.4. Soil Bacterial and Fungal Community Structure and Diversity
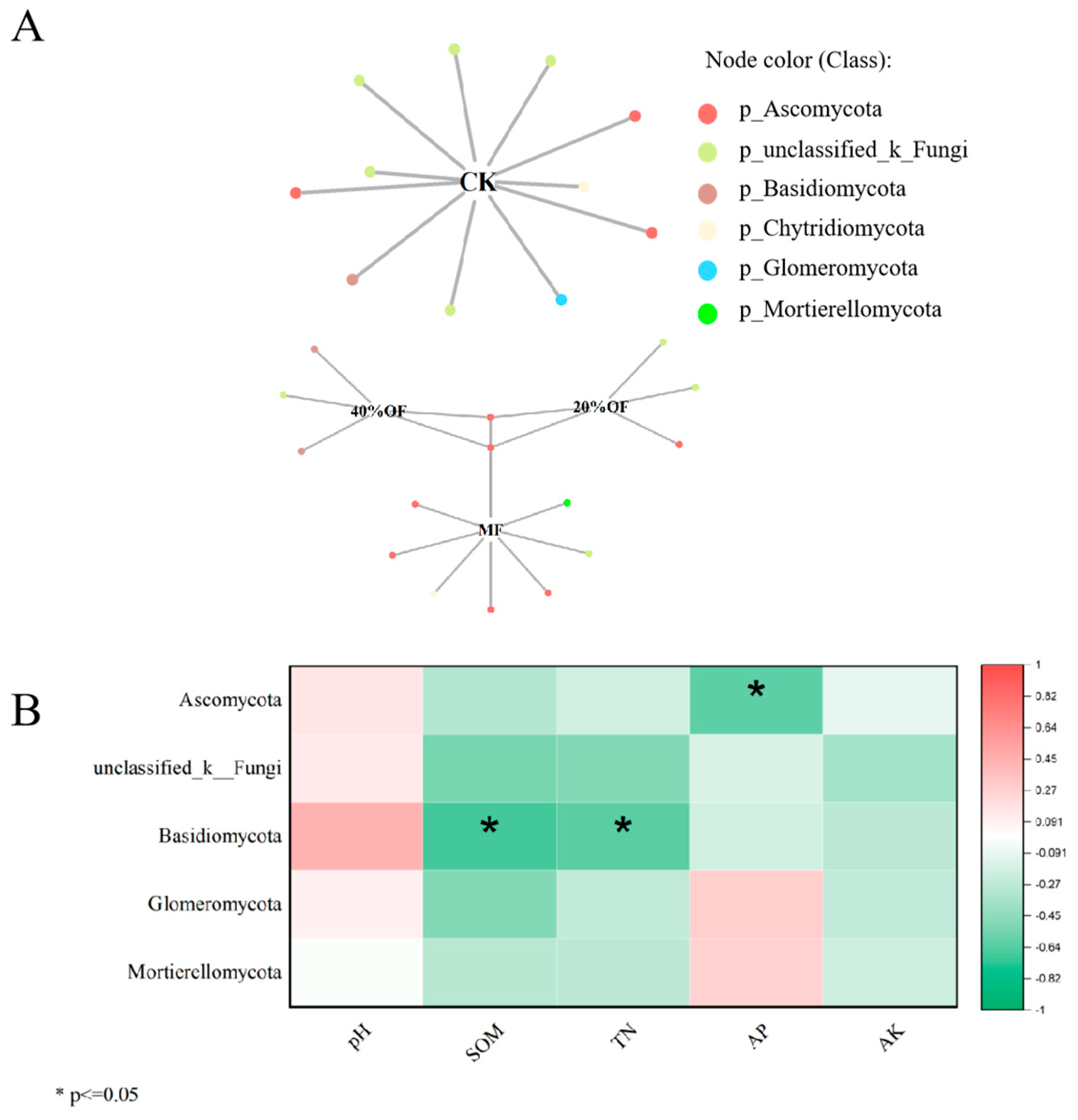
3.5. Soil Bacterial Bipartite Networks
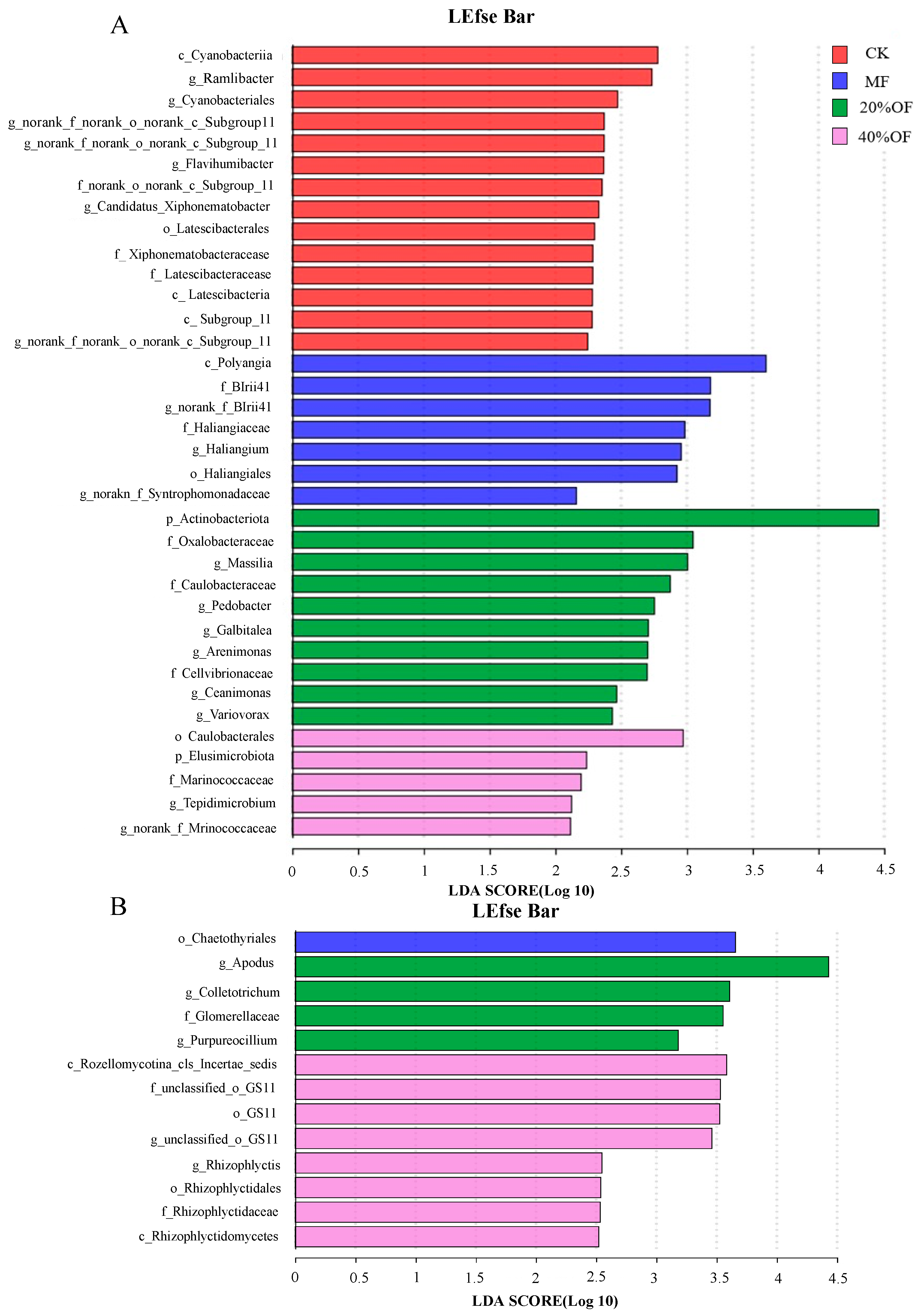
3.6. LEfSe
4. Discussion
4.1. Crop Yield and Soil Chemical Properties
4.2. Soil Enzyme Activity
4.3. Soil Bacterial and Fungal Community Diversity and Structure
4.4. Soil Bacterial and Fungal Community Structure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhong, W.H.; Gu, T.; Wang, W.; Zhang, B.; Lin, X.G.; Huang, Q.R.; Shen, W.S. The effects of mineral fertilizer and organic manure on soil microbial community and diversity. Plant Soil 2010, 326, 511–522. [Google Scholar] [CrossRef]
- Francioli, D.; Schulz, E.; Lentendu, G.; Wubet, T.; Buscot, F.; Reitz, T. Mineral vs. organic amendments: Microbial community structure, activity and abundance of agriculturally relevant microbes are driven by long-term fertilization strategies. Front. Microbiol. 2016, 7, 1446. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Jeong, S.T.; Das, S.; Kim, P.J. Composted cattle manure increases microbial activity and soil fertility more than composted swine manure in a submerged rice paddy. Front. Microbiol. 2017, 8, 1702. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, B.; Xia, L.L.; Fan, C.H.; Xiong, Z.Q. Organic-substitute strategies reduced carbon and reactive nitrogen footprints and gained net ecosystem economic benefit for intensive vegetable production. J. Clean. Prod. 2019, 225, 984–994. [Google Scholar] [CrossRef]
- Xia, L.L.; Lam, S.K.; Chen, D.L.; Wang, J.Y.; Tang, Q.; Yan, X.Y. Can knowledge-based N management produce more staple grain with lower greenhouse gas emission and reactive nitrogen pollution? A meta-analysis. Glob. Chang. Biol. 2017, 23, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Karlen, D.L.; Mausbach, M.J.; Doran, J.W.; Cline, R.G.; Harris, R.F.; Schuman, G.E. Soil quality: A concept, definition, and framework for evaluation. Soil Sci. Soc. Am. J. 1997, 61, 4–10. [Google Scholar] [CrossRef]
- Doran, J.W.; Zeiss, M.R. Soil health and sustainability: Managing the biotic component of soil quality. Appl. Soil Ecol. 2000, 15, 3–11. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, R.F.; Xue, C.; Xun, W.B.; Sun, L.; Xu, Y.C.; Shen, Q.R. Pyrosequencing reveals contrasting soil bacterial diversity and community structure of two main winter wheat cropping systems in China. Microb. Ecol. 2014, 67, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.C.; Li, K.J.; Zhou, W.; Qiu, S.J.; Huang, S.W.; He, P. Changes in soil microbial community, enzyme activities and organic matter fractions under long-term straw return in north-central China. Agric. Ecosyst. Environ. 2016, 216, 82–88. [Google Scholar] [CrossRef]
- Paterson, E.; Sim, A.; Osborne, S.M.; Murray, P.J. Long-term exclusion of plant-inputs to soil reduces the functional capacity of microbial communities to mineralise recalcitrant root-derived carbon sources. Soil Biol. Biochem. 2011, 43, 1873–1880. [Google Scholar] [CrossRef]
- Wu, G.H.; Chen, Z.H.; Jiang, D.Q.; Jiang, N.; Jiang, H.; Chen, L.J. Oxidases and hydrolases mediate soil organic matter accumulation in chernozem of northeastern China. Geoderma 2021, 403, 115206. [Google Scholar] [CrossRef]
- Zhou, J.; Guan, D.W.; Zhou, B.K.; Zhao, B.S.; Ma, M.C.; Qin, J.; Jiang, X.; Chen, S.F.; Cao, F.M.; Shen, D.L.; et al. Influence of 34-years of fertilization on bacterial communities in an intensively cultivated black soil in northeast China. Soil Biol. Biochem. 2015, 90, 42–51. [Google Scholar] [CrossRef]
- Pan, H.; Chen, M.M.; Feng, H.J.; Wei, M.; Song, F.P.; Lou, Y.H.; Cui, X.M.; Wang, H.; Zhuge, Y.P. Organic and inorganic fertilizers respectively drive bacterial and fungal community compositions in a fluvo-aquic soil in northern China. Soil Till. Res. 2020, 198, 104540. [Google Scholar] [CrossRef]
- Cui, X.W.; Zhang, Y.Z.; Gao, J.S.; Peng, F.Y.; Gao, P. Long-term combined application of manure and chemical fertilizer sustained higher nutrient status and rhizospheric bacterial diversity in reddish paddy soil of Central South China. Sci. Rep. 2018, 8, 16554. [Google Scholar] [CrossRef] [PubMed]
- Sarathchandra, S.U.; Ghani, A.; Yeates, G.W.; Burch, G.; Cox, N.R. Effect of nitrogen and phosphate fertilisers on microbial and nematode diversity in pasture soils. Soil Biol. Biochem. 2001, 33, 952–964. [Google Scholar] [CrossRef]
- Cui, J.W.; Zhu, R.L.; Wang, X.Y.; Xu, X.P.; Ai, C.; He, P.; Liang, G.Q.; Zhou, W.; Zhu, P. Effect of high soil C/N ratio and nitrogen limitation caused by the long-term combined organic-inorganic fertilization on the soil microbial community structure and its dominated SOC decomposition. J. Environ. Manag. 2022, 303, 114155. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Frey, B.; Mayer, J.; Mäder, P.; Widmer, F. Distinct soil microbial diversity under long-term organic and conventional farming. ISME J. 2015, 9, 1177–1194. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.M.; Li, C.; Xiao, X.P.; Shi, L.H.; Cheng, K.K.; Wen, L.; Li, W.Y. Effect of short-term manure nitrogen input on soil microbial community structure and diversity in a double-cropping paddy field. Sci. Rep. 2020, 10, 13540. [Google Scholar] [CrossRef]
- Yang, Y.J.; Lei, T.; Du, W.; Liang, C.L.; Li, H.D.; Lv, J.L. Substituting chemical fertilizer nitrogen with organic manure and comparing their nitrogen use efficiency and winter wheat yield. J. Agric. Sci. 2020, 158, 260–268. [Google Scholar] [CrossRef]
- Chesworth, W.; Camps, M.A.; Macías, F.; Spaargaren, O.; Spaargaren, O.; Mualem, Y.; Micheli, E. Classification of soils: World Reference Base (WRB) for soil resources. In Encyclopedia of Soil Science, 2008th ed.; Chesworth, W., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 120–122. [Google Scholar]
- Murphy, J.; Riley, J. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Walker, J.M.; Barber, S.A. Absorption of potassium and rubidium from the soil by corn roots. Plant Soil 1962, 17, 243–259. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Liu, S.B.; Wang, J.Y.; Pu, S.Y.; Blagodatskaya, E.; Kuzyakov, Y.; Razavi, B.S. Impact of manure on soil biochemical properties: A global synthesis. Sci. Total Environ. 2020, 745, 141003. [Google Scholar] [CrossRef] [PubMed]
- Gopal, M.; Gupta, A.; Arunachalam, V.; Magu, S.P. Impact of azadirachtin, an insecticidal allelochemical from neem on soil microflora, enzyme and respiratory activities. Bioresour. Technol. 2007, 98, 3154–3158. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, G.; Xue, S.; Song, Z. Rhizosphere soil microbial activity under different vegetation types on the Loess Plateau, China. Geoderma 2011, 161, 115–125. [Google Scholar] [CrossRef]
- Wang, B.; Chen, C.; Xiao, Y.M.; Chen, K.Y.; Wang, J.; Zhao, S.; Liu, N.; Li, J.N.; Zhou, G.Y. Trophic relationships between protists and bacteria and fungi drive the biogeography of rhizosphere soil microbial community and impact plant physiological and ecological functions. Microb. Res. 2024, 280, 127603. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Amato, K.R.; Yeoman, C.J.; Kent, A.; Righini, N.; Carbonero, F.; Estrada, A.; Gaskins, H.R.; Stumpf, R.M.; Yildirim, S.; Torralba, M.; et al. Habitat degradation impacts black howler monkey (Alouatta pigra) gastrointestinal microbiomes. ISME J. 2013, 7, 1344–1353. [Google Scholar] [CrossRef]
- Fan, T.L.; Stewart, B.A.; Wang, Y.; Luo, J.J.; Zhou, G.Y. Long-term fertilization effects on grain yield, water-use efficiency and soil fertility in the dryland of Loess Plateau in China. Agric. Ecosyst. Environ. 2005, 106, 313–329. [Google Scholar] [CrossRef]
- Garcíamantrana, I.; Selmaroyo, M.; Alcántara-Baena, C.; Collado, M.C. Shifts on gut microbiota associated to mediterranean diet adherence and specific dietary intakes on general adult population. Front. Microbiol. 2018, 9, 890. [Google Scholar]
- Urkurkar, J.S.; Tiwari, A.; Chitale, S.; Bajpai, R.K. Influence of longterm use of inorganic and organic manures on soil fertility and sustainable productivity of rice (Oryza sativa) and wheat (Triticum aestivum) in inceptisols. Indian J. Agric. Sci. 2010, 80, 208–212. [Google Scholar]
- Li, Y.; Wu, X.P.; He Gang Wang, Z.H. Benefits of yield, environment and economy from substituting fertilizer by manure for wheat production of China. Sci. Agric. Sin. 2020, 53, 4879–4890. (In Chinese) [Google Scholar]
- Liu, X.; Chen, Q.; Zhang, H.C.; Zhang, J.; Chen, Y.T.; Yao, F.C.; Chen, Y.T. Effects of exogenous organic matter addition on agricultural soil microbial communities and relevant enzyme activities in southern China. Sci. Rep. 2023, 13, 8045. [Google Scholar] [CrossRef]
- Caravaca, F.; Masciandaro, G.; Ceccanti, B. Land use in relation to soil chemical and biochemical properties in a semiarid Mediterranean environment. Soil Till. Res. 2002, 68, 22–30. [Google Scholar] [CrossRef]
- Saha, S.; Mina, B.L.; Gopinath, K.A.; Kundu, S.; Gupta, H.S. Organic amendments affect biochemical properties of a subtemperate soil of the Indian Himalayas. Nutr. Cycl. Agroecosyst. 2008, 80, 233–242. [Google Scholar] [CrossRef]
- Mendham, D.S.; O’Connell, A.M.; Grove, T.S.; Rance, S.J. Residue management effects on soil carbon and nutrient contents and growth of second rotation eucalypts. Forest Ecol. Manag. 2003, 181, 357–372. [Google Scholar] [CrossRef]
- Liu, Y.M.; Cao, W.Q.; Chen, X.X.; Yu, B.G.; Lang, M.; Chen, X.P.; Zou, C.Q. The responses of soil enzyme activities, microbial biomass and microbial community structure to nine years of varied zinc application rates. Sci. Total Environ. 2020, 737, 140245. [Google Scholar] [CrossRef]
- Jin, K.; Sleutel, S.; Buchan, D.; Neve, S.D.; Cai, D.X.; Gabriels, D.; Jin, J.Y. Changes of soil enzyme activities under different tillage practices in the Chinese Loess plateau. Soil Till. Res. 2009, 104, 115–120. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Carter, M.R.; Angers, D.A.; Monreal, C.M.; Ellert, B.H. Towards a minimum data set to assess soil organic matter quality in agricultural soils. Can. J. Soil Sci. 1994, 74, 367–385. [Google Scholar] [CrossRef]
- Souza, E.D.; Costa, S.E.V.G.A.; Anghinoni, I.; Carneiro, M.A.C.; Martins, A.P.; Bayer, C. Soil quality indicators in a Rhodic Paleudult under long term tillage systems. Soil Till. Res. 2014, 139, 28–36. [Google Scholar] [CrossRef]
- Chaer, G.; Fernandes, M.; Myrold, D.; Bottomley, P. Comparative resistance and resilience of soil microbial communities and enzyme activities in adjacent native forest and agricultural soils. Microb. Ecol. 2009, 58, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.N.; Waite, I.S.; Blackburn, A.; Husband, R.; Rushton, S.P.; Manning, D.C.; O’Donnell, A.G. Actinobacterial community dynamics in long term managed grasslands. Antonie Van Leeuwenhoek 2009, 95, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community composition at the continental scale. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef] [PubMed]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guo, Q.K.; Liu, D.H.; Hu, C.; Sun, J.W.; Wang, X.B.; Liang, G.Q.; Zhou, W. Composition, predicted functions, and co-occurrence networks of fungal and bacterial communities_links to soil organic carbon under long-term fertilization in a rice-wheat cropping system. Eur. J. Soil Biol. 2020, 100, 103226. [Google Scholar] [CrossRef]
- Gong, W.; Yan, X.Y.; Wang, J.Y.; Hu, T.X.; Gong, Y.B. Long-term manure and fertilizer effects on soil organic matter fractions and microbes under a wheat–maize cropping system in northern China. Geoderma 2009, 149, 318–324. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Jacquiod, S.; Franqueville, L.; Cecillon, S.; Vogel, T.M.; Simonet, P. Soil bacterial community shifts after chitin enrichment: An integrative metagenomic approach. PLoS ONE 2013, 8, e79699. [Google Scholar] [CrossRef]
- Guo, J.J.; Liu, W.B.; Zhu, C.; Luo, G.W.; Kong, Y.L.; Ling, N.; Wang, M.; Dai, J.Y.; Shen, Q.R.; Guo, S.W. Bacterial rather than fungal community composition is associated with microbial activities and nutrient-use efficiencies in a paddy soil with short-term organic amendments. Plant Soil 2018, 424, 335–349. [Google Scholar] [CrossRef]
- Pang, G.; Cai, F.; Li, R.X.; Zhao, Z.; Li, R.; Gu, X.L.; Shen, Q.R.; Chen, W. Trichoderma-enriched organic fertilizer can mitigate microbiome degeneration of monocropped soil to maintain better plant growth. Plant Soil 2017, 416, 181–192. [Google Scholar] [CrossRef]
- Lee, S.M.; Kong, H.G.; Song, G.C.; Ryu, C.M. Disruption of Firmicutes and Actinobacteria abundance in tomato rhizosphere causes the incidence of bacterial wilt disease. ISME J. 2021, 15, 330–347. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.C.; Fu, W.J.; Hu, C.W.; Chen, G.Q.; Xiao, Z.W.; Chen, Y.R.; Wang, Z.J.; Cheng, H.Y. Variation of rhizosphere microbial community in continuous mono-maize seed production. Sci. Rep. 2021, 11, 1544. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.X.; Bi, Q.F.; Hao, X.L.; Zhou, G.W.; Yang, X.R. Massilia phosphatilytica sp. nov., a phosphate solubilizing bacteria isolated from a long-term fertilized soil. Int. J. Syst. Evol. Microbiol. 2017, 67, 2514–2519. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, M.; Suarez, C.; Steffens, D.; Ratering, S.; Schnell, S. Effect of different soil phosphate sources on the active bacterial microbiota is greater in the rhizosphere than in the endorhiza of barley (Hordeum vulgare L.). Microb. Ecol. 2019, 77, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.F.A.; Pan, Y.; Bloem, J.; ten Berge, H.; Kuramae, E.E. Organic nitrogen rearranges both structure and activity of the soil-borne microbial seedbank. Sci. Rep. 2017, 7, 42634. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Wen, T.; Zhang, H.; Zhao, M.L.; Penton, C.R.; Thomashow, L.S.; Shen, Q.R. Predicting disease occurrence with high accuracy based on soil macroecological patterns of Fusarium wilt. ISME J. 2020, 14, 2936–2950. [Google Scholar] [CrossRef] [PubMed]
- Paungfoo-Lonhienne, C.; Yeoh, Y.K.; Kasinadhuni, N.R.P.; Lonhienne, T.G.; Robinson, N.; Hugenholtz, P.; Ragan, M.A.; Schmidt, S. Nitrogen fertilizer dose alters fungal communities in sugarcane soil and rhizosphere. Sci. Rep. 2015, 5, 8678. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.L.; Hu, W.J.; Tao, J.; Liu, Y.Z.; Kong, Z.Y.; Wu, L. Soil bacterial community structure and extracellular enzyme activities under different land use types in a long-term reclaimed wetland. J. Soils Sediments 2019, 19, 2543–2557. [Google Scholar] [CrossRef]
- Banerjee, S.; Walder, F.; Büchi, L.; Meyer, M.; Held, A.Y.; Gattinger, A.; Keller, T.; Charles, R.; van der Heijden, M.G.A. Agricultural intensification reduces microbial network complexity and the abundance of keystone taxa in roots. ISME J. 2019, 13, 1722–1736. [Google Scholar] [CrossRef]
- van Bruggen, A.H.C.; Finckh, M.R. Plant diseases and management approaches in organic farming systems. Annu. Rev. Phytopathol. 2016, 54, 25–54. [Google Scholar] [CrossRef]
- Berg, M.; Koskella, B. Nutrient-and dose-dependent microbiome-mediated protection against a plant pathogen. Curr. Biol. 2018, 28, 2487–2492. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, A.K.A.; Harkes, P.; van den Elsen, S.; Holterman, M.; Korthals, G.W.; Helder, J.; Kuramae, E.E. Organic amendment strengthens interkingdom associations in the soil and rhizosphere of barley (Hordeum vulgare). Sci. Total Environ. 2019, 695, 133885. [Google Scholar] [CrossRef]
- Lewis, J.A.; Fravel, D.R.; Papavizas, G.C. Cladorrhinum foecundissimum- a potential biological control agent for the reduction of Rhizoctonia solani. Soil Biol. Biochem. 1995, 27, 863–869. [Google Scholar] [CrossRef]
- Bonanomi, G.; Antignani, V.; Capodilupo, M.; Scala, F. Identifying the characteristics of organic soil amendments that suppress soilborne plant diseases. Soil Biol. Biochem. 2010, 42, 136–144. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Abdelnabby, H.; Xiao, Y. The role of a phospholipase (PLD) in virulence of Purpureocillium lilacinum (Paecilomyces lilacinum). Microb. Pathog. 2015, 85, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Talhinhas, P.; Baroncelli, R. Colletotrichum species and complexes: Geographic distribution, host range and conservation status. Fungal Divers. 2021, 110, 109–198. [Google Scholar] [CrossRef]
- Semenov, M.V.; Krasnov, G.S.; Semenov, V.M.; van Bruggen, A. Mineral and organic fertilizers distinctly affect fungal communities in the crop rhizosphere. J. Fungi 2022, 8, 251. [Google Scholar] [CrossRef]
- Wang, J.Q.; Shi, X.Z.; Zheng, C.Y.; Suter, H.; Huang, Z.Q. Different responses of soil bacterial and fungal communities to nitrogendeposition in a subtropical forest. Sci. Total Environ. 2021, 755, 142449. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, J.N.; Lu, M.; Qin, C.; Chen, Y.H.; Yang, L.; Huang, Q.W.; Wang, J.C.; Shen, Z.G.; Shen, Q.R. Microbial communities of an arable soil treated for 8 years with organic and inorganic fertilizers. Biol. Fertil. Soils 2016, 52, 455–467. [Google Scholar] [CrossRef]
- van Diepeningen, A.D.; de Vos, O.J.; Zelenev, W.; Semenov, A.M.; van Bruggen, A.H.C. DGGE fragments oscillate with or counter to fluctuations in cultivable bacteria along wheat roots. Microb. Ecol. 2005, 50, 506–517. [Google Scholar] [CrossRef] [PubMed]



| Cropping Season and Treatments | Fertilizer Source | N Rate | P2O5 Rate | K2O Rate | |
|---|---|---|---|---|---|
| Basal N | Topdressing N | ||||
| (kg ha−1) | |||||
| Wheat cropping season | |||||
| CK | CF | 0 | 0 | 105 | 75 |
| MF | CF | 97.5 | 97.5 | 105 | 75 |
| 20% OF | CF | 58.5 | 97.5 | 58.5 | 38.4 |
| OF | 39 | 0 | 46.5 | 36.6 | |
| 40% OF | CF | 19.5 | 97.5 | 12 | 1.8 |
| OF | 78 | 0 | 93 | 73.2 | |
| Maize cropping season | |||||
| CK | CF | 0 | 0 | 120 | 135 |
| MF | CF | 112.5 | 112.5 | 120 | 135 |
| 20% OF | CF | 112.5 | 112.5 | 120 | 135 |
| 40% OF | CF | 112.5 | 112.5 | 120 | 135 |
| Treatment | pH | Organic Matter (%) | Total N (g kg−1) | Available K (mg kg−1) | Available P (mg kg−1) |
|---|---|---|---|---|---|
| CK | 9.0 ± 0.18 a | 1.7 ± 0.38 b | 1.1 ± 0.08 b | 199 ± 13.11 a | 17.9 ± 2.73 a |
| MF | 8.9 ± 0.18 b | 2.0 ± 0.44 ab | 1.2 ± 0.21 ab | 200.3 ± 16.52 a | 18.9 ± 6.11 a |
| 20% OF | 8.8 ± 0.15 b | 2.1 ± 0.27 a | 1.3 ± 0.11 a | 202.0 ± 8.21 a | 20.9 ± 4.23 a |
| 40% OF | 8.9 ± 0.13 b | 2.2 ± 0.14 a | 1.3 ± 0.12 a | 201.7 ± 12.42 a | 19.5 ± 5.41 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xu, Y.; Jiang, L.; Yang, Y.; Shi, J.; Guan, X.; Sun, T.; Zhao, H.; Wang, Y.; Liu, Y. Effect of Mild Organic Substitution on Soil Quality and Microbial Community. Agronomy 2024, 14, 888. https://doi.org/10.3390/agronomy14050888
Wang Y, Xu Y, Jiang L, Yang Y, Shi J, Guan X, Sun T, Zhao H, Wang Y, Liu Y. Effect of Mild Organic Substitution on Soil Quality and Microbial Community. Agronomy. 2024; 14(5):888. https://doi.org/10.3390/agronomy14050888
Chicago/Turabian StyleWang, Yijun, Yu Xu, Lihua Jiang, Yan Yang, Jing Shi, Xilin Guan, Tao Sun, Huanyu Zhao, Yafei Wang, and Yumin Liu. 2024. "Effect of Mild Organic Substitution on Soil Quality and Microbial Community" Agronomy 14, no. 5: 888. https://doi.org/10.3390/agronomy14050888
APA StyleWang, Y., Xu, Y., Jiang, L., Yang, Y., Shi, J., Guan, X., Sun, T., Zhao, H., Wang, Y., & Liu, Y. (2024). Effect of Mild Organic Substitution on Soil Quality and Microbial Community. Agronomy, 14(5), 888. https://doi.org/10.3390/agronomy14050888






