Suppressing Ralstonia solanacearum and Bacterial Antibiotic Resistance Genes in Tomato Rhizosphere Soil through Companion Planting with Basil or Cilantro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Rhizosphere Soil Collection
2.3. DNA Extraction, Construction of Sequencing Libraries, and Metagenomic Sequencing
2.4. Raw Sequencing Data Processing and Taxonomy Profiling
2.5. Antibiotic Resistance Genes (ARG) Prediction
2.6. Determination of the Abundance of R. solanacearum
2.7. Statistical Analysis
3. Results
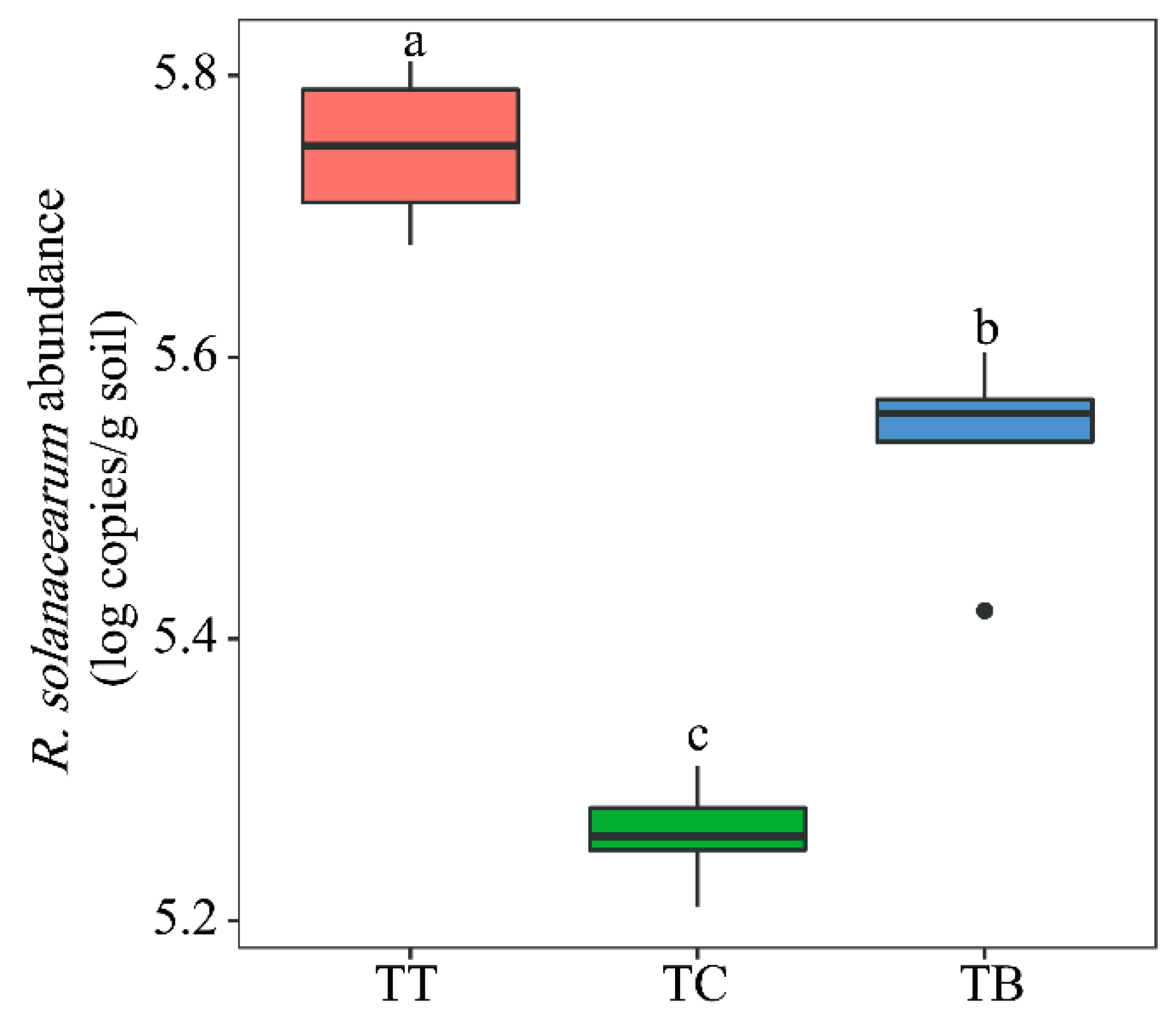
3.1. R. solanacearum Abundance
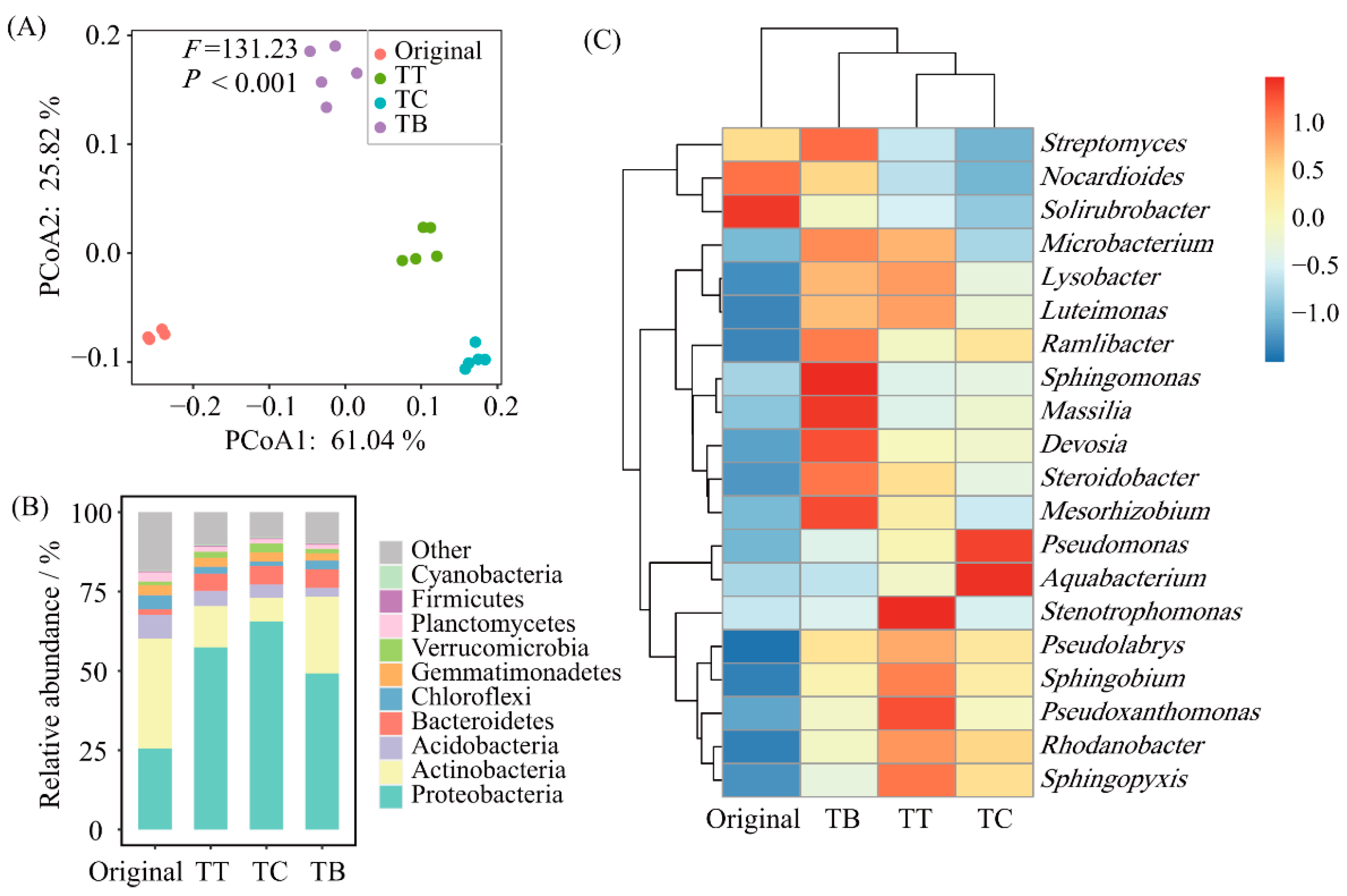
3.2. Soil Microbial Composition
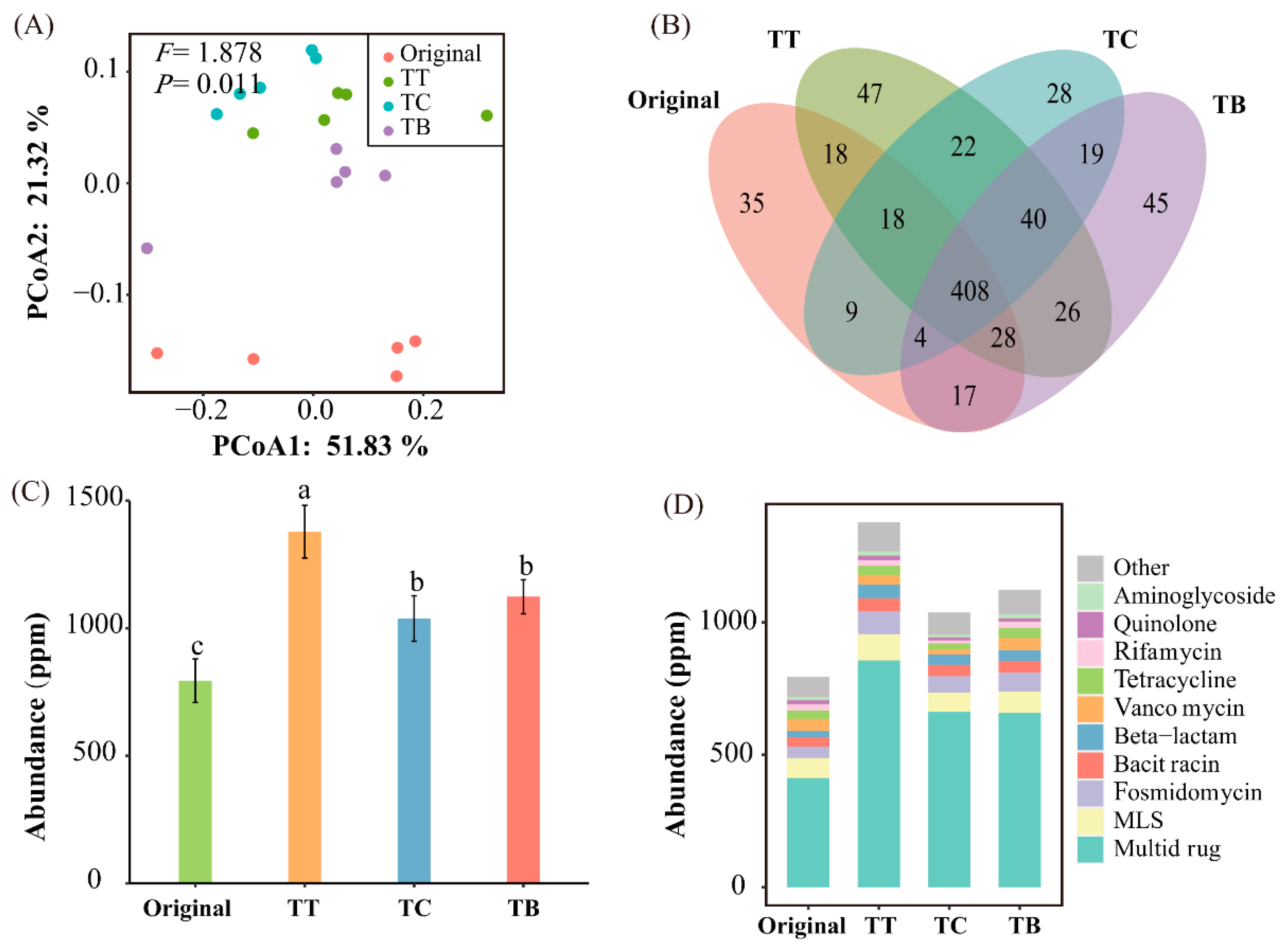
3.3. Abundance and Composition of ARGs
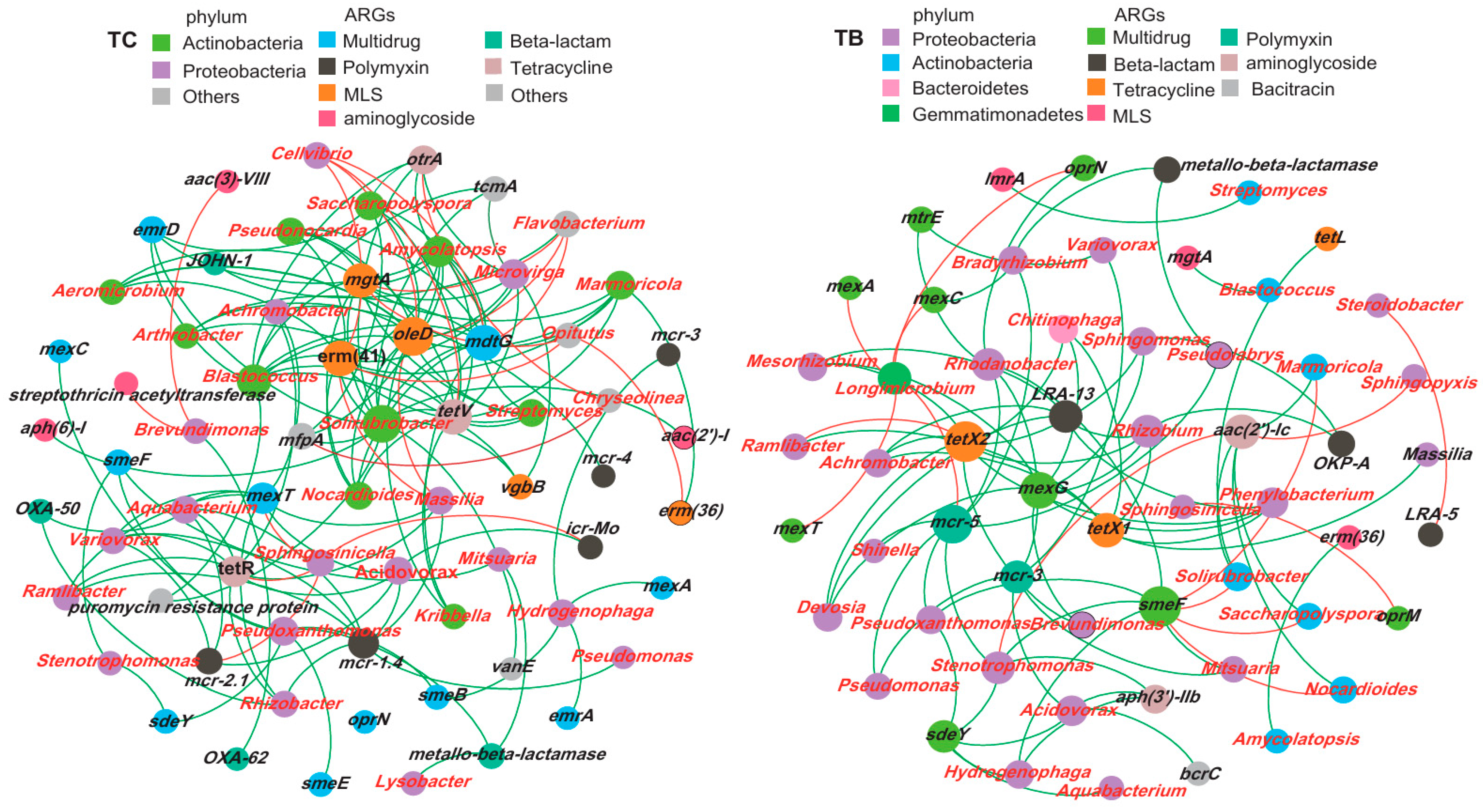
3.4. Relationships between Soil Bacterial Community Composition and ARGs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Abbas, M.; Rehman, M.U.; Huang, Y.; Zhou, R.; Gong, S.; Yang, H.; Chen, S.; Wang, M.; Cheng, A. Dissemination of Antibiotic Resistance Genes (ARGs) via Integrons in Escherichia coli: A Risk to Human Health. Environ. Pollut. 2020, 266, 115260. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Ellabaan, M.M.; Munck, C.; Porse, A.; Imamovic, L.; Sommer, M.O. Forecasting the Dissemination of Antibiotic Resistance Genes across Bacterial Genomes. Nat. Commun. 2021, 12, 2435. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Yin, G.; Liu, M.; Hou, L.; Yang, Y.; Van Boeckel, T.P.; Zheng, Y.; Li, Y. Global Biogeography and Projection of Soil Antibiotic Resistance Genes. Sci. Adv. 2022, 8, eabq8015. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Feng, T.; Yang, J.; Su, W.; Zhou, R.; Wang, Y.; Zhang, H.; Li, H. Seasonal Distribution of Antibiotic Resistance Genes in the Yellow River Water and Tap Water, and Their Potential Transmission from Water to Human. Environ. Pollut. 2022, 292, 118304. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Huo, L.; Guo, Y.; Gao, M.; Wang, G.; Hu, D.; Li, C.; Wang, Z.; Liu, G.; Wang, X. Metagenomic Assembly Reveals Hosts and Mobility of Common Antibiotic Resistome in Animal Manure and Commercial Compost. Environ. Microbiome 2022, 17, 42. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Li, H.; Zhou, J.; Wang, T. Seasonal Dissemination of Antibiotic Resistome from Livestock Farms to Surrounding Soil and Air: Bacterial Hosts and Risks for Human Exposure. J. Environ. Manag. 2023, 325, 116638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.M.; Liu, X.; Wang, S.L.; Fang, L.X.; Sun, J.; Liu, Y.H.; Liao, X.P. Distribution Patterns of Antibiotic Resistance Genes and Their Bacterial Hosts in Pig Farm Wastewater Treatment Systems and Soil Fertilized with Pig Manure. Sci. Total Environ. 2021, 758, 143654. [Google Scholar] [CrossRef] [PubMed]
- Ibekwe, A.M.; Bhattacharjee, A.S.; Phan, D.; Ashworth, D.; Schmidt, M.P.; Murinda, S.E.; Obayiuwana, A.; Murry, M.A.; Schwartz, G.; Lundquist, T.; et al. Potential Reservoirs of Antimicrobial Resistance in Livestock Waste and Treated Wastewater That Can Be Disseminated to Agricultural Land. Sci. Total Environ. 2023, 872, 162194. [Google Scholar] [CrossRef]
- Liao, H.; Li, X.; Yang, Q.; Bai, Y.; Cui, P.; Wen, C.; Liu, C.; Chen, Z.; Tang, J.; Che, J.; et al. Herbicide Selection Promotes Antibiotic Resistance in Soil Microbiomes. Mol. Biol. Evol. 2021, 38, 2337–2350. [Google Scholar] [CrossRef]
- Zhang, H.; Song, J.; Zheng, Z.; Li, T.; Shi, N.; Han, Y.; Zhang, L.; Yu, Y.; Fang, H. Fungicide Exposure Accelerated Horizontal Transfer of Antibiotic Resistance Genes via Plasmid-Mediated Conjugation. Water Res. 2023, 233, 119789. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, T.; Tang, J.; Ling, H.; Wu, X. Key Taxa and Mobilome-Mediated Responses Co-Reshape the Soil Antibiotic Resistome under Dazomet Fumigation Stress. Environ. Int. 2023, 182, 108318. [Google Scholar] [CrossRef] [PubMed]
- Cui, E.; Cui, B.; Fan, X.; Li, S.; Gao, F. Ryegrass (Lolium multiflorum L.) and Indian Mustard (Brassica juncea L.) Intercropping Can Improve the Phytoremediation of Antibiotics and Antibiotic Resistance Genes but Not Heavy Metals. Sci. Total Environ. 2021, 784, 147093. [Google Scholar] [CrossRef]
- Zhang, W.; Wen, T.; Liu, L.; Li, J.; Gao, Y.; Zhu, D.; He, J.; Zhu, Y. Agricultural Land-Use Change and Rotation System Exert Considerable Influences on the Soil Antibiotic Resistome in Lake Tai Basin. Sci. Total Environ. 2021, 771, 144848. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Li, Z.; Guo, S.; Li, K.; Xu, P.; Ok, Y.S.; Jones, D.L.; Zou, J. Antibiotics and Antibiotic Resistance Genes in Agricultural Soils: A systematic analysis. Crit. Rev. Env. Sci. Tec. 2023, 53, 847–864. [Google Scholar] [CrossRef]
- Mazzafera, P.; Favarin, J.L.; Andrade, S.A.L. Intercroping Systems in Sustainable Agriculture. Front. Sustain. Food Syst. 2021, 5, 634361. [Google Scholar] [CrossRef]
- Salaheen, S.; Biswas, D. Organic Farming Practices: Integrated Culture versus Monoculture. In Safety and Practice for Organic Food; Academic Press: Cambridge, MA, USA, 2019; pp. 23–32. [Google Scholar]
- De Corato, U. Soil Microbiota Manipulation and Its Role in Suppressing Soil-Borne Plant Pathogens in Organic Farming Systems under the Light of Microbiome-Assisted Strategies. Chem. Biol. Technol. Agric. 2020, 7, 17. [Google Scholar] [CrossRef]
- Jia, S.; Wu, J.; Ye, L.; Zhao, F.; Li, T.; Zhang, X. Metagenomic Assembly Provides a Deep Insight into the Antibiotic Resistome Alteration Induced by Drinking Water Chlorination and Its Correlations with Bacterial Host. J. Hazard. Mater. 2019, 379, 120841. [Google Scholar] [CrossRef] [PubMed]
- Artal, R.; Gopalakrishnan, C.; Thippeswamy, B. An Efficient Inoculation Method to Screen Tomato, Brinjal and Chilli Entries for Bacterial Wilt Resistance. Pest Manag. Hortic. Ecosyst. 2012, 18, 70–73. [Google Scholar]
- Elsayed, T.R.; Jacquiod, S.; Nour, E.H.; Sørensen, S.J.; Smalla, K. Biocontrol of Bacterial Wilt Disease Through Complex Interaction Between Tomato Plant, Antagonists, the Indigenous Rhizosphere Microbiota, and Ralstonia solanacearum. Front. Microbiol. 2020, 10, 2835. [Google Scholar] [CrossRef]
- Wind, L.; Keenum, I.; Gupta, S.; Ray, P.; Knowlton, K.; Ponder, M.; Hession, W.C.; Pruden, A.; Krometis, L.A. Integrated Metagenomic Assessment of Multiple Pre-Harvest Control Points on Lettuce Resistomes at Field-Scale. Front. Microbiol. 2021, 12, 683410. [Google Scholar] [CrossRef] [PubMed]
- Bomford, M.K. Do Tomatoes Love Basil but Hate Brussels Sprouts? Competition and Land-Use Efficiency of Popularly Recommended and Discouraged Crop Mixtures in Biointensive Agriculture Systems. J. Sustain. Agric. 2009, 33, 396–417. [Google Scholar] [CrossRef]
- Fu, X.; Wu, X.; Zhou, X.; Liu, S.; Shen, Y.; Wu, F. Companion Cropping with Potato Onion Enhances the Disease Resistance of Tomato against Verticillium Dahliae. Front. Plant Sci. 2015, 6, 726. [Google Scholar] [CrossRef] [PubMed]
- Hailu, G. A Review on the Comparative Advantage of Intercropping Systems. J. Biol. Agric. Healthc. 2015, 5, 28–38. [Google Scholar]
- Chen, X.; Cui, Z.; Fan, M.; Vitousek, P.; Zhao, M.; Ma, W.; Wang, Z.; Zhang, W.; Yan, X.; Yang, J.; et al. Producing More Grain with Lower Environmental Costs. Nature 2014, 514, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Salehi, Y.; Zarehaghi, D. The Effect of Intercropping and Deficit Irrigation on the Water Use Efficiency and Yield of Tomato (Lycopersicon esculentum Mill) and Basil (Ocimum basilicum). J. Agric. Sci. Sustain. Prod. 2018, 28, 209–220. [Google Scholar]
- Padala, V.K.; Kumar, P.S.; Ramya, N.; Jayanthi, P.D.K. Aromatic Plant Odours of Anethum Graveolens and Coriandrum Sativum Repel Whitefly, Bemisia Tabaci in Tomato. Curr. Sci. 2023, 124, 231–238. [Google Scholar]
- Girma, A. Yield Advantage and Economic Benefit of Maize Basil Intercropping under Different Spatial Arrangements and Nitrogen Rates. Sch. J. Agric. Sci. 2015, 5, 296–302. [Google Scholar]
- Bolger, A.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.; Luo, R.; Sadakane, K.; Lam, T. MEGAHIT: An Ultra-Fast Single-Node Solution for Large and Complex Metagenomics Assembly via Succinct de Bruijn Graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef]
- Zhu, W.; Lomsadze, A.; Borodovsky, M. Ab Initio Gene Identification in Metagenomic Sequences. Nucleic Acids Res. 2010, 38, e132. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Godzik, A. Cd-Hit: A Fast Program for Clustering and Comparing Large Sets of Protein or Nucleotide Sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, B.; Xie, C.; Huson, D. Fast and Sensitive Protein Alignment Using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Huson, D.H.; Mitra, S.; Ruscheweyh, H.-J.; Weber, N.; Schuster, S.C. Integrative Analysis of Environmental Sequences Using MEGAN4. Genome Res. 2011, 21, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, X.; Chai, B.; Ma, L.; Li, B.; Zhang, A.; Cole, J.R.; Tiedje, J.M.; Zhang, T. ARGs-OAP: Online Analysis Pipeline for Antibiotic Resistance Genes Detection from Metagenomic Data Using an Integrated Structured ARG-Database. Bioinformatics 2016, 32, 2346–2351. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, X.; Zhang, T. Evaluation of a Hybrid Approach Using UBLAST and BLASTX for Metagenomic Sequences Annotation of Specific Functional Genes. PLoS ONE 2014, 9, 110947. [Google Scholar] [CrossRef] [PubMed]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.; Tsang, K.K.; Bouchard, E.; Edalatmand, A.; Huynh, W.; Nguyen, A.-L.V.; Cheng, A.A.; Liu, S.; et al. CARD 2020: Antibiotic Resistome Surveillance with the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2020, 48, D517–D525. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Raphenya, A.R.; Alcock, B.; Waglechner, N.; Guo, P.; Tsang, K.K.; Lago, B.A.; Dave, B.M.; Pereira, S.; Sharma, A.N.; et al. CARD 2017: Expansion and Model-Centric Curation of the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2017, 45, D566–D573. [Google Scholar] [CrossRef]
- Liu, B.; Pop, M. ARDB—Antibiotic Resistance Genes Database. Nucleic Acids Res. 2009, 37, D443–D447. [Google Scholar] [CrossRef]
- Mcarthur, A.G.; Waglechner, N.; Nizam, F.; Yan, A.; Azad, M.A.; Baylay, A.J.; Bhullar, K.; Canova, M.J.; De Pascale, G.; Ejim, L.; et al. The Comprehensive Antibiotic Resistance Database. Antimicrob. Agents Chemother. 2013, 57, 3348–3357. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, J.A.; Vilgalys, R.; Jackson, R.B. Assessment of Soil Microbial Community Structure by Use of Taxon-Specific Quantitative PCR Assays. Appl. Environ. Microbiol. 2005, 71, 4117–4120. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, J.; Heuer, H.; Van Elsas, J.D.; Smalla, K. Specific and Sensitive Detection of Ralstonia solanacearum in Soil on the Basis of PCR Amplification of FliC Fragments. Appl. Environ. Microbiol. 2003, 69, 7248–7256. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, A.J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; Mcglinn, D.; Minchin, P.R.; Hara, R.B.O.; Simpson, G.L.; Solymos, P.; et al. Package ‘Vegan’. Community Ecol. Packag. 2019, 2, 1–295. [Google Scholar]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA). In Wiley StatsRef: Statistics Reference Online; Wiley: Hoboken, NJ, USA, 2014; pp. 1–15. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Johnson, T.A.; Su, J.-Q.; Qiao, M.; Guo, G.-X.; Stedtfeld, R.D.; Hashsham, S.A.; Tiedje, J.M. Diverse and Abundant Antibiotic Resistance Genes in Chinese Swine Farms. Natl. Acad. Sci. 2013, 110, 3435–3440. [Google Scholar] [CrossRef] [PubMed]
- Ghirardini, A.; Grillini, V.; Verlicchi, P. A Review of the Occurrence of Selected Micropollutants and Microorganisms in Different Raw and Treated Manure—Environmental Risk Due to Antibiotics after Application to Soil. Sci. Total Environ. 2020, 707, 136118. [Google Scholar] [CrossRef] [PubMed]
- Asaf, S.; Numan, M.; Khan, A.L.; Al-Harrasi, A. Sphingomonas: From Diversity and Genomics to Functional Role in Environmental Remediation and Plant Growth. Crit. Rev. Biotechnol. 2020, 40, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Shrestha, S.; Kunwar, S.; Tseng, T. Crop Diversification for Improved Weed Management: A Review. Agriculture 2021, 11, 461. [Google Scholar] [CrossRef]
- Ren, L.; Su, S.; Yang, X.; Xu, Y.; Huang, Q.; Shen, Q. Intercropping with Aerobic Rice Suppressed Fusarium Wilt in Watermelon. Soil Biol. Biochem. 2008, 40, 834–844. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Y.; Qi, L.; Mei, X.; Liao, J.; Ding, X.; Deng, W.; Fan, L.; He, X.; Vivanco, J.M.; et al. Plant-Plant-Microbe Mechanisms Involved in Soil-Borne Disease Suppression on a Maize and Pepper Intercropping System. PLoS ONE 2014, 9, e115052. [Google Scholar] [CrossRef]
- Ren, X.; Zhou, Z.; Liu, M.; Shen, Z.; Wang, B.; Jousset, A.; Geisen, S.; Ravanbakhsh, M.; Kowalchuk, G.A.; Li, R.; et al. Intercropping with Trifolium Repens Contributes Disease Suppression of Banana Fusarium Wilt by Reshaping Soil Protistan Communities. Agric. Ecosyst. Environ. 2024, 361, 108797. [Google Scholar] [CrossRef]
- Raza, S.M.J.; Akhter, A.; Wahid, F.; Hashem, A.; Abdallah, E.F. Rhizophagus Intraradices and Tomato-Basil Companionship Affect Root Morphology and Root Exudate Dynamics in Tomato under Fusarium Wilt Disease Stress. Appl. Ecol. Environ. Res. 2022, 20, 235–249. [Google Scholar] [CrossRef]
- Fierro-Coronado, R.; Castro-Moreno, M.; Ruelas-Ayala, R.; Apodaca-Sánchez, M.; Maldonado-Mendoza, I. Induced Protection by Rhizophagus Intraradices against Fusarium Wilt of Tomato. Interciencia 2013, 38, 48–53. [Google Scholar]
- Gómez-Rodrıguez, O.; Zavaleta-Mejıa, E.; González-Hernandez, V.; Livera-Muñoz, M.; Cárdenas-Soriano, E. Allelopathy and Microclimatic Modification of Intercropping with Marigold on Tomato Early Blight Disease Development. F. Crop. Res. 2003, 83, 27–34. [Google Scholar] [CrossRef]
- Yuan, S.; Li, M.; Fang, Z.; Liu, Y.; Shi, W.; Pan, B.; Wu, K.; Shi, J.; Shen, B.; Shen, Q. Biological Control of Tobacco Bacterial Wilt Using Trichoderma Harzianum Amended Bioorganic Fertilizer and the Arbuscular Mycorrhizal Fungi Glomus Mosseae. Biol. Control 2016, 92, 164–171. [Google Scholar] [CrossRef]
- Hage-Ahmed, K.; Krammer, J.; Steinkellner, S. The Intercropping Partner Affects Arbuscular Mycorrhizal Fungi and Fusarium oxysporum f. Sp. Lycopersici Interactions in Tomato. Mycorrhiza 2013, 23, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Kichu, L.T.; Prasad, V.M.; Das, K.S. Study of Intercropping in Tomato (Lycopersicon esculentum Mill.). Pharma Innov. J. 2022, 11, 2513–2515. [Google Scholar]
- Homulle, Z.; George, T.; Karley, A. Root Traits with Team Benefits: Understanding Belowground Interactions in Intercropping Systems. Plant Soil 2021, 471, 1–26. [Google Scholar] [CrossRef]
- Lv, J.; Dong, Y.; Dong, K.; Zhao, Q.; Yang, Z.; Chen, L. Intercropping with Wheat Suppressed Fusarium Wilt in Faba Bean and Modulated the Composition of Root Exudates. Plant Soil 2020, 448, 153–164. [Google Scholar] [CrossRef]
- Rahman, M.K.U.; Wang, X.; Gao, D.; Zhou, X.; Wu, F. Root Exudates Increase Phosphorus Availability in the Tomato/Potato Onion Intercropping System. Plant Soil 2021, 464, 45–62. [Google Scholar] [CrossRef]
- Zia, M.; Riaz, R.; Batool, A.; Yasmin, H.; Nosheen, A.; Naz, R.; Hassan, M. Glucanolytic Rhizobacteria Associated with Wheat-Maize Cropping System Suppress the Fusarium Wilt of Tomato (Lycopersicum esculentum L.). Sci. Hortic. 2021, 287, 110275. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, N.; Shen, Z.; Zhu, C.; Liu, H.; Xu, Z.; Li, R.; Shen, Q.; Salles, J.F. Soil Microbiome Manipulation Triggers Direct and Possible Indirect Suppression against Ralstonia Solanacearum and Fusarium Oxysporum. NPJ Biofilms Microbiomes 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Stefan, L.; Hartmann, M.; Engbersen, N.; Six, J.; Schöb, C. Positive Effects of Crop Diversity on Productivity Driven by Changes in Soil Microbial Composition. Front. Microbiol. 2021, 12, 660749. [Google Scholar] [CrossRef]
- Tian, X.L.; Wang, C.B.; Bao, X.G.; Wang, P.; Li, X.F.; Yang, S.C.; Ding, G.C.; Christie, P.; Li, L. Crop Diversity Facilitates Soil Aggregation in Relation to Soil Microbial Community Composition Driven by Intercropping. Plant Soil 2019, 436, 173–192. [Google Scholar] [CrossRef]
- Song, Y.N.; Zhang, F.S.; Marschner, P.; Fan, F.L.; Gao, H.M.; Bao, X.G.; Sun, J.H.; Li, L. Effect of Intercropping on Crop Yield and Chemical and Microbiological Properties in Rhizosphere of Wheat (Triticum aestivum L.), Maize (Zea mays L.), and Faba Bean (Vicia faba L.). Biol. Fertil. Soils 2007, 43, 565–574. [Google Scholar] [CrossRef]
- Laichao, S.; Zhanhai, N.; Shiliang, C.; Shilei, Z.; Ziyuan, Q.; Yu, W.; Xuewen, H.; Zhaotang, D.; Qingping, M. Effects of Pea-Tea Intercropping on Rhizosphere Soil Microbial Communities. Plant Soil 2023, 1–11. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, M.; Song, M.; Tian, J.; Song, B.; Hu, Y.; Zhang, J.; Yao, Y. Intercropping with Aromatic Plants Increased the Soil Organic Matter Content and Changed the Microbial Community in a Pear Orchard. Front. Microbiol. 2021, 12, 616932. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Richardson, A.E.; O’callaghan, M.; Deangelis, K.M.; Jones, E.E.; Stewart, A.; Firestone, M.K.; Condron, L.M. Effects of Selected Root Exudate Components on Soil Bacterial Communities. FEMS Microbiol. Ecol. 2011, 77, 600–610. [Google Scholar] [CrossRef]
- Hu, L.; Robert, C.A.M.; Cadot, S.; Zhang, X.; Ye, M.; Li, B.; Manzo, D.; Chervet, N.; Steinger, T.; Van Der Heijden, M.G.A.; et al. Root Exudate Metabolites Drive Plant-Soil Feedbacks on Growth and Defense by Shaping the Rhizosphere Microbiota. Nat. Commun. 2018, 9, 2738. [Google Scholar] [CrossRef] [PubMed]
- Sasse, J.; Martinoia, E.; Northen, T. Feed Your Friends: Do Plant Exudates Shape the Root Microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef]
- Wang, F.; Qiao, M.; Chen, Z.; Su, J.; Zhu, Y. Antibiotic Resistance Genes in Manure-Amended Soil and Vegetables at Harvest. J. Hazard. Mater. 2015, 299, 215–221. [Google Scholar] [CrossRef]
- Li, Y.; Deng, X.; Zhang, N.; Shen, Z.; Li, R.; Shen, Q.; Salles, J. Rhizosphere Suppression Hinders Antibiotic Resistance Gene (ARG) Spread under Bacterial Invasion. One Health 2023, 16, 100481. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gu, J.; Wang, X.; Song, Z.; Dai, X.; Guo, H.; Yu, J.; Zhao, W.; Lei, L. Enhanced Removal of Antibiotic Resistance Genes and Mobile Genetic Elements during Swine Manure Composting Inoculated with Mature Compost. J. Hazard. Mater. 2021, 411, 125135. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, R.; Cao, Y.; Tao, C.; Deng, X.; Ou, Y.; Liu, H.; Shen, Z.; Li, R.; Shen, Q. Soil Antibiotic Abatement Associates with the Manipulation of Soil Microbiome via Long-Term Fertilizer Application. J. Hazard. Mater. 2022, 439, 12970. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Fan, X.; Zhu, D.; An, X.; Su, J.; Cui, L. Effect of Biochar Amendment on the Alleviation of Antibiotic Resistance in Soil and Phyllosphere of Brassica chinensis L. Soil Biol. Biochem. 2018, 119, 74–82. [Google Scholar] [CrossRef]
- Han, X.; Hu, H.; Chen, Q.; Yang, L.; Li, H.; Zhu, Y.; Li, X.; Ma, Y. Antibiotic Resistance Genes and Associated Bacterial Communities in Agricultural Soils Amended with Different Sources of Animal Manures. Soil Biol. Biochem. 2018, 126, 91–102. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Ou, Y.; Ling, S.; Gao, M.; Deng, X.; Liu, H.; Li, R.; Shen, Z.; Shen, Q. Suppressing Ralstonia solanacearum and Bacterial Antibiotic Resistance Genes in Tomato Rhizosphere Soil through Companion Planting with Basil or Cilantro. Agronomy 2024, 14, 1129. https://doi.org/10.3390/agronomy14061129
Li T, Ou Y, Ling S, Gao M, Deng X, Liu H, Li R, Shen Z, Shen Q. Suppressing Ralstonia solanacearum and Bacterial Antibiotic Resistance Genes in Tomato Rhizosphere Soil through Companion Planting with Basil or Cilantro. Agronomy. 2024; 14(6):1129. https://doi.org/10.3390/agronomy14061129
Chicago/Turabian StyleLi, Tingting, Yannan Ou, Shuqin Ling, Ming Gao, Xuhui Deng, Hongjun Liu, Rong Li, Zongzhuan Shen, and Qirong Shen. 2024. "Suppressing Ralstonia solanacearum and Bacterial Antibiotic Resistance Genes in Tomato Rhizosphere Soil through Companion Planting with Basil or Cilantro" Agronomy 14, no. 6: 1129. https://doi.org/10.3390/agronomy14061129




