Evaluation of the Rhizosphere Resistome of Cultivated Soils Polluted with Antibiotics from Reclaimed Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soils
2.2. Crops
2.3. Antibiotic Analysis
2.3.1. Chemicals and Reagents
2.3.2. Antibiotics Extraction and Quantification
2.3.3. Soil DNA Extraction, PCR Amplification and High-Throughput Sequencing
2.4. Analysis and Processing of Sequencing Data
3. Results
3.1. Antibiotics Analysis
3.1.1. Soil
3.1.2. Fruits
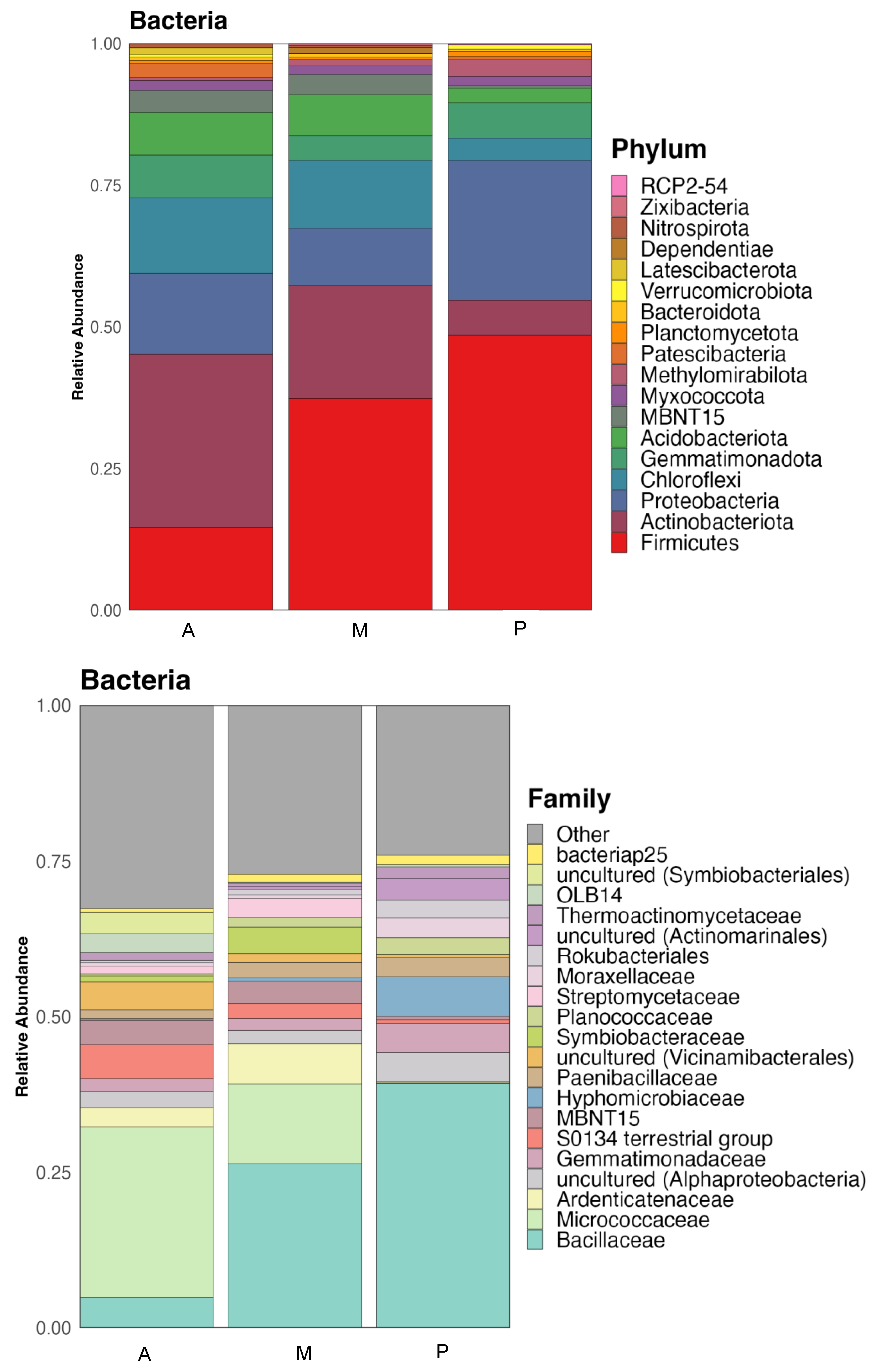
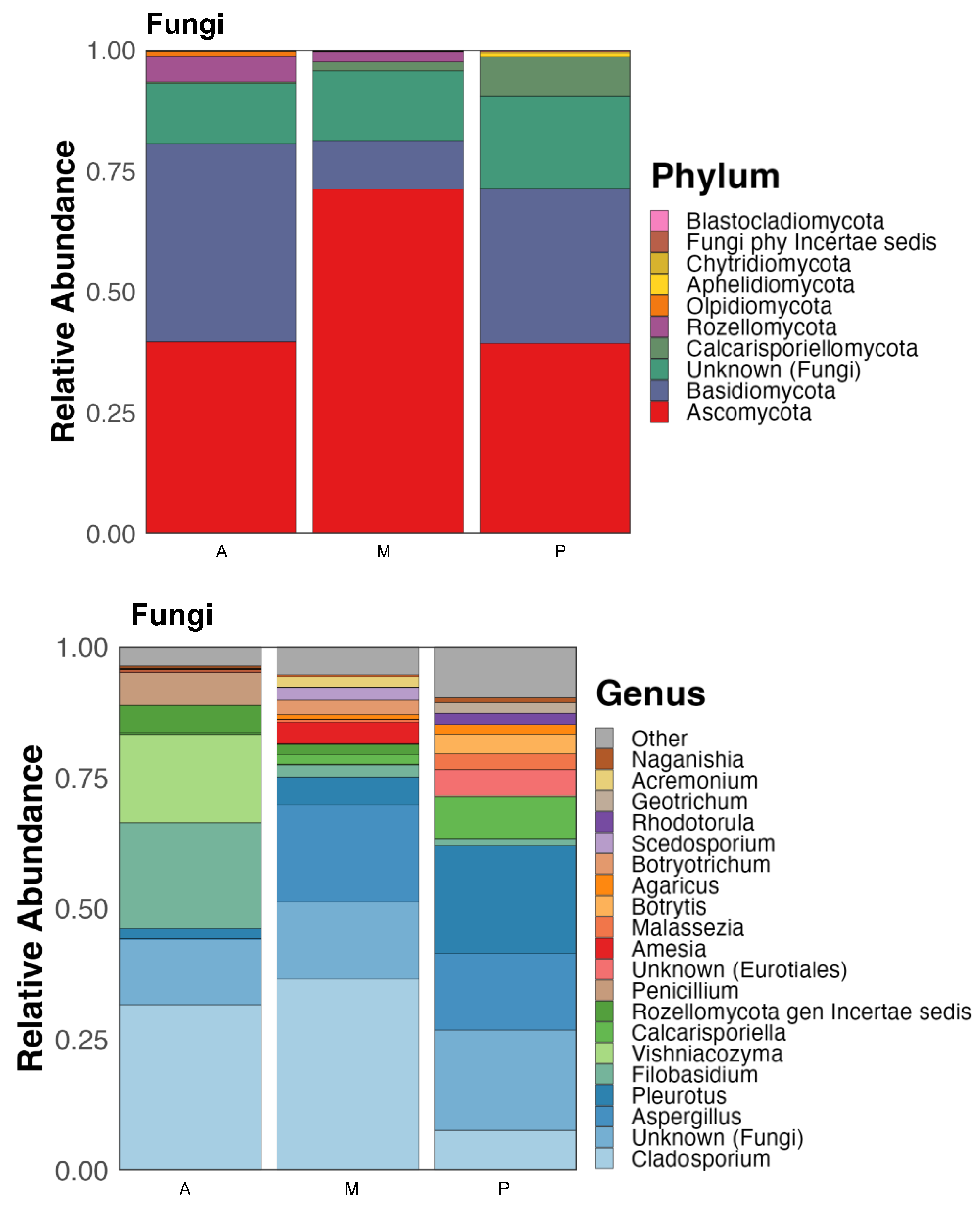
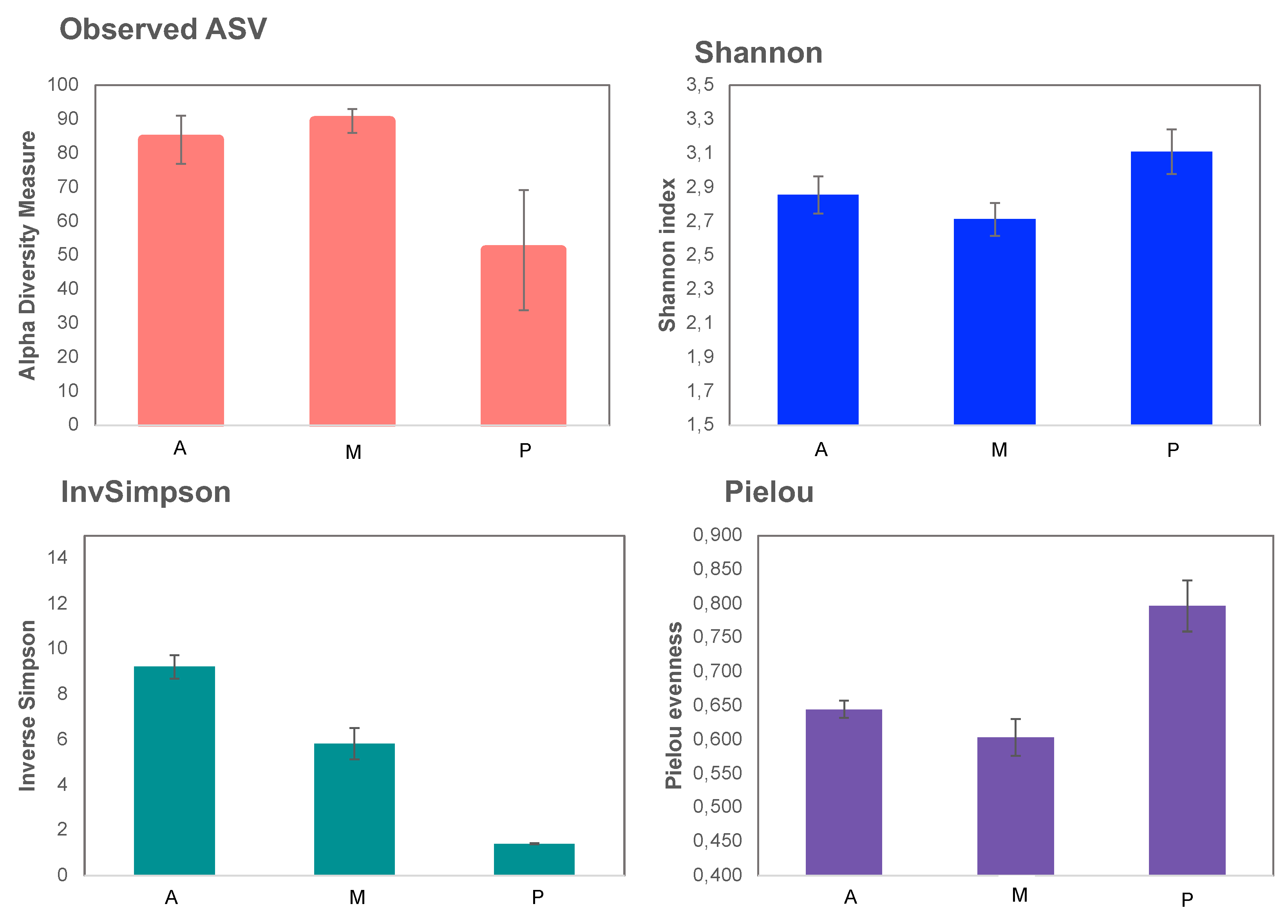
3.2. Bacterial and Fungal Communities of Rhizosphere Soil
3.2.1. Bacteria
3.2.2. Fungi
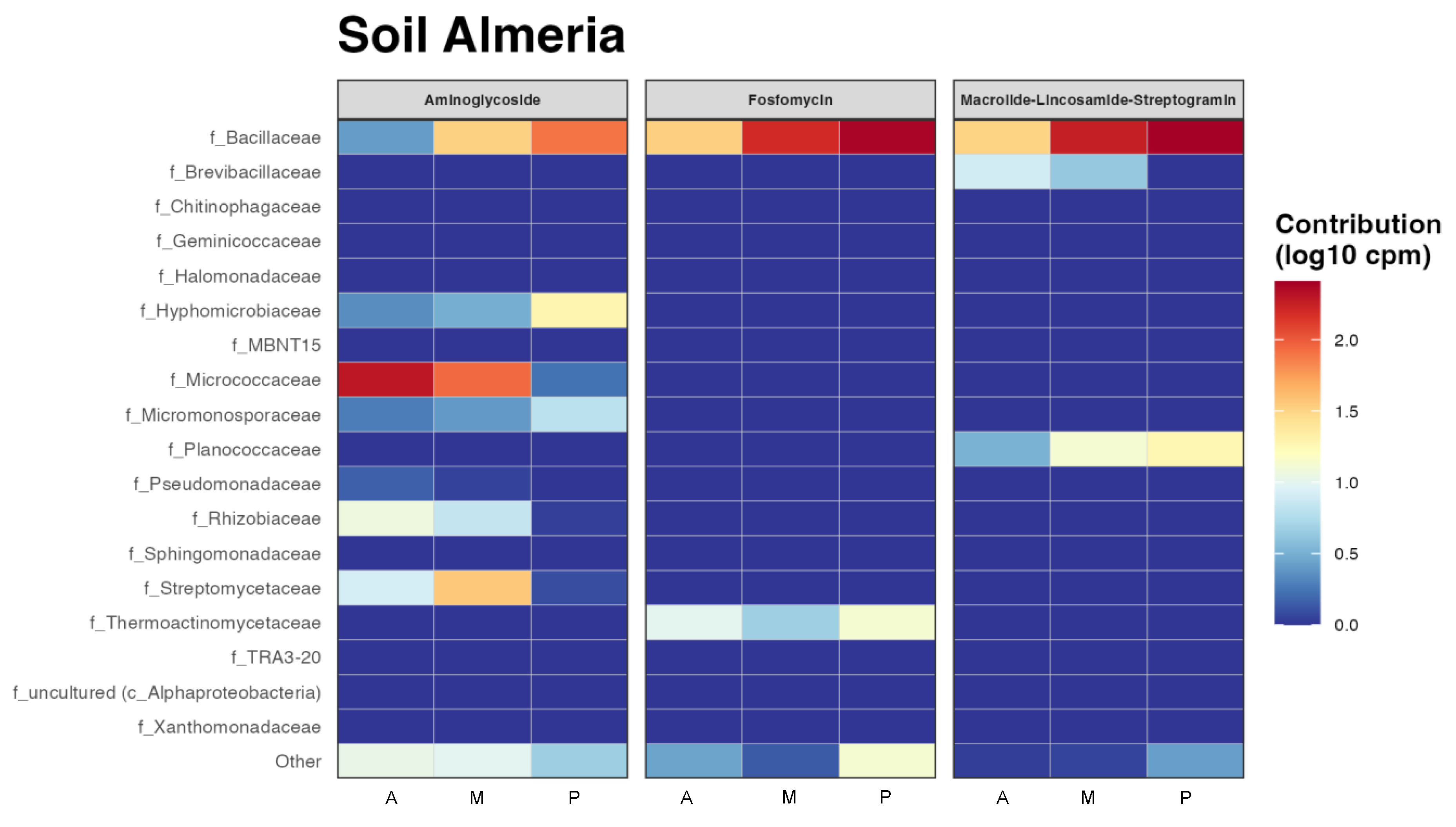
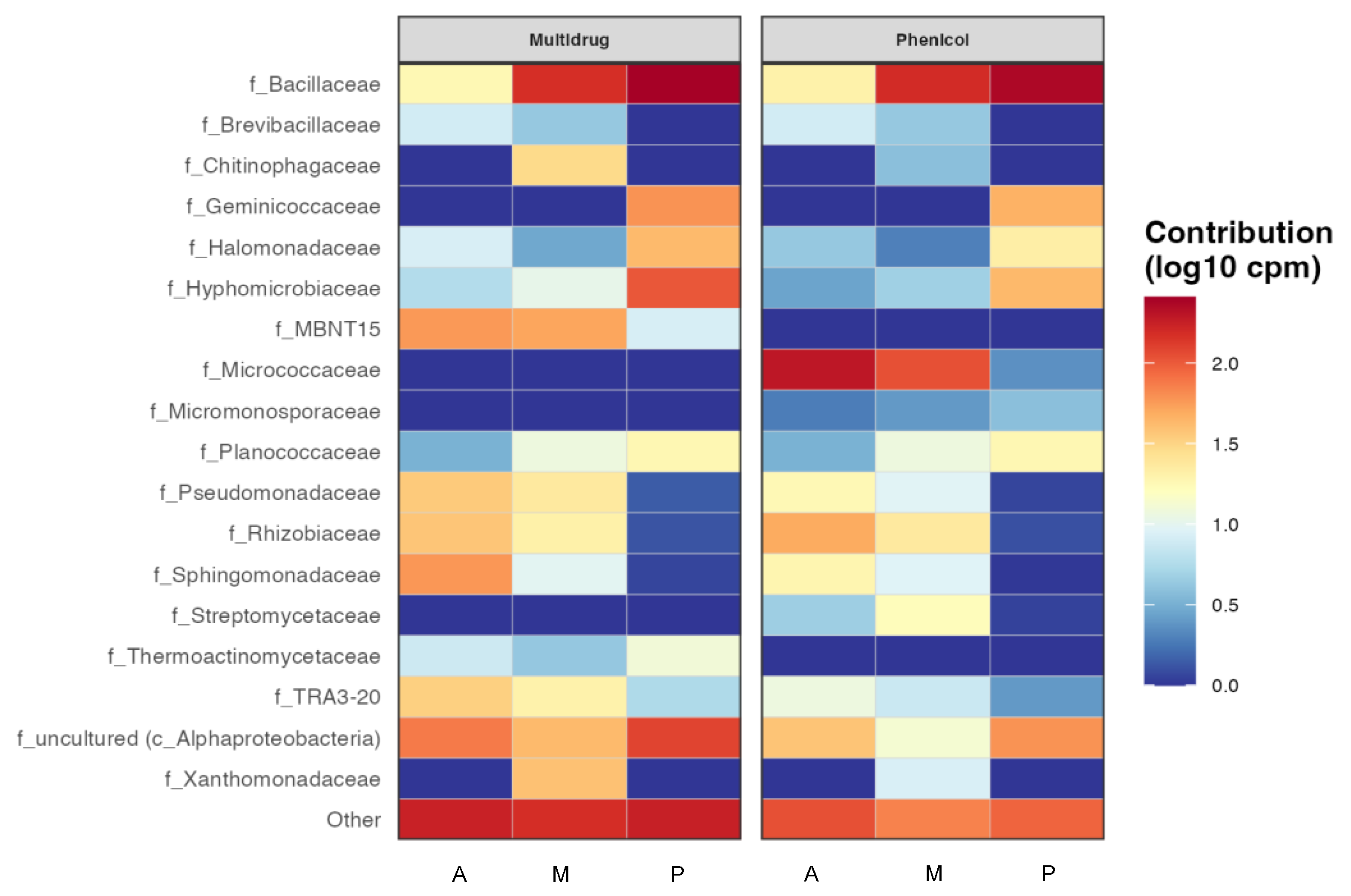
3.3. Predictive Functional Analysis of the Presence of Antibiotic Resistance Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fierer, N. Embracing the Unknown: Disentangling the Complexities of the Soil Microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.-L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant Growth-Promoting Rhizobacteria and Root System Functioning. Front. Plant Sci. 2013, 4, 57135. [Google Scholar] [CrossRef] [PubMed]
- Amoo, A.E.; Olanrewaju, O.S.; Babalola, O.O.; Ajilogba, C.F.; Chukwuneme, C.F.; Ojuederie, O.B.; Omomowo, O.I. The Functionality of Plant-Microbe Interactions in Disease Suppression. J. King Saud Univ. Sci. 2023, 35, 102893. [Google Scholar] [CrossRef]
- Berendonk, T.U.; Manaia, C.M.; Merlin, C.; Fatta-Kassinos, D.; Cytryn, E.; Walsh, F.; Bürgmann, H.; Sørum, H.; Norström, M.; Pons, M.-N.; et al. Tackling Antibiotic Resistance: The Environmental Framework. Nat. Rev. Microbiol. 2015, 13, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban Wastewater Treatment Plants as Hotspots for the Release of Antibiotics in the Environment: A Review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, Q.; Zhang, Z.; Zhou, S.; Jin, M.; Zhu, D.; Yang, X.; Qian, H.; Lu, T. Plants Select Antibiotic Resistome in Rhizosphere in Early Stage. Sci. Total Environ. 2023, 858, 159847. [Google Scholar] [CrossRef] [PubMed]
- Soler, N.; Forterre, P. Vesiduction: The Fourth Way of HGT. Environ. Microbiol. 2020, 22, 2457–2460. [Google Scholar] [CrossRef] [PubMed]
- Gaudin, M.; Gauliard, E.; Schouten, S.; Houel-Renault, L.; Lenormand, P.; Marguet, E.; Forterre, P. Hyperthermophilic Archaea Produce Membrane Vesicles That Can Transfer DNA. Environ. Microbiol. Rep. 2013, 5, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The Rhizosphere Microbiome and Plant Health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Umadevi, P.; Anandaraj, M.; Srivastav, V.; Benjamin, S. Trichoderma Harzianum MTCC 5179 Impacts the Population and Functional Dynamics of Microbial Community in the Rhizosphere of Black Pepper (Piper nigrum L.). Braz. J. Microbiol. 2018, 49, 463–470. [Google Scholar] [CrossRef]
- Gao, Z.; Han, M.; Hu, Y.; Li, Z.; Liu, C.; Wang, X.; Tian, Q.; Jiao, W.; Hu, J.; Liu, L.; et al. Effects of Continuous Cropping of Sweet Potato on the Fungal Community Structure in Rhizospheric Soil. Front. Microbiol. 2019, 10, 2269. [Google Scholar] [CrossRef]
- Forsberg, K.J.; Patel, S.; Gibson, M.K.; Lauber, C.L.; Knight, R.; Fierer, N.; Dantas, G. Bacterial Phylogeny Structures Soil Resistomes across Habitats. Nature 2014, 509, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Müller, D.B.; Srinivas, G.; Garrido-Oter, R.; Potthoff, E.; Rott, M.; Dombrowski, N.; Münch, P.C.; Spaepen, S.; Remus-Emsermann, M.; et al. Functional Overlap of the Arabidopsis Leaf and Root Microbiota. Nature 2015, 528, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, C.; Lang, L.; Wang, A.; Altendorf, K.; García, F.; Lipski, A. Lettuce for Human Consumption Collected in Costa Rica Contains Complex Communities of Culturable Oxytetracycline- and Gentamicin-Resistant Bacteria. Appl. Environ. Microbiol. 2006, 72, 5870–5876. [Google Scholar] [CrossRef]
- Goldstein, M.; Shenker, M.; Chefetz, B. Insights into the Uptake Processes of Wastewater-Borne Pharmaceuticals by Vegetables. Environ. Sci. Technol. 2014, 48, 5593–5600. [Google Scholar] [CrossRef]
- Sengupta, A.; Sarkar, D.; Das, P.; Panja, S.; Parikh, C.; Ramanathan, D.; Bagley, S.; Datta, R. Tetracycline Uptake and Metabolism by Vetiver Grass (Chrysopogon zizanioides L. Nash). Environ. Sci. Pollut. Res. 2016, 23, 24880–24889. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lu, S.; Jiao, W.; Wang, M.; Chang, A.C. Reclaimed water: A safe irrigation water source? Environ. Dev. 2013, 8, 74–83. [Google Scholar] [CrossRef]
- Gupta, S.K.; Shin, H.; Han, D.; Hur, H.-G.; Unno, T. Metagenomic Analysis Reveals the Prevalence and Persistence of Antibiotic- and Heavy Metal-Resistance Genes in Wastewater Treatment Plant. J. Microbiol. 2018, 56, 408–415. [Google Scholar] [CrossRef]
- Pallares-Vega, R.; Blaak, H.; van der Plaats, R.; de Roda Husman, A.M.; Hernandez Leal, L.; van Loosdrecht, M.C.M.; Weissbrodt, D.G.; Schmitt, H. Determinants of Presence and Removal of Antibiotic Resistance Genes during WWTP Treatment: A Cross-Sectional Study. Water Res. 2019, 161, 319–328. [Google Scholar] [CrossRef]
- Sun, L.; Gao, C.; He, N.; Yang, B.; Duan, X.; Chen, T. The Removal of Antibiotic Resistance Genes in Secondary Effluent by the Combined Process of PAC-UF. J. Environ. Sci. Health Part A 2019, 54, 1075–1082. [Google Scholar] [CrossRef]
- da Silva Rodrigues, D.A.; da Cunha, C.C.R.F.; Freitas, M.G.; de Barros, A.L.C.; e Castro, P.B.N.; Pereira, A.R.; de Queiroz Silva, S.; da Fonseca Santiago, A.; de Cássia Franco Afonso, R.J. Biodegradation of Sulfamethoxazole by Microalgae-Bacteria Consortium in Wastewater Treatment Plant Effluents. Sci. Total Environ. 2020, 749, 141441. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hu, W.; Zhang, H.; Wang, H.; Sun, P. Enhanced Degradation of Sulfonamide Antibiotics by UV Irradiation Combined with Persulfate. Processes 2021, 9, 226. [Google Scholar] [CrossRef]
- Mayans, B.; Camacho-Arévalo, R.; García-Delgado, C.; Antón-Herrero, R.; Escolástico, C.; Segura, M.L.; Eymar, E. An Assessment of Pleurotus Ostreatus to Remove Sulfonamides, and Its Role as a Biofilter Based on Its Own Spent Mushroom Substrate. Environ. Sci. Pollut. Res. 2021, 28, 7032–7042. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, S.K.; Nghiem, L.D.; van de Merwe, J.P.; Leusch, F.D.L.; Asif, M.B.; Hai, F.I.; Price, W.E. Degradation of Diclofenac, Trimethoprim, Carbamazepine, and Sulfamethoxazole by Laccase from Trametes versicolor: Transformation Products and Toxicity of Treated Effluent. Biocatal. Biotransform. 2019, 37, 399–408. [Google Scholar] [CrossRef]
- Čvančarová, M.; Moeder, M.; Filipová, A.; Cajthaml, T. Biotransformation of Fluoroquinolone Antibiotics by Ligninolytic Fungi—Metabolites, Enzymes and Residual Antibacterial Activity. Chemosphere 2015, 136, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Křesinová, Z.; Linhartová, L.; Filipová, A.; Ezechiáš, M.; Mašín, P.; Cajthaml, T. Biodegradation of Endocrine Disruptors in Urban Wastewater Using Pleurotus Ostreatus Bioreactor. New Biotechnol. 2018, 43, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Palli, L.; Castellet-Rovira, F.; Pérez-Trujillo, M.; Caniani, D.; Sarrà-Adroguer, M.; Gori, R. Preliminary Evaluation of Pleurotus ostreatus for the Removal of Selected Pharmaceuticals from Hospital Wastewater. Biotechnol. Prog. 2017, 33, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive Functional Profiling of Microbial Communities Using 16S RRNA Marker Gene Sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Arévalo, R.; García-Delgado, C.; Mayans, B.; Antón-Herrero, R.; Cuevas, J.; Segura, M.L.; Eymar, E. Sulfonamides in Tomato from Commercial Greenhouses Irrigated with Reclaimed Wastewater: Uptake, Translocation and Food Safety. Agronomy 2021, 11, 1016. [Google Scholar] [CrossRef]
- Galdeano-Gómez, E.; Aznar-Sánchez, J.A.; Pérez-Mesa, J.C.; Piedra-Muñoz, L. Exploring Synergies Among Agricultural Sustainability Dimensions: An Empirical Study on Farming System in Almería (Southeast Spain). Ecol. Econ. 2017, 140, 99–109. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An Examination of the Degtjareff Method for Determining Soil Organic Matter, and a Proposed Modification of the Chromic Acid Titration Method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Abarenkov, K.; Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; May, T.W.; Frøslev, T.G.; Pawlowska, J.; Lindahl, B.; Põldmaa, K.; Truong, C.; et al. The UNITE Database for Molecular Identification and Taxonomic Communication of Fungi and Other Eukaryotes: Sequences, Taxa and Classifications Reconsidered. Nucleic Acids Res. 2024, 52, D791–D797. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for Prediction of Metagenome Functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a Reference Resource for Gene and Protein Annotation. Nucleic Acids Res. 2016, 44, D457–D462. [Google Scholar] [CrossRef] [PubMed]
- Chicca, I.; Becarelli, S.; Di Gregorio, S. Microbial Involvement in the Bioremediation of Total Petroleum Hydrocarbon Polluted Soils: Challenges and Perspectives. Environments 2022, 9, 52. [Google Scholar] [CrossRef]
- Gauthier, J.; Derome, N. Evenness-Richness Scatter Plots: A Visual and Insightful Representation of Shannon Entropy Measurements for Ecological Community Analysis. mSphere 2021, 6, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Szczepanowski, R.; Linke, B.; Krahn, I.; Gartemann, K.-H.; Gützkow, T.; Eichler, W.; Pühler, A.; Schlüter, A. Detection of 140 Clinically Relevant Antibiotic-Resistance Genes in the Plasmid Metagenome of Wastewater Treatment Plant Bacteria Showing Reduced Susceptibility to Selected Antibiotics. Microbiology 2009, 155, 2306–2319. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, V.; Tripathi, P. Antibiotic Resistance Genes: An Emerging Environmental Pollutant. In Perspectives in Environmental Toxicology; Springer: Cham, Switzerland, 2017; pp. 183–201. [Google Scholar]
- Pan, M.; Chu, L.M. Transfer of Antibiotics from Wastewater or Animal Manure to Soil and Edible Crops. Environ. Pollut. 2017, 231, 829–836. [Google Scholar] [CrossRef]
- Xiao, R.; Huang, D.; Du, L.; Song, B.; Yin, L.; Chen, Y.; Gao, L.; Li, R.; Huang, H.; Zeng, G. Antibiotic Resistance in Soil-Plant Systems: A Review of the Source, Dissemination, Influence Factors, and Potential Exposure Risks. Sci. Total Environ. 2023, 869, 161855. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef]
- FAO. Microbiome: The Missing Link? Science and Innovation for Health, Climate and Sustainable Food Systems; FAO: Rome, Italy, 2020. [Google Scholar]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P.; et al. Dynamic Root Exudate Chemistry and Microbial Substrate Preferences Drive Patterns in Rhizosphere Microbial Community Assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–Microbiome Interactions: From Community Assembly to Plant Health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Badri, D.V.; Vivanco, J.M. Regulation and Function of Root Exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, S.; Sun, L.; Wang, H. Metabolic Profiling of Root Exudates from Two Ecotypes of Sedum Alfredii Treated with Pb Based on GC-MS. Sci. Rep. 2017, 7, 39878. [Google Scholar] [CrossRef]
- Mandic-Mulec, I.; Stefanic, P.; van Elsas, J.D. Ecology of Bacillaceae. Microbiol. Spectr. 2015, 3, 1–24. [Google Scholar] [CrossRef]
- Singh, B.K.; Trivedi, P.; Egidi, E.; Macdonald, C.A.; Delgado-Baquerizo, M. Crop Microbiome and Sustainable Agriculture. Nat. Rev. Microbiol. 2020, 18, 601–602. [Google Scholar] [CrossRef]
- Madigan, M.T.; Bender, K.S.; Buckley, D.H.; Stahl, D.A.; Sattley, M. Brock. Biology of Microorganisms, 15th ed.; Beauparlant, S., Ed.; Pearson: New York, NY, USA, 2019. [Google Scholar]
- Soares, F.L.; Melo, I.S.; Dias, A.C.F.; Andreote, F.D. Cellulolytic Bacteria from Soils in Harsh Environments. World J. Microbiol. Biotechnol. 2012, 28, 2195–2203. [Google Scholar] [CrossRef]
- Zhong, Y.; Xun, W.; Wang, X.; Tian, S.; Zhang, Y.; Li, D.; Zhou, Y.; Qin, Y.; Zhang, B.; Zhao, G.; et al. Root-Secreted Bitter Triterpene Modulates the Rhizosphere Microbiota to Improve Plant Fitness. Nat. Plants 2022, 8, 887–896. [Google Scholar] [CrossRef]
- Gao, H.; Tian, G.; Khashi u Rahman, M.; Wu, F. Cover Crop Species Composition Alters the Soil Bacterial Community in a Continuous Pepper Cropping System. Front. Microbiol. 2022, 12, 789034. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Hu, M.; Qian, D.; Xue, H.; Gao, N.; Lin, X. Microbial Deterioration and Restoration in Greenhouse-Based Intensive Vegetable Production Systems. Plant Soil 2021, 463, 1–18. [Google Scholar] [CrossRef]
- Yang, N.; Zhang, W.; Wang, D.; Cao, D.; Cao, Y.; He, W.; Lin, Z.; Chen, X.; Ye, G.; Chen, Z.; et al. A Novel Endophytic Fungus Strain of Cladosporium: Its Identification, Genomic Analysis, and Effects on Plant Growth. Front. Microbiol. 2023, 14, 1287582. [Google Scholar] [CrossRef] [PubMed]
- Răut, I.; Călin, M.; Capră, L.; Gurban, A.-M.; Doni, M.; Radu, N.; Jecu, L. Cladosporium Sp. Isolate as Fungal Plant Growth Promoting Agent. Agronomy 2021, 11, 392. [Google Scholar] [CrossRef]
- Hamayun, M.; Afzal Khan, S.; Ahmad, N.; Tang, D.-S.; Kang, S.-M.; Na, C.-I.; Sohn, E.-Y.; Hwang, Y.-H.; Shin, D.-H.; Lee, B.-H.; et al. Cladosporium Sphaerospermum as a New Plant Growth-Promoting Endophyte from the Roots of Glycine max (L.) Merr. World J. Microbiol. Biotechnol. 2009, 25, 627–632. [Google Scholar] [CrossRef]
- Meena, V.S.; Maurya, B.R.; Verma, J.P. Does a Rhizospheric Microorganism Enhance K+ Availability in Agricultural Soils? Microbiol. Res. 2014, 169, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Vadkertiová, R.; Dudášová, H.; Balaščáková, M. Yeasts in Agricultural and Managed Soils. In Yeasts in Natural Ecosystems: Diversity; Springer International Publishing: Cham, Switzerland, 2017; pp. 117–144. [Google Scholar]
- Hirose, D.; Degawa, Y.; Inaba, S.; Tokumasu, S. The Anamorphic Genus Calcarisporiella Is a New Member of the Mucoromycotina. Mycoscience 2012, 53, 256–260. [Google Scholar] [CrossRef]
- An, X.-L.; Chen, Q.-L.; Zhu, D.; Su, J.-Q. Distinct Effects of Struvite and Biochar Amendment on the Class 1 Integron Antibiotic Resistance Gene Cassettes in Phyllosphere and Rhizosphere. Sci. Total Environ. 2018, 631–632, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-J.; Hu, H.-W.; Chen, Q.-L.; Singh, B.K.; Yan, H.; Chen, D.; He, J.-Z. Transfer of Antibiotic Resistance from Manure-Amended Soils to Vegetable Microbiomes. Environ. Int. 2019, 130, 104912. [Google Scholar] [CrossRef]
- Chen, Q.-L.; An, X.-L.; Zhu, Y.-G.; Su, J.-Q.; Gillings, M.R.; Ye, Z.-L.; Cui, L. Application of Struvite Alters the Antibiotic Resistome in Soil, Rhizosphere, and Phyllosphere. Environ. Sci. Technol. 2017, 51, 8149–8157. [Google Scholar] [CrossRef]
- Wang, F.-H.; Qiao, M.; Chen, Z.; Su, J.-Q.; Zhu, Y.-G. Antibiotic Resistance Genes in Manure-Amended Soil and Vegetables at Harvest. J. Hazard. Mater. 2015, 299, 215–221. [Google Scholar] [CrossRef]
- Sun, J.; Jin, L.; He, T.; Wei, Z.; Liu, X.; Zhu, L.; Li, X. Antibiotic Resistance Genes (ARGs) in Agricultural Soils from the Yangtze River Delta, China. Sci. Total Environ. 2020, 740, 140001. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Ma, L.; Yu, Q.; Yang, J.; Su, W.; Hilal, M.G.; Li, X.; Zhang, S.; Li, H. The Source, Fate and Prospect of Antibiotic Resistance Genes in Soil: A Review. Front. Microbiol. 2022, 13, 976657. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Wang, J.; Singh, B.K.; Liu, Y.; Chen, Y.; Zhang, Y.; He, J. Diversity of Herbaceous Plants and Bacterial Communities Regulates Soil Resistome across Forest Biomes. Environ. Microbiol. 2018, 20, 3186–3200. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, J.; Chen, H.; Bond, P.L.; Yuan, Z. Metagenomic Analysis Reveals Wastewater Treatment Plants as Hotspots of Antibiotic Resistance Genes and Mobile Genetic Elements. Water Res. 2017, 123, 468–478. [Google Scholar] [CrossRef]
- Song, M.; Song, D.; Jiang, L.; Zhang, D.; Sun, Y.; Chen, G.; Xu, H.; Mei, W.; Li, Y.; Luo, C.; et al. Large-Scale Biogeographical Patterns of Antibiotic Resistome in the Forest Soils across China. J. Hazard. Mater. 2021, 403, 123990. [Google Scholar] [CrossRef]


| Gradient Elution of Antibiotics | |||
|---|---|---|---|
| Time (min) | Flow (μL min−1) | A1 (%) | B2 (%) |
| 0.0 | 0.500 | 8.0 | 92.0 |
| 5.00 | 0.500 | 15.0 | 85.0 |
| 9.00 | 0.500 | 55.0 | 45.0 |
| 12.0 | 0.500 | 8.0 | 92.0 |
| Antibiotic Concentrations in Crop Soil | |||
|---|---|---|---|
| Antibiotic | Aubergine | Melon | Pepper |
| Sulfadiazine | <DL | <DL | 0.330 ± 0.042 |
| Sulfathiazole | <DL | 0.784 ± 0.046 | <DL |
| Sulfapyridine | <DL | 0.369 ± 0.032 | <DL |
| Sulfamethazine | 0.661 ± 0.066 | 0.245 ± 0.030 | <DL |
| Sulfamethizole | 2.213 ± 0.079 | 1.449 ± 0.080 | 2.913 ± 0.184 |
| Sulfamethoxipyridazine | <DL | 0.696 ± 0.017 | 0.343 ± 0.052 |
| Sulfamonomethoxine | <DL | <DL | 0.303 ± 0.083 |
| Sulfamethoxazole | 1.590 ± 0.259 | 2.465 ± 0.246 | 2.700 ± 0.446 |
| Sulfisoxazole | 1.880 ± 0.333 | 4.844 ± 0.290 | 2.652 ± 0.375 |
| Sulfadimethoxine | 0.127 ± 0.017 | 0.166 ± 0.042 | 0.181 ± 0.014 |
| Tetracycline | 3.587 ± 0.612 | 6.764 ± 0.556 | 6.052 ± 0.531 |
| Doxycicline | 3.127 ± 0.395 | 2.397 ± 0.722 | 6.299 ± 0.387 |
| Oxolinic acid | 0.426 ± 0.032 | 0.644 ± 0.144 | 0.348 ± 0.058 |
| Antibiotic | Aubergine | Pepper |
|---|---|---|
| Sulfadiazine | <DL | 0.227 ± 0.040 |
| Sulfamethizole | 0.312 ± 0.087 | 0.751 ± 0.089 |
| Sulfametoxipyridazine | <DL | 0.407 ± 0.018 |
| Sulfamethoxazole | 0.947 ± 0.082 | 0.943 ± 0.084 |
| Sulfisoxazole | 0.485 ± 0.090 | 0.592 ± 0.084 |
| Tetracycline | 0.407 ± 0.036 | 0.281 ± 0.025 |
| Doxycicline | 0.223 ± 0.014 | 0.210 ± 0.018 |
| Oxolinic acid | 0.142 ± 0.024 | 0.127 ± 0.016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayans, B.; Zamora-Martin, S.; Antón-Herrero, R.; García-Delgado, C.; Delgado-Moreno, L.; Guirado, M.; Pérez-Esteban, J.; Segura, M.L.; Escolástico, C.; Eymar, E. Evaluation of the Rhizosphere Resistome of Cultivated Soils Polluted with Antibiotics from Reclaimed Wastewater. Agronomy 2024, 14, 1118. https://doi.org/10.3390/agronomy14061118
Mayans B, Zamora-Martin S, Antón-Herrero R, García-Delgado C, Delgado-Moreno L, Guirado M, Pérez-Esteban J, Segura ML, Escolástico C, Eymar E. Evaluation of the Rhizosphere Resistome of Cultivated Soils Polluted with Antibiotics from Reclaimed Wastewater. Agronomy. 2024; 14(6):1118. https://doi.org/10.3390/agronomy14061118
Chicago/Turabian StyleMayans, Begoña, Sergio Zamora-Martin, Rafael Antón-Herrero, Carlos García-Delgado, Laura Delgado-Moreno, María Guirado, Javier Pérez-Esteban, Mª Luz Segura, Consuelo Escolástico, and Enrique Eymar. 2024. "Evaluation of the Rhizosphere Resistome of Cultivated Soils Polluted with Antibiotics from Reclaimed Wastewater" Agronomy 14, no. 6: 1118. https://doi.org/10.3390/agronomy14061118







