Nanoparticles of Zinc Oxides Mitigated N2O Emissions in Tea Plantation Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Soil Sampling
2.2. Aerobic Incubation Trial
2.3. Sampling
2.4. Soil Analysis and N2O Measurement
2.5. Soil DNA Extraction and Gene Abundance Quantification
2.6. Emission Calculation and Statistical Analysis
3. Results
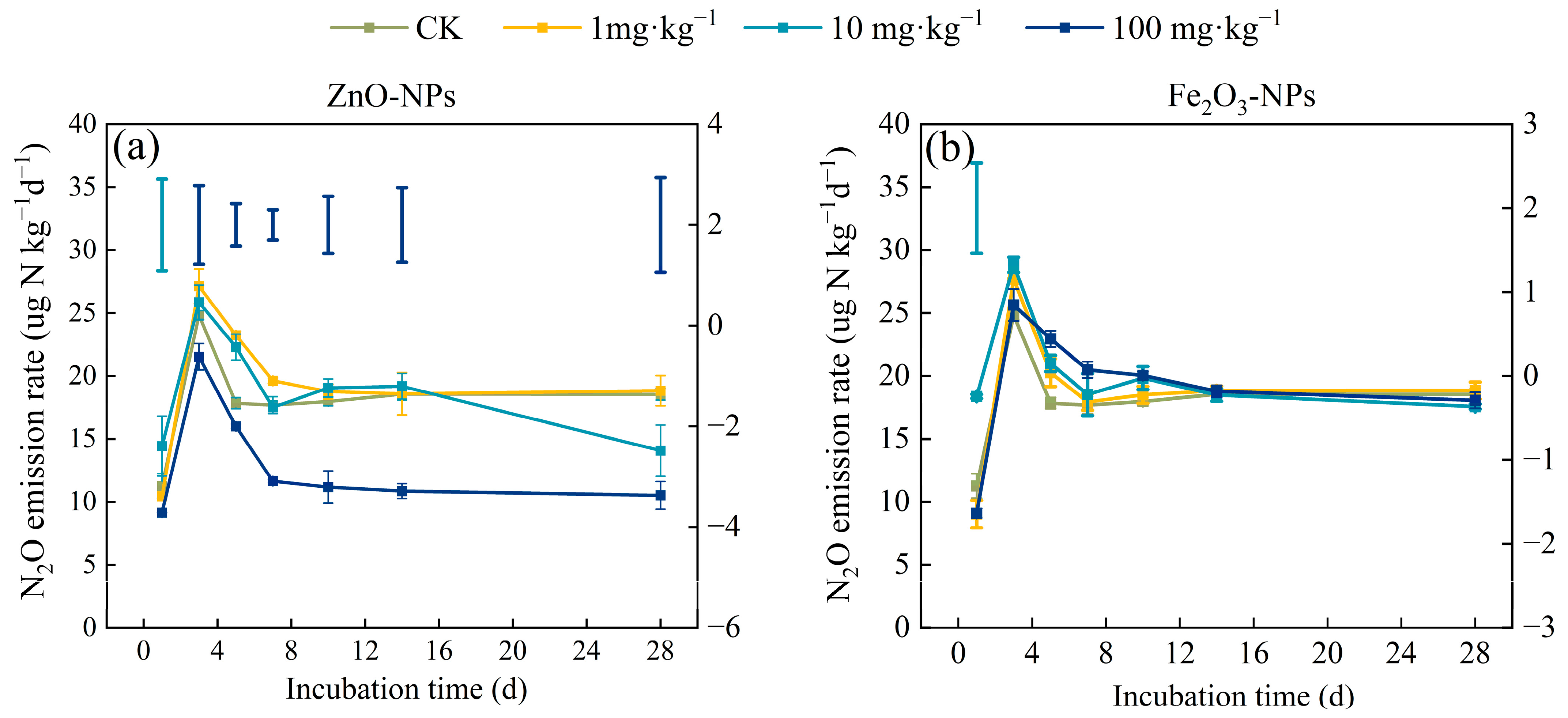
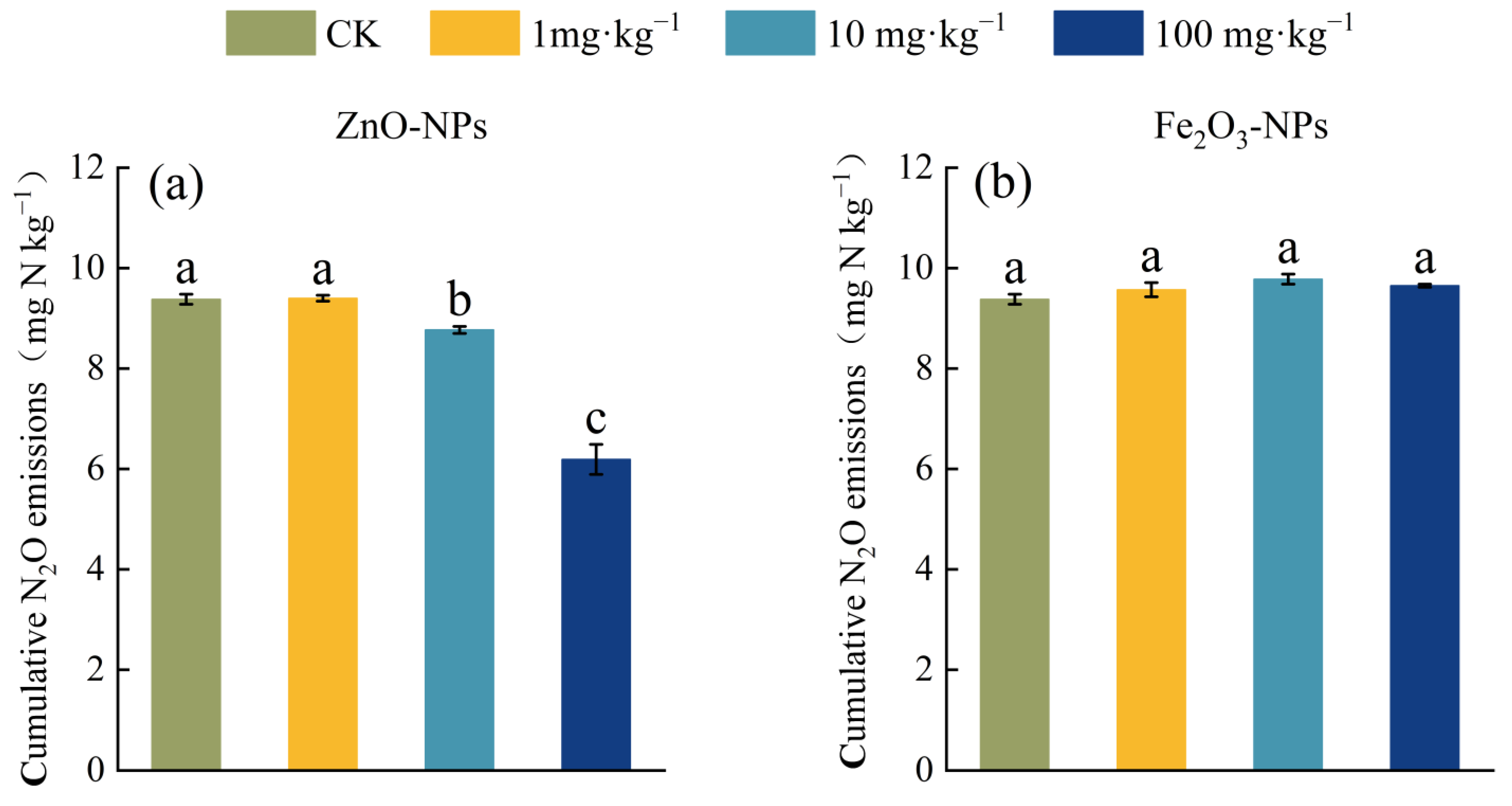
3.1. Soil N2O Emission
3.2. Changes in Soil pH and Inorganic N
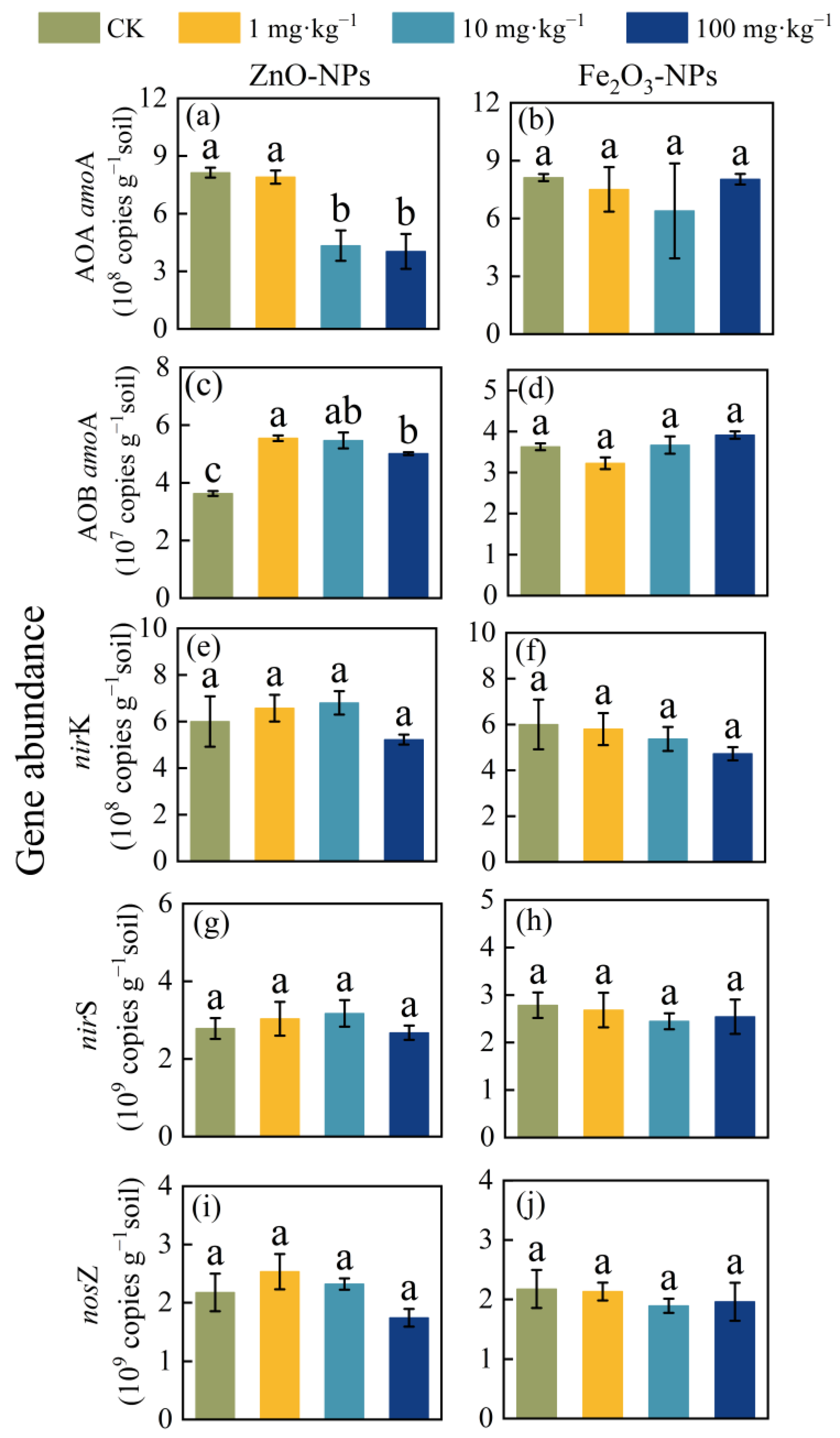
3.3. Expression of Soil Microbial Functional Genes
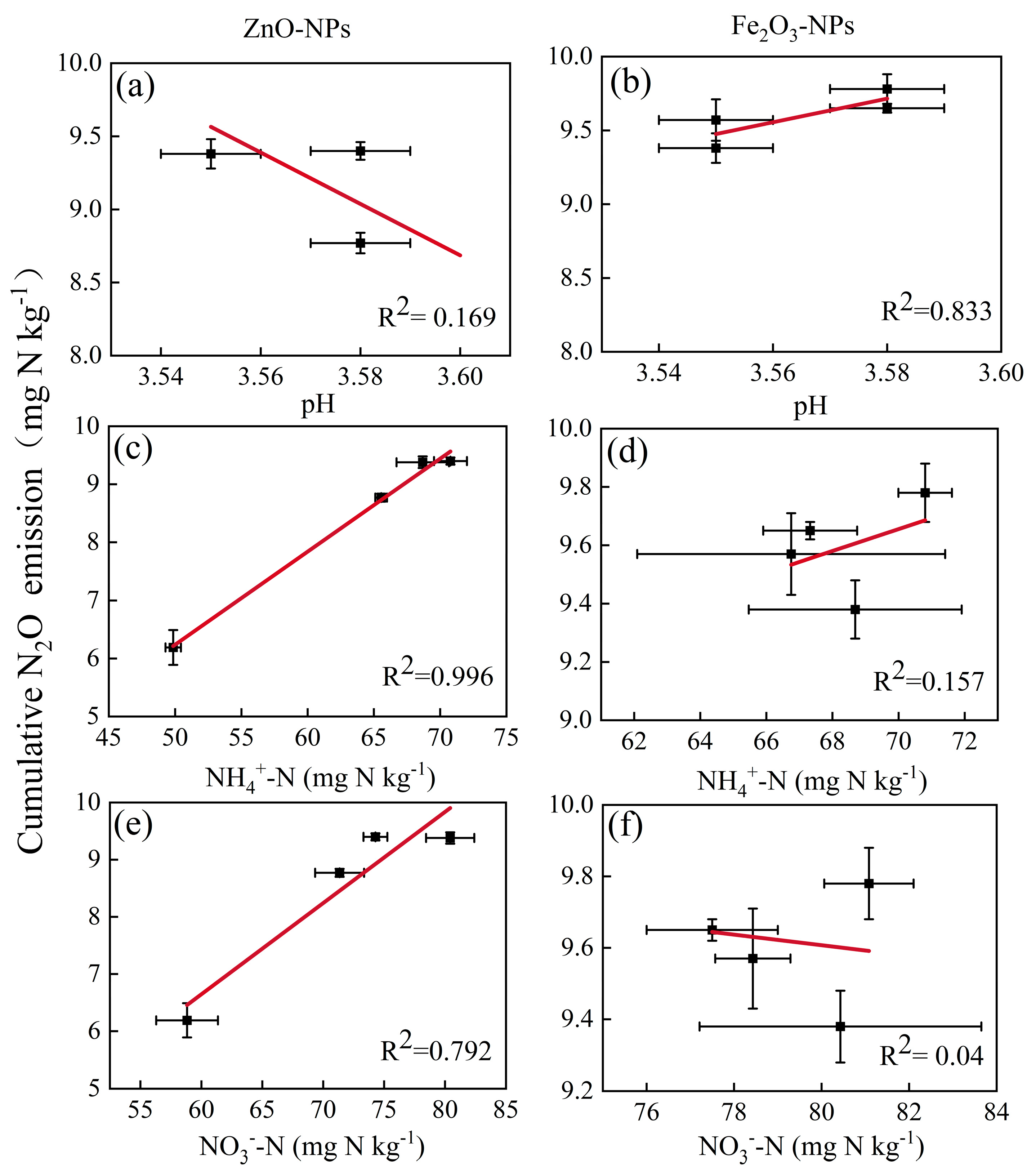
3.4. Relationships between Factors and Cumulative N2O Emissions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Xu, R.; Canadell, J.G.; Thompson, R.L.; Winiwarter, W.; Suntharalingam, P.; Davidson, E.A.; Ciais, P.; Jackson, R.B.; Janssens-Maenhout, G.; et al. A Comprehensive Quantification of Global Nitrous Oxide Sources and Sinks. Nature 2020, 586, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Xu, B.; Han, Y.; Wang, J.; Wang, X.; Liu, L.; Liu, W.; Wan, S.; Tan, H.; Liu, Y.; et al. Synthetic Nitrogen Fertilizers Alter the Soil Chemistry, Production and Quality of Tea. A Meta-Analysis. Agron. Sustain. Dev. 2018, 38, 10. [Google Scholar] [CrossRef]
- Wang, J.; Smith, P.; Hergoualc’h, K.; Zou, J. Direct N2O Emissions from Global Tea Plantations and Mitigation Potential by Climate-Smart Practices. Resour. Conserv. Recycl. 2022, 185, 106501. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, Z.; Pan, Z.; Wang, R.; Yan, G.; Liu, C.; Su, Y.; Zheng, X.; Butterbach-Bahl, K. Tea-Planted Soils as Global Hotspots for N2O Emissions from Croplands. Environ. Res. Lett. 2020, 15, 104018. [Google Scholar] [CrossRef]
- Preetha, P.S.; Balakrishnan, N. A Review of Nano Fertilizers and Their Use and Functions in Soil. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 3117–3133. [Google Scholar] [CrossRef]
- Kah, M.; Kookana, R.S.; Gogos, A.; Bucheli, T.D. A Critical Evaluation of Nanopesticides and Nanofertilizers against Their Conventional Analogues. Nat. Nanotechnol. 2018, 13, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Baggs, E.M. Soil Microbial Sources of Nitrous Oxide: Recent Advances in Knowledge, Emerging Challenges and Future Direction. Curr. Opin. Environ. Sustain. 2011, 3, 321–327. [Google Scholar] [CrossRef]
- Smith, K.A. Changing Views of Nitrous Oxide Emissions from Agricultural Soil: Key Controlling Processes and Assessment at Different Spatial Scales. Eur. J. Soil Sci. 2017, 68, 137–155. [Google Scholar] [CrossRef]
- Parada, J.; Rubilar, O.; Sousa, D.Z.; Martínez, M.; Fernández-Baldo, M.A.; Tortella, G.R. Short Term Changes in the Abundance of Nitrifying Microorganisms in a Soil-Plant System Simultaneously Exposed to Copper Nanoparticles and Atrazine. Sci. Total Environ. 2019, 670, 1068–1074. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Liu, W. Effect of Metal and Metal Oxide Engineered Nano Particles on Nitrogen Bio-Conversion and Its Mechanism: A Review. Chemosphere 2022, 287, 132097. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.I.; Shahzad, T.; Shahid, M.; Ismail, I.M.I.; Shah, G.M.; Almeelbi, T. Zinc Oxide Nanoparticles Affect Carbon and Nitrogen Mineralization of Phoenix Dactylifera Leaf Litter in a Sandy Soil. J. Hazard. Mater. 2017, 324, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Gao, H.; Wu, J.; Chang, Y.; Chen, Z.; Yu, R. Responses of Nitrogen Transformation Processes and N2O Emissions in Biological Nitrogen Removal System to Short-Term ZnO Nanoparticle Stress. Sci. Total Environ. 2020, 705, 135916. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, J.; Zhang, J.-B.; Müller, C.; Wang, S.-Q. Mechanistic Insights into the Effects of N Fertilizer Application on N2O-Emission Pathways in Acidic Soil of a Tea Plantation. Plant Soil 2015, 389, 45–57. [Google Scholar] [CrossRef]
- Beddow, J.; Stolpe, B.; Cole, P.A.; Lead, J.R.; Sapp, M.; Lyons, B.P.; Colbeck, I.; Whitby, C. Nanosilver Inhibits Nitrification and Reduces Ammonia-Oxidising Bacterial but Not Archaeal amoA Gene Abundance in Estuarine Sediments. Environ. Microbiol. 2017, 19, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zeng, J.; Zhao, D.; Yong, B.; Yu, Z. Co-Association of Two Nir Denitrifiers Under the Influence of Emergent Macrophytes. Microb. Ecol. 2020, 80, 809–821. [Google Scholar] [CrossRef]
- Throbäck, I.N.; Enwall, K.; Jarvis, Å.; Hallin, S. Reassessing PCR Primers Targeting nirS, nirK and nosZ Genes for Community Surveys of Denitrifying Bacteria with DGGE. FEMS Microbiol. Ecol. 2004, 49, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Yu, Y.; Yao, H.; Ge, C. Effect of Zinc Oxide Nanoparticles on Nitrous Oxide Emissions in Agricultural Soil. Agriculture 2021, 11, 730. [Google Scholar] [CrossRef]
- Guo, J.; Peng, Y.; Wang, S.; Ma, B.; Ge, S.; Wang, Z.; Huang, H.; Zhang, J.; Zhang, L. Pathways and Organisms Involved in Ammonia Oxidation and Nitrous Oxide Emission. Crit. Rev. Environ. Sci. Technol. 2013, 43, 2213–2296. [Google Scholar] [CrossRef]
- Prosser, J.I.; Nicol, G.W. Archaeal and Bacterial Ammonia-Oxidisers in Soil: The Quest for Niche Specialisation and Differentiation. Trends Microbiol. 2012, 20, 523–531. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, N.; Fu, H.; Chen, T.; Liu, S.; Zheng, S.; Zhang, J. Effect of Zinc Oxide Nanoparticles on Nitrogen Removal, Microbial Activity and Microbial Community of CANON Process in a Membrane Bioreactor. Bioresour. Technol. 2017, 243, 93–99. [Google Scholar] [CrossRef]
- Wu, Q.; Huang, K.; Sun, H.; Ren, H.; Zhang, X.; Ye, L. Comparison of the Impacts of Zinc Ions and Zinc Nanoparticles on Nitrifying Microbial Community. J. Hazard. Mater. 2018, 343, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Phan, D.C.; Pasha, A.B.M.T.; Carwile, N.; Kapoor, V. Effect of Zinc Oxide Nanoparticles on Physiological Activities and Gene Expression of Wastewater Nitrifying Bacteria. Environ. Eng. Sci. 2020, 37, 328–336. [Google Scholar] [CrossRef]
- Wright, C.L.; Lehtovirta-Morley, L.E. Nitrification and beyond: Metabolic Versatility of Ammonia Oxidising Archaea. ISME J. 2023, 17, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Tolar, B.B.; Herrmann, J.; Bargar, J.R.; van den Bedem, H.; Wakatsuki, S.; Francis, C.A. Integrated Structural Biology and Molecular Ecology of N-Cycling Enzymes from Ammonia-Oxidizing Archaea. Environ. Microbiol. Rep. 2017, 9, 484–491. [Google Scholar] [CrossRef]
- French, E.; Kozlowski, J.A.; Mukherjee, M.; Bullerjahn, G.; Bollmann, A. Ecophysiological Characterization of Ammonia-Oxidizing Archaea and Bacteria from Freshwater. Appl. Environ. Microbiol. 2012, 78, 5773–5780. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Hu, W.; Li, L.; Li, Y. Interactions between Organic Matter and Fe Oxides at Soil Micro-Interfaces: Quantification, Associations, and Influencing Factors. Sci. Total Environ. 2023, 855, 158710. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Zhou, Q.; Zheng, T.; Mo, F.; Ouyang, S. Iron Oxide Nanoparticles in the Soil Environment: Adsorption, Transformation, and Environmental Risk. J. Hazard. Mater. 2023, 459, 132107. [Google Scholar] [CrossRef]
- Wang, S.; Yang, X.; Meng, H.; Zhang, Y.; Li, X.; Xu, J. Enhanced Denitrification by Nano α-Fe2O3 Induced Self-Assembled Hybrid Biofilm on Particle Electrodes of Three-Dimensional Biofilm Electrode Reactors. Environ. Int. 2019, 125, 142–151. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, M.; Ding, K.; Zhou, S. Iron Oxides Affect Denitrifying Bacterial Communities with the NirS and NirK Genes and Potential N2O Emission Rates from Paddy Soil. Eur. J. Soil Biol. 2019, 93, 103093. [Google Scholar] [CrossRef]
- Gurung, R.B.; Ogle, S.M.; Breidt, F.J.; Parton, W.J.; Del Grosso, S.J.; Zhang, Y.; Hartman, M.D.; Williams, S.A.; Venterea, R.T. Modeling Nitrous Oxide Mitigation Potential of Enhanced Efficiency Nitrogen Fertilizers from Agricultural Systems. Sci. Total Environ. 2021, 801, 149342. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primers | Sequences | PCR Conditions | Reference |
|---|---|---|---|---|
| AOA | CrenamoA23f | ATGGTCTGGCTWAGACG | 95 °C 5 min 1 cycle, 95 °C-15 s, 55 °C 30 s, 72 °C-30 s | [15] |
| CrenamoA616r | GCCATCCATCTGTATGTCCA | 45 cycles, melt curve (95 °C, 60 °C, 95 °C) 15 s | ||
| AOB | amoA-1F | GGGGTTTCTACTGGTGGT | 95 °C 5 min 1 cycle, 95 °C-15 s, 55 °C 30 s, 72 °C-30 s | [15] |
| amoA-2R | CCCCTCKGSAAAGCCTTCTTC | 45 cycles, melt curve (95 °C, 60 °C, 95 °C) 15 s | ||
| nirK | Copper583F | TCATGGTGCTGCCGCGYGANGG | 95 °C 5 min 1 cycle, 95 °C-15 s, 55 °C 30 s, 72 °C-30 s | [16] |
| Copper909R | GAACTTGCCGGTKGCCCAGAC | 45 cycles, melt curve (95 °C, 60 °C, 95 °C) 15 s | ||
| nirS | nirS-Cd3aF | GTSAACGTSAAGGARACSGG | 95 °C 5 min 1 cycle, 95 °C-15 s, 37 °C 45 s, 72 °C-60 s | [17] |
| nirS-R3cd | GASTTCGGRTGSGTCTTGA | 45 cycles, melt curve (95 °C, 60 °C, 95 °C) 15 s | ||
| nosZ | nosZ-F | CGYTGTTCMTCGACAGCCAG | 95 °C 5 min 1 cycle, 95 °C-15 s, 55 °C 30 s, 72 °C-30 s | [17] |
| nosZ-1662R | CGSACCTTSTTGCCSTYGCG | 45 cycles, melt curve (95 °C, 60 °C, 95 °C) 15 s |
| Response Variable | N2O | pH | NH4+ | NO3− | AOA amoA | AOB amoA | nirK | nirS | nosZ |
|---|---|---|---|---|---|---|---|---|---|
| T | <0.001 | <0.001 | <0.001 | <0.001 | 0.061 | <0.001 | 0.067 | 0.814 | 0.066 |
| D | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.084 | 0.997 | 0.212 |
| T × D | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.740 | 0.262 | 0.134 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Guo, L.; Yang, F.; Xiang, J.; Long, L.; Ni, K. Nanoparticles of Zinc Oxides Mitigated N2O Emissions in Tea Plantation Soil. Agronomy 2024, 14, 1113. https://doi.org/10.3390/agronomy14061113
Wang J, Guo L, Yang F, Xiang J, Long L, Ni K. Nanoparticles of Zinc Oxides Mitigated N2O Emissions in Tea Plantation Soil. Agronomy. 2024; 14(6):1113. https://doi.org/10.3390/agronomy14061113
Chicago/Turabian StyleWang, Jing, Linfang Guo, Fengmin Yang, Jian Xiang, Lizhi Long, and Kang Ni. 2024. "Nanoparticles of Zinc Oxides Mitigated N2O Emissions in Tea Plantation Soil" Agronomy 14, no. 6: 1113. https://doi.org/10.3390/agronomy14061113
APA StyleWang, J., Guo, L., Yang, F., Xiang, J., Long, L., & Ni, K. (2024). Nanoparticles of Zinc Oxides Mitigated N2O Emissions in Tea Plantation Soil. Agronomy, 14(6), 1113. https://doi.org/10.3390/agronomy14061113










