Impacts of Farming Activities on Nitrogen Degradability under a Temperate Continental Monsoon Climate
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Effective Soil Depth and Sample Collection
2.2. Soil Fertility Measurement
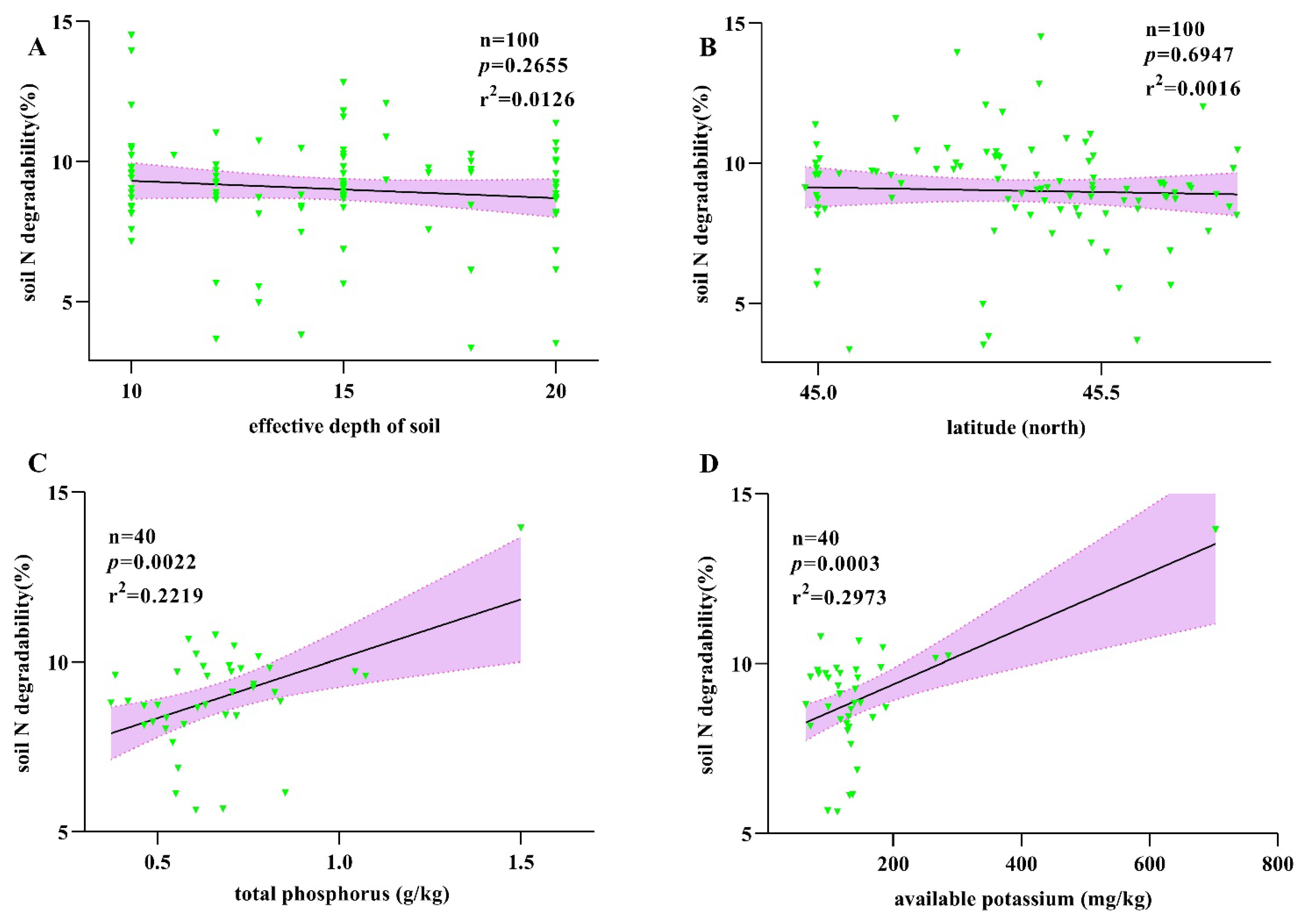
2.3. Correlation Analysis of Soil N Degradability
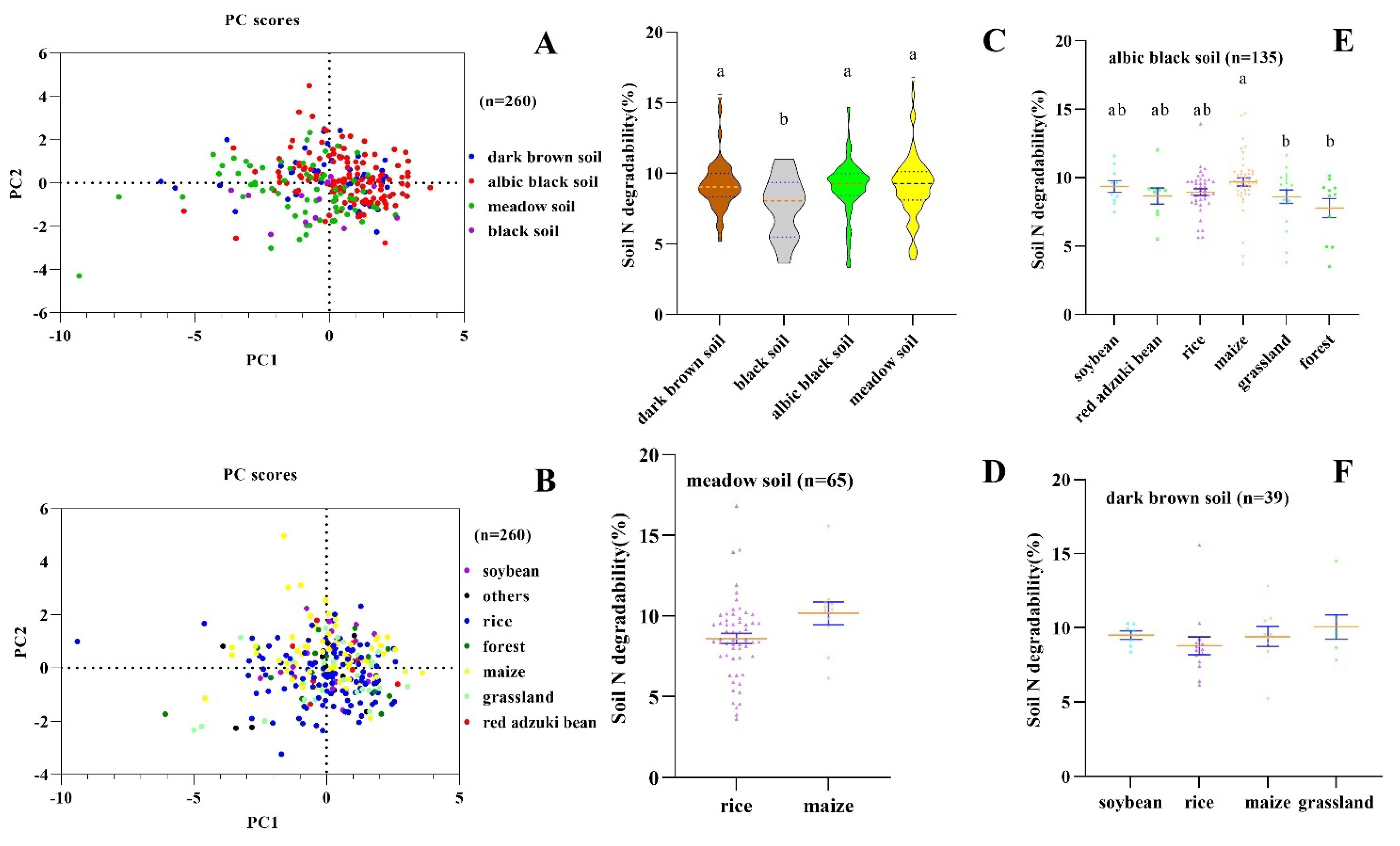
2.4. Principal Component Analysis (PCA) of Soil N Degradability
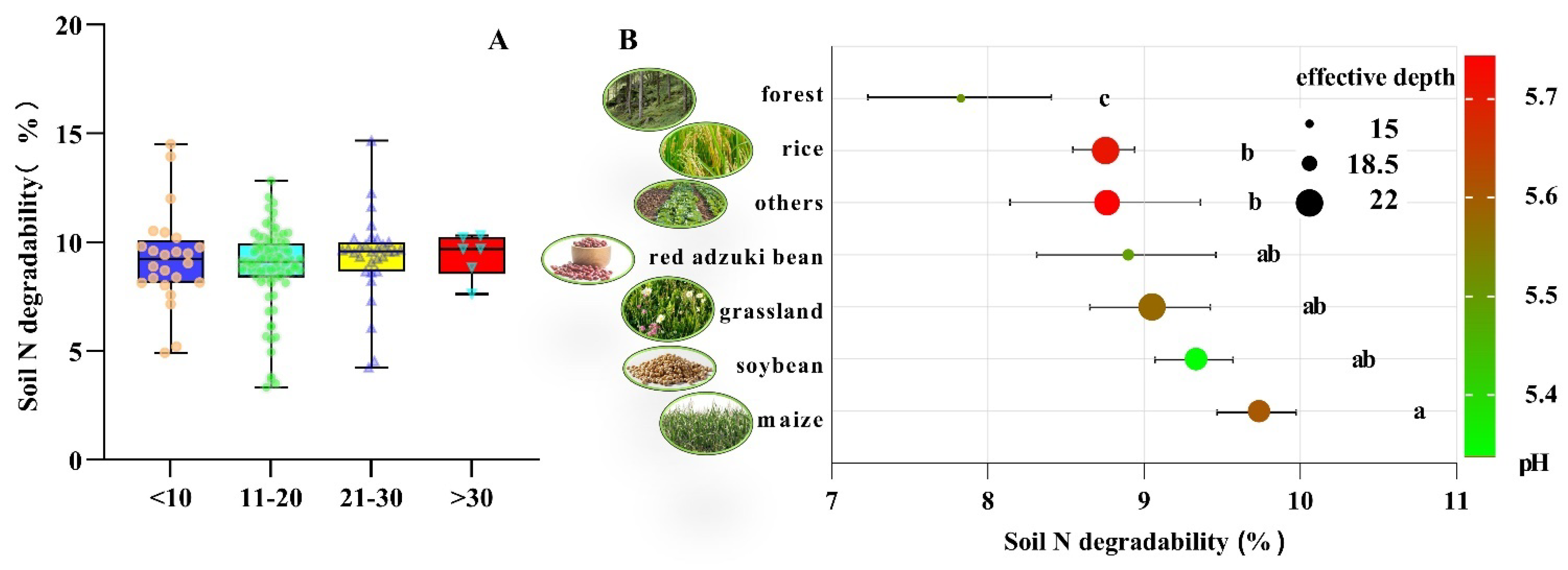
2.5. One-Way ANOVA of Soil N Degradability
2.6. Statistical Analysis
3. Results
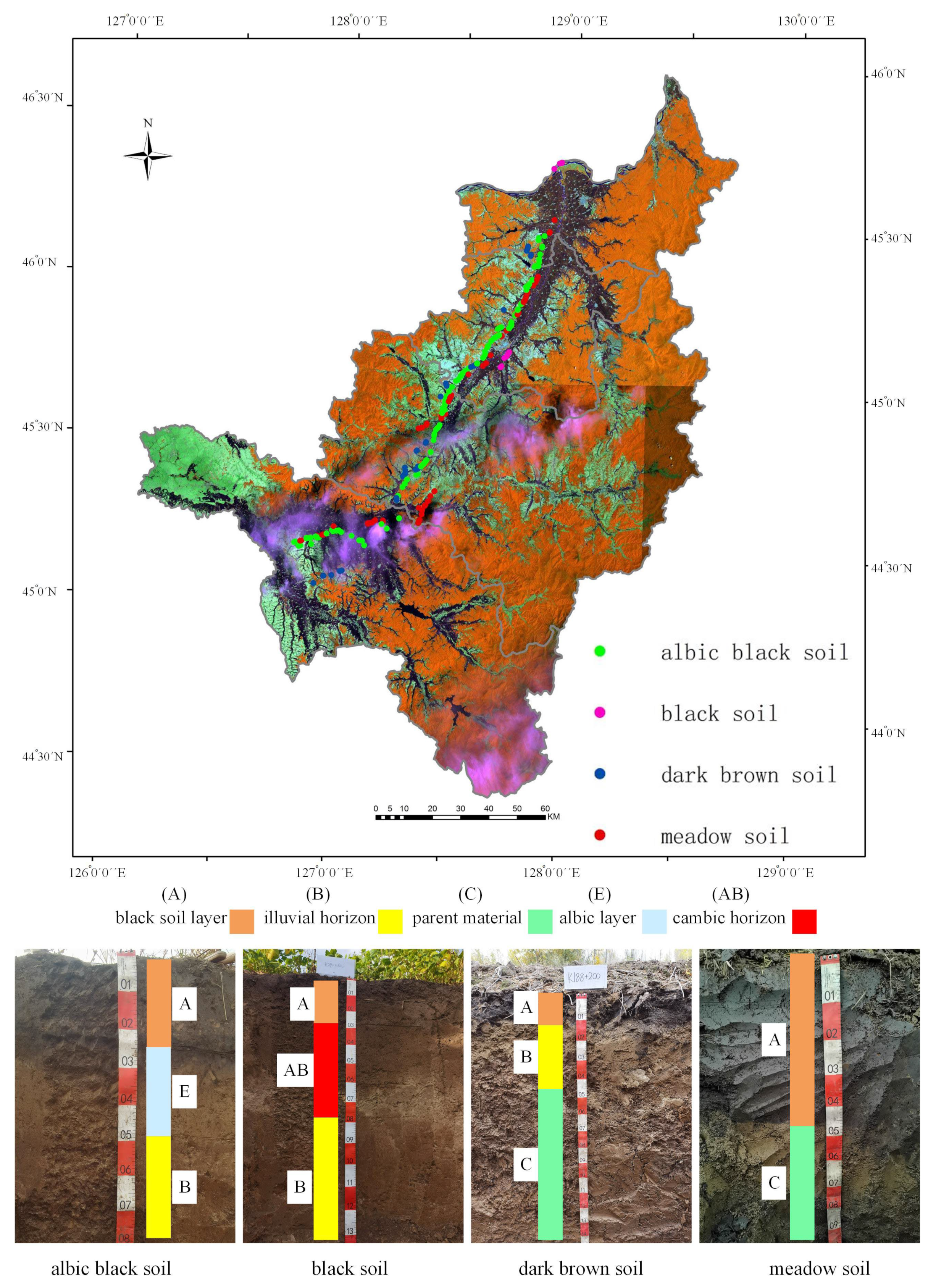
3.1. Recognition of Four Soil Types and the Effective Soil Depth
3.2. The Effect of Tillage Activities on Soil N Degradability
4. Discussion
4.1. Ground Vegetation Affects Soil N
4.2. Farming Activities Affect N
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Flores, B.M.; Montoya, E.; Sakschewski, B.; Nascimento, N.; Staal, A.; Betts, R.A.; Levis, C.; Lapola, D.M.; Esquivel-Muelbert, A.; Jakovac, C.; et al. Critical transitions in the Amazon forest system. Nature 2024, 626, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, A.; Shakoor, S.; Rehman, A.; Ashraf, F.; Abdullah, M.; Shahzad, S.M.; Farooq, T.H.; Ashraf, M.; Manzoor, M.A.; Altaf, M.M.; et al. Effect of animal manure, crop type, climate zone, and soil attributes on greenhouse gas emissions from agricultural soils—A global meta-analysis. J. Clean. Prod. 2021, 278, 124019. [Google Scholar] [CrossRef]
- Xu, Q.; Dai, L.; Zhou, Y.; Dou, Z.; Gao, W.; Yuan, X.; Gao, H.; Zhang, H. Effect of nitrogen application on greenhouse gas emissions and nitrogen uptake by plants in integrated rice-crayfish farming. Sci. Total Environ. 2023, 905, 167629. [Google Scholar] [CrossRef]
- Li, M.; Li, S.; Meng, Q.; Chen, S.; Wang, J.; Guo, X.; Ding, F.; Shi, L. Feedstock optimization with rice husk chicken manure and mature compost during chicken manure composting: Quality and gaseous emissions. Bioresour. Technol. 2023, 387, 129694. [Google Scholar] [CrossRef]
- Iboko, M.P.; Dossou-Yovo, E.R.; Obalum, S.E.; Oraegbunam, C.J.; Diedhiou, S.; Brummer, C.; Teme, N. Paddy rice yield and greenhouse gas emissions: Any trade-off due to co-application of biochar and nitrogen fertilizer? A systematic review. Heliyon 2023, 9, e22132. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Liu, L.; Wang, S.; Wu, X.; Zhang, W.; Zhao, L.; Lu, X.; Zhao, X. Evaluating the effects of nitrogen deposition on rice ecosystems across China. Agric. Ecosyst. Environ. 2019, 285, 106617. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, L.; Li, P.; He, H.; Ren, X. Effects of atmospheric nitrogen deposition on carbon allocation and vegetation carbon turnover time of forest ecosystems in China. Agric. For. Meteorol. 2024, 345, 109853. [Google Scholar] [CrossRef]
- Ma, T.-Y.; Liu, X.-Y.; Xu, S.-Q.; Guo, H.-R.; Huang, H.; Hu, C.-C.; Wu, D.; Sun, Z.-C.; Chen, C.-J.; Song, W. Levels and variations of soil organic carbon and total nitrogen among forests in a hotspot region of high nitrogen deposition. Sci. Total Environ. 2020, 713, 136620. [Google Scholar] [CrossRef]
- Storkey, J.; Macdonald, A.J.; Poulton, P.R.; Scott, T.; Kohler, I.H.; Schnyder, H.; Goulding, K.W.; Crawley, M.J. Grassland biodiversity bounces back from long-term nitrogen addition. Nature 2015, 528, 401–404. [Google Scholar] [CrossRef]
- Xie, L.; Gao, X.; Liu, Y.; Yang, B.; Yuan, H.; Li, X.; Song, J.; Zhao, J.; Xing, Q. Atmospheric deposition as a direct source of particulate organic carbon in region coastal surface seawater: Evidence from stable carbon and nitrogen isotope analysis. Sci. Total Environ. 2023, 854, 158540. [Google Scholar] [CrossRef]
- Fernandez-Martinez, M.; Penuelas, J.; Chevallier, F.; Ciais, P.; Obersteiner, M.; Rodenbeck, C.; Sardans, J.; Vicca, S.; Yang, H.; Sitch, S.; et al. Diagnosing destabilization risk in global land carbon sinks. Nature 2023, 615, 848–853. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Li, W.; Chen, Y.; Zhang, Q.; Zhang, K. Soil carbon and nitrogen accumulation during long-term natural vegetation restoration following agricultural abandonment in Qingling Mountains. Ecol. Eng. 2024, 201, 107212. [Google Scholar] [CrossRef]
- Walton, C.R.; Zak, D.; Audet, J.; Petersen, R.J.; Lange, J.; Oehmke, C.; Wichtmann, W.; Kreyling, J.; Grygoruk, M.; Jabłońska, E.; et al. Wetland buffer zones for nitrogen and phosphorus retention: Impacts of soil type, hydrology and vegetation. Sci. Total Environ. 2020, 727, 138709. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Yu, G.; Jiang, C.; Yan, J.; Liu, Y.; Wang, S.; Wang, B.; Zhang, J.; Wang, C.; Zhou, M.; et al. Monitoring nitrogen deposition in typical forest ecosystems along a large transect in China. Environ. Monit. Assess. 2013, 185, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Guerrieri, R.; Vanguelova, E.I.; Michalski, G.; Heaton, T.H.; Mencuccini, M. Isotopic evidence for the occurrence of biological nitrification and nitrogen deposition processing in forest canopies. Glob. Chang. Biol. 2015, 21, 4613–4626. [Google Scholar] [CrossRef]
- Kosonen, Z.; Thimonier, A.; Schnyder, E.; Thoni, L. Nitrogen concentration in moss compared with N load in precipitation and with total N deposition in Switzerland. Environ. Pollut. 2018, 239, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Hernández, D.; Esch, E.; Alster, C.; McKone, M.; Camill, P. Rapid accumulation of soil carbon and nitrogen in restored prairie is not predicted using a chronosequence approach. Soil Sci. Soc. Am. J. 2012, 77, 2029. [Google Scholar] [CrossRef]
- McDonough, A.M.; Watmough, S.A. Interactive effects of precipitation and above canopy nitrogen deposition on understorey vascular plants in a jack pine (Pinus banksiana) forest in northern Alberta, Canada. Sci. Total Environ. 2023, 855, 158708. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Zhao, Y.; He, S.; Wu, Z.; Yue, M.; Hong, M. Metagenomics reveals the response of desert steppe microbial communities and carbon-nitrogen cycling functional genes to nitrogen deposition. Front. Microbiol. 2024, 15, 1369196. [Google Scholar] [CrossRef]
- Ochoa-Hueso, R. Nonlinear disruption of ecological interactions in response to nitrogen deposition. Ecology 2016, 97, 2802–2814. [Google Scholar] [CrossRef]
- Abdullaeva, Y.; Ratering, S.; Rosado-Porto, D.; Ambika Manirajan, B.; Glatt, A.; Schnell, S.; Cardinale, M. Domestication caused taxonomical and functional shifts in the wheat rhizosphere microbiota, and weakened the natural bacterial biocontrol against fungal pathogens. Microbiol. Res. 2024, 281, 127601. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Xie, B.; Cagle, G.A.; Wang, X.; Han, G.; Wang, X.; Hou, A.; Guan, B. Effects of simulated nitrogen deposition on soil microbial community diversity in coastal wetland of the Yellow River Delta. Sci. Total Environ. 2021, 757, 143825. [Google Scholar] [CrossRef]
- Lafuente, A.; Recio, J.; Ochoa-Hueso, R.; Gallardo, A.; Perez-Corona, M.E.; Manrique, E.; Duran, J. Simulated nitrogen deposition influences soil greenhouse gas fluxes in a Mediterranean dryland. Sci. Total Environ. 2020, 737, 139610. [Google Scholar] [CrossRef] [PubMed]
- Renau-Morata, B.; Jimenez-Benavente, E.; Gil-Villar, D.; Cebolla-Cornejo, J.; Romero-Hernandez, G.; Carrillo, L.; Vicente-Carbajosa, J.; Medina, J.; Molina, R.V.; Nebauer, S.G. Arabidopsis CDF3 transcription factor increases carbon and nitrogen assimilation and yield in trans-grafted tomato plants. Plant Physiol. Biochem. 2024, 210, 108607. [Google Scholar] [CrossRef] [PubMed]
- Djalovic, I.; Prasad, P.V.V.; Akhtar, K.; Paunovic, A.; Riaz, M.; Dugalic, M.; Katanski, S.; Zaheer, S. Nitrogen Fertilization and Cultivar Interactions Determine Maize Yield and Grain Mineral Composition in Calcareous Soil under Semiarid Conditions. Plants 2024, 13, 844. [Google Scholar] [CrossRef]
- Biradar, S.S.; Patil, M.K.; Desai, S.A.; Singh, S.K.; Naik, V.R.; Lamani, K.; Joshi, A.K. Nitrogen use efficiency in bread wheat: Genetic variation and prospects for improvement. PLoS ONE 2024, 19, e0294755. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Sraw, P.K.; Kang, J.S.; Kaur, J.; Sharma, V.; Pathania, N.; Kalia, A.; Al-Ansari, N.; Alataway, A.; Dewidar, A.Z.; et al. Interactive effects of long-term management of crop residue and phosphorus fertilization on wheat productivity and soil health in the rice-wheat. Sci. Rep. 2024, 14, 1399. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Butterbach-Bahl, K.; Vereecken, H.; Bruggemann, N. A meta-analysis of soil salinization effects on nitrogen pools, cycles and fluxes in coastal ecosystems. Glob. Chang. Biol. 2017, 23, 1338–1352. [Google Scholar] [CrossRef]
- Yang, L.; Wu, Q.; Liang, H.; Yin, L.; Shen, P. Integrated analyses of transcriptome and metabolome provides new insights into the primary and secondary metabolism in response to nitrogen deficiency and soil compaction stress in peanut roots. Front. Plant Sci. 2022, 13, 948742. [Google Scholar] [CrossRef]
- Maier, R.; Hortnagl, L.; Buchmann, N. Greenhouse gas fluxes (CO(2), N(2)O and CH(4)) of pea and maize during two cropping seasons: Drivers, budgets, and emission factors for nitrous oxide. Sci. Total Environ. 2022, 849, 157541. [Google Scholar] [CrossRef]
- Saud, S.; Wang, D.; Fahad, S. Improved Nitrogen Use Efficiency and Greenhouse Gas Emissions in Agricultural Soils as Producers of Biological Nitrification Inhibitors. Front. Plant Sci. 2022, 13, 854195. [Google Scholar] [CrossRef] [PubMed]
- Fornara, D.; Tilman, D. Soil carbon sequestration in prairie grasslands increased by chronic nitrogen addition. Ecology 2012, 93, 2030–2036. [Google Scholar] [PubMed]
- Liu, H.; Wang, J.; Sun, X.; McLaughlin, N.B.; Jia, S.; Liang, A.; Zhang, S. The driving mechanism of soil organic carbon biodegradability in the black soil region of Northeast China. Sci. Total Environ. 2023, 884, 163835. [Google Scholar] [CrossRef] [PubMed]
- Hosogoe, Y.; Nguyen-Sy, T.; Tang, S.; Bimantara, P.O.; Sekikawa, Y.; Kautsar, V.; Kimani, S.M.; Xu, X.; Tawaraya, K.; Cheng, W. Five-year vegetation conversion from pasture to C(3) and C(4) plants affects dynamics of SOC and TN and their natural stable C and N isotopes via mediating C input and N leaching. Sci. Total Environ. 2024, 912, 169481. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, S.; Abbadie, L.; Aubert, M.; Barot, S.; Bloor, J.M.G.; Derrien, D.; Duchene, O.; Gross, N.; Henneron, L.; Le Roux, X.; et al. Plant-soil synchrony in nutrient cycles: Learning from ecosystems to design sustainable agrosystems. Glob. Chang. Biol. 2024, 30, e17034. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, X.; Li, J.; Li, P.; Zuo, X.; Chang, B.; Liu, Y.; Zhang, N.; Yu, H.; Miao, L.; et al. Temporal variation in soil carbon in various paddy soil types in a cold temperate continental monsoon climate. Soil Use Manag. 2023, 39, 122–133. [Google Scholar] [CrossRef]
- Shi, X.; Yang, G.; Yu, D.; Xu, S.; Warner, E.D.; Petersen, G.W.; Sun, W.; Zhao, Y.; Easterling, W.E.; Wang, H. A WebGIS system for relating genetic soil classification of China to soil taxonomy. Comput. Geosci. 2010, 36, 768–775. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, T.; Chang, R. An increasing trend of inorganic nitrogen deposition across montane regions of China. Atmos. Environ. 2023, 304, 119780. [Google Scholar] [CrossRef]
- Gilliam, F.S.; Burns, D.A.; Driscoll, C.T.; Frey, S.D.; Lovett, G.M.; Watmough, S.A. Chapter 12—Responses of forest ecosystems to decreasing nitrogen deposition in eastern North America. In Atmospheric Nitrogen Deposition to Global Forests; Du, E., de Vries, W., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 205–225. [Google Scholar]
- Liu, X.J.; Xu, W.; Du, E.Z.; Tang, A.H.; Zhang, Y.; Zhang, Y.Y.; Wen, Z.; Hao, T.X.; Pan, Y.P.; Zhang, L.; et al. Environmental impacts of nitrogen emissions in China and the role of policies in emission reduction. Philos. Trans. A Math. Phys. Eng. Sci. 2020, 378, 20190324. [Google Scholar] [CrossRef]
- VanZomeren, C.M.; Bhomia, R.K.; Tfaily, M.M.; Inglett, K.S.; Cooper, W.T.; White, J.R.; Reddy, K.R. Influence of vegetation on soil organic nitrogen composition and mineralization in a subtropical wetland. Ecol. Eng. 2024, 200, 107186. [Google Scholar] [CrossRef]
- Henneron, L.; Kardol, P.; Wardle, D.A.; Cros, C.; Fontaine, S. Rhizosphere control of soil nitrogen cycling: A key component of plant economic strategies. New Phytol. 2020, 228, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Maire, V.; Wright, I.J.; Prentice, I.C.; Batjes, N.H.; Bhaskar, R.; van Bodegom, P.M.; Cornwell, W.K.; Ellsworth, D.; Niinemets, Ü.; Ordonez, A.; et al. Global effects of soil and climate on leaf photosynthetic traits and rates. Glob. Ecol. Biogeogr. 2015, 24, 706–717. [Google Scholar] [CrossRef]
- Shahzad, T.; Chenu, C.; Genet, P.; Barot, S.; Perveen, N.; Mougin, C.; Fontaine, S. Contribution of exudates, arbuscular mycorrhizal fungi and litter depositions to the rhizosphere priming effect induced by grassland species. Soil Biol. Biochem. 2015, 80, 146–155. [Google Scholar] [CrossRef]
- Trap, J.; Bonkowski, M.; Plassard, C.; Villenave, C.; Blanchart, E. Ecological importance of soil bacterivores for ecosystem functions. Plant Soil 2016, 398, 1–24. [Google Scholar] [CrossRef]
- Lu, J.; Dijkstra, F.A.; Wang, P.; Cheng, W. Roots of non-woody perennials accelerated long-term soil organic matter decomposition through biological and physical mechanisms. Soil Biol. Biochem. 2019, 134, 42–53. [Google Scholar] [CrossRef]
- Fuller, D.Q.; Denham, T.; Allaby, R. Plant domestication and agricultural ecologies. Curr. Biol. 2023, 33, R636–R649. [Google Scholar] [CrossRef]
- Fontaine, S.; Barot, S. Size and functional diversity of microbe populations control plant persistence and long-term soil carbon accumulation. Ecol. Lett. 2005, 8, 1075–1087. [Google Scholar] [CrossRef]
- Henneron, L.; Cros, C.; Picon-Cochard, C.; Rahimian, V.; Fontaine, S. Plant economic strategies of grassland species control soil carbon dynamics through rhizodeposition. J. Ecol. 2020, 108, 528–545. [Google Scholar] [CrossRef]
- Fukudome, M.; Uchiumi, T. Regulation of nitric oxide by phytoglobins in Lotus japonicus is involved in mycorrhizal symbiosis with Rhizophagus irregularis. Plant Sci. 2024, 340, 111984. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, X.; Xiang, Y.; Liang, J.; Guo, J.; Li, W.; Lu, J.; Du, R.; Li, Z.; Zhang, F. Application of hyperspectral technology for leaf function monitoring and nitrogen nutrient diagnosis in soybean (Glycine max L.) production systems on the Loess Plateau of China. Eur. J. Agron. 2024, 154, 127098. [Google Scholar] [CrossRef]
- Atta-Darkwa, T.; Asare, A.; Asampana, K.A.; Budu, M.; Amerh, A.A.; Kwesi, S.A.; Bessah, E.; Owusu, P.A.; Quansah, A.; Kwasi, E.N.; et al. Effects of varying tillage practices and weed control methods on the efficacy of infiltration models. PLoS ONE 2024, 19, e0293507. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Lv, Q.; Wang, P.; Xie, H. Changes in the degree of lateral root trait plasticity and trade-offs of maize under long-term no tillage. Front. Plant Sci. 2024, 15, 1345189. [Google Scholar] [CrossRef] [PubMed]
- Shiono, K.; Matsuura, H. Exogenous abscisic acid induces the formation of a suberized barrier to radial oxygen loss in adventitious roots of barley (Hordeum vulgare). Ann. Bot. 2024, 133, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Sakata, K.; Watanabe, Y. Water-soluble and water-insoluble organic nitrogen in the dry and wet deposition. Atmos. Environ. 2019, 218, 117022. [Google Scholar] [CrossRef]

| pH | OM (%) | TN (g/kg) | TP (g/kg) | TK (g/kg) | TSN (mg/kg) | AP (mg/kg) | AK (mg/kg) | ||
|---|---|---|---|---|---|---|---|---|---|
| Dark brown soil | Mean | 5.53 | 3.31 | 1.92 | 0.72 | 15.53 | 178.67 | 26.71 | 127.51 |
| SD | 0.33 | 1.84 | 1.08 | 0.23 | 1.38 | 106.43 | 26.34 | 36.82 | |
| Albic black soil | Mean | 5.59 | 2.88 | 1.62 | 0.67 | 16.25 | 145.65 | 30.80 | 119.80 |
| SD | 0.38 | 1.26 | 0.68 | 0.19 | 1.00 | 62.47 | 22.56 | 65.68 | |
| Meadow soil | Mean | 5.74 | 4.31 | 2.19 | 0.94 | 15.80 | 201.00 | 28.68 | 168.56 |
| SD | 0.39 | 1.83 | 0.84 | 0.32 | 1.30 | 92.72 | 23.23 | 111.91 | |
| Black soil | Mean | 5.70 | 3.96 | 1.98 | 1.03 | 15.33 | 155.55 | 18.51 | 136.45 |
| SD | 0.35 | 1.24 | 0.59 | 0.32 | 1.47 | 71.90 | 17.47 | 35.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Zou, J.; Liu, Y.; Li, J.; Liu, X.; Zhang, H.; Jiao, F.; Meng, Q.; Guo, Z. Impacts of Farming Activities on Nitrogen Degradability under a Temperate Continental Monsoon Climate. Agronomy 2024, 14, 1094. https://doi.org/10.3390/agronomy14061094
Wang Q, Zou J, Liu Y, Li J, Liu X, Zhang H, Jiao F, Meng Q, Guo Z. Impacts of Farming Activities on Nitrogen Degradability under a Temperate Continental Monsoon Climate. Agronomy. 2024; 14(6):1094. https://doi.org/10.3390/agronomy14061094
Chicago/Turabian StyleWang, Qiuju, Jiahe Zou, Yanxia Liu, Jingyang Li, Xin Liu, Haibin Zhang, Feng Jiao, Qingying Meng, and Zhenhua Guo. 2024. "Impacts of Farming Activities on Nitrogen Degradability under a Temperate Continental Monsoon Climate" Agronomy 14, no. 6: 1094. https://doi.org/10.3390/agronomy14061094





