Abstract
Trialeurodes vaporariorum Westwood (greenhouse whitefly) are worldwide polyphagous pests of economic importance that damage solanaceous vegetables. Neonicotinoid pesticides and parasitoid Encarsia formmosa Gahan are the main management strategies applied worldwide, but precise control methods in greenhouse vegetables need to be developed to reduce the application amounts of pesticides and improve the suppression of whitefly populations. Therefore, we assessed the indoor acute toxicities and risk assessment of neonicotinoids for T. vaporariorum and E. formosa adults and pupae and compared the control effects of E. formosa and neonicotinoid acetamiprid. According to the acute toxicities results, most neonicotinoid insecticides were more toxic to E. formosa than T. vaporariorum, and pupae were much less susceptible than adults of both species. Moreover, acetamiprid had a low risk effect on E. formosa. Sole application of E. formosa and acetamiprid could effectively control T. vaporariorum, but their combined application resulted in antagonistic effects on the control of T. vaporariorum. The results showed that a combined application or sole use of E. formosa could reduce the use of insecticides, slow down the development of insecticide resistance in whiteflies, and improve the efficiency of controlling the growth of whitefly populations in greenhouse vegetable production.
1. Introduction
Pest management has always been an important scientific issue in food production and agricultural ecology, as 20–40% of crop production is lost to pests annually [1,2]. Whiteflies, including the tobacco whitefly (Bemisia tabaci Gennadius) and the greenhouse whitefly (Trialeurodes vaporariorum Westwood, Hemiptera: Aleyrodidae), are the most economically important agricultural pests in the world, infesting many agricultural and ornamental plants grown under greenhouse and field conditions [3]. In particular, solanaceous vegetables, such as tomatoes, cucumbers, eggplants, and pepper, which accounted for 32% of global total vegetable production in 2020 [4], are mainly infested by whiteflies. Whiteflies cause devastating damage by direct phloem feeding and honeydew excretion, and indirectly acting as a medium for sooty mold fungi and vectors of plant pathogens [5,6]. Although many novel control strategies such as semiochemicals, endosymbionts, RNA interference, and genetic modifications of plants for the expression of anti-whitefly proteins [7,8,9], chemical pesticides and parasitoid Encarsia spp. are still the main management strategies applied worldwide [10].
It is a fact that the use of chemical pesticides has negative effects on environmental and ecological safety and food security [11,12]. Furthermore, the excessive and continuous application of pesticides has led to the development of resistance in whiteflies [13,14,15,16,17]. Thus, strictly controlling the types and amounts of insecticides used is crucial for managing pesticide resistance and minimizing ecological side effects. Neonicotinoid insecticides are most commonly used to control sucking pests; they had a 25% share of the global insecticide market in 2014 [18]. They are broad spectrum insecticides with high target specificities and toxicities and long persistence times [19,20]. However, whiteflies have developed a high level of resistance to neonicotinoids imidacloprid and thiamethoxam [21], and the European Union prohibited the outdoor use of three neonicotinoids (clothianidin, imidacloprid, and thiamethoxam) in 2018 with concern about their effects on pollinators [22,23,24]. However, other neonicotinoids like acetamiprid and thiacloprid are friendly to pollinators [25,26], and using low doses of insecticides in combination with natural enemies can be an effective strategy for controlling pests such as Myzus persicae (green peach aphid) and Frankliniella occidentalis (western flower thrips) [27,28]. Whether low-dose neonicotinoids combined with Encarsia spp. can effectively control whiteflies needs further investigation.
Encarsia formosa Gahan, which can feed on first and second instar nymphs and oviposit in second to fourth instar nymphs of whiteflies [29,30,31], is the most important and successful biological control agent for the control of whiteflies under protected cultivation [32]. Both inundative and seasonal inoculative release methods are applied to control greenhouse whiteflies with E. formosa. When managing whiteflies, it is important to consider the host plant species and the development stage of the whiteflies to determine the most effective control methods [33]. However, the developmental rate and daily parasitism of E. formosa are influenced by the temperature of the environment, and are disadvantaged at lower temperatures [34]. Under these conditions, it is better to utilize pesticides to control whiteflies. Although it is known that E. formosa adults are extremely sensitive to the neonicotinoids imidacloprid, thiamethoxam, nitenpyram, and acetamiprid [35], it is still necessary to test the toxicity of neonicotinoid insecticides to the different developmental stages of E. formosa and evaluate the possibility of combining neonicotinoids and E. formosa.
In this study, we estimated the susceptibility of T. vaporariorum and E. formosa to neonicotinoid pesticides and compared the control effects of E. formosa and neonicotinoid pesticides applied separately or combined in low doses. The main aim was to develop precise whitefly control methods in greenhouse vegetables, reduce the application amounts of pesticides, and improve the suppression of whitefly populations.
2. Materials and Methods
2.1. Insects and Insecticides
Laboratory colonies of T. vaporariorum and E. formosa were collected from tomato fields in Jinan, Shandong Province, China (36.40° N, 117.00° E). The T. vaporariorum were reared on tobacco seedlings, and the E. formosa were reared on tobacco seedlings infected with T. vaporariorum nymphs growing on tobacco leaves. All laboratory experiments were conducted in an artificial climate chamber (YCRC-300C, Jiangnan Instrument Factory, Ningbo, China) at 26 ± 1 °C, relative humidity of 70 ± 5% RH, and photoperiod of 14 L:10 D, in the Institute of Plant Protection, Shandong Academy of Agricultural Sciences.
Eight neonicotinoid insecticides were tested in this study, including imidacloprid WG (70%, Bayer Crop Science, Leverkusen, Germany), nitenpyram AS (10%, Zhejiang Shijia Technology Co. Ltd., Huzhou, China), acetamiprid SP (20%, Jiangsu Longdeng Chemical Co. Ltd., Changzhou, China), thiacloprid SC (2%, Shandong Guorun Biological Pesticide Co. Ltd., Taian, China), thiamethoxam WG (25%, Syngenta Crop Protection, Basel, Switzerland), clothianidin SC (20%, FMC Corporation, Philadelphia, PA, USA), dinotefuran SG (20%, Mitsui Chemicals AGRO, Tokyo, Japan), and flupyradifurone SL (17%, Bayer Crop Science, Monsanto, St. Louis, MO, USA). All pesticide stock solutions were prepared with distilled water (without a carrier solvent) immediately prior to use.
2.2. Acute Toxicity Bioassays
Leaf-dip bioassay [36] was used to detect the toxicity of neonicotinoid insecticides on whitefly adults. Leaf discs (20 mm in diameter) collected from tomato leaves were dipped into distilled water or different concentrations of insecticides (Table 1) for 10 s and dried with filter paper in a ventilated hood for 20 min. Subsequently, the leaf discs were placed on agar beds (2 mL, 20 g/L) in glass tubes with their adaxial surface downwards. About 20 adult whiteflies were added into each tube and the tube was covered with a black cloth. The mortality was scored after 48 h.

Table 1.
Neonicotinoid insecticides and concentrations used in this study.
Larval-dip bioassay following Elbert and Nauen [37] with minor modifications was carried out to detect the whitefly nymphs. Tomato plants with four leaves were prepared for adult whiteflies to lay eggs for 24 h. When the 3rd instar nymphs appeared on the infested tomato seedings, the leaves with about 40 3rd nymphs were picked and dipped into distilled water or different concentrations of insecticides (Table 1) for 10 s. The bottom stems of leaves were immersed in water after the solution of insecticides on the leaves dried. The number of total treated nymphs and emerged adults were counted after adult emergence (about 15 days post-treatment).
The adult E. formosa bioassay was conducted following the dry film residual contact method [38]. Different concentrations of insecticides (Table 1) or distilled water were added into a glass tubes (3 cm in diameter, 12 cm in height), respectively, and the glass tubes were rolled for approximately 2 h to generate a homogenous film on the surface of the vials. About 50 ± 10 newly emerged adults (after 24 h) in glass tubes were allowed to crawl to each treated tube in the light. Small streaks of 10% honey solution scribed with cotton swabs were added as food sources. The glass tubes were sealed with black cloth. Mortalities were recorded after 24 h. The adults were considered dead if their feet failed to move upon a light touch.
For E. formosa pupae, pupae-dip bioassay was conducted following Simmonds et al. [39] with minor modifications. Pupae sticking to cards were dipped into distilled water or different concentrations of insecticides (Table 1) for 10 s when they turned black for 3 days. The cards were then placed in glass tubes after they were dried. The number of treated pupae and emerged adults were counted after 15 days of treatment.
Five concentration gradients were set for each insecticide, and each treatment had three biological replications. All treatments were kept at 25 ± 2 °C, 70 ± 5% RH, and a photoperiod of 16 L:8 D (h). The obtained data were corrected by the mortality of control (which was never larger than 10%) using Abbott’s formula [13] prior to analysis. The slope, 50% lethal concentration (LC50), 95% confidence interval, and the application rate for 50% mortality (LR50) were estimated using SPSS 18.0 (Chicago, IL, USA).
2.3. Risk Assessment
The risk assessment for E. formosa was based on the environmental risk assessment guidelines for non-target arthropods, which was described by Lin et al. (2020) [27]. The in-field predicted exposure rate (PER in-field) = the application rate × the multiple application factor (MAF) and the hazard quotient (HQ in-field) = PER in-field/the application rate for 50% mortality (LR50). HQ (in-field) < 2 indicated low risk; higher tier tests were needed if HQ (in-field) ≥ 2 [27].
2.4. Greenhouse Efficacy Trial
The greenhouse efficacy trials were conducted in a greenhouse from 12 January to 17 April 2020. Eight cages (90 × 90 × 90 cm3) were set in a greenhouse and 20 tomato seedlings (15–20 cm in height) were in each cage. On 12 January, about 100 adult whiteflies were released into each cage and the number of whiteflies on each seeding was surveyed on 18 January. The following four treatments were established: (I) control group: 10 mL water sprayed only; (II) biological group: 20 E. formosa pupae stuck on a paper card were introduced to each seedling; (III) chemical group: 10 mL acetamiprid solution with recommended concentration (64 mg a.i./L) was sprayed; (IV) integrated group: 10 mL acetamiprid (10.31 mg a.i./L, the LC20 for adult T. vaporariorum) was sprayed and 10 E. formosa pupae stuck on a paper card were introduced to each seeding. Each group contained three repetitions. In all groups, water and acetamiprid solution was sprayed evenly on the back and top sides of the leaves. Acetamiprid or water was sprayed on 21 January and 31 January, and the E. formosa pupae were introduced on 22 January, 1 February, and 11 February. The number of nymphs and adults of T. vaporariorum and black pupae of T. vaporariorum that were parasitized with E. formosa in each treatment were counted every 10 days from January 18. The control effect (%) was computed per Lin et al. (2021) [28].
2.5. Statistical Analysis
The population reduction rate for T. vaporariorum and the control effect of each treatment were calculated. A two-way ANOVA was used to analyze the control efficiency of T. vaporariorum under different treatments. The Student–Newman–Keuls test was used as a post hoc test (p < 0.05) when significant differences were detected among different treatments on the same day.
3. Results
3.1. Acute Toxicity of Neonicotinoids on T. vaporariorum and E. formosa
The acute toxicities of the eight neonicotinoid insecticides to adults and nymphs of T. vaporariorum and E. formosa are shown in Table 2 and Table 3, respectively. The LC50 values of the neonicotinoids on T. vaporariorum adults ranged from 6.82 mg a.i.·L−1 for nitenpyram to 59.22 mg a.i.·L−1 for thiacloprid, which were much higher than the LC50 values of most neonicotinoids on adult E. formosa ranging from 0.043 mg a.i.·L−1 for thiamethoxam to 3.28 mg a.i.·L−1 for flupyradifurone, except for the confusingly high LC50 value of imidacloprid on E. formosa adults of 140,034.5 mg a.i.·L−1 (Table 2). The T. vaporariorum nymphs were much less sensitive to neonicotinoids than the E. formosa pupae. The LC50 values of T. vaporariorum ranged from 32.71 mg a.i.·L−1 for nitenpyram to 377,488.75 mg a.i.·L−1 for acetamiprid, while LC50 values of E. formosa ranged from 1.99 mg a.i.·L−1 for dinotefuran to 22.19 mg a.i.·L−1 for thiacloprid (Table 3). In addition, the T. vaporariorum nymphs and E. formosa pupae were less sensitive to neonicotinoids than the adults, except for imidacloprid.

Table 2.
Acute toxicity data of eight neonicotinoid pesticides tested on adult whiteflies T. vaporariorum and parasitoid E. formosa.

Table 3.
Acute toxicity data of eight neonicotinoid pesticides tested on T. vaporariorum nymphs and E. formosa pupae.
3.2. Risk Assessments of the Pesticides to E. formosa in Field
We conducted an in-field risk assessment since the application of E. formosa was limited to the greenhouse. As seen in Table 4, the HQs of imidacloprid and acetamiprid for adult E. formosa were less than 2, and the two neonicotinoids were considered low-risk insecticides. HQs of other neonicotinoids ranging from 2.97 to 192.63 indicated that the risks of these neonicotinoids were high risk without further higher tier tests. As for E. Formosa pupae, only the HQs of flupyradifurone was higher than 2, indicating a high risk. Other neonicotinoids were low risk to E. Formosa pupae since all HQs of these neonicotinoids were less than 2 (Table 4). Thus, only imidacloprid and acetamiprid were low risk to both E. Formosa adults and pupae. However, considering the high resistance to imidacloprid in whiteflies and side effects on pollinators, we selected acetamiprid for further research.

Table 4.
Risk assessment of eight neonicotinoid pesticides to E. formosa adults and pupae based on acute toxicity data and field exposure levels (DT50—50% of pesticide degradation time (usually the default is 10d). MAF—multiple application factor. PER—predicted exposure rate. LR50—application rate for 50% mortality. HQ—hazard quotient).
3.3. Greenhouse Efficacy Trial
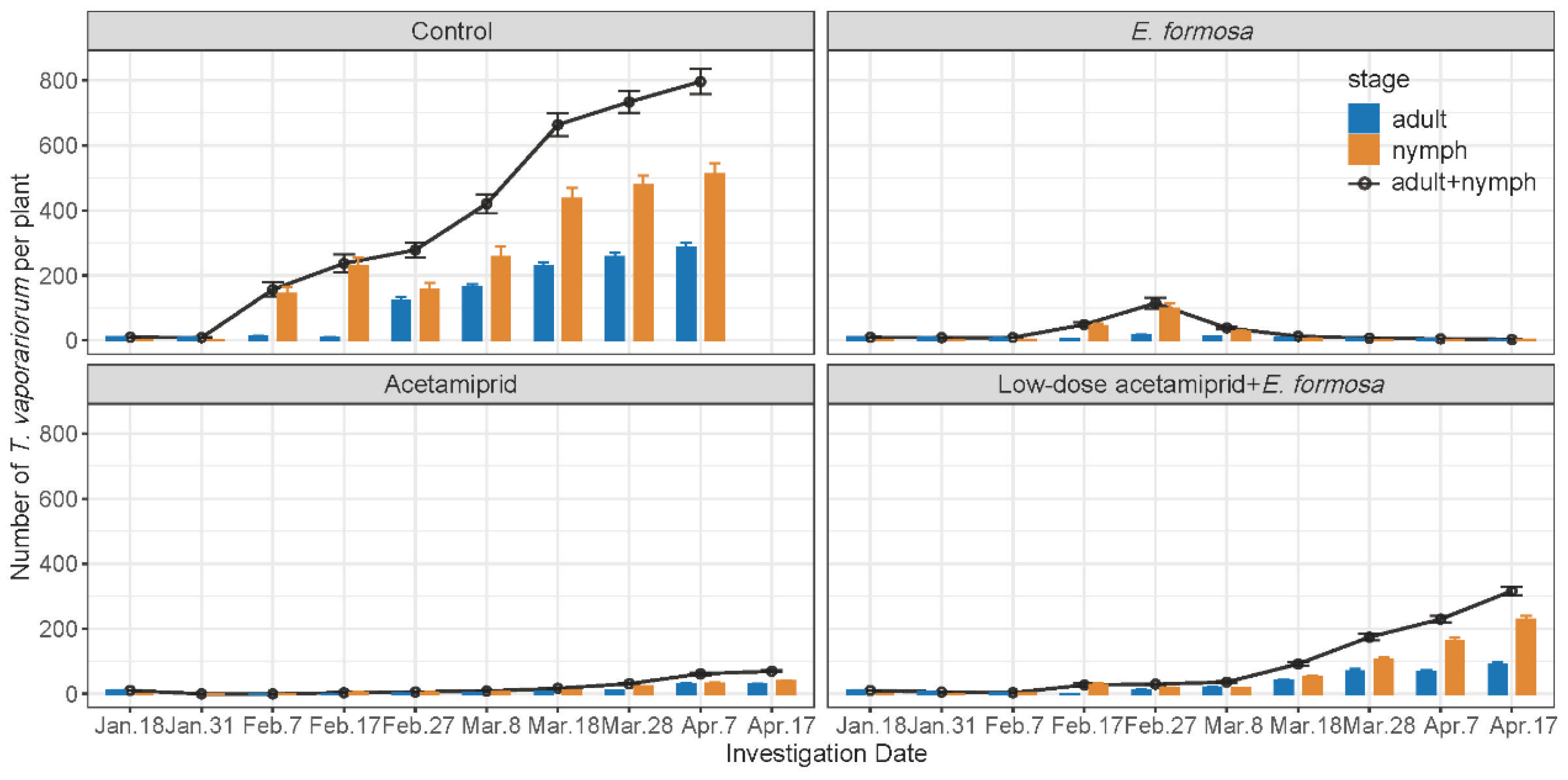
The population of T. vaporariorum kept increasing throughout the entire investigation period, with the total population number increasing from 10 to 795.75 per plant (Figure 1). The number of T. vaporariorum adults in the biological group was always below 17.23 per plant, while the highest number of T. vaporariorum pupae was 97.33, observed on 27 February. The highest population number of T. vaporariorum in the chemical group was observed on 17 April, with 29.78 adults and 39.5 nymphs per plant. The total population of T. vaporariorum in the integrated group was 3.65 per plant on 7 February, and began to increase after 17 February, reaching 316.48 per plant on 17 April (Figure 1). The control effects of E. Formosa were always over 90%, except on 31 January (−2.21%) and 27 February (56.84%). In contrast, the control effects of the chemical group were all above 92.5% during the investigation (Table 5). The integrated group demonstrated the highest control effect of 97.74% on 7 February, followed by a decreased control effect of 72.2% on 7 April. Among these three groups, the control effects of the chemical group were significantly higher than those of the biological group and the integrated group from 31 January to 8 March. However, the control effect of the biological group became the highest (Table 5).
Figure 1.
Number of T. vaporariorum (mean ± SE) under different treatments on tomato seedlings grown in field cages of 90 × 90 × 90 cm set up within a greenhouse.

Table 5.
Control effect of T. vaporariorum populations under different treatments compared with the untreated control on tomato seedlings set up in field cages of 90 × 90 × 90 cm within a greenhouse. Assigned lowercase letters to each group of data (same day) indicate the results from Student–Newman–Keuls multiple comparison tests; the same letters in the same column indicate that values are not significantly different.
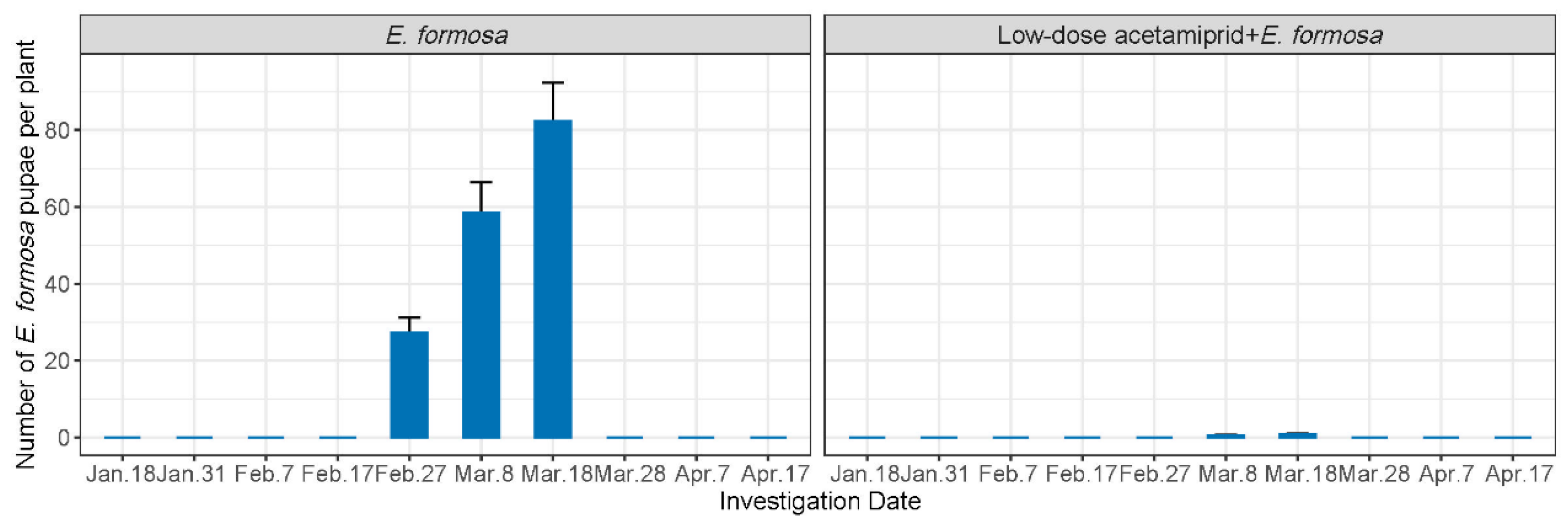
The number of E. Formosa pupae was investigated in both the biological and the integrated group (Figure 2). In the biological group, E. Formosa pupae only emerged between 27 February and 18 March, with the population number increasing from 27.28 to 82.4 per plant. On the other hand, the population numbers in the integrated group were significantly lower, ranging from 0.53 to 0.8 E. Formosa pupae per plant from 8 March to 18 March.
Figure 2.
Number of E. Formosa pupae (mean ± SE) in each treatment.
4. Discussion
Whiteflies are economically important pests that induce havoc on a wide range of crop plants. They have a high reproduction rate and can achieve an exceptionally high population size within a few generations [10]. The management of whiteflies is difficult due to the tiny body size, covert nymph, and population explosion. In this research, we found that the sensitivity of third instar nymphs of whiteflies to neonicotinoids is lower than that of adults, and the LC50 values of some neonicotinoids (imidacloprid, acetamiprid, thiamethoxam, and clothianidin) for nymphs far exceed the recommended dosage. Field trial data also show that the continuous application of neonicotinoid acetamiprid twice in the early stage of whitefly occurrence effectively controls the population of whiteflies for 3 months. We also found that the sensitivity of E. Formosa pupae to neonicotinoids is lower than adults, and the use of low doses of acetamiprid and E. Formosa can effectively control the number of whiteflies for 45 days. In the early stages of whitefly occurrence, the release of E. Formosa can continuously control the number of adult whiteflies at a lower level.
The continuous and effective control of whiteflies with neonicotinoid acetamiprid in this research was due to the sensitive population of whiteflies, and the application of acetamiprid during the period when only adults and young nymphs were present. Here, we found that neonicotinoid toxicity toward adults was high, but was relatively low toward third instar T. vaporariorum nymphs. Furthermore, first and second instar whitefly nymphs are more sensitive to neonicotinoids than adults, while third instar nymphs are the least sensitive [15,40,41]. Thus, application of acetamiprid significantly reduces the number of adults and early aged nymphs. However, whiteflies have tremendous potential to develop resistance to insecticide [13,14,21], and increasing the resistance levels to neonicotinoids also increases the LC50s for first instar nymph in whitefly field strains [38]. Therefore, in order to slow down the development rate of resistance to insecticides and keep the high control effects of insecticides, it is necessary to control the application time of insecticides and reduce the application amount. In addition, the population size of whiteflies began to increase after March 28, and would develop into an outbreak without taking measures. This means that the effectiveness of applying insecticides to control whiteflies can persist for 3 months at the most, and other methods need to be considered afterwards.
In this research, the application of E. Formosa could continuously control the number of adult whiteflies at a lower level, while the number of T. vaporariorum nymphs increased to 97.3 per plant on 27 February. We believe that these nymphs may have been parasitized due to the subsequent decrease in the population of whiteflies and the increase in E. Formosa pupae. However, in the last month of the experiment, both populations of whiteflies and E. Formosa decreased to the lowest level, meaning the population growth could not be controlled by E. Formosa after new whiteflies moved in. Furthermore, most greenhouse vegetables grown in winter are infested by T. vaporariorum, while control by E. formosa is delayed due to the significantly prolonged development duration at the low winter temperatures (<15 °C) [42,43]. At these times, applying insecticides or increasing the amount of E. formosa may be helpful for improving control effects on whiteflies. In order to not weaken the control effects of E. formosa when applying insecticides during the use of E. formosa, a feasible approach is to improve the insecticide resistance in E. formosa. The development of insecticide resistance in parasitoids could be affected either directly by exposure to the spray or indirectly by factors such as the insecticide penetrating the body of host insects and exposure to plants when host insects feed on them [44]. The resistance of parasitoids Tetrastichus brontispae and Asecodes hispinarum to acetamiprid has been increased by using the resistance found in Brontispa longissimi [45]. In this research, E. formosa adults showed a high level of resistance to imidacloprid. Therefore, it may be feasible to develop resistant populations of E. formosa to reduce the negative effect of neonicotinoid insecticides.
In this research, the control effects of a combination of low dose acetamiprid and E. formosa could last for 45 days. The early control of the number of whitefly adults and nymphs may have been caused by low doses of insecticide acetamiprid, affecting the number and fertility of T. vaporariorum adults. Another possibility is that E. formosa fed on the T. vaporariorum nymphs. Previous research has shown that E. formosa feeds on all stages of T. vaporariorum, especially more frequently on second and late fourth instars than on first and third instars [46], while preferring third and early fourth instars for oviposition [47]. Previous studies have focused on combining insecticides with biocontrol agents, applied as low-dose insecticides and predators, achieving good control effects [24,25,48,49]. However, in this research, the number of E. formosa pupae never exceeded one per plant, and the whitefly population was gradually out of control after March 18. Sublethal insecticide applications induce adverse effects, such as prolonged development duration, reduced emergence, decreased reproduction, and weakened parasitic behavior [50,51], therefore, in order to achieve persistent integrated control effect, it is better to delay the release timing of E. formosa and increase the release frequency one or two more times. Whether this advice can improve the control effect of combination of low dose acetamiprid and E. formosa needs further validation.
5. Conclusions
This research analyzed the advantages and disadvantages of neonicotinoids and E. formosa in controlling whiteflies based on indoor toxicity testing and semi-field experiments, and preliminarily explored the effectiveness of using low-dose insecticides and E. formosa in combination to control whiteflies. In order to reduce the use of insecticides, slow down the development of insecticide resistance in whiteflies, and improve the efficiency of controlling the growth of whitefly populations in greenhouse vegetable production, further studies based on precise application timing and E. formosa resistance development are needed to improve the efficiency of combined E. formosa and acetamiprid treatments.
Author Contributions
Formal analysis, data curation, writing—review and editing, X.D. and Q.L.; data curation, writing—review and editing, H.C.; formal analysis, writing—review and editing, Y.L.; sampling and investigation, data curation, R.W.; writing—review and editing., formal analysis, conceptualization, L.Z. (Lisheng Zhang) and Y.Z.; sampling and investigation, data curation, L.S.; sampling and investigation, data curation, F.Z. and L.Z. (Li Zheng); conceptualization, funding acquisition, Z.Y. and S.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Key R&D Program of China (2023YFE0123000); Shandong Provincial Natural Science Foundation (ZR2021YQ21, ZR2021QC218); Shandong Provincial Agriculture Research System (SDAIT-24); Taishan Scholar program of Shandong Province (tsqn202312293); Shandong Provincial Key R&D Program (2023LZGC017).
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Editorial, R. Pathogens, precipitation and produce prices. Nat. Clim. Change 2021, 11, 635. [Google Scholar] [CrossRef]
- Heeb, L.; Jenner, E.J.; Cock, M.J.W. Climate-smart pest management: Building resilience of farms and landscapes to changing pest threats. J. Pest. Sci. 2019, 92, 951–969. [Google Scholar] [CrossRef]
- Krause-Sakate, R.; Watanabe, L.F.M.; Gorayeb, E.S.; da Silva, F.B.; Alvarez, D.d.L.; Bello, V.H.; Nogueira, A.M.; de Marchi, B.R.; Vicentin, E.; Ribeiro-Junior, M.R.; et al. Population dynamics of whiteflies and associated viruses in South America: Research progress and perspectives. Insects 2020, 11, 847. [Google Scholar] [CrossRef] [PubMed]
- Shahbandeh, M. Vegetables Production Worldwide by Type 2020. Global-Production-of-Vegetables-by-Type. 2022. Available online: https://www.statista.com/statistics/264065/ (accessed on 6 February 2024).
- Byrne, D.N.; Bellows, T.S. Whitefly biology. Annu. Rev. Entomol. 1991, 36, 431–457. [Google Scholar] [CrossRef]
- Kanakala, S.; Ghanim, M. Advances in the Genomics of the Whitefly Bemisia tabaci: An insect pest and a virus vector. In Short Views on Insect Genomics and Proteomics; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Milenovic, M.; Ghanim, M.; Hoffmann, L.; Rapisarda, C. Whitefly endosymbionts: IPM opportunity or tilting at windmills? J. Pest. Sci. 2022, 95, 543–566. [Google Scholar] [CrossRef] [PubMed]
- Saurabh, S.; Mishra, M.; Rai, P.; Pandey, R.; Singh, J.; Khare, A.; Jain, M.; Singh, P.K. Tiny Flies: A mighty pest that threatens agricultural productivity—A case for next-generation control strategies of whiteflies. Insects 2021, 12, 585. [Google Scholar] [CrossRef] [PubMed]
- Schlaeger, S.; Pickett, J.A.; Birkett, M.A. Prospects for management of whitefly using plant semiochemicals, compared with related pests. Pest Manag. Sci. 2018, 74, 2405–2411. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, M.; Koul, B.; Chandrashekar, K.; Raut, A.; Yadav, D. Whitefly (Bemisia tabaci) management (WFM) strategies for sustainable agriculture: A Review. Agriculture 2022, 12, 1317. [Google Scholar] [CrossRef]
- Morillo, E.; Villaverde, J. Advanced technologies for the remediation of pesticide-contaminated soils. Sci. Total Environ. 2017, 586, 576–597. [Google Scholar] [CrossRef] [PubMed]
- Philippe, V.; Neveen, A.; Marwa, A.; Ahmad Basel, A.Y. Occurrence of pesticide residues in fruits and vegetables for the Eastern Mediterranean Region and potential impact on public health. Food Control 2021, 119, 107457. [Google Scholar] [CrossRef]
- Pappas, M.L.; Migkou, F.; Broufas, G.D. Incidence of resistance to neonicotinoid insecticides in greenhouse populations of the whitefly, Trialeurodes vaporariorum (Hemiptera: Aleyrodidae) from Greece. Appl. Entomol. Zool. 2013, 48, 373–378. [Google Scholar] [CrossRef]
- Shah, R.; Al-Sadi, A.; Scott, I.; AlRaeesi, A.; Al-Jahdhami, A. Insecticide resistance monitoring in whitefly (Bemisia tabaci) (Hemiptera: Aleyrodidae) in Oman. J. Asia. Pac. Entomol. 2020, 23, 1248–1254. [Google Scholar] [CrossRef]
- Erdogan, C.; Velioglu, A.S.; Gurkan, M.O.; Denholm, I.; Moores, G.D. Detection of resistance to pyrethroid and neonicotinoid insecticides in the greenhouse whitefly, Trialeurodes vaporariorum (Westw.) (Hemiptera: Aleyrodidae). Crop Prot. 2021, 146, 105661. [Google Scholar] [CrossRef]
- Gorman, K.; Devine, G.; Bennison, J.; Coussons, P.; Punchard, N.; Denholm, I. Report of resistance to the neonicotinoid insecticide imidacloprid in Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). Pest Manag. Sci. 2007, 558, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, M.; Jia, Z.; Ahmat, T.; Xie, L.; Jiang, W. Resistance to neonicotinoid insecticides and expression changes of eighteen cytochrome P450 genes in field populations of Bemisia tabaci from Xinjiang, China. Entomol. Res. 2020, 50, 205–211. [Google Scholar] [CrossRef]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Bonmatin, J.; Giorio, C.; Girolami, V.; Goulson, D.; Kreutzweiser, D.P. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. 2015, 22, 35–67. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P.; Nauen, R. Neonicotinoids–from zero to hero in insecticide chemistry. Pest Manag. Sci. 2008, 64, 1084–1098. [Google Scholar] [CrossRef]
- Naveen, N.C.; Chaubey, R.; Kumar, D.; Rebijith, K.B.; Rajagopal, R.; Subrahmanyam, B.; Subramanian, S. Insecticide resistance status in the whitefly, Bemisia tabaci genetic groups Asia-I, Asia-II-1 and Asia-II-7 on the Indian subcontinent. Sci. Rep. 2017, 7, 40634. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2018/783 of 29 May 2018 amending Implementing Regulation (EU) No 540/2011 as regards the conditions of approval of the active substance imidacloprid. Off. J. Eur. Union 2018, 132, 31–34. [Google Scholar]
- European Commission. Commission Implementing Regulation (EU) 2018/784 of 29 May 2018 amending Implementing Regulation (EU) No 540/2011 as regards the conditions of approval of the active substance clothianidin. Off. J. Eur. Union 2018, 132, 35–39. [Google Scholar]
- European Commission. Commission Implementing Regulation (EU) 2018/785 of 29 May 2018 amending Implementing Regulation (EU) No 540/2011 as regards the conditions of approval of the active substance thiamethoxam. Off. J. Eur. Union 2018, 132, 40–44. [Google Scholar]
- Manjon, C.; Troczka, B.J.; Zaworra, M.; Beadle, K.; Randall, E.; Hertlein, G.; Singh, K.S.; Zimmer, C.T.; Homem, R.A.; Lueke, B.; et al. Unravelling the molecular determinants of bee sensitivity to neonicotinoid insecticides. Curr. Biol. 2018, 28, 1137–1143.e5. [Google Scholar] [CrossRef]
- Troczka, B.J.; Homem, R.A.; Reid, R.; Beadle, K.; Kohler, M.; Zaworra, M.; Field, L.M.; Williamson, M.S.; Nauen, R.; Bass, C.; et al. Identification and functional characterisation of a novel N-cyanoamidine neonicotinoid metabolising cytochrome P450, CYP9Q6, from the buff-tailed bumblebee Bombus terrestris. Insect Biochem. Mol. Biol. 2019, 111, 103171. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.C.; Chen, H.; Babendreier, D.; Zhang, J.; Zhang, F.; Dai, X.Y.; Sun, Z.; Shi, Z.; Dong, X.L.; Wu, G.A.; et al. Improved control of Frankliniella occidentalis on greenhouse pepper through the integration of Orius sauteri and neonicotinoid insecticides. J. Pest Sci. 2020, 94, 101–109. [Google Scholar] [CrossRef]
- Lin, Q.C.; Chen, H.; Dai, X.Y.; Yin, S.; Shi, C.H.; Yin, Z.J.; Zhang, J. Myzus persicae management through combined use of beneficial insects and thiacloprid in pepper seedlings. Insects 2021, 12, 791. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, Y.; Wang, K.; Zhang, Y.; Wu, Q.; Wang, S. Development and fitness of the parasitoid, Encarsia formosa (Hymenoptera: Aphelinidae), on the B and Q of the sweetpotato whitefly (Hemiptera: Aleyrodidae). Biol. Microb. Control J. 2019, 112, 2597–2603. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Xie, W.; Wu, Q.; Wang, S. The suitability of biotypes Q and B of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) at different nymphal instars as hosts for Encarsia formosa Gahan (Hymenoptera: Aphelinidae). Peer J. 2016, 4, e1863. [Google Scholar] [CrossRef]
- Walia, A.; Verma, S.C.; Sharma, P.L.; Sharma, N.; Palial, S. Relative preference and demographic parameters of Encarsia formosa Gahan against Trialeurodes vaporariorum (Westwood). Egypt. J. Biol. Pest Control 2021, 31, 79. [Google Scholar] [CrossRef]
- Liu, T.; Stansly, P.A.; Gerling, D. Whitefly parasitoids: Distribution, life history, bionomics, and utilization. Annu. Rev. Entomol. 2015, 60, 273–392. [Google Scholar] [CrossRef]
- Lenteren, J.C.; Roermund, H.J.W.; Sütterlin, S. Biological control of greenhouse whitefly (Trialeurodes vaporariorum) with the parasitoid Encarsia formosa: How does it work? Biol. Control 1996, 6, 1–10. [Google Scholar] [CrossRef]
- Qiu, Y.; Lenteren, J.C.; Drost, Y.C.; Posthuma-Doodeman, C.J.A.M. Life-history parameters of Encarsia formosa, Eretmocerus eremicus and E. mundus, aphelinid parasitoids of Bemisia argentifolii (Hemiptera: Aleyrodidae). Eur. J. Entomol. 2004, 101, 83–94. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, P.; Yang, X.; Ruan, C.; Biondi, A.; Zang, L. Selectivity of novel and traditional insecticides used for management of whiteflies on the parasitoid Encarsia formosa. Pest Manag. Sci. 2019, 75, 2716–2724. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.T.; Wu, Q.J.; Xu, B.Y.; Wang, S.L.; Chang, X.L.; Xie, W.; Zhang, Y.J. Fitness costs and morphological change of laboratory-selected thiamethoxam resistance in the B-type Bemisia tabaci (Hemiptera: Aleyrodidae). J. Appl. Entomol. 2009, 133, 466–472. [Google Scholar] [CrossRef]
- Elbert, A.; Nauen, R. Resistance of Bemisia tabaci (Homoptera: Aleyrodidae) to insecticides in southern Spain with special reference to neonicotinoids. Pest Manag. Sci. 2000, 56, 60–64. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, X.; Huang, X.; Yu, X.; Zhang, W.; Zhang, X.; Mu, W. Comparative ecotoxicity of neonicotinoid insecticides to three species of Trichogramma parasitoid wasps (Hymenoptera: Trichogrammatidae). Ecotoxicol. Environ. Saf. 2019, 183, 109587. [Google Scholar] [CrossRef]
- Simmonds, M.S.J.; Manlove, J.D.; Blaney, W.M.; Khambay, B.P.S. Effects of selected botanical insecticides on the behaviour and mortality of the glasshouse whitefly Trialeurodes vaporariorum and the parasitoid Encarsia formosa. Entomol. Exp. Appl. 2002, 102, 39–47. [Google Scholar] [CrossRef]
- Bi, J.L.; Toscano, N.C. Current status of the greenhouse whitefly, Trialeurodes vaporariorum, susceptibility to neonicotinoid and conventional insecticides on strawberries in southern California. Pest Manag. Sci. 2007, 752, 747–752. [Google Scholar] [CrossRef]
- Karatolos, N.; Denholm, I.; Williamson, M.; Gorman, K. Incidence and characterisation of resistance to neonicotinoid insecticides and pymetrozine in the greenhouse whitefly, Trialeurodes vaporariorum Westwood (Hemiptera: Aleyrodidae). Pest Manag. Sci. 2014, 66, 1304–1307. [Google Scholar] [CrossRef]
- Gamarra, H.; Sporleder, M.; Carhuapoma, P.; Kroschel, J.; Kreuze, J. A temperature-dependent phenology model for the greenhouse whitefly Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). Virus Res. 2020, 289, 198107. [Google Scholar] [CrossRef]
- Manzano, M.R.; Lenteren, J.V. Life history parameters of Trialeurodes vaporariorum (Westwood) (Hemiptera: Aleyrodidae) at different environmental conditions on two bean cultivars. Neotrop. Entomol. 2009, 38, 452–458. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, G.; Jiang, S. Advances in evolution of insecticide resistance in parasitoid wasps. Acta Entomol. Sin. 2004, 47, 515–521. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Jin, T.; Jin, Q.A.; Wen, H.B.; Peng, Z.Q. Differential susceptibilities of Brontispa longissima (Coleoptera: Hispidae) to insecticides in southeast Asia. J. Economic Entomol. 2012, 105, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Nell, H.; Sevenster-van der Lelie, L.; Woets, J.; Lenteren, J. The parasite-host relationship between Encarsia formosa (Hymenoptera: Aphelinidae) and Trialeurodes vaporariorum (Homoptera: Aleyrodidae) II. Selection of host stages for oviposition and feeding by the parasite. J. Appl. Entomol. 1976, 81, 372–376. [Google Scholar] [CrossRef]
- Hu, J.S.; Gelman, D.B.; Blackburn, M.B. Growth and development of Encarsia formosa (Hymenoptera: Aphelinidae) in the greenhouse whitefly, Trialeurodes vaporariorum (Homoptera: Aleyrodidae): Effect of host age. Arch. Insect. Biochem. Physiol. 2002, 49, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Beatriz, D.; Colomer, I.; Medina, P.; Vi, E. Compatibility of early natural enemy introductions in commercial pepper and tomato greenhouses with repeated pesticide applications. Insect Sci. 2019, 27, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Colomer, I.; Aguado, P.; Medina, P.; Heredia, M.; Belda, E.; Vi, E. Field trial measuring the compatibility of methoxyfenozide and flonicamid with Orius laevigatus Fieber (Hemiptera: Anthocoridae) and Amblyseius swirskii (Athias-Henriot) (Acari: Phytoseiidae) in a commercial pepper greenhouse. Pest Manag. Sci. 2011, 67, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Drobnjaković, T.; Marčić, D.; Prijović, M.; Milenković, S. Toxic and sublethal effects of buprofezin on the whitefly parasitoid Encarsia formosa Gahan. Pestic. Fitomed. 2019, 34, 201–209. [Google Scholar] [CrossRef]
- Yang, S.; Li, M.; Shang, H.; Liu, Y.; Li, X.; Jiang, Z.; Chen, G.; Zhang, X. Effect of sublethal spirotetramat on host locating and parasitic behavior of Encarsia formosa Gahan. Pest Manag. Sci. 2021, 78, 329–335. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).