Fertilising Maize with Bio-Based Mineral Fertilisers Gives Similar Growth to Conventional Fertilisers and Does Not Alter Soil Microbiome
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Bio-Based Fertilisers
2.2. Microcosm Assay Design
2.3. Sampling and Plant and Soil Chemical Analysis
2.4. Metataxonomic Analysis
2.5. Data Analysis
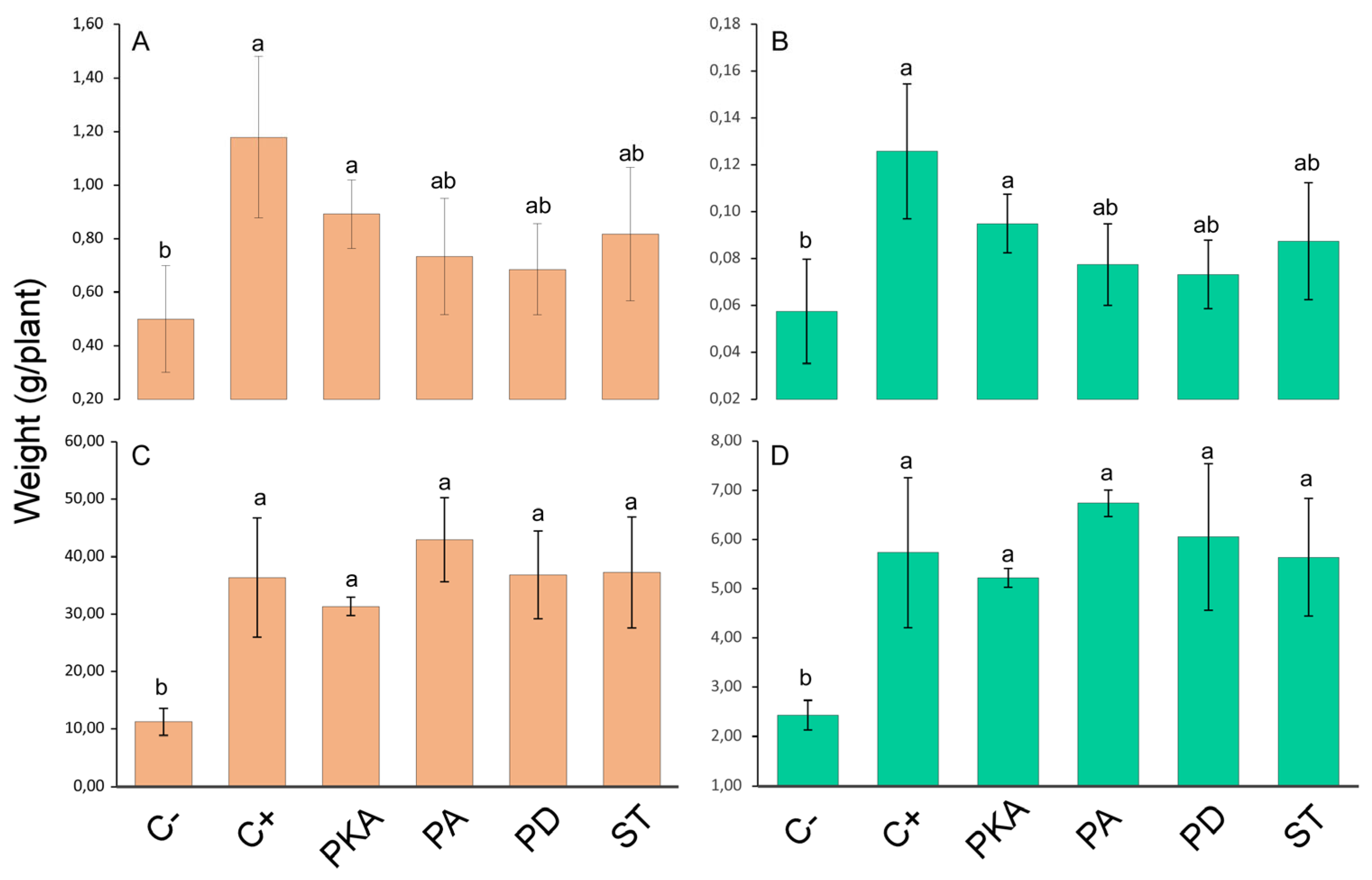
3. Results
3.1. Plant Growth Parameters
3.2. Plant and Soil Nutrient Content
3.2.1. Plant Nutrient Content
3.2.2. Soil Nutrient Content
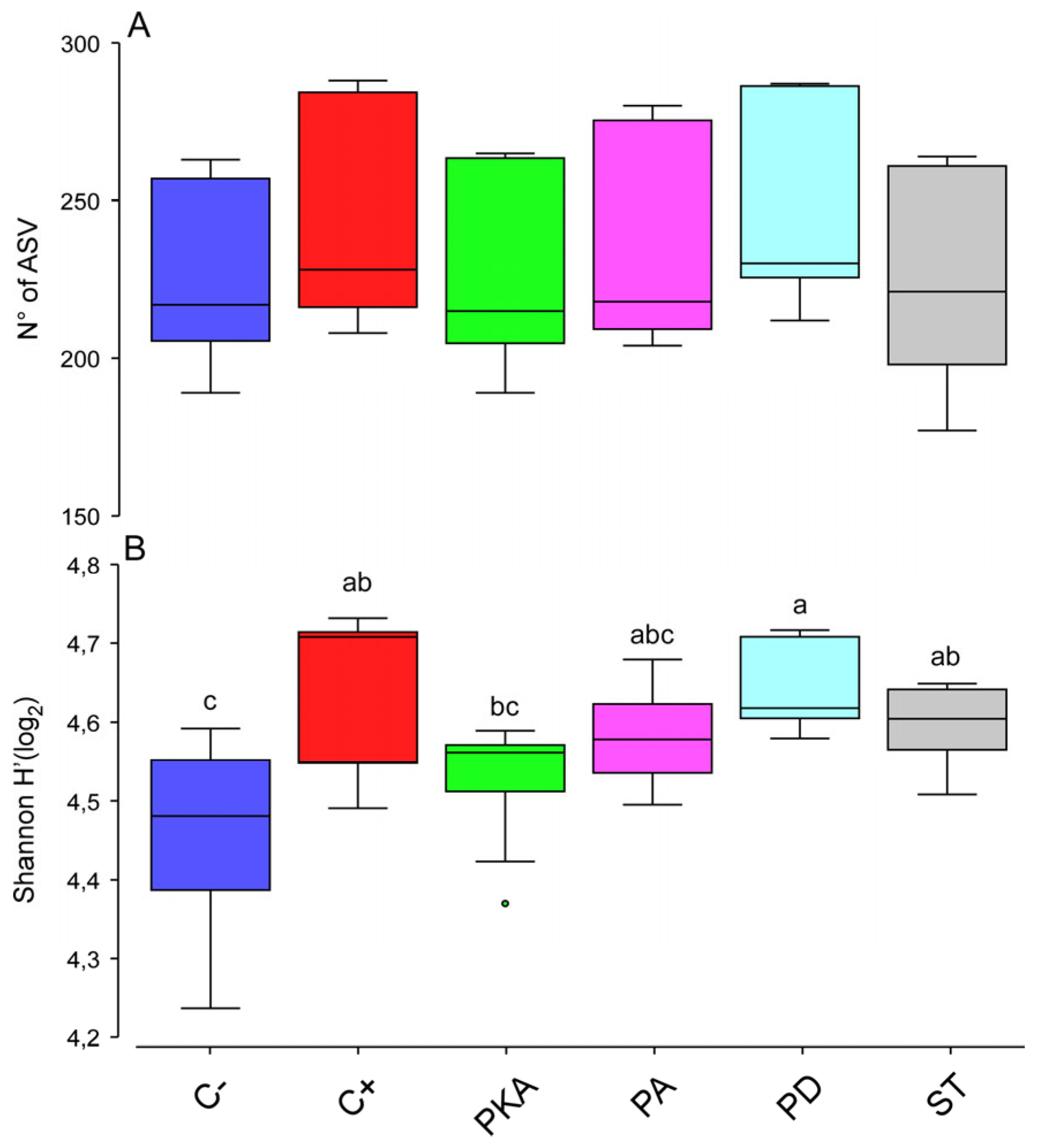
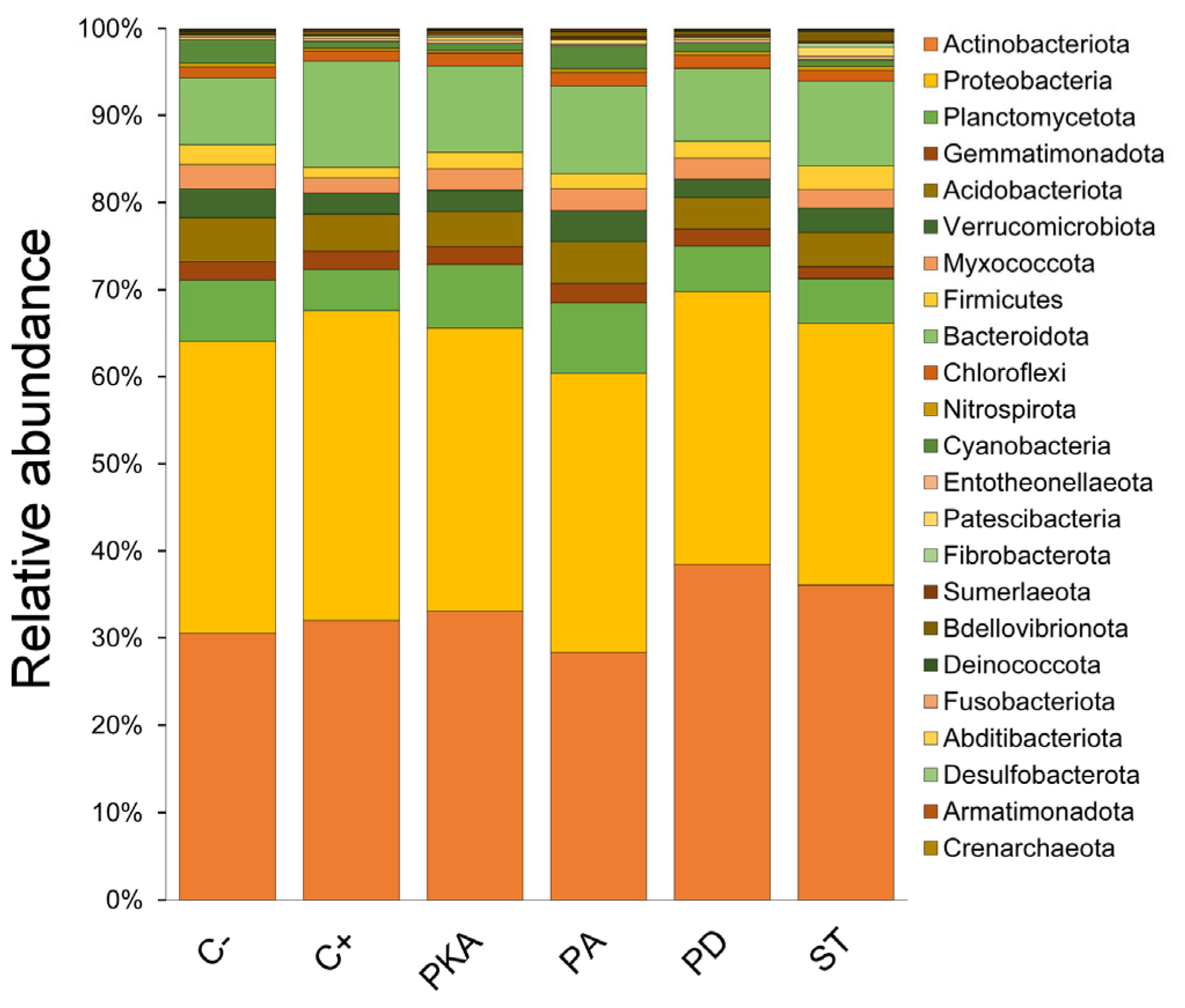
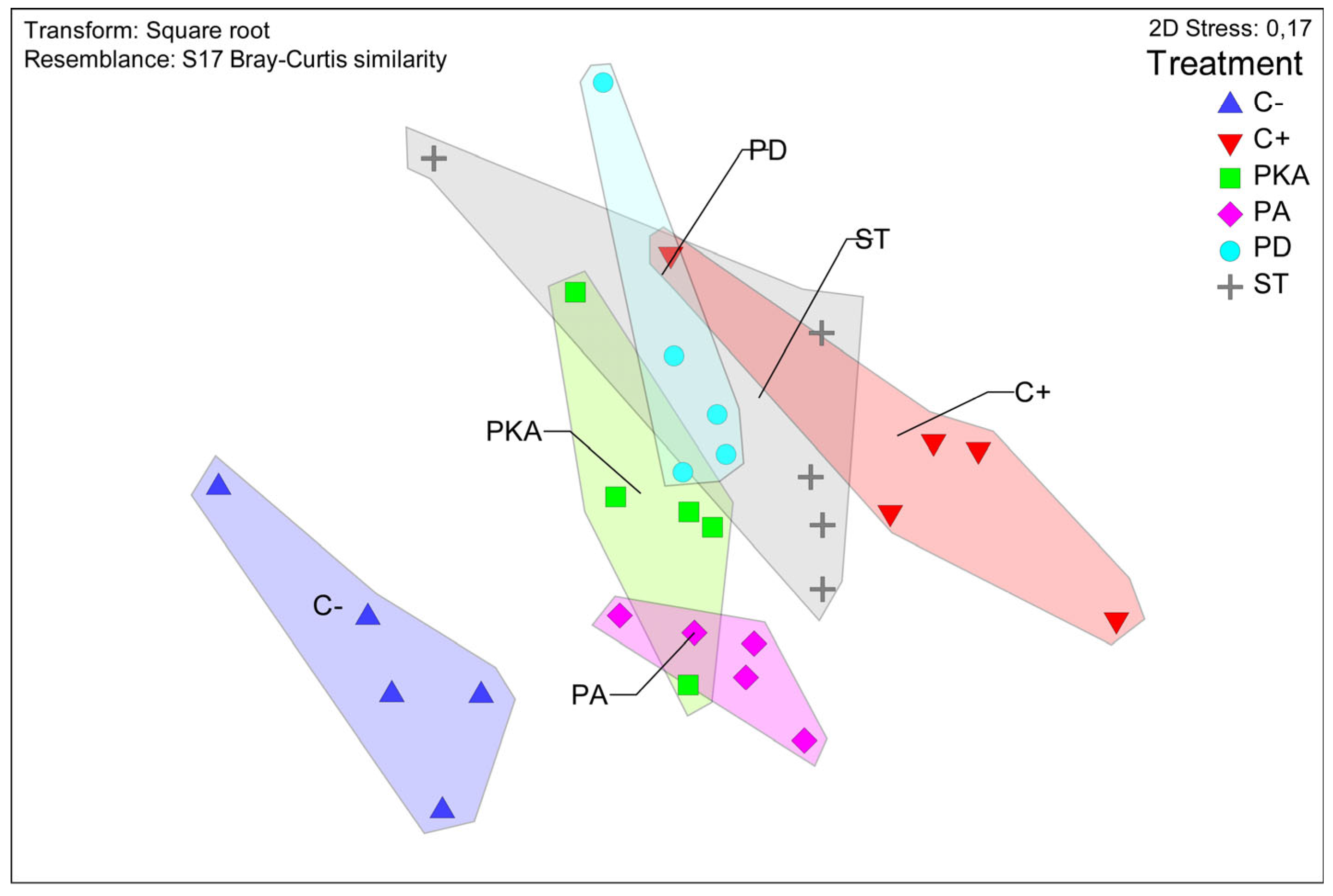
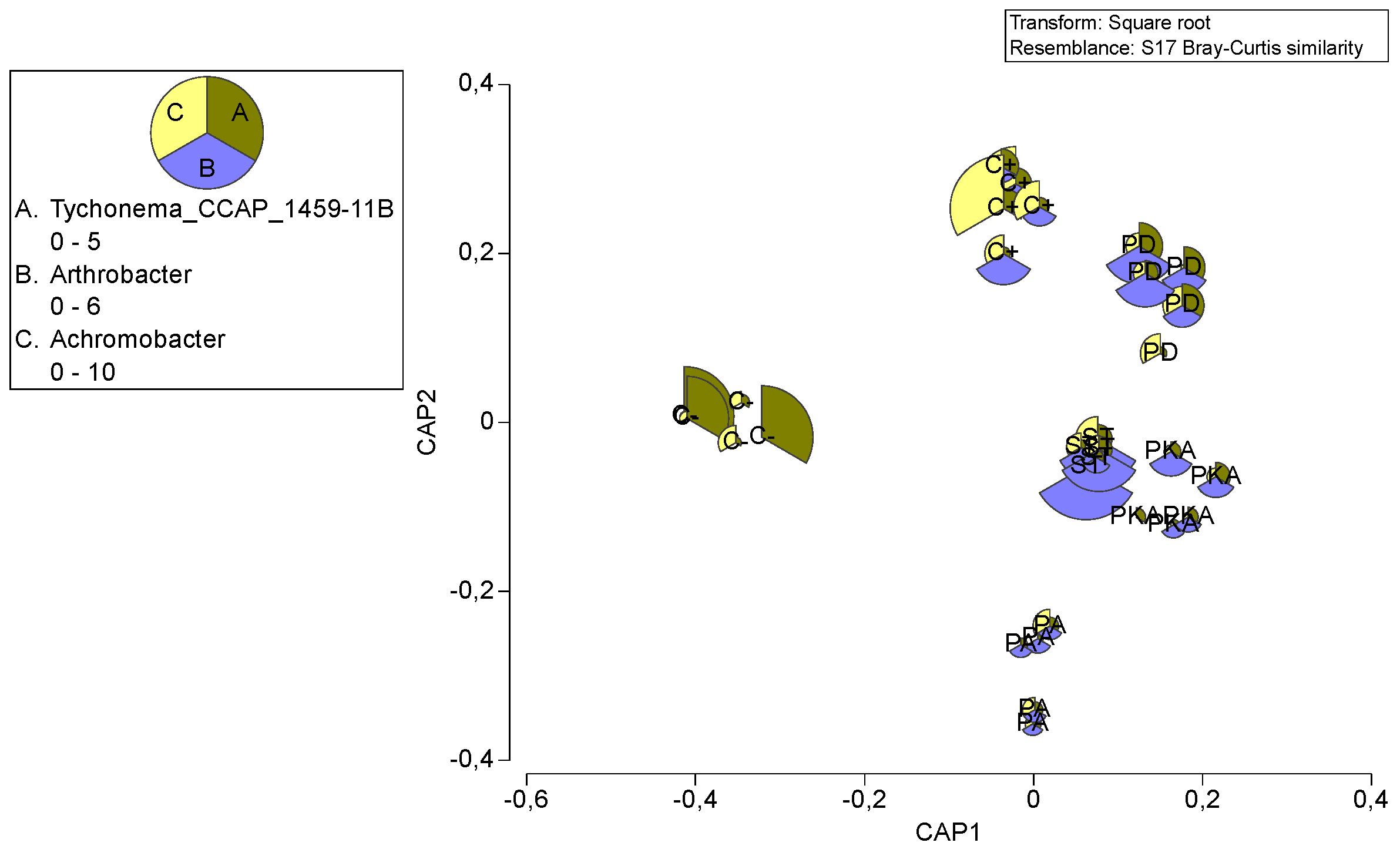
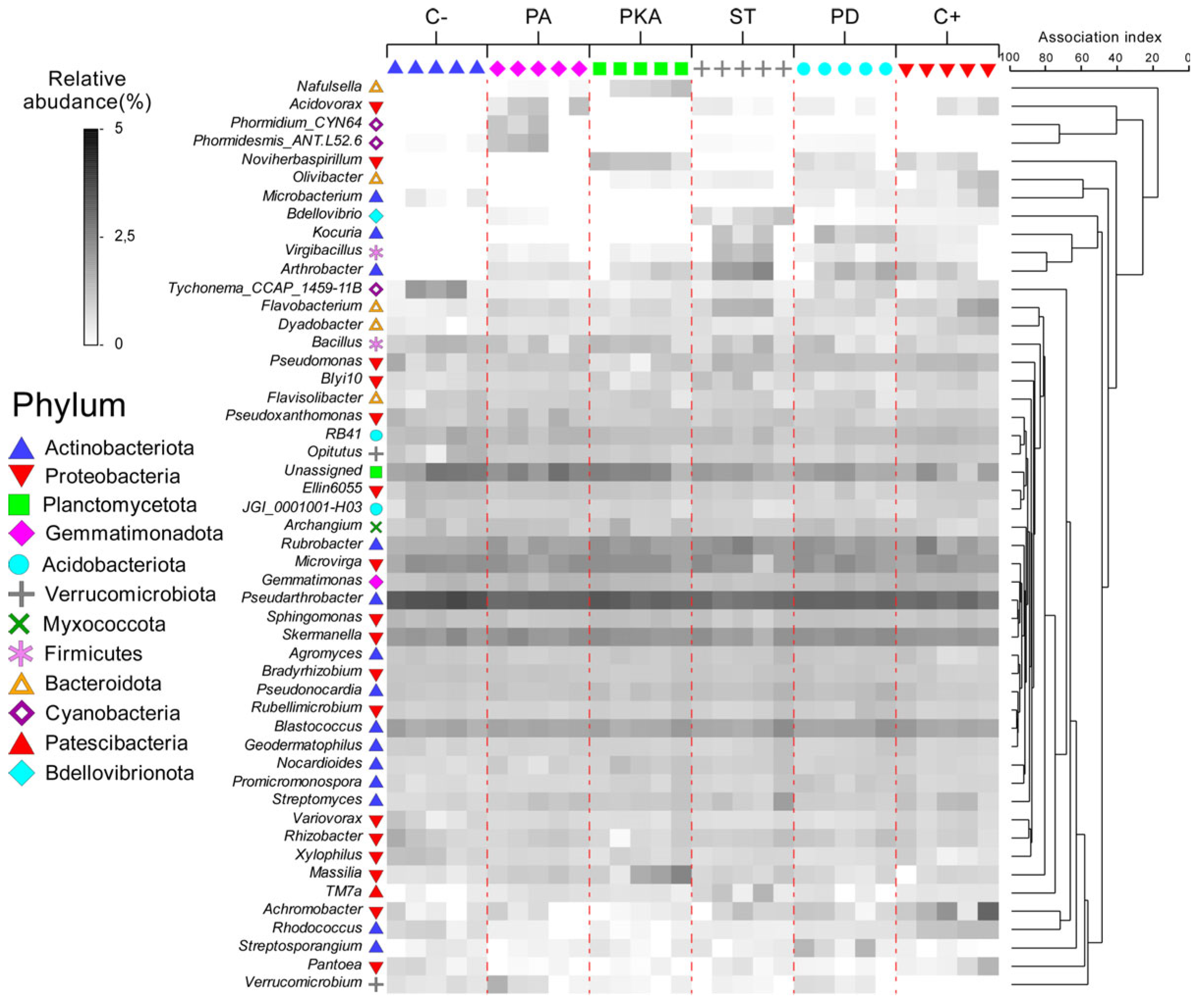
3.3. Metataxonomic Analysis
Effect of Fertiliser Application on Soil Bacterial Diversity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- European Union. Directive 2008/98/EC of the European Parliament and the Council of 19 November 2008 on Waste and Repealing Certain Directives. Off. J. Eur. Union 2008, 312, 3–30. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008L0098 (accessed on 15 January 2024).
- Van der Linden, A.; Reichel, A. Bio-Waste in Europe—Turning Challenges into Opportunities—European Environment Agency. Available online: https://www.eea.europa.eu/publications/bio-waste-in-europe (accessed on 16 January 2024).
- Lin, C.S.K.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.H.; Koutinas, A.A.; Kopsahelis, N.; Stamatelatou, K.; Dickson, F.; et al. Food Waste as a Valuable Resource for the Production of Chemicals, Materials and Fuels. Current Situation and Global Perspective. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar] [CrossRef]
- Vea, E.B.; Romeo, D.; Thomsen, M. Biowaste Valorisation in a Future Circular Bioeconomy. Procedia CIRP 2018, 69, 591–596. [Google Scholar] [CrossRef]
- Luque, R.; Clark, J.H. Valorisation of Food Residues: Waste to Wealth Using Green Chemical Technologies. Sustain. Chem. Process 2013, 1, 10. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, S.E.; Lee, D.W. Current Status and Future Prospects of Biological Routes to Bio-Based Products Using Raw Materials, Wastes, and Residues as Renewable Resources. Crit. Rev. Environ. Sci. Technol. 2022, 52, 2453–2509. [Google Scholar] [CrossRef]
- Langeveld, J.W.A.; Dixon, J.; Jaworski, J.F. Development Perspectives of The Biobased Economy: A Review. Crop. Sci. 2010, 50, S-142–S-151. [Google Scholar] [CrossRef]
- Kyttä, V.; Helenius, J.; Tuomisto, H.L. Carbon Footprint and Energy Use of Recycled Fertilizers in Arable Farming. J. Clean Prod. 2021, 287, 125063. [Google Scholar] [CrossRef]
- Wesseler, J. The EU’s Farm-to-Fork Strategy: An Assessment from the Perspective of Agricultural Economics. Appl. Econ. Perspect. Policy 2022, 44, 1826–1843. [Google Scholar] [CrossRef]
- Billen, G.; Aguilera, E.; Einarsson, R.; Garnier, J.; Gingrich, S.; Grizzetti, B.; Lassaletta, L.; Le Noë, J.; Sanz-Cobena, A. Beyond the Farm to Fork Strategy: Methodology for Designing a European Agro-Ecological Future. Sci. Total Environ. 2024, 908, 168160. [Google Scholar] [CrossRef]
- Jagtap, S.; Trollman, H.; Trollman, F.; Garcia-Garcia, G.; Parra-López, C.; Duong, L.; Martindale, W.; Munekata, P.E.S.; Lorenzo, J.M.; Hdaifeh, A.; et al. The Russia-Ukraine Conflict: Its Implications for the Global Food Supply Chains. Foods 2022, 11, 2098. [Google Scholar] [CrossRef]
- Sigurnjak, I.; Brienza, C.; Snauwaert, E.; De Dobbelaere, A.; De Mey, J.; Vaneeckhaute, C.; Michels, E.; Schoumans, O.; Adani, F.; Meers, E. Production and Performance of Bio-Based Mineral Fertilizers from Agricultural Waste Using Ammonia (Stripping-) Scrubbing Technology. Waste Manag. 2019, 89, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Vaneeckhaute, C.; Lebuf, V.; Michels, E.; Belia, E.; Vanrolleghem, P.A.; Tack, F.M.G.; Meers, E. Nutrient Recovery from Digestate: Systematic Technology Review and Product Classification. Waste Biomass Valoriz. 2017, 8, 21–40. [Google Scholar] [CrossRef]
- Hansen, J. EU Must Get Serious about Promoting the Circular Economy. 2018. Available online: https://www.theparliamentmagazine.eu/articles/partner_article/fertilizers-europe/eu-must-get-serious-about-promoting-circular-economy (accessed on 21 February 2024).
- Chojnacka, K.; Moustakas, K.; Witek-Krowiak, A. Bio-Based Fertilizers: A Practical Approach towards Circular Economy. Bioresour. Technol. 2020, 295, 122223. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Mikula, K.; Skrzypczak, D.; Izydorczyk, G.; Gorazda, K.; Kulczycka, J.; Kominko, H.; Moustakas, K.; Witek-Krowiak, A. Practical aspects of biowastes conversion to fertilizers. Biomass Convers. Biorefin. 2022, 14, 1515–1533. [Google Scholar] [CrossRef]
- Tur-Cardona, J.; Bonnichsen, O.; Speelman, S.; Verspecht, A.; Carpentier, L.; Debruyne, L.; Marchand, F.; Jacobsen, B.H.; Buysse, J. Farmers’ Reasons to Accept Bio-Based Fertilizers: A Choice Experiment in Seven Different European Countries. J. Clean Prod. 2018, 197, 406–416. [Google Scholar] [CrossRef]
- Hertzberger, A.J.; Cusick, R.D.; Margenot, A.J. A review and meta-analysis of the agricultural potential of struvite as a phosphorus fertilizer. Soil Sci. Soc. Am. J. 2020, 84, 653–671. [Google Scholar] [CrossRef]
- Silva, F.C.; Cruz, N.C.; Tarelho, L.A.C.; Rodrigues, S.M. Use of Biomass Ash-Based Materials as Soil Fertilisers: Critical Review of the Existing Regulatory Framework. J. Clean. Prod. 2019, 214, 112–124. [Google Scholar] [CrossRef]
- European Union. Regulation (EU) 2019/1009 of the European Parliament and the Council of 5 June 2019 on Biostimulants. Off. J. Eur. Union 2019, 170, 1–114. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32019R1009 (accessed on 16 April 2024).
- Thomas, R.F.; Booth, R.L. Selective Electrode Measurement of Ammonia in Water and Wastes. Environ. Sci. Technol. 1973, 7, 523–526. [Google Scholar] [CrossRef]
- Norman, R.J.; Stucki, J.W. The Determination of Nitrate and Nitrite in Soil Extracts by Ultraviolet Spectrophotometry. Soil Sci. Soc. Am. J. 1981, 45, 347–353. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, R.; Edwards, R. Quality Control and Preprocessing of Metagenomic Datasets. Bioinformatics 2011, 27, 863–864. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of RRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.R.; Gorley, R.N. Getting Started with PRIMER V7. In PRIMER-E: Plymouth; Plymouth Marine Laboratory: Devon, UK, 2015. [Google Scholar]
- Kurniawati, A.; Toth, G.; Ylivainio, K.; Toth, Z. Opportunities and Challenges of Bio-Based Fertilizers Utilization for Improving Soil Health. Org. Agr. 2023, 13, 335–350. [Google Scholar] [CrossRef]
- Garmendia-Lemus, S.; Moshkin, E.; Hung, Y.; Tack, J.; Buysse, J. European Farmers’ Perceptions and Intentions to Use Bio-Based Fertilisers: Insights from the Theory of Planned Behaviour and Perceived Utility. J. Clean Prod. 2024, 434, 139755. [Google Scholar] [CrossRef]
- Vaneeckhaute, C.; Ghekiere, G.; Michels, E.; Vanrolleghem, P.A.; Tack, F.M.G.; Meers, E. Assessing Nutrient Use Efficiency and Environmental Pressure of Macronutrients in Biobased Mineral Fertilizers: A Review of Recent Advances and Best Practices at Field Scale. Adv. Agron. 2014, 128, 137–180. [Google Scholar] [CrossRef]
- Nkoa, R. Agricultural Benefits and Environmental Risks of Soil Fertilization with Anaerobic Digestates: A Review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef]
- Sayara, T.; Basheer-Salimia, R.; Hawamde, F.; Sánchez, A. Recycling of Organic Wastes through Composting: Process Performance and Compost Application in Agriculture. Agronomy 2020, 10, 1838. [Google Scholar] [CrossRef]
- Wierzbowska, J.; Sienkiewicz, S.; Zarczyński, P.; Krzebietke, S. Environmental Application of Ash from Incinerated Biomass. Agronomy 2020, 10, 482. [Google Scholar] [CrossRef]
- Sniatala, B.; Kurniawan, T.A.; Sobotka, D.; Makinia, J.; Othman, M.H.D. Macro-Nutrients Recovery from Liquid Waste as a Sustainable Resource for Production of Recovered Mineral Fertilizer: Uncovering Alternative Options to Sustain Global Food Security Cost-Effectively. Sci. Total Environ. 2023, 856, 159283. [Google Scholar] [CrossRef] [PubMed]
- Rizzioli, F.; Bertasini, D.; Bolzonella, D.; Frison, N.; Battista, F. A Critical Review on the Techno-Economic Feasibility of Nutrients Recovery from Anaerobic Digestate in the Agricultural Sector. Sep. Purif. Technol. 2023, 306, 122690. [Google Scholar] [CrossRef]
- Mclaughlin, M.J.; Mcbeath, T.M.; Smernik, R.; Stacey, S.P.; Ajiboye, B.; Guppy, C.; Mclaughlin, M.J.; Mcbeath, T.M.; Smernik, R.; Stacey, S.P.; et al. The Chemical Nature of P Accumulation in Agricultural Soils—Implications for Fertiliser Management and Design: An Australian Perspective. Plant Soil 2011, 349, 69–87. [Google Scholar] [CrossRef]
- Hart, M.R.; Quin, B.F.; Nguyen, M.L. Phosphorus Runoff from Agricultural Land and Direct Fertilizer Effects: A Review. J. Environ. Qual. 2004, 33, 1954–1972. [Google Scholar] [CrossRef] [PubMed]
- Arenberg, M.R.; Arai, Y. Uncertainties in Soil Physicochemical Factors Controlling Phosphorus Mineralization and Immobilization Processes. Adv. Agron. 2019, 154, 153–200. [Google Scholar] [CrossRef]
- Saliu, T.D.; Oladoja, N.A. Nutrient Recovery from Wastewater and Reuse in Agriculture: A Review. Environ. Chem. Lett. 2021, 19, 2299–2316. [Google Scholar] [CrossRef]
- Schiemenz, K.; Kern, J.; Paulsen, H.-M.; Bachmann, S.; Eichler-Löbermann, B. Phosphorus Fertilizing Effects of Biomass Ashes. In Recycling of Biomass Ashes; Insam, H., Knapp, B.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 17–31. [Google Scholar] [CrossRef]
- Talboys, P.J.; Heppell, J.; Roose, T.; Healey, J.R.; Jones, D.L.; Withers, P.J.A. Struvite: A Slow-Release Fertiliser for Sustainable Phosphorus Management? Plant Soil 2016, 401, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Doran, J.W.; Parkin, T.B. Defining and assessing soil quality. In Defining Soil Quality for a Sustainable Environment; Doran, J.W., Coleman, D.C., Bezdicek, D.F., Stewart, B.A., Eds.; Soil Science Society of America: Madison, WI, USA, 1994; pp. 3–21. [Google Scholar] [CrossRef]
- Lehmann, J.; Bossio, D.A.; Kögel-Knabner, I.; Rillig, M.C. The Concept and Future Prospects of Soil Health. Nat. Rev. Earth Environ. 2020, 1, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Dincă, L.C.; Grenni, P.; Onet, C.; Onet, A. Fertilization and Soil Microbial Community: A Review. Appl. Sci. 2022, 12, 1198. [Google Scholar] [CrossRef]
- Sabir, M.S.; Shahzadi, F.; Ali, F.; Shakeela, Q.; Niaz, Z.; Ahmed, S. Comparative Effect of Fertilization Practices on Soil Microbial Diversity and Activity: An Overview. Curr. Microbiol. 2021, 78, 3644–3655. [Google Scholar] [CrossRef]
- Semenov, M.V.; Krasnov, G.S.; Semenov, V.M.; van Bruggen, A.H.C. Long-Term Fertilization Rather than Plant Species Shapes Rhizosphere and Bulk Soil Prokaryotic Communities in Agroecosystems. Appl. Soil Ecol. 2020, 154, 103641. [Google Scholar] [CrossRef]
- Santoni, M.; Verdi, L.; Imran Pathan, S.; Napoli, M.; Dalla Marta, A.; Dani, F.R.; Pacini, G.C.; Ceccherini, M.T. Soil Microbiome Biomass, Activity, Composition and CO2 Emissions in a Long-Term Organic and Conventional Farming Systems. Soil Use Manag. 2023, 39, 588–605. [Google Scholar] [CrossRef]
- Poveda, J. Cyanobacteria in Plant Health: Biological Strategy against Abiotic and Biotic Stresses. Crop. Prot. 2021, 141, 105450. [Google Scholar] [CrossRef]
- Capelli, C.; Cerasino, L.; Boscaini, A.; Salmaso, N. Molecular Tools for the Quantitative Evaluation of Potentially Toxigenic Tychonema Bourrellyi (Cyanobacteria, Oscillatoriales) in Large Lakes. Hydrobiologia 2018, 824, 109–119. [Google Scholar] [CrossRef]
- Salmaso, N.; Cerasino, L.; Boscaini, A.; Capelli, C. Planktic Tychonema (Cyanobacteria) in the Large Lakes South of the Alps: Phylogenetic Assessment and Toxigenic Potential. FEMS Microbiol. Ecol. 2016, 92, fiw155. [Google Scholar] [CrossRef]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial Features of Plant Growth-Promoting Rhizobacteria for Improving Plant Growth and Health in Challenging Conditions: A Methodical Review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef]
- Santoyo, G.; Urtis-Flores, C.A.; Loeza-Lara, P.D.; Orozco-Mosqueda, M.D.C.; Glick, B.R. Rhizosphere Colonization Determinants by Plant Growth-Promoting Rhizobacteria (PGPR). Biology 2021, 10, 475. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Beneficial Plant-Bacterial Interactions, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–383. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd-Allah, E.F.; Hashem, A. Phytohormones and Beneficial Microbes: Essential Components for Plants to Balance Stress and Fitness. Front. Microbiol. 2017, 8, 2104. [Google Scholar] [CrossRef]
- Khan, M.A.; Ullah, I.; Waqas, M.; Hamayun, M.; Khan, A.L.; Asaf, S.; Kang, S.M.; Kim, K.M.; Jan, R.; Lee, I.J. Halo-Tolerant Rhizospheric Arthrobacter woluwensis AK1 Mitigates Salt Stress and Induces Physio-Hormonal Changes and Expression of GmST1 and GmLAX3 in Soybean. Symbiosis 2019, 77, 9–21. [Google Scholar] [CrossRef]
- Santana, M.M.; Rosa, A.P.; Zamarreño, A.M.; García-Mina, J.M.; Rai, A.; Cruz, C. Achromobacter xylosoxidans and Enteromorpha intestinalis Extract Improve Tomato Growth under Salt Stress. Agronomy 2022, 12, 934. [Google Scholar] [CrossRef]
- Singh, P.; Pandey, S.S.; Dubey, B.K.; Raj, R.; Barnawal, D.; Chandran, A.; Rahman, L.U. Salt and Drought Stress Tolerance with Increased Biomass in Transgenic Pelargonium graveolens through Heterologous Expression of ACC Deaminase Gene from Achromobacter xylosoxidans. Plant. Cell. Tiss. Organ. Cult. 2021, 147, 297–311. [Google Scholar] [CrossRef]
- Vanissa, T.T.G.; Berger, B.; Patz, S.; Becker, M.; Turečková, V.; Novák, O.; Tarkowská, D.; Henri, F.; Ruppel, S. The Response of Maize to Inoculation with Arthrobacter sp. and Bacillus sp. in Phosphorus-Deficient, Salinity-Affected Soil. Microorganisms 2020, 8, 1005. [Google Scholar] [CrossRef] [PubMed]
- Samain, E.; Duclercq, J.; Ait Barka, E.; Eickermann, M.; Ernenwein, C.; Mazoyon, C.; Sarazin, V.; Dubois, F.; Aussenac, T.; Selim, S. PGPR-Soil Microbial Communities’ Interactions and Their Influence on Wheat Growth Promotion and Resistance Induction against Mycosphaerella graminicola. Biology 2023, 12, 1416. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.H.; Yoon, A.R.; Oh, H.E.; Park, Y.G. Plant Growth-Promoting Microorganism Pseudarthrobacter sp. NIBRBAC000502770 Enhances the Growth and Flavonoid Content of Geum aleppicum. Microorganisms 2022, 10, 1241. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Deng, X.; Ma, L.; Wang, H.; Wang, X.; Feng, L.; Zhu, F.; Xue, S.; Mohammad, A. Standardized Framework for Assessing Soil Quality at Antimony Smelting Site by Considering Microbial-Induced Resilience and Heavy Metal Contamination. J. Environ. Sci. 2024, 148, 306–320. [Google Scholar] [CrossRef]
- Wongkiew, S.; Chaikaew, P.; Takrattanasaran, N.; Khamkajorn, T. Evaluation of Nutrient Characteristics and Bacterial Community in Agricultural Soil Groups for Sustainable Land Management. Sci. Rep. 2022, 12, 7368. [Google Scholar] [CrossRef]
- Korkar, M.H.; Magdy, M.; Rizk, S.M.; Fiteha, Y.G.; Atta, A.H.; Rashed, M.A.S. Rhizosphere-Associated Microbiome Profile of Agricultural Reclaimed Lands in Egypt. Agronomy 2022, 12, 2543. [Google Scholar] [CrossRef]
- Cavite, H.J.M.; Mactal, A.G.; Evangelista, E.V.; Cruz, J.A. Growth and Yield Response of Upland Rice to Application of Plant Growth-Promoting Rhizobacteria. J. Plant Growth Regul. 2021, 40, 494–508. [Google Scholar] [CrossRef]
- Koo, Y.M.; Heo, A.Y.; Choi, H.W. Isolation and Identification Antagonistic Bacterium Paenibacillus tianmuensis YM002 against Acidovorax citrulli. Front. Plant Sci. 2023, 14, 1173695. [Google Scholar] [CrossRef]
- Davydov, D.; Vilnet, A. Review of the Cyanobacterial Genus Phormidesmis (Leptolyngbyaceae) with the Descrition of Apatinema gen. nov. Diversity 2022, 14, 731. [Google Scholar] [CrossRef]
- Múnera-Porras, L.M.; García-Londoño, S.; Ríos-Osorio, L.A. Action Mechanisms of Plant Growth Promoting Cyanobacteria in Crops in Situ: A Systematic Review of Literature. Int. J. Agron. 2020, 2020, 2690410. [Google Scholar] [CrossRef]
- Dhawi, F. How Can We Stabilize Soil Using Microbial Communities and Mitigate Desertification? Sustainability 2023, 15, 863. [Google Scholar] [CrossRef]
- Do Rêgo Barros, F.M.; Pedrinho, A.; Mendes, L.W.; Freitas, C.C.G.; Andreote, F.D. Interactions between Soil Bacterial Diversity and Plant-Parasitic Nematodes in Soybean Plants. Appl. Environ. Microbiol. 2022, 88, e00963-22. [Google Scholar] [CrossRef]
- Rodríguez-Berbel, N.; Ortega, R.; Lucas-Borja, M.E.; Solé-Benet, A.; Miralles, I. Long-Term Effects of Two Organic Amendments on Bacterial Communities of Calcareous Mediterranean Soils Degraded by Mining. J. Environ. Manag. 2020, 271, 110920. [Google Scholar] [CrossRef]
- Zotti, M.; Bonanomi, G.; Mancinelli, G.; Barquero, M.; De Filippis, F.; Giannino, F.; Mazzoleni, S.; González-Andrés, F. Riding the Wave: Response of Bacterial and Fungal Microbiota Associated with the Spread of the Fairy Ring Fungus Calocybe Gambosa. Appl. Soil Ecol. 2021, 163, 103963. [Google Scholar] [CrossRef]
- Navarro-Noya, Y.E.; Martínez-Romero, E.; Hernández-Rodríguez, C. Potential Plant-Growth-Promoting and Nitrogen-Fixing Bacteria Associated with Pioneer Plants Growing on Mine Tailings. In Molecular Microbial Ecology of the Rhizosphere; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; Volume 2, pp. 1003–1011. [Google Scholar] [CrossRef]
- De Tender, C.; Vandecasteele, B.; Verstraeten, B.; Ommeslag, S.; Kyndt, T.; Debode, J. Biochar-Enhanced Resistance to Botrytis cinerea in Strawberry Fruits (But Not Leaves) Is Associated with Changes in the Rhizosphere Microbiome. Front. Plant Sci. 2021, 12, 700479. [Google Scholar] [CrossRef]
- Martins, S.J.; Taerum, S.J.; Triplett, L.; Emerson, J.B.; Zasada, I.; de Toledo, B.F.; Kovac, J.; Martin, K.; Bull, C.T. Predators of Soil Bacteria in Plant and Human Health. Phytobiomes J. 2022, 6, 184–200. [Google Scholar] [CrossRef]
- Li, X.; Sun, P.; Zhang, Y.; Jin, C.; Guan, C. A Novel PGPR Strain Kocuria rhizophila Y1 Enhances Salt Stress Tolerance in Maize by Regulating Phytohormone Levels, Nutrient Acquisition, Redox Potential, Ion Homeostasis, Photosynthetic Capacity and Stress-Responsive Genes Expression. Environ. Exp. Bot. 2020, 174, 104023. [Google Scholar] [CrossRef]
- Renoud, S.; Abrouk, D.; Prigent-Combaret, C.; Wisniewski-Dyé, F.; Legendre, L.; Moënne-Loccoz, Y.; Muller, D. Effect of Inoculation Level on the Impact of the PGPR Azospirillum lipoferum CRT1 on Selected Microbial Functional Groups in the Rhizosphere of Field Maize. Microorganisms 2022, 10, 325. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Liu, M.; Xu, J.; Bian, H.; Chen, T.; You, E.; Deng, C.; Wei, Y.; Yang, T.; et al. Effects of Different Fertilization Conditions and Different Geographical Locations on the Diversity and Composition of the Rhizosphere Microbiota of Qingke (Hordeum Vulgare L.) Plants in Different Growth Stages. Front. Microbiol. 2023, 14, 1094034. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, X.; Ma, C.; Wu, F.; Jin, X.; Dini-Andreote, F.; Wei, Z. Biochar Amendment Reduces Cadmium Uptake by Stimulating Cadmium-Resistant PGPR in Tomato Rhizosphere. Chemosphere 2022, 307, 136138. [Google Scholar] [CrossRef]
- Andrews, M.; Andrews, M.E. Specificity in Legume-Rhizobia Symbioses. Int. J. Mol. Sci. 2017, 18, 705. [Google Scholar] [CrossRef]
- Ardley, J.K.; Parker, M.A.; De Meyer, S.E.; Trengove, R.D.; O’Hara, G.W.; Reeve, W.G.; Yates, R.J.; Dilworth, M.J.; Willems, A.; Howieson, J.G. Microvirga Lupini sp. nov., Microvirga lotononidis sp. nov. and Microvirga zambiensis sp. nov. are Alphaproteobacterial Root-Nodule Bacteria That Specifically Nodulate and Fix Nitrogen with Geographically and Taxonomically Separate Legume Hosts. Int. J. Syst. Evol. Microbiol. 2012, 62, 2579–2588. [Google Scholar] [CrossRef]
- Zhang, L.N.; Jiang, C.H.; Si, F.; Song, N.; Yang, W.; Zhu, Y.; Luo, Y.; Guo, J.H. Long-Term Field Application of a Plant Growth-Promoting Rhizobacterial Consortium Suppressed Root-Knot Disease by Shaping the Rhizosphere Microbiota. Plant Dis. 2024, 108, 94–103. [Google Scholar] [CrossRef]
- Viaene, T.; Langendries, S.; Beirinckx, S.; Maes, M.; Goormachtig, S. Streptomyces as a Plant’s Best Friend? FEMS Microbiol. Ecol. 2016, 92, fiw119. [Google Scholar] [CrossRef]
- Yao, X.; Huang, K.; Zhao, S.; Cheng, Q.; Zhang, S.; Yang, L.; Ding, W.; Zhang, Y. Identification and Verification of Rhizosphere Indicator Microorganisms in Tobacco Root Rot. Agron. J. 2021, 113, 1480–1491. [Google Scholar] [CrossRef]
- Essghaier, B.; Mrah, S.; ben Jalloul, A.; Ghazghazi, H.; Ahmed, H. Ben Ability of Virgibacillus marismortui and Salinococcus roseus for Plant Growth Promotion by Evaluating Their Effect on Physiological and Morphological Parameters in Vitro and in Soilless System. Biologia 2022, 77, 2257–2267. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, R.N.; Song, X.P.; Singh, R.K.; Guo, D.J.; Singh, P.; Verma, K.K.; Li, Y.R. Genome Analysis of a Halophilic Virgibacillus halodenitrificans ASH15 Revealed Salt Adaptation, Plant Growth Promotion, and Isoprenoid Biosynthetic Machinery. Front. Microbiol. 2023, 14, 1229955. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, X.; Shen, Z.; Zhu, C.; Jiao, Z.; Li, R.; Shen, Q. Pre-Colonization of PGPR Triggers Rhizosphere Microbiota Succession Associated with Crop Yield Enhancement. Plant Soil 2019, 439, 553–567. [Google Scholar] [CrossRef]
- Bi, W.X.; Wang, K.; Weng, B.S.; Yan, D.H.; Liu, S.Y. Does the Returning Farmland to Forest Program Improve the Ecosystem Stability of Rhizosphere in Winter in Alpine Regions? Appl. Soil Ecol. 2021, 165, 104011. [Google Scholar] [CrossRef]
- Meena, K.K.; Bitla, U.M.; Sorty, A.M.; Singh, D.P.; Gupta, V.K.; Wakchaure, G.C.; Kumar, S. Mitigation of Salinity Stress in Wheat Seedlings Due to the Application of Phytohormone-Rich Culture Filtrate Extract of Methylotrophic Actinobacterium Nocardioides sp. NIMMe6. Front. Microbiol. 2020, 11, 2091. [Google Scholar] [CrossRef]
- Amy, C.; Avice, J.C.; Laval, K.; Bressan, M. Are Native Phosphate-Solubilizing Bacteria a Relevant Alternative to Mineral Fertilizations for Crops? Part II: PSB Inoculation Enables a Halving of P Input and Improves the Microbial Community in the Rapeseed Rhizosphere. Rhizosphere 2022, 21, 100480. [Google Scholar] [CrossRef]
- Kang, S.M.; Khan, A.L.; Hamayun, M.; Hussain, J.; Joo, G.J.; You, Y.H.; Kim, J.G.; Lee, I.J. Gibberellin-Producing Promicromonospora sp. SE188 Improves Solanum lycopersicum Plant Growth and Influences Endogenous Plant Hormones. J. Microbiol. 2012, 50, 902–909. [Google Scholar] [CrossRef]
- Kang, S.M.; Khan, A.L.; Waqas, M.; You, Y.H.; Kim, J.H.; Kim, J.G.; Hamayun, M.; Lee, I.J. Plant Growth-Promoting Rhizobacteria Reduce Adverse Effects of Salinity and Osmotic Stress by Regulating Phytohormones and Antioxidants in Cucumis sativus. J. Plant Int. 2014, 9, 673–682. [Google Scholar] [CrossRef]
- Francioli, D.; Schulz, E.; Lentendu, G.; Wubet, T.; Buscot, F.; Reitz, T. Mineral vs. Organic Amendments: Microbial Community Structure, Activity and Abundance of Agriculturally Relevant Microbes Are Driven by Long-Term Fertilization Strategies. Front. Microbiol. 2016, 7, 1446. [Google Scholar] [CrossRef]
- Beltran-Garcia, M.J.; Martínez-Rodríguez, A.; Olmos-Arriaga, I.; Valdes-Salas, B.; Di Mascio, P.; White, J.F. Nitrogen Fertilization and Stress Factors Drive Shifts in Microbial Diversity in Soils and Plants. Symbiosis 2021, 84, 379–390. [Google Scholar] [CrossRef]

| Fertiliser | Total Nutrients Content | Nutrients Content from Renewable Bio-Based Origin | Bio-Based Source | Nutrients Content from Mineral Conventional Origin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N (% w:w) | P2O5 (% w:w) | K2O (% w:w) | N (% w:w) | P2O5 (% w:w) | K2O (% w:w) | N (% w:w) | P2O5 (% w:w) | K2O (% w:w) | ||
| Control (C+) | 8 | 15 | 15 | 0.00 | 0.00 | 0.00 | - | 8.00 | 15.00 | 15.00 |
| PKA | 8 | 15 | 15 | 0.00 | 3.16 | 3.33 | Ash | 8.00 | 11.84 | 11.67 |
| PA | 8 | 15 | 15 | 0.00 | 3.91 | 0.00 | Ash | 8.00 | 11.09 | 15.00 |
| PD | 8 | 15 | 15 | 0.00 | 5.35 | 0.00 | CaHPO4 from the patented process DMPhos (EP17382535) | 8.00 | 9.65 | 15.00 |
| ST | 8 | 15 | 15 | 0.22 | 5.00 | 0.00 | Struvite | 7.78 | 10.00 | 15.00 |
| Treatment | N (%) | P (mg/kg) | K (cmol(+)/kg) | |||
|---|---|---|---|---|---|---|
| 60 Days | 90 Days | 60 Days | 90 Days | 60 Days | 90 Days | |
| C− | 3.24 ± 0.24 b | 1.40 ± 0.32 b | 4278.95 ± 342.37 c | 721.25 ± 79.13 c | 42,332.08 ± 939.85 | 17,106.51 ± 161.71 b |
| C+ | 4.16 ± 0.50 a | 2.19 ± 0.37 a | 5286.88 ± 433.75 bc | 1268.67 ± 87.98 a | 44,682.54 ± 4383.45 | 20,352.58 ± 701.28 a |
| PKA | 4.55 ± 0.22 a | 2.13 ± 0.06 a | 6052.44 ± 106.39 ab | 1079.36 ± 12.18 ab | 44,988.06 ± 1336.85 | 19,168.08 ± 1963.77 ab |
| PA | 4.18 ± 0.10 a | 1.94 ± 0.13 ab | 6346.77 ± 624.66 ab | 1092.45 ± 42.94 ab | 45,517.50 ± 2913.02 | 18,433.63 ± 1207.63 ab |
| PD | 4.03 ± 0.20 ab | 2.17 ± 0.05 a | 6630.11 ± 190.03 a | 1133.62 ± 22.57 ab | 44,159.85 ± 6904.36 | 19,020.83 ± 139.28 ab |
| ST | 3.96 ± 0.33 ab | 1.87 ± 0.26 ab | 6685.29 ± 435.16 a | 1218.19 ± 99.55 ab | 45,875.72 ± 4835.01 | 19,038.87 ± 794.80 ab |
| ANOVA Mean square | 0.56 | 0.27 | 2,624,348.29 | 1416.67 | 4,778,125.48 | 0.10 |
| F value | 6.60 | 4.90 | 16.89 | 25.38 | 0.28 | 3.13 |
| Significance | ** | * | *** | *** | ns | ** |
| Treatment | N-NH4+ (mg/kg) | N-NO3− (mg/kg) | P (mg/kg) | K (mg/kg) | ||||
|---|---|---|---|---|---|---|---|---|
| 60 Days | 90 Days | 60 Days | 90 Days | 60 Days | 90 Days | 60 Days | 90 Days | |
| C− | 0.38 ± 0.20 | 0.25 ± 0.06 | 14.05 ± 1.67 b | 5.68 ± 0.54 b | 13.95 ± 2.98 b | 11.29 ± 1.49 c | 1.15 ± 0.05 | 1.05 ± 0.03 |
| C+ | 0.41 ± 0.11 | 0.25 ± 0.06 | 41.18 ± 0.71 a | 40.48 ± 0.10 a | 19.28 ± 4.12 b | 48.58 ± 4.35 b | 1.67 ± 0.38 | 1.38 ± 0.21 |
| PKA | 0.70 ± 0.36 | 0.32 ± 0.01 | 41.32 ± 0.76 a | 35.18 ± 3.2 a | 32.35 ± 6.91 b | 76.84 ± 6.74 a | 1.80 ± 0.36 | 1.47 ± 0.20 |
| PA | 0.56 ± 0.30 | 0.32 ± 0.07 | 40.58 ± 0.92 a | 35.81 ± 0.18 a | 64.16 ± 13.70 a | 52.91 ± 6.36 b | 1.80 ± 0.33 | 1.52 ± 0.17 |
| PD | 0.68 ± 0.33 | 0.31 ± 0.07 | 40.53 ± 0.80 a | 37.41 ± 2.16 a | 81.11 ± 17.32 a | 47.65 ± 11.52 b | 1.93 ± 0.22 | 1.37 ± 0.23 |
| ST | 0.63 ± 0.32 | 0.27 ± 0.07 | 40.13 ± 1.43 a | 39.65 ± 1.55 a | 31.22 ± 6.67 b | 61.26 ± 9.82 ab | 1.97 ± 0.42 | 1.56 ± 0.26 |
| ANOVA mean square | 0.06 | 0.003 | 356.85 | 525.75 | 2109.71 | 1416.67 | 0.27 | 0.10 |
| F value | 0.71 | 0.98 | 290.25 | 176.97 | 20.90 | 25.29 | 2.66 | 2.61 |
| Significance | ns | ns | *** | *** | *** | *** | ns | ns |
| Groups | Number of ASV | H’ | ||
|---|---|---|---|---|
| Pseudo-F/t | p-Values | Pseudo-F/t | p-Values | |
| Comparisons between groups | 0.406 | 0.8365 | 3.1656 | 0.0194 |
| C−, C+ | - | - | 2.383 | 0.0469 |
| C−, PKA | - | - | 0.98671 | 0.3699 |
| C−, PA | - | - | 1.8228 | 0.0779 |
| C−, PD | - | - | 2.8406 | 0.0153 |
| C−, ST | - | - | 2.1237 | 0.0485 |
| C+, PKA | - | - | 1.8045 | 0.0925 |
| C+, PA | - | - | 1.0618 | 0.3164 |
| C+, PD | - | - | 0.097895 | 0.9131 |
| C+, ST | - | - | 0.82197 | 0.4327 |
| PKA, PA | - | - | 1.0373 | 0.3519 |
| PKA, PD | - | - | 2.4278 | 0.0179 |
| PKA, ST | - | - | 1.4378 | 0.1907 |
| PA, PD | - | - | 1.5995 | 0.1178 |
| PA, ST | - | - | 0.39919 | 0.7048 |
| PD, ST | - | - | 1.3391 | 0.2205 |
| Groups | PERMANOVA | Dissimilarity Percentages (SIMPER Analysis) | |
|---|---|---|---|
| Pseudo-F/t | p-Values | ||
| Comparison between groups | 4.8455 | 0.0001 | |
| C−/C+ | 2.585 | 0.0076 | 27.71 |
| C−/PA | 2.5267 | 0.0095 | 24.97 |
| C−/PD | 2.6287 | 0.0083 | 26.12 |
| C−/PKA | 2.5729 | 0.0066 | 25.74 |
| C−/ST | 2.3397 | 0.0072 | 27.04 |
| C+/PA | 2.3017 | 0.0088 | 24.59 |
| C+/PD | 2.0196 | 0.0084 | 23.18 |
| C+/PKA | 2.1465 | 0.0077 | 23.98 |
| C+/ST | 1.8722 | 0.0087 | 24.69 |
| PA/PD | 2.4263 | 0.0079 | 23.37 |
| PA/ST | 1.8639 | 0.0068 | 22.82 |
| PD/ST | 1.8012 | 0.0093 | 22.84 |
| PKA/PA | 2.1037 | 0.0075 | 21.57 |
| PKA/PD | 1.9288 | 0.0075 | 20.98 |
| PKA/ST | 1.78 | 0.0077 | 22.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barquero, M.; Cazador, C.; Ortiz-Liébana, N.; Zotti, M.; Brañas, J.; González-Andrés, F. Fertilising Maize with Bio-Based Mineral Fertilisers Gives Similar Growth to Conventional Fertilisers and Does Not Alter Soil Microbiome. Agronomy 2024, 14, 916. https://doi.org/10.3390/agronomy14050916
Barquero M, Cazador C, Ortiz-Liébana N, Zotti M, Brañas J, González-Andrés F. Fertilising Maize with Bio-Based Mineral Fertilisers Gives Similar Growth to Conventional Fertilisers and Does Not Alter Soil Microbiome. Agronomy. 2024; 14(5):916. https://doi.org/10.3390/agronomy14050916
Chicago/Turabian StyleBarquero, Marcia, Cinta Cazador, Noemí Ortiz-Liébana, Maurizio Zotti, Javier Brañas, and Fernando González-Andrés. 2024. "Fertilising Maize with Bio-Based Mineral Fertilisers Gives Similar Growth to Conventional Fertilisers and Does Not Alter Soil Microbiome" Agronomy 14, no. 5: 916. https://doi.org/10.3390/agronomy14050916
APA StyleBarquero, M., Cazador, C., Ortiz-Liébana, N., Zotti, M., Brañas, J., & González-Andrés, F. (2024). Fertilising Maize with Bio-Based Mineral Fertilisers Gives Similar Growth to Conventional Fertilisers and Does Not Alter Soil Microbiome. Agronomy, 14(5), 916. https://doi.org/10.3390/agronomy14050916







